Abstract
Lysosome-associated membrane protein 3 (LAMP3) was first identified as a cell surface marker of mature dendritic cells and specifically expressed in lung tissues. Recently studies demonstrated that LAMP3 plays a critical role in several cancers, and regulated by hypoxia. However, whether LAMP3 expressed in the heart and cardiomyocytes and changed its expression level in the hearts with cardiac remodelling was largely unknown. In this study, we first cultured H9C2 (a clonal muscle cell line from rat heart) and stimulated with 1 μM angiotensin II (Ang II), or 100 μM isoproterenol (ISO), or 100 μM phenylephrine (PE) for indicated times. We found that LAMP3 expression level was significantly increased after these stimulation. Next, the pressure overload-induced cardiac remodelling mouse model was performed in the wild type C57BL/6J mice. After 4 and 8 weeks of transverse aortic constriction (TAC), obvious cardiac remodelling was observed in the wild type mice compared with sham group. Importantly, LAMP3 expression level was gradually elevated from 2 weeks to 8 weeks after TAC surgery. Furthermore, in human dilated cardiomyopathy (DCM) hearts, severe cardiac remodelling was observed, as evidenced by remarkably increased cardiomyocytes cross sectional area and collagen deposition. Notably, the mRNA and protein level of LAMP3 were significantly increased in the DCM hearts compared with donor hearts. Immunohistochemistry assay showed that LAMP3 was expression in the cardiomyocytes and responsible for its increased expression in the hearts. Our data indicated that LAMP3 might have a potential role in the process of cardiac remodelling.
Keywords: LAMP3, cardiac remodelling, TAC surgery
Introduction
Heart failure, the end events of dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy and ischemic cardiomyopathy, is the leading cause of death all over the world [1,2]. Irreversible cardiac remodelling, initial a adaptive reponse to multiple physiological or pathological stimuli, is the primary cause resulting in heart failure. A vast array of signaling, transcriptional, metabolic, structural and functional events were changed during the process of cardiac remodelling [3-5]. It is difficult to improve the cardiac function of patient with heart failure because there is no good therapeutic targets to ameliorate pathological cardiac remodelling. Therefore, to further ascertain the mechanisms of cardiac remodelling is the pivotal approach to overcome heart failure.
Recent years, an increasing number of studies link autophagy with cardiac remodelling [6-9]. During the process of autophagy, autophagosome fuses with a lysosome to develop autolysosome and the contents are degraded and recycled. Therefore, the lysosome plays a critical role in the process of autophagy [10,11]. Lysosome-associated membrane protein 3 (LAMP3, also known as DC-LAMP, TSC-403 or CD208), predominantly in the lysosomal membrane, was first ascertained in 1998 independently by two research groups [12,13]. LAMP3 was originally isolated as a gene specifically expressed in lung tissues and also a cell surface marker of mature dendritic cells [12,13]. Recently, Pihlstrøm et al. identified that chromosomal locus MCCC1/LAMP3 was associated with increased risk for Parkinson’s disease [14]. More importantly, ample evidences demonstrated that LAMP3 is overexpressed in various human cancers, including cervix tumors [15], breast cancer [16], colon, esophagus, and ovary cancer [13]. As we known, hypoxia has been recognized as a hallmark of solid tumors, and LAMP3 was shown to be regulated by hypoxia via the unfolded protein response (UPR) in several kinds of tumor cells [15,16]. During cardiac hypertrophy, the cardiomyocytes were in a relative lack of oxygen (hypoxia) and nutrients status. However, whether LAMP3 expressed in the heart and cardiomyocytes has not yet investigated. Furthermore, whether the expression level of LAMP3 was altered in the heart with remodelling was also unknown. In current study, we found that LAMP3 is expressed in the heart and cardiomyocytes, and its mRNA and protein level are increased in the human DCM hearts and hypertrophic mouse hearts.
Materials and methods
Human heart samples
The remodelling human heart samples were collected from the left ventricles of patients with heart failure. The patients were having the heart transplantation because of irreversible heart failure. Control samples were obtained from the left ventricles of normal heart donors which were not suitable for transplantation for noncardiac reasons [17]. Informed consent was obtained from the families of prospective heart donors. All the procedures related to human samples were conformed to the principles outlined in the Declaration of Helsinki and were approved by the Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology Review Board in Wuhan, China.
Transverse aortic constriction in mice
In the present study, transverse aortic constriction (TAC) was conducted in mice for pressure overload-induced cardiac remodelling and heart failure. Aged from 10 to 12 weeks adult male mice are anesthetized in an induction chamber, and then placed in a supine position atop heating pad; rubber band is placed over the front teeth to extend the neck. After the fur from the neckline to mid chest level shaved, endotracheal intubation is performed using PE 90 tubing, and then connected to a MouseVent Automatic Ventilator (Kent Scientific). During the surgical procedure, anesthesia is maintained at 2% isoflurane with 0.6~0.8 L/min 100% O2. Thoracotomy is performed; the sternum retracted using a chest retractor, the thymus and fat tissue separated from the aortic arch. Between the right innominate and left carotid artery, a 6.0 silk suture and a 27 gauge blunt needle are placed, the first knot is fastly tied against the needle, followed by the second and the needle promptly removed which yielded a constriction of approximately 0.3 mm in the outer diameter. According to previous studies [18], this procedure imposed a 60~80% aortic constriction on the mice. As sham control mice, a sham operation without occlusion was performed on age-matched and weight-matched mice.
