Abstract
The prevention of doxorubicin (Dox) induced cardiotoxicity may be co-operative to recover future Dox treatment. The aim of this study was to explore the cardioprotective effects of oleanolic acid (OA), an antioxidant agent, on Dox induced cardiotoxicity. OA is a triterpenoid compound, which exist widely in plant kingdom in free acid form or as a glycosidic triterpenoids saponins. Cardiotoxicity was induced in Wistar rats with single intravenous injection of doxorubicin at dose of 67.75 mg/kg i.v for 48 hrs. At 12 hrs of interval following Dox administration the cardioprotective effect of OA (1.5 mg/kg, i.v.) and Amifostine (AMF) (90 mg/kg i.v., single dose prior 30 min) were evaluated. Induction of cardiotoxicity was confirmed by increase in systolic, diastolic, mean arterial pressures, maximal positive rate of developed left ventricular pressure (+LVdP/dtmax, an indicator of myocardial contraction), maximal negative rate of developed left ventricular pressure (-LVdP/dtmax, a meter of myocardial relaxation) and an increase in left ventricular end-diastolic pressure (LVEDP, a marker of pre-load). Cardiac markers in such as CK-MB, LDH and alterations in ECG. Dox administration showed alteration in Biochemical parameters and endogenous antioxidants. Administration of OA Showed maximal protection against Dox induced cardiac toxicity as observed by reduction in blood pressure, prevention of left ventricular function and attenuation of biochemical and antioxidant parameters. Based on the findings, its concluded that OA can be used as an adjuvant with Dox therapy in treating cancers.
Keywords: Doxorubicin, oleanolic acid, antioxidant, cardiotoxicity, lipid peroxidation
Introduction
Doxorubicin (Dox) is an effective anthracycline chemotherapeutic mediator clinically used for haematological malignancy and variety of solid tumour [1-3]. The therapeutic utility is narrow Since it causes serious dose limiting cardiotoxicity, which leads to congestive heart failure [4]. The molecular mechanisms in Dox-induced cardiotoxicity include the formation of free radicals, Ca2+ overloading, increased lipid peroxidation and activation of apoptotic factor [5,6]. It is apparent that oxidative stress augmentation is a key aspect in development of myocardial infarction and also in other cardiovascular diseases [7]. Dox has been shown to enhance the activity of xanthine oxidase, which is considered as one of the mechanisms responsible for the generation of free radicals [8,9]. Recently, increasing evidence suggested that Dox can be catalysed into a secondary alcohol metabolites, doxorubicinol through two-electron carbonyl reduction [10]. Dox also changes the blood pressure and heart rate as well loss of contractility of myocardium [11]. Oxidative damage to membrane lipids and other cellular components is believed to be a major factor in Dox toxicity [12]. Dox causes myocardial damage via the formation of mitochondrial reactive oxygen species (ROS), with resulting mitochondrial injury and apoptotic cell death [13,14]. In addition, the ROS-dependent c-Jun N-terminal kinase (JNK) contributes to Dox-induced apoptosis [11,15-18]. Free oxygen radicals are efficiently detoxified by antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase in normal healthy conditions. However, when there is an imbalance between the production of oxidants and the respective defence systems of the organism, the induction of lipid peroxidation and alteration in intracellular homeostasis occurred, as seen in Dox induced cardiotoxicity [19]. By understanding the free radical mechanism of anthracycline cardiotoxicity, it has become possible to develop nominal strategies to prevent or modify anticipated damage. Overexpression of the naturally occurring antioxidant proteins, catalase and superoxide dismutase, is an interesting approach in circumventing Dox-induced cardiotoxicity.
