Abstract
The purpose of this study was to verify the efficacy and safety of high intensity focused ultrasound therapy (HIFU) combined with S-1 in the treatment of metastatic pancreatic cancer after failure of gemcitabine (GEM). In total, 120 patients with GEM-refractory PC who received HIFU and S-1 between Aug 2012 and December 2014 were randomly assigned to 2 groups. The patients in group A (n = 61) received HIFU in combination with S-1 and those in group B (n = 59) were given S-1 alone. S-1 was administered orally twice a day on days 1-14. Cycles were repeated every 21 days. The follow up time was 3~19 months in both groups. The median overall survival (OS) time and progression free survival (PFS) were analysed by Kaplan-Meier method and Logrank test. The pain remission rate of the two groups was compared by χ2 test. Patient characteristics and prognostic factors were compared. Patient characteristics did not significantly differ between the 2 groups. Median OS was significantly longer in group A (10.3 months) than in group B (6.6 months, P = 0.000). Median PFS was also significantly longer in group A than in group B (5.1 months vs 2.3 months, P = 0.000). Meanwhile, the pain remission rate was markedly higher in group A than in group B (57% vs. 20%, P = 0.000). There were mild side effects and no significant difference was observed between the two groups. The treatment effect was independently associated with a good outcome. HIFU in combination with S-1 might be effective and well tolerated as salvage chemotherapy in the treatment for metastatic pancreatic cancer.
Keywords: Pancreatic neoplasm, high intensity focused ultrasound therapy, S-1
Introduction
Most pancreatic cancer patients are diagnosed with advanced stage, which the 5 year survival rate is only 2% [1]. Gemcitabine (GEM) based systemic chemotherapy has been regarded as the standard method for advanced PC because Burris et al. [2] found its superior efficacy over 5-fluorouracil. However, after disease progression during GEM-based chemotherapy, further treatment methods are very limited. No standard salvage chemotherapy has been established.
In Japan, the clinical trials of S-1 in advanced PC have been reported [3]. Compared with the gemcitabine monotherapy, the efficacy of S-1 is about the same as that in the treatment of advanced PC [1]. In recent years, studies have identified that S-1 achieve favorable therapeutic effect in GEM resistant PC [4,5].
High-intensity focused ultrasound (HIFU) therapy, accumulating energy at a targeted area and inducing coagulation necrosis without harming surrounding tissue, has been used to treat different solid tumors. Recently clinical studies have confirmed that HIFU is a promising method for the treatment of pancreatic cancer [6]. In terms of HIFU treatment for pancreatic cancer, not only can local tumor control be achieved by ultrasound absorption, cancer pain can also be relieved, perhaps by destroying peripancreatic nerves and portions of the celiac plexus [7].
As noted above, treatment with S-1 or HIFU can provide good antitumor activity and tolerable side effects for pancreatic cancer patients. However, the efficacy and safety of the combined treatment for the treatment of metastatic pancreatic cancer after failure of gemcitabine are not well known. In this study, we reviewed the HIFU and S-1 combined treatment outcomes to find the impact of HIFU and S-1 on the prognosis of GEM-refractory metastatic PC.
Patients and methods
Patients
Patients with metastatic GEM-refractory PC received HIFU and S-1 treatment between Aug 2012 to December 2014 as second-line treatment at Xinhua Hospital Affiliated to Shanghai Jiaotong University and Fudan University Cancer Hospital. All patients had a pathological diagnosis of pancreatic ductal adenocarcinoma. The patients were divided into 2 groups. The patients in group A (n = 61) received HIFU in combination with S-1 and those in group B (n = 59) were given S-1 alone. We retrospectively collected their medical records. Each patient signed a written informed consent form, and this retrospective study was approved by the independent ethics committees/IRBs of Xinhua Hospital and Fudan University Cancer Hospital. The characteristics of the patients are shown in Table 1. The baseline prognostic characteristics were balanced between the two groups.
Table 1.