Cardiac function by transthoracic echocardiography in mice
For cardiac function measurements, a Vevo2100 High-Resolution Micro-Imaging System (VisualSonics, Toronto, ON, Canada) was used. Male mice were anesthetized and then placed in a supine position atop heating pad; body hair was removed with hair removal cream. Apply a layer of preheated ultrasound gel without air bubbles to the chest, primarily the area overlying the heart. Placed the probe onto the thorax, at the level of the papillary muscles, we firstly performed two-dimensional (2D) imaging (‘B-mode’) to obtain a view along the parasternal short axis, and then M-mode echocardiography was performed to obtain fine measurements of cardiac dimensions and contractility. Later, we could conduct evaluation of parameters of systolic left ventricular function including LVEDd (left ventricular end-diastolic diameter), LVESd (left ventricular end-systolic dimension), and %FS (fractional shortening).
Western blot
The Total protein were extracted from heart tissues or H9C2 cells by RIPA lysis buffer (900 ul RIPA, 20 ul PMSF, 10 ul protease and phosphatase inhibitor cocktail (Thermo fisher scientific, 78440), 10 ul EDTA solution (Thermofisher scientific, 78440), 50 ul NaF, 10 ul Na3VO4). The Pierce™ BCA Protein Assay Kit (Thermofisher scientific, 23225) was used to determine protein concertration. Twenty micrograms of denaturated protein were loaded and separated by SDS-PAGE, and then transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, IPVH00010). The membrane was incubated primary antibodies (GAPDH, Cat No. 5174, Cell signaling technology; LAMP3, Cat No. AP1827A, Abgent) overnight at 4°C after blocked with 5% non-fat milk for 90 min at room temperature. Next day, the membrane was incubated with the Peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, 111-035-003, at 1:25000 dilution) for 1 hour at room temperature. The ChemiDocTM Touch Imaging System (Bio-Rad) was used to detect the protein signals and then analyzed by Image lab software (version 5.2.1, Bio-Rad).
Real-time PCR
Total mRNA were extracted from human, mouse left ventricles and H9C2 cells by using TRI Reagent® Solution (AM9738, ThermoFisher Scientific). The precipitated mRNA was dissolved by nuclease-free water and the RNA concertration was determined by Nanodrop2000 (ThermoFisher Scientific). Then, 2 ug of total RNA was reverse transcripted into cDNA by using Transcriptor First Strand cDNA Synthesis Kit (4896866001, Roche). The selected gene mRNA levels were detected by CFX Connect™ Real-Time PCR Detection System (Bio-Rad) using iQ™SYBR® Green Supermix (1708884, Bio-Rad) and results were normalized against GAPDH gene expression. The primers were as follows (Table 2):
Table 2.
The primers’ list
| Primer Name | Foward Primer (5’--3’) | Reverse Primer (5’--3’) |
|---|---|---|
| Lamp3-human | GTGGCACCCGAAAATCCAAC | TGTCTGGAACATCACCACCG |
| ANP-human | CAGCAAGCAGTGGATTGCTCCT | TCTGCGTTGGACACGGCATTGT |
| BNP-human | TGGAAACGTCCGGGTTACAGGA | TCCGGTCCATCTTCCTCCCAAA |
| GAPDH-human | GAGTCAACGGATTTGGTCGT | TTGATTTTGGAGGGATCTCG |
| Lamp3-mouse | TGGAGCATATTTGACCATCTCA | CAAAGGCCTGAAGGTGGATA |
| ANP-mouse | ACCTGCTAGACCACCTGGAG | CCTTGGCTGTTATCTTCGGTACCGG |
| BNP-mouse | GAGGTCACTCCTATCCTCTGG | GCCATTTCCTCCGACTTTTCTC |
| GAPDH-mouse | ACTCCACTCACGGCAAATTC | TCTCCATGGTGGTGAAGACA |
| Lamp3-rat | CCCAGAGGCACTTCAACATT | TGAGATGGTCAAATAAGCTCCA |
| ANP-rat | AAAGCAAACTGAGGGCTCTGCTCG | TTCGGTACCGGAAGCTGTTGCA |
| BNP-rat | CAGCAGCTTCTGCATCGTGGAT | TTCCTTAATCTGTCGCCGCTGG |
| GAPDH-rat | GACATGCCGCCTGGAGAAAC | AGCCCAGGATGCCCTTTAGT |
Immunohistochemistry analysis
The immunohistochemistry analysis for LAMP3 was followed the standard protocol with minor changes. In brief, the paraffin sections were deparaffinized by xylene and then hydrated from 100% ethanol, 95% ethanol to dH2O. After that, the slides were put into a boil in 10 mM sodium citrate buffer (pH 6.0) and maintain at a sub-boiling temperature for 10 min. The slides were blocked with blocking buffer (5% normal goat serum (16210-064, ThermoFisher Scientific) in TBST) for 1 hour at room temperature after treated with 3% hydrogen peroxide for 10 min. The LAMP3 primary antibody (1:50 dilution) was added to the slides for incubating overnight at 4°C after removing blocking solution. The peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, 111-035-003, at 1:2500 dilution) was incubated for 1 hour at room temperature. The DAB kit was used to developing colour, and then counterstain sections with hematoxylin. The slices were mounted with mounting solution after dehydration.