Oleanolic acid (OA) is a triterpenoids compound that exists widely in food and herbs [20]. It has a variety of biological effects such, as antioxidants [21], antifungal, anti-inflammatory, anti-hyperlipidaemia, hepatoprotective, tumour prevention, immunomodulatory [22,23], anti-HIV [24], anti-arrhythmic and cardiotonic [25]. It will provide an accessible and cheap traditional medicine source for treatment of myocardial ischemia and Dox induced cardiotoxicity. Oleuropein shows prevention against cardiac remodelling after myocardial infarction in Wistar rat through inhibiting angiotensin-converting enzyme activity [26]. OA modulates the immune-inflammatory response in mice with experimental autoimmune myocarditis and protects from cardiac injury Martin, et al. [27]. Therefore, the unprejudiced of study was to evaluate the effect of OA in an animal model of Dox-induced cardiomyopathy and compare it with amifostine (AMF) a well-known organ protective agent.
Materials and methods
Animals
Wistar rats (150-200 g) were obtained from the central animal house facility of R. C. Patel Institute of Pharmaceutical Education and Research, Shirpur, India. The study protocol was reviewed and approved by the Institutional Animal Ethics Committee (Protocol approval no. 651/2015/CPCSEA). Animals were kept in the departmental animal house under controlled conditions of temperature at 25±2°C, relative humidity of 60±5% and light-dark cycle of 12:12 h. They were fed with standard food pellets (India) and water ad libitum. Animals were maintained in polypropylene cages, each containing a maximum of four animals.
Drugs and chemicals
Doxorubicin lyophilized powder (Biochem, India), sterile saline, oleanolic acid (Sigma Aldrich, USA), CK-MB, LDH, alkaline phosphatase and albumin kits (Span diagnostics, Mumbai, India), SGOT and SGPT kits (ERBA diagnostics, Germany) were purchased. All other chemicals were of analytical grade.
Experimental protocol for Dox induced myocardial toxicity in rats
Doxorubicin 67.75 mg/kg, prepared in 0.9% normal saline solution was injected i.v. in rats after 48 hrs of interval to induce experimental myocardial infarction [28].
Experimental group
Twenty four rats were randomized and allotted to four treatment groups (n=6 per group) as follows:
Group 1 (Vehicle treated): Received vehicle (2.5 ml/kg i.v.); Group 2 (Dox treated group): Received Dox (67.75 mg/kg i.v.); Group 3 (OA + Dox): OA (1.5 mg/kg i.v., 12 hrs. interval up to 48 h) + Dox (67.75 mg/kg i.v.); Groups 4 STD (AMF): AMF (90 mg/kg i.v.) + Dox (67.75 mg/kg i.v.).
Pharmacological evaluation
Surgical procedures for recording hemodynamic parameters: ECG Monitoring
Animals were placed in supine position on a board and ECG was continuously recorded with standard 3 lead skin electrodes, with 2 electrodes towards the heart on right and left forelimbs and the neutral 3rd electrode on the hind limb facing the heart. The electrodes connected to the data acquisition system power lab (AD Instruments, Australia) and ECG were recorded.
Estimation of biochemical parameters
Myocardial tissue homogenate was prepared and an aliquot was used to estimate the content of malondialdehyde (MDA) [29] and reduced glutathione (GSH) [30]. lactate dehydrogenase (LDH) [31], catalase [32], superoxide dismutase (SOD) [33], protein content [34], creatinine kinase (CKMB) [35]. SGOT and SGPT was estimated using commercial kit (ERBA diagnostics, Germany), alkaline phosphatase and Albumin was estimated using commercial kit (Span diagnostic Ltd. India).
Histopathological examination
Hematoxylin and eosin staining was performed and the sections were examined under the light microscope and Photographs were taken. A minimum of 10 fields per slide were examined and graded for severity of changes using scores on a scale of severe (+++), moderate (++), mild (+) and nil (-). The pathologist performing histopathological evaluation was masked to the treatment assigned to different study groups.
Statistical analysis
The data is offered as mean ± SEM, One-way ANOVA followed by Dunnet Post-hoc test using a graph pad, prism software, version 5.0, USA was applied for significance of hemodynamic and biochemical data of different groups. The value of P<0.05 was considered as a significant.