Clinicopathological characters of metastatic pancreatic cancer
| Group A | Group B | P value | |
|---|---|---|---|
| Gender (male/female) | 32/29 | 28/31 | 0.588 |
| Age (years) | 50.13±19.87 | 55.22±18.12 | 0.742 |
| Primary tumor location (head/other) | 31/30 | 32/27 | 0.711 |
| Previous Whipple procedure (yes/no) | 31/30 | 26/33 | 0.463 |
| Differentiation (well+moderate/poor) | 24/37 | 23/36 | 0.968 |
| Metastatic sites | |||
| Peritoneal lymph node | 30 | 27 | 0.711 |
| Liver | 37 | 39 | 0.540 |
| Lung | 27 | 32 | 0.278 |
| Number of Metastatic site | |||
| 1-3 | 34 | 30 | |
| > 3 | 27 | 29 | 0.595 |
| CEA (U/ml) | 8.61±7.16 | 9.12±8.33 | 0.482 |
| CA199 (U/ml) | 1215±997 | 1510±1120 | 0.347 |
| Performance status (median) | 1.0 | 2.0 | 0.472 |
| VAS (median) | 6.0 | 7.0 | 0.094 |
| S1 treatment (mean) | 4.35 | 3.88 | 0.554 |
VAS: visual analog scale.
Eligibility
The main inclusion criteria were: metastatic pancreatic cancer; disease progression under gemcitabine based chemotherapy; age ≥ 18 years; Eastern Cooperative Oncology Group (ECOG) performance status of 0-2. Main exclusion criteria were: severe complications, such as active concomitant malignancy, active infection, uncontrolled diabetes, massive pleural effusion or ascites, reduced haematological, hepatic, or renal functions, any disease that may increase the risk of adverse effects, or severe drug hypersensitivity; patients with central nervous system (CNS) metastases; Severe disability, ECOG score of more than 3.
Chemotherapy
S-1 was administered orally twice daily on day 1-14, then rest for a week. The dosage was determined according to the body surface area (BSA): BSA < 1.25 m2, 80 mg/day; 1.25 m2 ≤ BSA < 1.50 m2, 100 mg/day; and BSA ≥ 1.50 m2, 120 mg/day. The treatment course was repeated every 3 weeks until the disease progression or intolerance to toxicity. Each patient received 2-6 cycles of chemotherapy, the median treatment was 4 cycles.
HIFU
High intensity focused ultrasound treatment was performed using the JC HIFU system (Chongqing Haifu Tech Co. Ltd, Chongqing, China). All patients underwent a preliminary evaluation 2 weeks before HIFU treatment to further assess the treatment target and determine the targeted areas. Before the HIFU procedure, the skin in the treatment area was cleaned. The patients were fasted overnight. All patients signed written informed consent before treatment.
The patient was placed in a prone posture. The HIFU therapeutic energy is delivered in a pulsed mode. HIFU is palliative for those with advanced cancer, impeding tumor growth and improving the quality of life, so the principle of target selection is mainly the largest tumor lesions. Real-time ultrasound was used to comprehensively detect tumor targets. The target area was scanned by continuous high intensity focused ultrasound beam, and the targeted regions of tumor were completely ablated. Every patient in the study received only one session of HIFU treatment.
Evaluation of the efficacy and side effects of the treatment
Contrast-enhanced CT was used before and after treatment to assess the tumor response according to the RECIST version 1.1. Contrast-enhanced MRI was also used to evaluate the therapeutic effect of locoregional control of HIFU. OS was calculated from the start of treatment to the date of last follow-up or death, and PFS was calculated from the start of treatment to the date of disease progression or death. We also carried evaluation of pain relief rate according to previous reports [8]. Before and after every treatment cycle, the pain was evaluated with a visual analog scale (VAS). Complete remission of pain was defined as 0 pain score and no need for opioid analgesics), partial remission of pain was defined as decrease in pain score by 2 or more. We evaluated treatment-related toxicities according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Statistical analysis
SPSS 16.0 software package (SPSS Inc., Chicago, IL, USA) was used to analyze all the data. Objective responses were analysed with Pearson’s χ2 test. The median overall survival (OS) time and progression free survival (PFS) were analysed by Kaplan-Meier method and Logrank test. Multivariate logistic regression model was used to evaluate the prognostic variables. The results are presented as the median survival in months with the hazard risk (HR) with 95% confidence interval (CI). A p value less than 0.05 was considered to be statistically significant as indicated.
Results
Survival benefit
OS for the HIFU and S-1 combination therapy was 10.3 months compared with 6.6 months for S-1 monotherapy (HR 0.194, 95% CI, 0.112-0.336, P = 0.000) (Figure 1). PFS for the HIFU and S-1 combination therapy was 5.1 months compared with 2.3 months for S-1 monotherapy (HR 0.151, 95% CI, 0.083-0.273, P = 0.000) (Figure 2).
