Abstract
CPA4 belongs to a member of the metallocarboxypeptidase family, and its expression in pancreatic cancer samples and clinical significance are still not investigated until now. In this study, we aimed to evaluate the level of CPA4 in pancreatic cancer samples and study its clinical implications as a diagnostic marker for pancreatic cancer. The levels of CPA4 in pancreatic cancer tissues and serum samples were measured by immunohistochemistry (IHC) and enzyme-linked immunosorbent assay (ELISA), respectively. Among 150 pancreatic cancer tissues examined, 86.7% (130/150) of cases showed positive staining for CPA4. Clinicopathological relevance analysis showed that CPA4 expression was correlated with advanced clinical stage and lymph node metastasis. Also, we found that the levels of CPA4 in serum samples were significantly high in cases whose expression was also high in paired tissue samples (N=50). In a larger sample set, we found that serum CPA4 in pancreatic cancer patients was significantly higher than for healthy controls (P<0.05). In addition, high serum CPA4 was significantly associated with the TNM stage, Lymph node involvement and distant metastasis. At a cutoff value of 0.3 ng/ml, CPA4 might be a better diagnostic biomarker of pancreatic cancer than CA199. In conclusion, CPA4 overexpression is associated with pancreatic cancer progression, and it might be a potential diagnostic serum marker for pancreatic cancer.
Keywords: CPA4, pancreatic cancer, marker, immunohistochemistry, ELISA
Introduction
Pancreatic cancer (PC) is one of the most lethal malignancies with a 5-year survival rate of only 6% [1]. Because of the asymptomatic nature in early stage, aggressive disease course, and limitations of current detection technologies, fewer than 20% of pancreatic cancer patients are diagnosed with localized resectable disease. Early detection of pancreatic cancer can offer patients the best chances for survival and can increase five-year survival rates from ~5% to 20-30% [2]. Serum-based assays are the most used tests for the early detection of cancer. Although CA199 is the gold-standard serum biomarker for monitoring and evaluating prognosis of pancreatic cancer, it is also elevated in other benign conditions and multiple cancer types with limited sensitivity [3,4]. Consequently, the discovery of novel biomarkers involved in the diagnosis and progression of pancreatic cancer is of great value.
Carboxypeptidase A4 (CPA4) is a member of the metallocarboxypeptidase family [5].
CPA4 is a zinc-containing exopeptidase that catalyzes the release of carboxy-terminal amino acids. It was proved to be secreted from cells as a soluble proenzyme (pro-CPA4) that is activated by proteolytic cleavage [5]. CPA4 was originally found in a screen of mRNAs up-regulated by sodium butyrate-induced differentiation of cancer cells. There was a relation between CPA4 and prostate cancer aggressiveness [10]. However, the clinical significance of CPA4 expression in pancreatic cancer still remains unclear.
In this study, we firstly demonstrated that CPA4 level was significantly elevated in pancreatic cancer tissues as well as serum samples, and is closely associated with tumor progression. From a clinical standpoint, it might be a potential diagnostic serum marker for pancreatic cancer.
Materials and methods
Clinical samples
All tissue and blood specimens were collected from patients in the Department of Pathology in Cancer Hospital, Chinese Academy of Medical Sciences, Beijing, China. Patients did not receive any treatment prior to surgery, and signed informed consent forms for sample collection. All tissue samples were taken by experienced surgeons and examined independently by two experienced pathologists. For immunohistochemistry analysis, 150 paraffin-embedded pancreatic cancer tumors and paired adjacent normal pancreatic tissues were randomly obtained from patients during 2008-2012. For ELISA study, preoperative peripheral blood samples were obtained from 100 pancreatic cancer patients (median age at 53.6 with a range of 21 to 77 years) during 2010-2012. Total of 50 specimens of healthy individuals (median age at 47.1 with a range of 21 to 65 years) were donated on a voluntary basis. For all the specimens, clinicopathological information (age, gender, and pathology, differentiation, and TNM stage) was available. The study was approved by the medical ethics committee of Cancer Institute and Hospital, CAMS.