Results
Effect of OA on mortality, body weight and heart weight variations
The 5% mortality was observed throughout the study protocol. The lashing of animals was due to bleeding or unseemly carotid artery cannulation whilst performing the surgery. No significant change in body weights was observed in unrelated untried clusters as compared to their normal values. Heart weight was significantly decrease in the Dox treated group as compared with control group (0.5379±0.29 gm; P<0.001) and it was significantly attenuated by OA and AMF as compared with the Dox group (0.6590±0.63 gm and 0.7176±0.29 gm respectively; P<0.001) (Table 1).
Table 1.
Effect of OA on body weight and heart weight in doxorubicin induced cardiac toxicity
| Groups | Body weight (gm) | Heart weight (gm) |
|---|---|---|
| Normal | 182.7±14.38 | 0.66±0.04 |
| Dox control | 169.2±7.99 | 0.53±0.029# |
| OA + Dox | 181.3±9.97 | 0.65±0.063* |
| AMF + Dox | 178.5±10.78 | 0.71±0.029** |
Data was analysed by one way analysis of variance (ANOVA) followed by Dunnett’s post hoc test.
P<0.05 as compared to normal;
P<0.05 as compared to Dox, Dox: Doxorubicin;
P<0.01 as compared to Dox, Dox: Doxorubicin;
OA: Oleanolic acid; AMF: Amifostine.
Effect of OA on hemodynamic and ECG parameter
Figure 1 exhibits the effect of OA on arterial pressure. Dox treated rats showed a significant increase insystolic arterial pressure (SAP), diastolic arterial pressure (DAP) and mean arterial pressure (MAP) (P>0.001) as compared with the vehicle treated group. While, Dox does not altered heart rate as compared to normal rats. Fourdays treatment with the OA at the dose of 1.5 mg/kg normalized systolic, diastolic as well as mean arterial pressure (P>0.001) as compared to vehicle treated group. OA does not have any effect on heart rate, and which is found to be normal. Figure 1D displays the harmful effect of Doxon left ventricular end diastolic pressure (LVEDP) and their restoration near to the normal values by treatment with OA. Doxdealing occasioned in ventricular dysfunction as indicated by augmented in the LVEDP (P>0.001) as associated to vehicle treated group, which was expressively lessened through entire treatment groups (P>0.001) as linked to Dox treated group. Figure 1E and 1F showed the consequence of Dox scheduled the peak positive pressure ([+] LVdp/dt) and negative pressure ([-] LVdp/dt) of left ventricle. Administration of Dox in rats presented noteworthy diminished (P>0.001) in the level of (+) LVdp/dt and (-) LVdp/dt as related to vehicle treated group, while dealing with the OA and AMF exposed momentous (P>0.001) reduction of peak pressures as compared to the Dox administered rats. ECG ticker tapes and waveform were found to be anomalous in Dox treated rats as linked to normal rats. In Dox treated rats exhibited decrease in ST height and increase in the QT interval as shown in Figure 2. The treatment with OA and AMF knowingly attenuated the ECG patterntoo the ST height and QT interval.
Figure 1.