Figure 1.
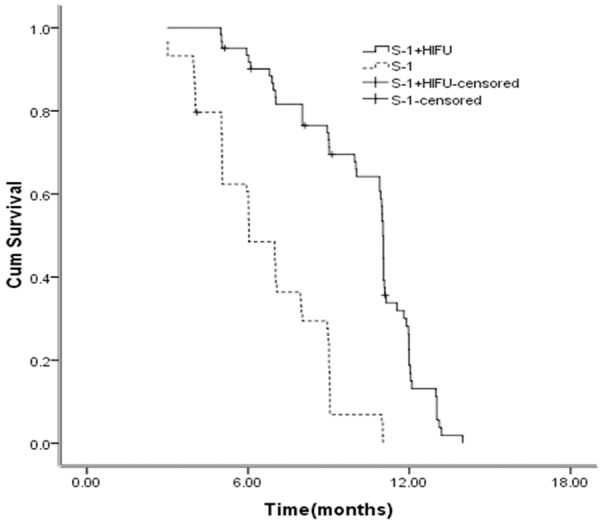
Kaplan-Meier curves for OS between the two groups.
Figure 2.
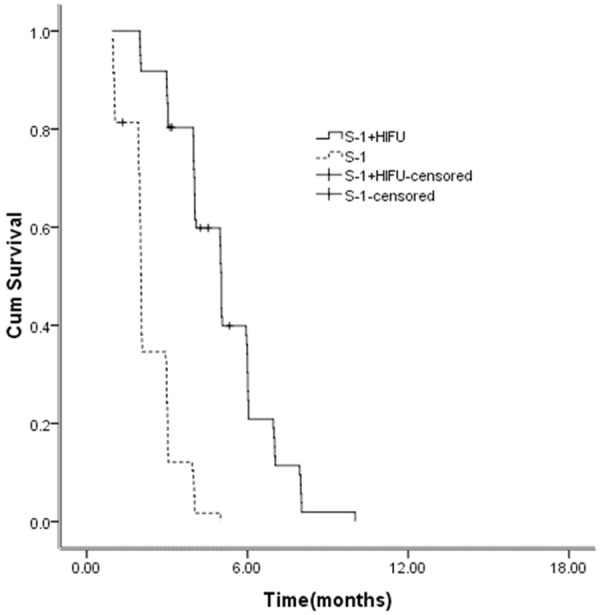
Kaplan-Meier curves for PFS between the two groups.
Efficacy
Of the 61 patients assigned to Group A, one patient had a complete response and 15 patients had partial responses, ie, 26.2% showed an objective response. The therapeutic effect of locoregional control of HIFU was also evaluated, one patient had a complete response and 8 patients had partial responses. Of the 59 patients assigned to Group B, five patients (8.5%) showed partial response and no patient showed complete response. There was significant difference between the two groups in the proportions of responders (P = 0.000).
Pancreatic cancer related abdominal pain was relieved obviously at 1 month after the HIFU procedure. The remission rate of pain was 57% and 20% respectively in Group A and Group B, the difference was significant (P = 0.000).
Side effects
The most common side effects in Group A were nausea, vomiting, anorexia, diarrhea; they were transient and quickly recovered in all patients. Slight skin burns were also noted in Group A. We didn’t record grade 3 or 4 adverse events in both groups (Table 2). There were mild side effects and no significant difference was observed between the two groups.
Table 2.
Side effects between the two groups
| Group A | Group B | P | |
|---|---|---|---|
| Anaemia | 15 | 13 | 0.743 |
| Neutropenia | 11 | 12 | 0.751 |
| Thrombocytopenia | 10 | 8 | 0.667 |
| Vomiting | 13 | 10 | 0.548 |
| Diarrhea | 8 | 9 | 0.739 |
| Hand foot syndrome | 7 | 6 | 0.820 |
| Neurotoxicity | 4 | 5 | 0.693 |
Prognostic factors
Median OS consistently favored Group A across most subgroups (Table 3). In a multivariate Cox model of OS, adjusting for the stratification factors, the treatment effect remained significant, with a similar magnitude of decease in the risk of death compared with the primary analysis (HR 0.193, 95% CI, 0.105-0.354, P = 0.000) (Table 4). As observed with OS, the median PFS by treatment group consistently favored Group A across most subgroups (Table 3). In the multivariate analyses of PFS, treatment effect remained significant after adjustment for stratification factors and favored Group A (HR 0.172, 95% CI, 0.093-0.319, P = 0.000) (Table 5).