Histological analysis
Hematoxylin-eosin (HE) for histopathology and myocyte cross sectional area analysis or picrosirius red (PSR) for collagen deposition performed as described before [4,17,19-21]. In brief, Hearts were fixed in 10% formalin after excised from anesthetized (pentobarbital sodium; 50 mg/kg, i.p.) mice. Then, the hearts were dehydrated and embedded in paraffin using standard histological procedures. Subsequently, these hearts were sectioned transversely or longitudinally at 5 μm. The slices were stained HE or PSR for analysis.
Cell culture and treatment
H9C2 cells were cultured in a plating medium consisted of DMEM basic medium (C11995500BT, Gibco) supplemented with 10% fetal bovine serum (10099-141, ThermoFisher Scientific), and 1% penicillin-streptomycin (15140-122, ThermoFisher Scientific). The cells were passaged when the cell density reached 80% of the culture dish. After starvation for 24 h, the cells of third passage were stimulated with 1 μM angiotensin II (Ang II, 17150, Cayman Chemical), or 100 μM isoproternol (ISO, 15592, Cayman Chemical), or 100 μM phenylephrine (PE, AB120761, Abcam) for 24 hours or 48 hours, respectively and were used for further experiments.
Statistical analysis
Values are showed as mean ± SD. For statistical comparisons, a two-tailed Student’s t-test was used to compare the means of two groups of samples, and one-way ANOVA followed by Bonferroni post hoc test was applied for multiple comparisons. A level of P<0.05 was considered statistical significance.
Results
The LAMP3 expression level was increased in the H9C2 cells treated with hypertrophic stimuli
Previously published literature showed that LAMP3 is expressed in the human mast cells [22], endothelial cells [23], and lung epithelial cells [24]. However, we still didn’t know whether LAMP3 is expressed in the heart, especially cardiomyocytes. To identify LAMP3 expression level in the cardiomyocytes, we first cultured H9C2 cells, a clonal muscle cell line from rat heart [25], and stimulated with PE (100 μM), ISO (100 μM), or Ang II (1 μM), respectively. The H9C2 cells were increased in its cell size, and the mRNA levels of hypertrophic markers atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) were elevated after treated with 100 μM of PE or ISO for 24 hours or 48 hours (Figure 1A-D). Similarly, upon 1 μM of Ang II stimulation for indicated times, the H9C2 cells surface area were enlarged, and the mRNA levels of ANP and BNP were profoundly augmented (Figure 1E and 1F).
Figure 1.

The phenotype of H9C2 after stimulated by PE, ISO, or Ang II for indicated times. (A, C and E) The morphology of H9C2 cells under optical microscope after treated with 100 μM of PE (A), 100 μM of ISO (C), or 1 μM of Ang II (E) for indicated times, scale bar, 60 μm (n=3). (B, D and F) Real-time PCR assay was performed to detect the relative mRNA levels of hypertrophic markers ANP and BNP after PE (B), ISO (D) or Ang II (F) stimulation (n=3). *p<0.05 vs DMSO 48 h or PBS 48 h.
Subsequently, we examed the LAMP3 expression level in the H9C2 cells after PE, ISO, or Ang II stimulation. The results showed that there was no significant changed in LAMP3 mRNA level in PE treated H9C2 cells both 24 hours and 48 hours treatment (Figure 2A), but the LAMP3 protein level was increased (Figure 2D). In the ISO induced H9C2 cells, the LAMP3 mRNA and protein level were increased after 24 hours treatment, and then decreased to normal levels compared with DMSO control group (Figure 2B and 2E). The mRNA and protein level of LAMP3 were significantly elevated in the H9C2 cells treated with 1 μM of Ang II for 48 hours compared with PBS control (Figure 2C and 2F).
Figure 2.

LAMP3 expression level was increased in PE, ISO, or Ang II treated H9C2 cells. (A-C) The relative mRNA level of LAMP3 in H9C2 cells under 100 μM of PE (A), 100 μM of ISO (B), or 1 μM of Ang II (C) treatment was assessed by real-time PCR (n=3). (D-F) The protein level of LAMP3 in PE (D), ISO (E), or Ang II (F) treated H9C2 cells was detected by western blot (n=3). *p<0.05 vs DMSO 48 h or PBS 48 h; n.s. indicated no significance.