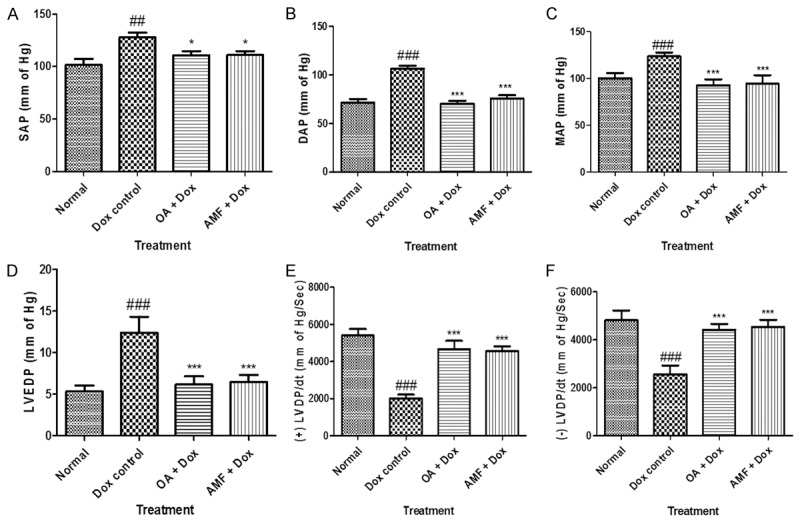
A. Systolic arterial pressure (SAP); B. Diastolic arterial pressure (DAP); C. Mean arterial pressure (MAP); D. LVEDP; E. Peak positive pressure ([+] LVdp/dt); F. Peak negative pressure ([-] LVdp/dt). Data was analysed by using one way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. #P<0.05, ##p<0.01, ###p<0.001 as compared to normal, *p<0.05, **p<0.01, ***p<0.001 as compared to Dox, Dox: Doxorubicin; OA: oleanolic acid; AMF: amifostine.
Figure 2.
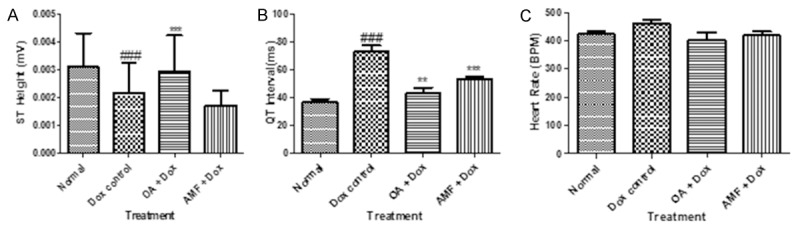
A. ST Height; B. QT interval; C. Heart rate. Data was analysed by using one way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. #P<0.05, ##p<0.01, ###p<0.001 as compared to normal, *p<0.05, **p<0.01, ***p<0.001 as compared to Dox, Dox: Doxorubicin; OA: oleanolic acid; AMF: amifostine.
Effect of OA on cardiac injury markers
A noteworthy deterioration in cardiac injury marker enzymes, CK-MB and LDH were pragmatic in the myocardium of Dox treated rats (P>0.001) as compared to normal rats (Figure 3). Treatment with OA and AMF ominously (P>0.001) attenuated the exhaustion of myocardial enzymes as compared to Dox treated group.
Figure 3.
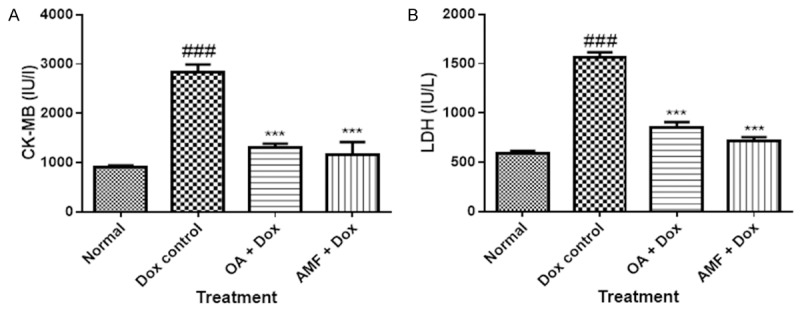
A. CK-MB; B. LDH. Data was analysed by using one way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. #P<0.05, ##p<0.01, ###p<0.001 as compared to normal, *p<0.05, **p<0.01, ***p<0.001 as compared to Dox, Dox: Doxorubicin; OA: oleanolic acid; AMF: amifostine.