Table 3.
Univaraite analysis of OS and PFS in pancreatic cancer
| Patient subgroups | N | OS | PFS | ||
|---|---|---|---|---|---|
|
| |||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Treatment (A/B) | 61/59 | 0.194 (0.112-0.336) | 0.000 | 0.151(0.083-0.273) | 0.000 |
| Age (< 65/≥ 65) | 55/65 | 0.512 (0.318-0.824) | 0.006 | 0.766 (0.489-1.144) | 0.249 |
| Sex (male/female) | 60/60 | 1.216 (0.772-1.916) | 0.399 | 1.255 (0.798-1.974) | 0.325 |
| PS (0-1/2) | 55/65 | 0.468 (0.290-0.756) | 0.002 | 0.728 (0.460-1.152) | 0.175 |
| Primary location (head/other) | 63/57 | 1.019 (0.650-1.599) | 0.933 | 1.007 (0.645-1.572) | 0.976 |
| Previous Whipple procedure (yes/no) | 57/63 | 0.446 (0.283-0.702) | 0.001 | 0.534 (0.341-0.835) | 0.006 |
| Differentiation (Well+moderately/poorly) | 47/73 | 0.408 (0.246-0.676) | 0.001 | 0.607 (0.372-0.990) | 0.045 |
| Presence of liver metastasis (yes/no) | 76/44 | 2.335 (1.475-3.695) | 0.000 | 1.856 (1.180-2.917) | 0.007 |
| Presence of lung metastasis (yes/no) | 59/61 | 1.513 (0.965-2.371) | 0.071 | 1.457 (0.930-2.282) | 0.101 |
| Peritoneal lymph node metastasis (yes/no) | 57/63 | 1.061 (0.676-1.666) | 0.798 | 1.094 (0.696-1.719) | 0.696 |
| Number of metastatic sites (1-3/> 3) | 64/56 | 0.573 (0.362-0.905) | 0.017 | 0.729 (0.465-1.144) | 0.169 |
Abbreviations: A/B: HIFU+S-1/S-1; PS: performance status.
Table 4.
Multivariate analysis of OS in pancreatic cancer
| Hazard Ratio | 95% CI | P Value | |
|---|---|---|---|
| Treatment (A/B) | 0.193 | 0.105-0.354 | 0.000 |
| Age (< 65/≥ 65) | 0.929 | 0.468-1.843 | 0.833 |
| PS (0-1/2) | 0.686 | 0.400-1.179 | 0.172 |
| Previous Whipple procedure (yes/no) | 0.662 | 0.400-1.096 | 0.109 |
| Liver metastasis (yes/no) | 1.249 | 0.728-2.145 | 0.419 |
| Lung metastasis (yes/no) | 0.917 | 0.547-1.536 | 0.741 |
| Number of metastatic sites (1-3/> 3) | 0.642 | 0.342-1.206 | 0.168 |
| Differentiation (well+moderately/poorly) | 0.452 | 0.226-0.904 | 0.025 |
Abbreviations: A/B: HIFU+S-1/S-1; PS: performance status.
Table 5.
Multivariate analysis of PFS in pancreatic cancer
| Hazard Ratio | 95% CI | P Value | |
|---|---|---|---|
| Treatment (A/B) | 0.172 | 0.093-0.319 | 0.000 |
| Previous Whipple procedure (yes/no) | 0.711 | 0.448-1.129 | 0.148 |
| Liver metastasis (yes/no) | 1.178 | 0.734-1.891 | 0.498 |
| Differentiation (well+moderately/poorly) | 0.691 | 0.419-1.140 | 0.148 |
Abbreviations: A/B: HIFU+S-1/S-1; PS: performance status.