CPA4 immunostaining of formalin-fixed tumor tissues
Formalin-fixed paraffin-embedded normal tissues and tumor tissues were evaluated using standard immunohistochemical staining for CPA4 expression. Tissue microarrays were prepared from archival formalin-fixed, paraffin-embedded tissue blocks. For each tumor, a representative tumor area was carefully selected from a H&E-stain section. Sections 5 μm in thickness were obtained and mounted on positively charged slides for immunohistochemical analysis. Standard avidin-biotin complex peroxidase immunohistochemical staining was performed. Briefly, after deparaffinizationin xylene and graded alcohols, heated antigen retrieval was done in citrate buffer (10 mmol/L pH 6.0) by water-bath kettle heating for 30 min. Endogenous peroxidase was blocked in 0.3% hydrogen peroxide for 10 min. Nonspecific binding was blocked by incubation in 10% normal animal serum for 10 min. Sections were incubated at 4°C for 24 h with a primary antibody for CPA4 (HPA021030, Sigma-Aldrich, 1:300). Expression levels of proteins were scored by malignant/epithelial cells staining intensity and the percentage of immunoreactive cells. Tissues with no staining were rated as 0, with faint staining or moderate to strong staining in 20% of cells as 1, with moderate staining or strong staining in 20% to 40% of cells as 2, and with strong staining in >40% of cells as 3. Pancreatic cancer tissues that registered levels 0 and 1 were defined as negative for expression, whereas samples at levels 2 or 3 were defined as positive.
Measurement of human plasma levels of CPA4
A commercially available ELISA kit (SEF317Hu, Cloud-Clone Corp.) was used to measure CPA4 level. The sensitivities of the immunoassays for CPA4 were 0.156 ng/mL-10 ng/mL respectively. Each serum sample was run in duplicate. Briefly, 100 ml of serum (1:2 dilution) were placed into each well of the ELISA plate and incubated for 45 min at 37°C. The plates were washed four times with buffer and incubated with 100 ml of detection antibody at 37°C for 45 min. After four washes, the plates were incubated with substrate solution for 15 min at room temperature, then the reaction was stopped and the plates were read by a spectrophotometer at wavelength 450 nm. A standard curve was generated with the provided standards and used to calculate the quantity of CPA4 in each serum sample.
Statistical analysis
The SPSS 15 software package (SPSS, Inc., Chicago, IL) was used for statistical analysis. Mean between-group values were compared using the χ2-test. The independent sample t test was used for ELISA group analysis. The association between the markers and clinicopathologic features was analyzed using χ2-test or two-sided t-test as appropriate. Receiver operating characteristics (ROC) curves were generated to compare the predictive sensitivity and specificity, and the area under the curve (AUC). All comparisons were two-tailed, and p values of <0.05 were considered significant.
Results
CPA4 expression in pancreatic cancer tissues by immunohistochemistry
To determine the level of CPA4 in pancreatic cancer and analyze the clinical significance, we evaluated its expression in a TMA of pancreatic tissues containing 150 pancreatic cancer tissues and adjacent normal tissues. IHC scoring showed that 130 of 150 of the primary lesions exhibited positive staining for CPA4, but there was no detectable staining of CPA4 in the normal tissues. As a secreted protein, we also identified specific expression of CPA4 in interstitial tissues of pancreatic cancer (Figure 1). Statistical analysis indicated that CPA4 expression in pancreatic cancer was correlated with Depth of invasion, Lymph Node Metastasis and Stage (P<0.05). There was no significant association with other clinicopathologic variables (Table 1). Taken together, these observations demonstrated that over-expression of CPA4 is significantly associated with pancreatic cancer progression, which can be released into tumor surroundings.
Figure 1.
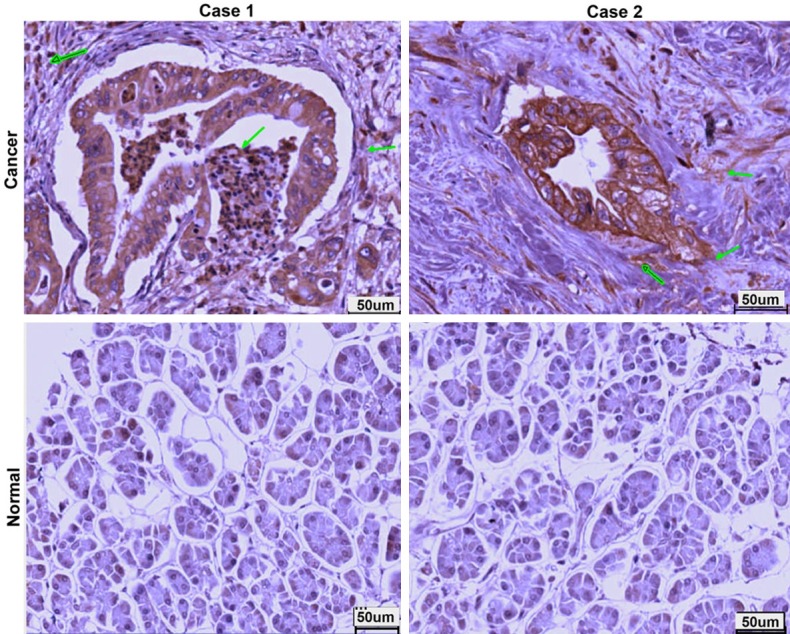
Expression of CPA4 in human primary pancreatic cancer tissues and normal adjacent tissues.