Effect of OA on biochemical parameters
Administration of Dox in rats showed significant decrease (p<0.001) in the level of albumin in myocardium and increase in the level of other biochemical parameters as observed by significantly increase (p<0.001) in the levels of alkaline phosphatase (ALP), serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT). Treatment with OA and AMF significantly attenuated the increase in the levels of ALP, SGOT, and SGPT in myocardium and showed its cardioprotective effect in DOX induced cardiac toxicity as shown in Figure 4.
Figure 4.
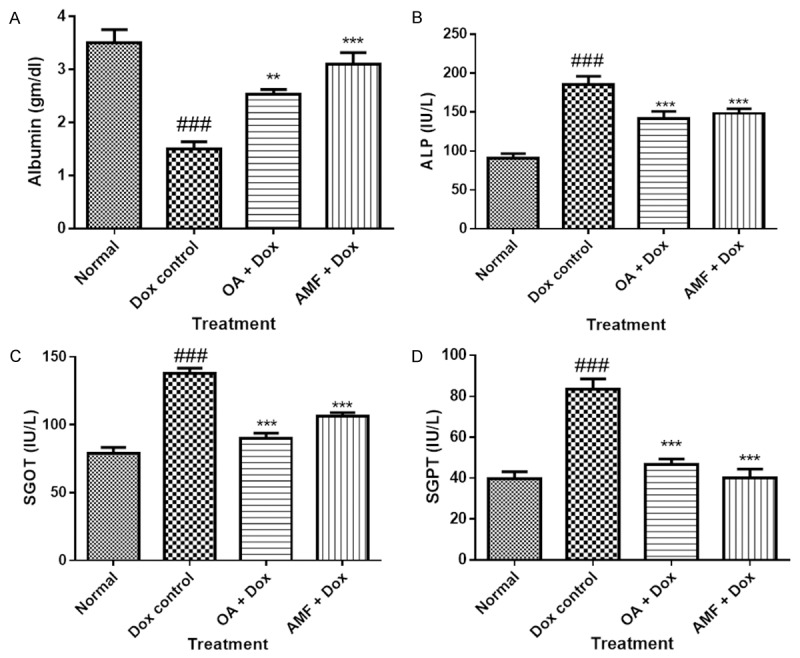
A. Albumin; B. ALP; C. SGOT; D. SGPT. Data was analysed by using one way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. #P<0.05, ##p<0.01, ###p<0.001 as compared to normal, *p<0.05, **p<0.01, ***p<0.001 as compared to Dox, Dox: Doxorubicin; OA: oleanolic acid; AMF: amifostine.
Effect of OA on oxidative stress
Cardiotoxicity-induced with Dox unveiled a significant (P<0.05) decrease in activities of reduced glutathione (GSH), superoxide dismutase (SOD) and catalase as compared tocontrol or normal rats. OA and AMF counteracted the deleterious effect of Dox by snowballing the content of these antioxidants. Rats challenged with Dox showed a significant increase (P<0.001) in the level of malondialdehyde (MDA), when compared to normal or vehicle treated rats. Treatment with OA and AMF showed highest guard of myocardium and attenuated the level of MDA (P<0.001) as compared with Dox treated rats as shown in Figure 5.
Figure 5.