Discussion
The prognosis of pancreatic cancer is very poor. Most ongoing clinical research was to investigate the more effective first-line protocols. Study results on second-line treatment in PC are rare [9]. In regard to treatment for GEM-refractory PC, oxaliplatin, fluorouracil, and folinic acid (OFF) indicated the benefit of second-line chemotherapy was significantly better than best supportive care (BSC) alone [10]. However, OFF has not been recognized as standard salvage chemotherapy in advanced PC patients for the patient number was small. Kasuga et al. [4] carried a retrospective analysis to evaluate the efficacy and safety of gemcitabine combined with S-1 in the treatment of pancreatic cancer after failure of first-line treatment. Results showed that GS might be effective and well tolerated as salvage chemotherapy in a practical setting.
S-1, a fluoropyrimidine derivative, was designed to improve the antitumor effect of 5-FU [11]. S-1 offered an alternative to traditional intravenous 5-FU. Several clinical trials of S-1 monotherapy have been conducted in the treatment of advanced pancreatic cancer [12-16]. The median OS and PFS time of S-1 monotherapy were 4.5-7.6 and 2.1-4.1 months respectively. There would be a need to evaluate its efficacy for patients following GEM failure in further RCTs.
The most obvious advantage of HIFU in the treatment of cancer is noninvasive. There are a lot of reports about HIFU in the treatment of advanced pancreatic cancer. Most of these reports come from China. It is recommended to use HIFU as monotherapy or combined with chemotherapy [17-22]. The objective response rate was 14.6%~74%. No obvious side effects were found in these reports. Ultrasound hyperthermia can reduce the dosage required and adverse effects of chemotherapy. Combined HIFU and chemotherapy can result in a better outcome, high pain relief and longer survival. Recent studies have also demonstrated that hyperthermia mediated doxorubicin release using HIFU improves intratumoural distributions of bioavailable drug and total drug levels [23]. However, randomized controlled studies have not rigorously designed to show that HIFU has a significant survival benefit in the treatment of advanced pancreatic cancer.
Previous studies of HIFU combined with gemcitabine based chemotherapy in the treatment of advanced pancreatic cancer, but HIFU combined with S-1 in the treatment of pancreatic carcinoma is rarely reported. In our reports, OS and PFS for the HIFU and S-1 combination therapy was 10.3 and 6.6 months respectively, which is significantly better than the S-1 monotherapy. The results of this analysis confirm and identify treatment with HIFU plus S-1 as independent predictors of survival.
Pain is an important factor seriously affectting the quality of life of patients with advanced PC. How to relieve the cancer pain is still a serious question. Most of the studies reported that pancreatic cancer patients’ pain was significantly relieved after HIFU treatment, although the mechanism is still unclear. In our study, 57% patients with varying degrees of pain relieved and their quality of life improved.
Zhao’s [8] study showed that the major grade 3 and 4 toxicities were haematological and gastrointestinal toxicities when gemcitabine was combined with HIFU therapy. In our study, the patients in group A only showed a mild skin burn at the time of treatment, serious complications such as bleeding, organ perforation and peritonitis didn’t appear. Regarding toxicity, grade 3-4 side effects weren’t found. The safety profile in this study suggests that HIFU and S-1 can be safely administered to patients with PC even as a salvage therapy.
It is important to point out the limitations of this retrospective study. This study included patients after failure of gemcitabine based chemotherapy. Considering the patients’ backgrounds were poor, we modified the S-1 regimen which was S-1 was administered orally twice daily on day 1-14, then rest for a week.
In conclusion, the combination of HIFU and S-1 in the treatment of advanced pancreatic cancer after failure of gemcitabine might be safe and effective. Nowadays the research of HIFU mainly focused on: (1) the safety monitoring during HIFU treatment; (2) the evaluation system after the treatment of HIFU such as MRI, CDFI, PET-CT and so on; (3) combining with thermal medium to enhance the therapeutic efficacy [24,25]. We need more clinical study of a larger sample size to further explore the application of HIFU combined with S-1 in the treatment of pancreatic cancer, and further optimize the dosage, duration time, treatment intervals.
Disclosure of conflict of interest
None.