The LAMP3 expression level was elevated in the pressure overload-induced hypertrophic mouse hearts
We next curious about that whether LAMP3 expression level was changed in the mouse heart after TAC treatment for indicated times. The wild-type (WT, C57BL/6 background) mice were subjected to TAC, and the results of anatomy showed that the heart weight (HW)/body weight (BW), lung weight (LW)/BW, and HW/tibia length (TL) ratios were gradually increased from 2 weeks to 8 weeks of TAC, which indicated that WT mice developed massive cardiac hypertrophy (Figure 3A-C). Consistent with this, echocardiographic results demonstrated that the WT mice exhibited decreased cardiac function, as evidenced by enlarged left ventricle end-diastolic dimension (LVEDd), left ventricle end-systolic dimension (LVESd), and decreased fractional shortening (FS%) (Figure 3D-F). Furthermore, as showed in the Figure 3G-I, the mouse gross heart, cardiomyocytes cross sectional area, left ventricle collagen deposition were gradually increased from 2 weeks to 8 weeks of TAC.
Figure 3.

The phenotype of pressure overload-induced cardiac remodelling in the wild type mice. (A-C) Statistical results for the heart weight (HW)/body weight (BW) (A), HW/tibia length (TL) (B), and lung weight (LW)/BW (C) ratios of wild type mice for sham, 2 weeks, 4 weeks, or 8 weeks of TAC (n=8 mice per experimental group). (D-F) Echocardiography was used to evluated the cardiac function of wild type mice (n=8 mice per experimental group). LVEDd indicates left ventricle end-diastolic dimension (D), LVESd indicates left ventricle end-systolic dimension (E), FS indicates fractional shortening (F). (G) The gross hearts (first panel, scale bar, 20 mm), hematoxylin and eosin (HE)-staining (second panel, scale bar, 50 μm), picrosirius red staining (third and forth panel, scale bar, 100 μm) of histological sections of the left ventricle in the indicated groups (n=4 mice per experimental group). (H) Quantification of cardiomyocytes cross sectional area in wild type mice after Sham or TAC surgery for indicated times (n=4 mice per experimental group). (I) Quantification of left ventricle total collagen deposition in wild type mice after Sham or TAC operation (n=4 mice per experimental group). *p<0.05 vs Sham; n.s. indicated no significance.
We next sought to examine whether the LAMP3 expression level changed in the hypertrophic mouse hearts. The real-time PCR results showed that pressure overload significantly promoted the LAMP3, ANP and BNP mRNA level elevation in the mouse hearts (Figure 4A). Importantly, compared with sham operation, the protein level of LAMP3 was increased in the hypertrophic hearts (Figure 4B) which indicated that LAMP3 might contribute to pressure overload induced cardiac remodelling.
Figure 4.

Pressure overload stimuli promoted LAMP3 expression in the heart of wild type mice. A. The mRNA level of LAMP3, ANP and BNP in the heart of wild type mice (n=4 mice per experimental group). B. The protein level of LAMP3 in the heart of wild type mice detected by using western blot (n=4 mice per experimental group). *p<0.05 vs Sham.
High LAMP3 expression was observed in the human DCM hearts
The aforementioned results showed that LAMP3 expression level was augment in H9C2 cells upon PE, ISO, or Ang II stimulation and in hypertrophic mouse hearts induced by pressure overload. To ascertain whether LAMP3 expressed in the human hearts and its expression level changed in the DCM human hearts, we collected normal donor hearts and DCM human hearts. As showed in the Table 1, the DCM patients were having severe cardiac dysfunction, as evidenced by poor ejection fraction and enlarged left ventricle diameter. HE and PSR staining demonstrated that the cardiomyocytes cross sectional area and collagen deposition were remarkably increased in the DCM hearts (Figure 5). Compared with normal counterparts, the mRNA levels of cardiac hypertrophic markers ANP and BNP were significantly elevated in the DCM hearts (Figure 6A), which indicated that the DCM hearts developed severe cardiac remodelling. More importantly, the mRNA level of LAMP3 concomitantly augmented with ANP and BNP (Figure 6A). The protein level of LAMP3 also remarkably increased in the DCM hearts, which was evidenced by western blot and immunohistochemistry using specific LAMP3 antibody (Figure 6B-D). As we known, the human hearts were consisting of several kinds of cells, such as cardiomyocytes, fibroblasts, endothelial cells and immune cells. Therefore, it is critical to uncover which cell type was contributed to increased LAMP3 expression level. Immunohistochemistry assay showed that relative lower LAMP3 expressed in the normal cardiomyocytes, and the LAMP3 was located in the cytoplasm (Figure 6D). In the DCM hearts, LAMP3 expression level significantly elevated in the cytoplasm of cardiomyocytes but not other cell types (Figure 6D). Taken together, these results indicate that LAMP3 might play a critical role in the pathological process of DCM.
Table 1.
The information of human DCM hearts and donor hearts
| Parameter | Donor Hearts | DCM Hearts |
|---|---|---|
| No. | 8 | 8 |
| Age, y | 33.9±9.9 | 45.8±12.0 |
| Sex, male/total (%) | 5/8 (62.5) | 6/8 (75) |
| BMI (kg/m2) | N/A | 22.7±1.0 |
| LVEDD (mm) | N/A | 76±13.1 |
| LVEF (%) | N/A | 27.3±6.7 |
| Heart rate, /min | N/A | 83±10 |
| Blood pressure, mmHg | ||
| Systolic | N/A | 111.1±13.3 |
| Diastolic | N/A | 68.4±9.7 |
BMI: body mass index; LVEDD: left ventricular end-diastolic dimension; LVEF: left ventricular ejection fraction; N/A: not available.