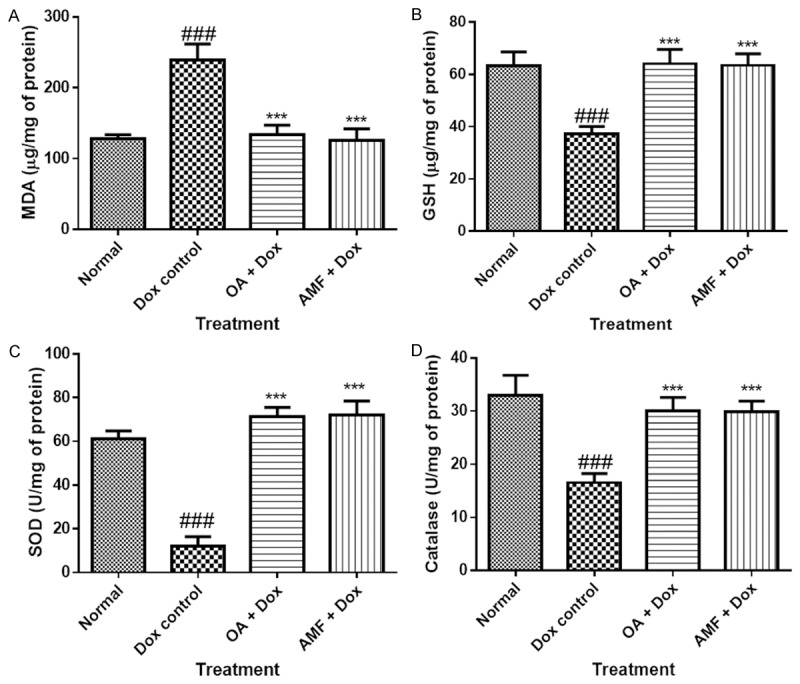
A. MDA; B. GSH; C. SOD; D. Catalase. Data was analysed by using one way analysis of variance (ANOVA) followed by Dunnett’s post hoc test. #P<0.05, ##p<0.01, ###p<0.001 as compared to normal, *p<0.05, **p<0.01, ***p<0.001 as compared to Dox, Dox: Doxorubicin; OA: oleanolic acid; AMF: amifostine.
Effect of OA on histopathology of myocardium
The architecture of normal or vehicle treated group showed normal structure and architecture of myocardium and no histological lesions were found. The rats treated with Dox showed severe histological lesions in which cytoplasmic vacuolization, myofibrillar loss were observed. Treatment with OA showed mild lesions in which cytoplasmic vacuolization’s are less than Dox treated group and the rats treated with AMF showed fewer lesions than OA treated group as shown in Figure 6 and Table 2.
Figure 6.
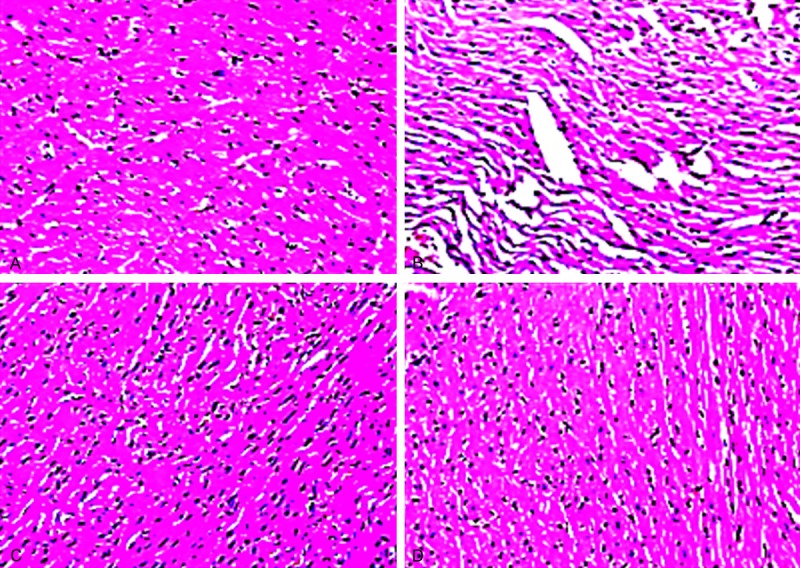
A. Normal rat’s showed normal structure and architecture of myocardium; B. Dox treated rats showed severe histological lesions increase in cytoplasmic vacuolization’s and inflammation; C. OA + Dox treated rats showed mild vacuolations and normal structure; D. AMF + Dox treated rats showed decreased in the inflammation and the vacuolations.
Table 2.