References
- 1.Tschoep-Lechner KE, Milani V, Berger F, Dieterle N, Abdel-Rahman S, Salat C, Issels RD. Gemcitabine and cisplatin combined with regional hyperthermia as second-line treatment in patients with gemcitabine-refractory advanced pancreatic cancer. Int J Hyperthermia. 2013;29:8–16. doi: 10.3109/02656736.2012.740764. [DOI] [PubMed] [Google Scholar]
- 2.Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi K, Imaizumi T, Uchida K, Kuramochi H, Takasaki K. High response rates in patients with pancreatic cancer using the novel oral fluoropyrimidine S-1. Oncol Rep. 2002;9:1355–61. [PubMed] [Google Scholar]
- 4.Kasuga A, Okano N, Naruge D, Kitamura H, Takasu A, Nagashima F, Furuse J. Retrospective analysis of fixed dose rate infusion of gemcitabine and S-1 combination therapy (FGS) as salvage chemotherapy in patients with gemcitabine-refractory advanced pancreatic cancer: inflammation-based prognostic score predicts survival. Cancer Chemother Pharmacol. 2015;75:457–64. doi: 10.1007/s00280-014-2665-8. [DOI] [PubMed] [Google Scholar]
- 5.Kawashima H, Itoh A, Ohno E, Nakamura M, Miyahara R, Ohmiya N, Hara K, Kanamori A, Itoh T, Taki T, Hirai T, Hashimoto S, Takeda K, Goto H, Hirooka Y. Prospective multicenter study to investigate the introduction rate of second-line S-1 in gemcitabine-refractory unresectable pancreatic cancer. Cancer Chemother Pharmacol. 2011;68:677–83. doi: 10.1007/s00280-010-1531-6. [DOI] [PubMed] [Google Scholar]
- 6.Xiaoping L, Leizhen Z. Advances of high intensity focused ultrasound (HIFU) for pancreatic cancer. Int J Hyperthermia. 2013;29:678–82. doi: 10.3109/02656736.2013.837199. [DOI] [PubMed] [Google Scholar]
- 7.Sofuni A, Moriyasu F, Sano T, Itokawa F, Tsuchiya T, Kurihara T, Ishii K, Tsuji S, Ikeuchi N, Tanaka R, Umeda J, Tonozuka R, Honjo M, Mukai S, Fujita M, Itoi T. Safety trial of high-intensity focused ultrasound therapy for pancreatic cancer. World J Gastroenterol. 2014;20:9570–7. doi: 10.3748/wjg.v20.i28.9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao H, Yang G, Wang D, Yu X, Zhang Y, Zhu J, Ji Y, Zhong B, Zhao W, Yang Z, Aziz F. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs. 2010;21:447–52. doi: 10.1097/CAD.0b013e32833641a7. [DOI] [PubMed] [Google Scholar]
- 9.Ohkawa S, Okusaka T, Isayama H, Fukutomi A, Yamaguchi K, Ikeda M, Funakoshi A, Nagase M, Hamamoto Y, Nakamori S, Tsuchiya Y, Baba H, Ishii H, Omuro Y, Sho M, Matsumoto S, Yamada N, Yanagimoto H, Unno M, Ichikawa Y, Takahashi S, Watanabe G, Wakabayashi G, Egawa N, Tsuda M, Hosotani R, Hamada C, Hyodo I. Randomised phase II trial of S-1 plus oxaliplatin vs S-1 in patients with gemcitabine-refractory pancreatic cancer. Br J Cancer. 2015;112:1428–34. doi: 10.1038/bjc.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelzer U, Schwaner I, Stieler J, Adler M, Seraphin J, Dorken B, Riess H, Oettle H. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer. 2011;47:1676–81. doi: 10.1016/j.ejca.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Sudo K, Nakamura K, Yamaguchi T. S-1 in the treatment of pancreatic cancer. World J Gastroenterol. 2014;20:15110–8. doi: 10.3748/wjg.v20.i41.15110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge F, Xu N, Bai Y, Ba Y, Zhang Y, Li F, Riess H, Oettle H. S-1 as monotherapy or in combination with leucovorin as second-line treatment in gemcitabine-refractory advanced pancreatic cancer: a randomized, open-label, multicenter, phase II study. Oncologist. 2014;19:1133–4. doi: 10.1634/theoncologist.2014-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahara N, Isayama H, Nakai Y, Sasaki T, Ishigami H, Yamashita H, Yamaguchi H, Hamada T, Uchino R, Mizuno S, Miyabayashi K, Mohri D, Kawakubo K, Kogure H, Yamamoto N, Sasahira N, Hirano K, Ijichi H, Tateishi K, Tada M, Kitayama J, Watanabe T, Koike K. Intravenous and intraperitoneal paclitaxel with S-1 for refractory pancreatic cancer with malignant ascites: an interim analysis. J Gastrointest Cancer. 2014;45:307–11. doi: 10.1007/s12029-014-9603-1. [DOI] [PubMed] [Google Scholar]