Figure 5.
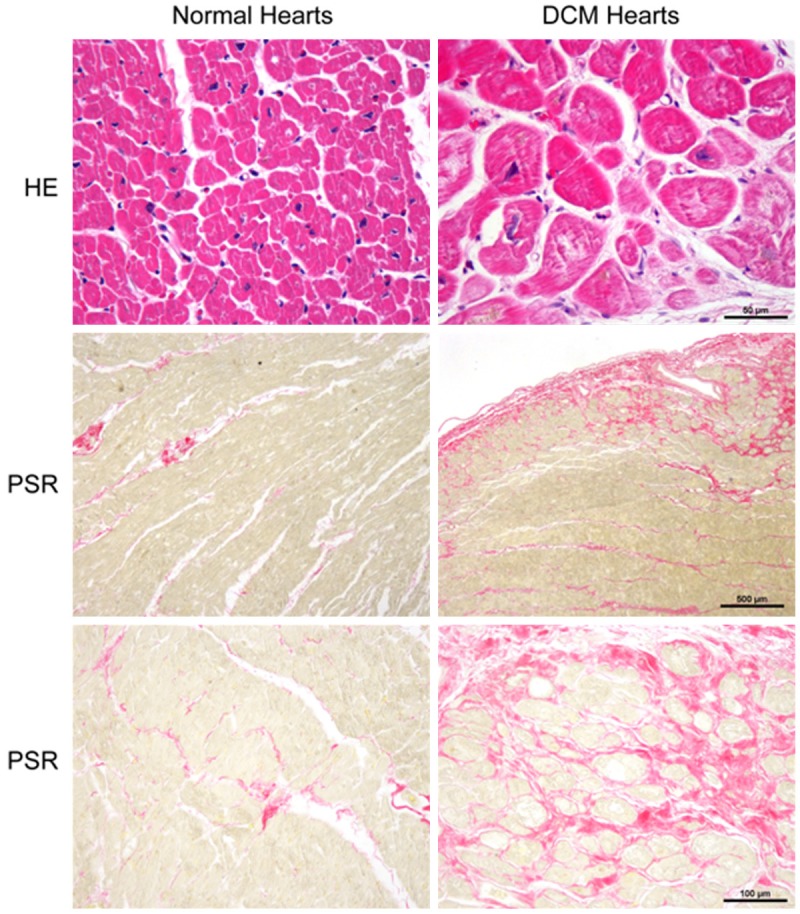
The human DCM hearts having severe cardiac remodelling. The hematoxylin and eosin (HE)-staining (first panel, scale bar, 50 μm) was performed to evaluate cardiomyocytes morphology and cell size, and picrosirius red (PSR) staining was used to measure the total collagen deposition (red staining) (second panel, scale bar, 500 μm; third panel, scale bar, 100 μm).
Figure 6.

The LAMP3 expression level was elevated in the human DCM heart. (A) The mRNA levels of LAMP3, ANP, and BNP in the hearts of normal donors and DCM patients (n=4). (B) The protein level of LAMP3 in the human donor and DCM hearts (n=8). (C) The statistical results of pannel (B). (D) The immunohistochemistry assay shows LAMP3 (brown) expression and localization in the cytoplasm of cardiomyocytes (n=4), first panel, scale bar, 100 μm; second and third panel, scale bar, 50 μm. *p<0.05 vs normal hearts.
Discussion
LAMP3 expressed in various cell types, including endothelial cells [23], lung epithelial cells [24], and mature dendritic cells [12]. Recent researches showed that LAMP3 was regulated by hypoxia and play an important role in the several types of cancer [15,16]. However, the expression pattern of LAMP3 in the hearts has remained unclear. In the present study, we utilized in vitro and in vivo studies to decipher LAMP3 expression pattern in the H9C2 cells, mouse hearts, and human hearts. Our findings demonstrated that compared with PBS or DMSO control, the LAMP3 protein level significantly increased in the H9C2 cells treated with PE, ISO, or Ang II for indicated times. More LAMP3 was expressed in the pressure overload-induced mouse hearts than Sham group. Importantly, we first found that LAMP3 was expressed in the human heart and cardiomyocyte, and its expression level was elevated in the human DCM hearts with severe cardiac remodelling. To our knowledge, this study is the first to identify LAMP3 as a potential regulator of cardiac remodelling.