Effect of OA on histopathology in doxorubicin induced cardiac toxicity
| Treatment groups | Cytoplasmic vacuolization | Inflammatory cells | Oedema |
|---|---|---|---|
| Normal | - | - | - |
| Dox control | +++ | +++ | +++ |
| OA + Dox | ++ | + | + |
| AMF + Dox | + | + | + |
Severe (+++); Moderate (++); Mild (+); Nil (-), Dox: Doxorubicin; OA: oleanolic acid; AMF: amifostine.
Discussion
The present study validates the cardioprotectivepotential of oleanolic acid by improving hemodynamic, biochemical, and histopathologicalchanges in Dox-induced myocardial toxicity. Acute Dox-induced cardiotoxicity alters the organization of the cardiomyocytes and encourages apoptosis, which is a hypothetically variable and avoidable form of myocardial tissue damage [36]. Alterations in the physiological and biochemical processes in the human body may produce oxygen-centered free radicals and reactive oxygen species as by-products. Overproduction of such free radicals can cause oxidative damage to biomolecules in due course leading to many diseases [37]. In living organism, there is an intricate balance between production and demolition of reactive oxygen species (ROS). The body has developed an antioxidant defense system against the harmful effects of ROS. Antioxidants function through several mechanisms, one of which is scavenging of ROS and free radicals. In order to evaluate free radical-scavenging activity of OA, Dox is one of the agents among top 15 essential chemotherapeutic agent [36]. Unfortunately, the conventional and cardiac toxicities of Dox are among the main factors that limit their use. That’s why Dox is also called as “Double Edged Swords” [38]. The clinical use of Dox is limited by dose-dependent cardiomyopathy, leading to congestive heart failure and death [39]. Doxorubicin cardiotoxicity has been demonstrated in cultured cells, isolated heart preparation, animal model and human. It has quinone and hydroquinone moieties, which undergoes one electron reduction by NADH dehydrogenase to form a semiquinone free radical or two electron reduction by carbonyl reductase to form Dox-ol [38]. Dox semiquinone in turn is oxidized to the parent compound by reducing molecular oxygen to O2. Which attack DNA and oxidize DNA bases and shows myocardial damage. The present study was evaluated to provide better therapeutic value of Dox in the treatment of cancer by reducing its deleterious effects on myocardium. Dox showed acute and chronic cardiotoxicity in rats, in this study acute toxicity achieved by single intravenous injection of doxorubicin at dose 67. 75 mg/kg. This dose is selected at the basis of cumulative dose toxicity of Dox. The single dose administration of Dox (67.75 mg/kg) in rats showed significant cardiac damage and increase in oxidative stress. It is reported that administration of Dox in rats showed marked increase in systolic as well as diastolic arterial pressure [40]. The present study confirms that Dox treated rats showed increase in systolic and diastolic arterial pressures. Treatment with OA normalized the arterial pressures and showed protective effect. Dox is well known to affect the left ventricular end diastolic pressure as observed in the present study. Treatment with OA showed improved left ventricular end-diastolic function by increasing inotropic (+LVdP/dtmax, sign of myocardial contraction) and lusitropic (-LVdP/dtmax, sign of myocardial relaxation) situations of the heart. It also ameliorated Dox induced increase in LVEDP, a sign of pre-load that again reflects an improvement of left ventricular function. OA is reported to have strong anti-oxidant activity through reduction in lipid peroxidation [21]. As observed in the existing study marked increase in malondialdehyde content in Dox treated rats, and treatment with OA reduced the increase in malondialdehyde. In this attempt OA a strong protector against Dox induced increase in the content of malondialdehyde. OA protects against myocardial injury by enhancing mitochondrial anti-oxidant mechanism mediated by glutathione and α-tocopherol in rats [41]. Free radical scavenging endogenous antioxidants such