- 14.Takahara N, Isayama H, Nakai Y, Sasaki T, Hamada T, Uchino R, Mizuno S, Miyabayashi K, Kogure H, Yamamoto N, Sasahira N, Hirano K, Ijichi H, Tateishi K, Tada M, Koike K. A retrospective study of S-1 and oxaliplatin combination chemotherapy in patients with refractory pancreatic cancer. Cancer Chemother Pharmacol. 2013;72:985–90. doi: 10.1007/s00280-013-2278-7. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Yun J, Kim HJ, Kim KH, Kim SH, Lee TH, Lee SC, Bae SB, Kim CK, Lee NS, Moon JH, Park SH, Lee KT, Park SK, Won JH, Park HS, Hong DS. Phase II study of palliative S-1 in combination with cisplatin as second-line chemotherapy for gemcitabine-refractory pancreatic cancer patients. Oncol Lett. 2012;3:1314–8. doi: 10.3892/ol.2012.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morizane C, Okusaka T, Ueno H, Kondo S, Ikeda M, Furuse J, Shinichi O, Nakachi K, Mitsunaga S, Kojima Y, Suzuki E, Ueno M, Yamaguchi T. Phase I/II study of gemcitabine as a fixed dose rate infusion and S-1 combination therapy (FGS) in gemcitabine-refractory pancreatic cancer patients. Cancer Chemother Pharmacol. 2012;69:957–64. doi: 10.1007/s00280-011-1786-6. [DOI] [PubMed] [Google Scholar]
- 17.Luo J, Ren X, Yu T. Efficacy of extracorporeal ultrasound-guided high intensity focused ultrasound: An evaluation based on controlled trials in China. Int J Radiat Biol. 2015;91:480–5. doi: 10.3109/09553002.2015.1021962. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y. High-intensity focused ultrasound treatment for advanced pancreatic cancer. Gastroenterol Res Pract. 2014;2014:205325. doi: 10.1155/2014/205325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao HF, Wang K, Meng ZQ, Chen Z, Lin JH, Zhou ZH, Wang P, Shi WD, Sheng YH. High intensity focused ultrasound treatment for patients with local advanced pancreatic cancer. Hepatogastroenterology. 2013;60:1906–10. doi: 10.5754/hge13498. [DOI] [PubMed] [Google Scholar]
- 20.Khokhlova TD, Hwang JH. HIFU for palliative treatment of pancreatic cancer. J Gastrointest Oncol. 2011;2:175–84. doi: 10.3978/j.issn.2078-6891.2011.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orgera G, Monfardini L, Della VP, Zhang L, Bonomo G, Arnone P, Padrenostro M, Orsi F. High-intensity focused ultrasound (HIFU) in patients with solid malignancies: evaluation of feasibility, local tumour response and clinical results. Radiol Med. 2011;116:734–48. doi: 10.1007/s11547-011-0634-4. [DOI] [PubMed] [Google Scholar]
- 22.Wang K, Chen Z, Meng Z, Lin J, Zhou Z, Wang P, Chen L, Liu L. Analgesic effect of high intensity focused ultrasound therapy for unresectable pancreatic cancer. Int J Hyperthermia. 2011;27:101–7. doi: 10.3109/02656736.2010.525588. [DOI] [PubMed] [Google Scholar]
- 23.Staruch RM, Hynynen K, Chopra R. Hyperthermia-mediated doxorubicin release from thermosensitive liposomes using MR-HIFU: therapeutic effect in rabbit Vx2 tumours. Int J Hyperthermia. 2015;31:118–33. doi: 10.3109/02656736.2014.992483. [DOI] [PubMed] [Google Scholar]
- 24.Li CC, Wang YQ, Li YP, Li XL. High-intensity focused ultrasound for treatment of pancreatic cancer: a systematic review. J Evid Based Med. 2014;7:270–81. doi: 10.1111/jebm.12128. [DOI] [PubMed] [Google Scholar]
- 25.Wu F. High intensity focused ultrasound: a noninvasive therapy for locally advanced pancreatic cancer. World J Gastroenterol. 2014;20:16480–8. doi: 10.3748/wjg.v20.i44.16480. [DOI] [PMC free article] [PubMed] [Google Scholar]