Table 1.
Correlation between CPA4 expression in pancreatic cancer tissues and clinicopathological parameters
| CPA4 | |||
|---|---|---|---|
|
|
|||
| Negative | Positive | p-value | |
| Sex (Male: Female) | 14:8 | 60:68 | 0.146 |
| Age | 59±4.8 | 60.2±9.2 | 0.567 |
| Depth of invasion | 0.006* | ||
| T1 | 4 | 4 | |
| T2 | 18 | 112 | |
| T3 | 0 | 12 | |
| T4 | 0 | 0 | |
| Lymph node involvement | 0.000* | ||
| N0 | 22 | 76 | |
| N1 | 0 | 48 | |
| N2 | 0 | 0 | |
| Liver metastasis | 0.228 | ||
| M0 | 22 | 120 | |
| M1 | 0 | 8 | |
| Stage | 0.000* | ||
| 1 | 22 | 60 | |
| 2 | 0 | 60 | |
| 3 | 0 | 0 | |
| 4 | 0 | 8 | |
| Grade | 0.367 | ||
| 1 | 0 | 6 | |
| 2 | 18 | 88 | |
| 3 | 4 | 34 | |
p-value is statistically significant.
Correlation between CPA4 level in serum and cancer tissues
To investigate the correlation, we harvested the 50 paired pancreatic cancer tissue and serum samples from Cancer Hospital, and evaluated the expression of CPA4. In pancreatic cancer tissues, the expression of CPA4 was high in 27 cancer tissues (27/50, 54%) by IHC. Similarly, 24 cancer serum sample (24/50, 48%) can be detected the existence of CPA4 by ELISA. Furthermore, correlation analysis using Spearman’s rho test revealed a significant correlation between CPA4 in tissue and serum samples (Spearman rho =0.887; P<0.01) (Table 2).
Table 2.
Association between CPA4 expression in tissue and in serum of pancreatic cancer patients
| CPA4 (tissue) | p-value | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| 0 | 1 | 2 | 3 | |||
| CPA4 (in serum) | 0 | 7 | 16 | 3 | 0 | 0.000* |
| 1 | 0 | 0 | 6 | 18 | ||
p-value is statistically significant.
Serum CPA4 in pancreatic cancer patients was significantly higher than that in healthy people
To evaluate the potential of CPA4 as serum pancreatic markers, we examined their levels in sera from pancreatic cancer patients (n=100) and healthy controls (n=50). The serum levels of CPA4 in patients with Pancreatic cancer were significantly greater than those of the healthy group (CPA4: 1.695±2.093 vs 0.123±0.251 ng/mL, P=0.000). ROC curve for serum CPA4 concentration was constructed to determine the cutoff values. The best cut-off value of CPA4 for distinguishing between pancreatic cancer patients and controls was 0.3 ng/ml, with a sensitivity and specificity of 61% and 90%, respectively (Figure 2A). The approximate area under the ROC curve assessing serum CPA4 as a diagnostic tool for the detection of pancreatic cancer against normal controls was 0.783.
Figure 2.
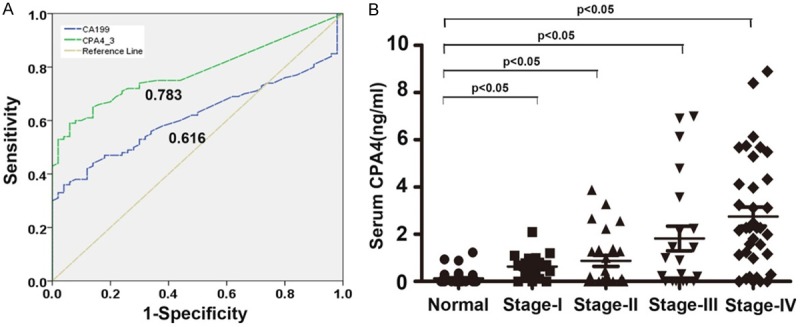
Elevated CPA4 levels in pancreatic cancer serum samples. A. ROC curve analysis of the diagnostic efficacy of CPA4 for pancreatic cancer. B. Serum levels of CPA4 in healthy controls and pancreatic cancer patients with different Stage.