Cardiac remodelling is occurred in response to chronic alterations in loading conditions. The most prominent features of cardiac remodelling are collagen deposition in the ventricle, cardiomyocytes hypertrophy and death [19]. Previously studies showed that cardiomyocytes died of a variety of mechanisms, such as necrosis, apoptosis, and autophagy, with autophagy reported as the most prominent in the failing human hearts [26]. The majority of studies demonstrated that autophagy activation in the heart is cardioprotective, while autophagy inhibition is related with cardiac injury and heart failure [27]. Nakai et al. showed that cardiac-specific Atg5 knockout early in cardiogenesis shows no pathological cardiac aberrant at baseline, but the knockout mice developed left ventricular dilatation and severe cardiac dysfunction 1 week after TAC operation [28]. Furthermore, inhibited autophagy was observed in the hearts of 6-, 14-, and 26-month-old Atg5 knockout mice which also showed shortened lifespan and aging-related cardiomyopathy [29]. In addition, Gsk3α knockout mice displayed suppressed autophagy and developed age related cardiac hypertrophy and contractile dysfunction [30]. Cathepsin-L deficiency accelerated TAC-induced ventricular remodeling and heart failure through impairing autophagosomal content degradation [31]. These results indicate that autophagy plays a protective role in pressure overload-induced cardiac hypertrophy. However, another several studies draw an opposite conclusion. For example, in TAC-induced pressure overload, autophagic activity was increased from 1 day till 3 weeks, and TAC-induced pathologic cardiac remodeling was ameliorated in beclin 1 haploinsufficient mice [32]. Conversely, cardiomyocyte-specific overexpression of beclin 1 aggravated autophagy and cardiac remodelling in response to TAC [32]. These studies indicated that TAC-induced cardiac autophagy is a maladaptive response.
As we known, in the process of autophagy, lysosome fuses with autophagosomes to degradation its contents [27]. Lysosome-associated membrane protein (LAMP) family proteins are thought to play the critical role in the fusion process of the autophagosome with the lysosome [33,34]. Tanaka at al. showed that accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice [33], which indicates LAMP proteins closely associated with cardiomyopathy. However, the role of LAMP3, one of the important LAMP family members, on cardiac remodelling remains unknown. In the current study, we found that LAMP3 expression level was increased in the H9C2 cell treated with ISO, PE or Ang II, and in TAC-induced hypertrophic mouse hearts, and in the human hearts with remodelling. Nagelkerke et al. showed that in breast cancer cells, LC3 (marker of autophagy) expression level was increased in response to LAMP3 knockdown [35]. This study indicates LAMP3 paly a negative role on autophagy occurrence. More recently, Kassiotis’s research group demonstrated that autophagy is inhibited in failing human hearts [36]. Therefore, according to our results and published literatures, we speculated that LAMP3 might affect cardiac remodelling through inhibiting autophagy. However, further gain-of-function and loss-of-function studies were needed to perform to clarify the critical role of LAMP3 in cardiac remodelling.
In this study, we found that LAMP3 expression level was elevated in the human hearts with heart failure. But the mechanism underlying increased LAMP3 in the hearts with remodelling is largely unclear. Previously published articles showed that LAMP3 mRNA and protein levels were strongly induced by hypoxia in several human tumor cell lines (e.g. HT-29, MCF7, DU 145, A549), and the activation of the PERK/eIF2a/ATF4 arm of the unfolded protein response (UPR) was response for increased LAMP3 expression level [15,37,38]. In human DCM hearts or hypertrophic mouse hearts, the cardiomyocytes were in a relative oxygen-deficient environment, which might one of the reasons for elevated LAMP3 expression in the human and mouse hearts with cardiac remodelling. However, further studies would be performed to ascertain the mechanism regulated LAMP3 expression in the hearts.
In conclusion, the present study provides in vitro and in vivo evidences that LAMP3, a member of LAMP family, increased its expression level in the H9C2 cells with PE, ISO, or Ang II treatment, in the mouse hearts treated with TAC surgery (pressure overload), and in the human DCM hearts. These observations indicated that LAMP3 may have the potential role in the cardiac remodelling and might be a novel therapeutic target for pathological cardiac remodelling.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (NO. 81370201), National key Scientific Instrument Special Program of China (No. 2013YQ030923-0607).
Disclosure of conflict of interest
None.