Clinical significance and prognostic value of CPA4 as a serologic biomarker for pancreatic cancer
The clinical profile of pancreatic patients with a serum CPA4 level above the cut-off level (0.3 ng/ml) is shown in Table 3. Serum CPA4 levels were significantly positively correlated with TNM stage (P=0.036), lymph node involvement (P=0.016), and distant metastases (P=0.004). However, no significant correlation was observed between serum CPA4 level and other clinicopathologic parameters, including age (P=0.793), gender (P=0.213) and Depth of invasion (P=0.973). Further analysis showed that a gradual increase in serum levels of CPA4 at different stages of pancreatic cancer was observed (Figure 2B). Thus, the elevation of serum CPA4 levels appears to be closely associated with pancreatic cancer progression.
Table 3.
Correlation between CPA4 level in pancreatic cancer serum and clinicopathological parameters
| Serum CPA4 | |||
|---|---|---|---|
|
|
|||
| Negative | Positive | p value | |
| Sex (Male: Female) | 13:26 | 28:33 | 0.213 |
| Age | 50.85±11.4 | 55.44±12.4 | 0.793 |
| Stage | 0.036* | ||
| 1 | 9 | 9 | |
| 2 | 14 | 11 | |
| 3 | 10 | 11 | |
| 4 | 8 | 28 | |
| Depth of invasion | 0.973 | ||
| T1 | 13 | 19 | |
| T2 | 21 | 29 | |
| T3 | 7 | 11 | |
| T4 | |||
| Lymph node involvement | 0.016* | ||
| N0 | 26 | 23 | |
| N1 | 15 | 36 | |
| Distant Metastasis | 0.004* | ||
| M0 | 33 | 31 | |
| M1 | 8 | 28 | |
p-value is statistically significant.
Discussion
Pancreatic cancer is one of the most lethal human cancers with a very low survival rate of 5 years. Most pancreatic cancer patients can not be early diagnosed because of the latent onset and lack of good biomarkers [6]. If pancreatic cancer is localized without invading local structures or metastasis, the survival is better after surgical resection [7]. Therefore, it is necessary to find the biomarkers for early detection of pancreatic cancer to achieve the best clinical outcomes.
Previous studies showed that sodium butyrate up-regulated the mRNA level of CPA4 in androgen-independent prostate cancer cells, which is involved in the Histone Hyperacetylation signaling pathway [8]. Moreover, they demonstrated that CPA4 mRNA level was extremely low in normal prostate tissues. These findings implied that CPA4 may play important roles in tumor progression.
In pancreatic tissues, Carboxypeptidase family (CPA1, CPA2, and CPB) act in the degradation of dietary proteins in the digestive tract [5]. CPA4 is a zinc-containing exopeptidase that catalyzes the release of carboxy-terminal amino acids. Despite the potential importance of CPA4 toward many types of cancer, no previous studies have examined the level and clinical significance in pancreatic cancer. Thus, we applied the IHC and ELISA to evaluate the CPA4 level and study its clinical significance.
Firstly, we illustrated that CPA4 was highly expressed in pancreatic cancer tissues, and the positive rate was 86.7%. Further study indicated that CPA4 expression in pancreatic cancer was correlated with Lymph Node Metastasis and stage (P<0.05). There was no significant association with other clinicopathologic variables. Moreover, we also identified its expression in cancer interstitial tissues. The results indicated that CPA4 as a secreted protein might be released by cancer tissues and play important roles in cancer progression.
Serum biomarker tests have great potential to facilitate the early detection. Although CA199 has been used for pancreatic cancer prognosis monitoring, it is proved to be neither sensitive nor specific [9]. Therefore, we investigated the expression correlation in 50 pancreatic cancer tissue and serum paired samples. The result showed that the level of CPA4 in pancreatic cancer serum sample was significantly elevated in cases whose expression was also high. Secondly, we collected blood samples including 100 cancer patients and 50 healthy ones to detect the CPA4 serum level by ELISA. For the first time, we showed that serum levels of CPA4 in patients with pancreatic cancer were significantly greater than those of the healthy group. Moreover, we also found that serum levels of CPA4 were significantly positively correlated with TNM stage (P=0.036), lymph node involvement (P=0.016), and distant metastases (P=0.004). Further analysis showed that a gradual increase in serum levels of CPA4 at different stages of pancreatic cancer was observed. ROC curves demonstrated that the best cut-off value of CPA4 for distinguishing between pancreatic cancer patients and controls was 0.3 ng/mL, with a sensitivity and specificity of 61% and 90%, respectively. The approximate area under the ROC curve assessing serum CPA4 as a diagnostic tool for the detection of pancreatic cancer against normal controls was 0.748.
Conclusions
In summary, CPA4 level was significantly elevated in pancreatic cancer tissues and serum samples, and it might be a potential diagnostic serum marker for pancreatic cancer.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 81101625), and National High-tech R&D Program of China for Young Scholars (Grant No. 2014AA020537).
Disclosure of conflict of interest
None.
References
- 1.Pandol S, Gukovskaya A, Edderkoui M, Dawson D, Eibl G, Lugea A. Epidemiology, risk factors, and the promotion of pancreatic cancer: Role of the stellate cell. J Gastroenterol Hepatol. 2012;27:127–134. doi: 10.1111/j.1440-1746.2011.07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu F, Du F, Chen X. Multiple tumor marker protein chip detection system in diagnosis of pancreatic cancer. World J Surg Oncol. 2014;12:333. doi: 10.1186/1477-7819-12-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hata S, Sakamoto Y, Yamamoto Y, Nara S, Esaki M, Shimada K, Kosuge T. Prognostic Impact of Postoperative Serum CA 19-9 Levels in Patients with Resectable Pancreatic Cancer. Ann Surg Oncol. 2012;19:636–641. doi: 10.1245/s10434-011-2020-9. [DOI] [PubMed] [Google Scholar]
- 4.Hess V, Glimelius B, Grawe P, Dietrich D, Bodoky G, Ruhstaller T, Bajetta E, Saletti P, Figer A, Scheithauer W, Herrmann R. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol. 2008;9:132–138. doi: 10.1016/S1470-2045(08)70001-9. [DOI] [PubMed] [Google Scholar]
- 5.Tanco S, Zhang X, Morano C, Avilés FX, Lorenzo J, Fricker LD. Characterization of the Substrate Specificity of Human Carboxypeptidase A4 and Implications for a Role in Extracellular Peptide Processing. J Biol Chem. 2010;285:18385–18396. doi: 10.1074/jbc.M109.060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eguia V, Gonda TA, Saif MW. Early detection of pancreatic cancer. JOP. 2012;13:131–134. [PubMed] [Google Scholar]
- 7.Barugola G, Partelli S, Marcucci S, Sartori N, Capelli P, Bassi C, Pederzoli P, Falconi M. Resectable Pancreatic Cancer: Who Really Benefits From Resection? Ann Surg Oncol. 2009;16:3316–3322. doi: 10.1245/s10434-009-0670-7. [DOI] [PubMed] [Google Scholar]
- 8.Huang H, Reed CP, Zhang JS, Shridhar V, Wang L, Smith DI. Carboxypeptidase A3 (CPA3): A Novel Gene Highly Induced by Histone Deacetylase Inhibitors during Differentiation of Prostate Epithelial Cancer Cells. Cancer Res. 1999;59:2981–2988. [PubMed] [Google Scholar]
- 9.Chan A, Prassas I, Dimitromanolakis A, Brand RE, Serra S, Diamandis EP, Blasutig IM. Validation of Biomarkers That Complement CA19.9 in Detecting Early Pancreatic Cancer. Clin Cancer Res. 2014;20:5787–5795. doi: 10.1158/1078-0432.CCR-14-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kayashima T, Yamasaki K, Yamada T, Sakai H, Miwa N, Ohta T, Yoshiura K, Matsumoto N, Nakane Y, Kanetake H, Ishino F, Niikawa N, Kishino T. The novel imprinted carboxypeptidase A4 gene ( CPA4) in the 7q32 imprinting domain. Hum Genet. 2003;112:220–226. doi: 10.1007/s00439-002-0891-3. [DOI] [PubMed] [Google Scholar]