References
- 1.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Schiattarella GG, Hill JA. Inhibition of hypertrophy is a good therapeutic strategy in ventricular pressure overload. Circulation. 2015;131:1435–1447. doi: 10.1161/CIRCULATIONAHA.115.013894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang DS, Bian ZY, Zhang Y, Zhang SM, Liu Y, Zhang R, Chen Y, Yang Q, Zhang XD, Fan GC, Li H. Role of interferon regulatory factor 4 in the regulation of pathological cardiac hypertrophy. Hypertension. 2013;61:1193–1202. doi: 10.1161/HYPERTENSIONAHA.111.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang DS, Li L, Huang L, Gong J, Xia H, Liu X, Wan N, Wei X, Zhu X, Chen Y, Chen X, Zhang XD, Li H. Interferon regulatory factor 1 is required for cardiac remodeling in response to pressure overload. Hypertension. 2014;64:77–86. doi: 10.1161/HYPERTENSIONAHA.114.03229. [DOI] [PubMed] [Google Scholar]
- 6.Halapas A, Armakolas A, Koutsilieris M. Autophagy: a target for therapeutic interventions in myocardial pathophysiology. Expert Opin Ther Targets. 2008;12:1509–1522. doi: 10.1517/14728220802555554. [DOI] [PubMed] [Google Scholar]
- 7.Nemchenko A, Chiong M, Turer A, Lavandero S, Hill JA. Autophagy as a therapeutic target in cardiovascular disease. J Mol Cell Cardiol. 2011;51:584–593. doi: 10.1016/j.yjmcc.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurent AC, Bisserier M, Lucas A, Tortosa F, Roumieux M, De Regibus A, Swiader A, Sainte-Marie Y, Heymes C, Vindis C, Lezoualc’h F. Exchange protein directly activated by cAMP 1 promotes autophagy during cardiomyocyte hypertrophy. Cardiovasc Res. 2015;105:55–64. doi: 10.1093/cvr/cvu242. [DOI] [PubMed] [Google Scholar]
- 9.Gatica D, Chiong M, Lavandero S, Klionsky DJ. Molecular mechanisms of autophagy in the cardiovascular system. Circ Res. 2015;116:456–467. doi: 10.1161/CIRCRESAHA.114.303788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godar RJ, Ma X, Liu H, Murphy JT, Weinheimer CJ, Kovacs A, Crosby SD, Saftig P, Diwan A. Repetitive stimulation of autophagy-lysosome machinery by intermittent fasting preconditions the myocardium to ischemia-reperfusion injury. Autophagy. 2015;11:1537–1560. doi: 10.1080/15548627.2015.1063768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teng AC, Miyake T, Yokoe S, Zhang L, Rezende LM Jr, Sharma P, MacLennan DH, Liu PP, Gramolini AO. Metformin increases degradation of phospholamban via autophagy in cardiomyocytes. Proc Natl Acad Sci U S A. 2015;112:7165–7170. doi: 10.1073/pnas.1508815112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Saint-Vis B, Vincent J, Vandenabeele S, Vanbervliet B, Pin JJ, Ait-Yahia S, Patel S, Mattei MG, Banchereau J, Zurawski S, Davoust J, Caux C, Lebecque S. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9:325–336. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- 13.Ozaki K, Nagata M, Suzuki M, Fujiwara T, Ueda K, Miyoshi Y, Takahashi E, Nakamura Y. Isolation and characterization of a novel human lung-specific gene homologous to lysosomal membrane glycoproteins 1 and 2: significantly increased expression in cancers of various tissues. Cancer Res. 1998;58:3499–3503. [PubMed] [Google Scholar]
- 14.Pihlstrom L, Axelsson G, Bjornara KA, Dizdar N, Fardell C, Forsgren L, Holmberg B, Larsen JP, Linder J, Nissbrandt H, Tysnes OB, Ohman E, Dietrichs E, Toft M. Supportive evidence for 11 loci from genome-wide association studies in Parkinson’s disease. Neurobiol Aging. 2013;34:1708, e1707–1713. doi: 10.1016/j.neurobiolaging.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Mujcic H, Nagelkerke A, Rouschop KM, Chung S, Chaudary N, Span PN, Clarke B, Milosevic M, Sykes J, Hill RP, Koritzinsky M, Wouters BG. Hypoxic activation of the PERK/eIF2alpha arm of the unfolded protein response promotes metastasis through induction of LAMP3. Clin Cancer Res. 2013;19:6126–6137. doi: 10.1158/1078-0432.CCR-13-0526. [DOI] [PubMed] [Google Scholar]
- 16.Nagelkerke A, Mujcic H, Bussink J, Wouters BG, van Laarhoven HW, Sweep FC, Span PN. Hypoxic regulation and prognostic value of LAMP3 expression in breast cancer. Cancer. 2011;117:3670–3681. doi: 10.1002/cncr.25938. [DOI] [PubMed] [Google Scholar]
- 17.Jiang DS, Zhang XF, Gao L, Zong J, Zhou H, Liu Y, Zhang Y, Bian ZY, Zhu LH, Fan GC, Zhang XD, Li H. Signal regulatory protein-alpha protects against cardiac hypertrophy via the disruption of toll-like receptor 4 signaling. Hypertension. 2014;63:96–104. doi: 10.1161/HYPERTENSIONAHA.113.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yue X, Yang X, Lin X, Yang T, Yi X, Dai Y, Guo J, Li T, Shi J, Wei L, Fan GC, Chen C, Chang J. Rnd3 haploinsufficient mice are predisposed to hemodynamic stress and develop apoptotic cardiomyopathy with heart failure. Cell Death Dis. 2014;5:e1284. doi: 10.1038/cddis.2014.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang DS, Liu Y, Zhou H, Zhang Y, Zhang XD, Zhang XF, Chen K, Gao L, Peng J, Gong H, Chen Y, Yang Q, Liu PP, Fan GC, Zou Y, Li H. Interferon regulatory factor 7 functions as a novel negative regulator of pathological cardiac hypertrophy. Hypertension. 2014;63:713–722. doi: 10.1161/HYPERTENSIONAHA.113.02653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang DS, Luo YX, Zhang R, Zhang XD, Chen HZ, Zhang Y, Chen K, Zhang SM, Fan GC, Liu PP, Liu DP, Li H. Interferon regulatory factor 9 protects against cardiac hypertrophy by targeting myocardin. Hypertension. 2014;63:119–127. doi: 10.1161/HYPERTENSIONAHA.113.02083. [DOI] [PubMed] [Google Scholar]
- 21.Jiang DS, Wei X, Zhang XF, Liu Y, Zhang Y, Chen K, Gao L, Zhou H, Zhu XH, Liu PP, Bond Lau W, Ma X, Zou Y, Zhang XD, Fan GC, Li H. IRF8 suppresses pathological cardiac remodelling by inhibiting calcineurin signalling. Nat Commun. 2014;5:3303. doi: 10.1038/ncomms4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grutzkau A, Smorodchenko A, Lippert U, Kirchhof L, Artuc M, Henz BM. LAMP-1 and LAMP-2, but not LAMP-3, are reliable markers for activation-induced secretion of human mast cells. Cytometry A. 2004;61:62–68. doi: 10.1002/cyto.a.20068. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi T, Vischer UM, Rosnoblet C, Lebrand C, Lindsay M, Parton RG, Kruithof EK, Gruenberg J. The tetraspanin CD63/lamp3 cycles between endocytic and secretory compartments in human endothelial cells. Mol Biol Cell. 2000;11:1829–1843. doi: 10.1091/mbc.11.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weichert N, Kaltenborn E, Hector A, Woischnik M, Schams A, Holzinger A, Kern S, Griese M. Some ABCA3 mutations elevate ER stress and initiate apoptosis of lung epithelial cells. Respir Res. 2011;12:4. doi: 10.1186/1465-9921-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimes BW, Brandt BL. Properties of a clonal muscle cell line from rat heart. Exp Cell Res. 1976;98:367–381. doi: 10.1016/0014-4827(76)90447-x. [DOI] [PubMed] [Google Scholar]
- 26.Lavandero S, Chiong M, Rothermel BA, Hill JA. Autophagy in cardiovascular biology. J Clin Invest. 2015;125:55–64. doi: 10.1172/JCI73943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orogo AM, Gustafsson AB. Therapeutic targeting of autophagy: potential and concerns in treating cardiovascular disease. Circ Res. 2015;116:489–503. doi: 10.1161/CIRCRESAHA.116.303791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 29.Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, Oka T, Tamai T, Oyabu J, Murakawa T, Nishida K, Shimizu T, Hori M, Komuro I, Takuji Shirasawa TS, Mizushima N, Otsu K. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6:600–606. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 30.Zhou J, Freeman TA, Ahmad F, Shang X, Mangano E, Gao E, Farber J, Wang Y, Ma XL, Woodgett J, Vagnozzi RJ, Lal H, Force T. GSK-3alpha is a central regulator of age-related pathologies in mice. J Clin Invest. 2013;123:1821–1832. doi: 10.1172/JCI64398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun M, Ouzounian M, de Couto G, Chen M, Yan R, Fukuoka M, Li G, Moon M, Liu Y, Gramolini A, Wells GJ, Liu PP. Cathepsin-L ameliorates cardiac hypertrophy through activation of the autophagy-lysosomal dependent protein processing pathways. J Am Heart Assoc. 2013;2:e000191. doi: 10.1161/JAHA.113.000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lullmann-Rauch R, Janssen PM, Blanz J, von Figura K, Saftig P. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature. 2000;406:902–906. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- 34.Eskelinen EL, Illert AL, Tanaka Y, Schwarzmann G, Blanz J, Von Figura K, Saftig P. Role of LAMP-2 in lysosome biogenesis and autophagy. Mol Biol Cell. 2002;13:3355–3368. doi: 10.1091/mbc.E02-02-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagelkerke A, Sieuwerts AM, Bussink J, Sweep FC, Look MP, Foekens JA, Martens JW, Span PN. LAMP3 is involved in tamoxifen resistance in breast cancer cells through the modulation of autophagy. Endocr Relat Cancer. 2014;21:101–112. doi: 10.1530/ERC-13-0183. [DOI] [PubMed] [Google Scholar]
- 36.Kassiotis C, Ballal K, Wellnitz K, Vela D, Gong M, Salazar R, Frazier OH, Taegtmeyer H. Markers of autophagy are downregulated in failing human heart after mechanical unloading. Circulation. 2009;120:S191–197. doi: 10.1161/CIRCULATIONAHA.108.842252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagelkerke A, Bussink J, Mujcic H, Wouters BG, Lehmann S, Sweep FC, Span PN. Hypoxia stimulates migration of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded protein response. Breast Cancer Res. 2013;15:R2. doi: 10.1186/bcr3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mujcic H, Rzymski T, Rouschop KM, Koritzinsky M, Milani M, Harris AL, Wouters BG. Hypoxic activation of the unfolded protein response (UPR) induces expression of the metastasis-associated gene LAMP3. Radiother Oncol. 2009;92:450–459. doi: 10.1016/j.radonc.2009.08.017. [DOI] [PubMed] [Google Scholar]