as SOD, catalase and GSH are the first line of cellular defense in contradiction of oxidative injury [42,43]. We observed the reduction in the levels of these endogenous antioxidants in the myocardium following administration of Dox. OA showed more pronouncing effects and increase the levels of these antioxidants leads to proven the strong antioxidant activity and one of the reason to provide beneficial effects on the hemodynamic parameters. Deficiency of oxygen supply or glucose supply may cause damage to the myocardial cell membrane leading to permeable and ruptures the myocardium so that the enzymes leaks out. These enzymes are also called as specific biomarkers, can be estimated to square the damage [44]. Dox is known to have damage the myocardium leads to release the membrane bound enzymes in blood stream and decrease the levels in the myocardium [45] as observed in the present study there is decrease in the activity of CK-MB, LDH and Albumin in myocardium showed deleterious effect of Dox on myocardium. Treatment with the OA showed attenuation of these cardiac markers and leads to protect the myocardium to release the CK-MB, LDH and Albumin. Doxtreated rats showed increase in the level of ALP, SGOT and SGPT in the myocardium confirms the toxicity and treatment with the OA leads to decrease the levels of ALP, SGOT and SGPT enzymes showed the cardioprotective role of OA in Dox-induced cardiac toxicity. Histopathological examination of myocardial tissueattained from normal animal exhibited clear integrity and architecture of myocardium. The heart sections obtained from Dox treated animals showed disruption of several subcellularelements including loss of myofibrils, vacuolization of the cytoplasm, swelling of mitochondria, formation of lysosomal bodies and dilation of the sarcotubular system [6]. Treatment with OA showed protection as does not observed the loss of myofibrils, vacuolization of the cytoplasm, swelling of mitochondria in the heart.
The present study backers the cardioprotective effect of oleanolic acid on Dox-induced cardiac toxicity by improving the endogenous antioxidant provisions, diminishing the oxidative stress as reference with amifostine. Relieve from cardiac injury and maintain the hemodynamic parameters also confirms the cardioprotective potential. It can be useful for the conjunction with the chemotherapeutic treatment as an organ protective drug.
Acknowledgements
The authors gratefully acknowledge the financial support received from DBT- Bio-CARe, New Delhi, India to Dr. Yogeeta O. Agrawal. The authors also acknowledge the financial support to the research works in the laboratory of Dr. Shreesh Ojha from United Arab Emirates University, United Arab Emirates.
Disclosure of conflict of interest
None.
References
- 1.Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology. 2010;115:155–162. doi: 10.1159/000265166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carvalho FS, Burgeiro A, Garcia R, Moreno AJ, Carvalho RA, Oliveira PJ. Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med Res Rev. 2014;34:106–135. doi: 10.1002/med.21280. [DOI] [PubMed] [Google Scholar]
- 3.Jean SR, Tulumello DV, Riganti C, Liyanage SU, Schimmer AD, Kelley SO. Mitochondrial Targeting of Doxorubicin Eliminates Nuclear Effects Associated with Cardiotoxicity. ACS Chem Biol. 2015;10:2007–15. doi: 10.1021/acschembio.5b00268. [DOI] [PubMed] [Google Scholar]
- 4.Singal PK, Deally CM, Weinberg LE. Subcellular effects of adriamycin in the heart: a concise review. J Mol Cell Cardiol. 1987;19:817–828. doi: 10.1016/s0022-2828(87)80392-9. [DOI] [PubMed] [Google Scholar]
- 5.Menna P, Recalcati S, Cairo G, Minotti G. An introduction to the metabolic determinants of anthracycline cardiotoxicity. Cardiovasc Toxicol. 2007;7:80–85. doi: 10.1007/s12012-007-0011-7. [DOI] [PubMed] [Google Scholar]
- 6.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330–352. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Ziaee M, Khorrami A, Ebrahimi M, Nourafcan H, Amiraslanzadeh M, Rameshrad M, Garjani M, Garjani A. Cardioprotective Effects of Essential Oil of Lavandula angustifolia on Isoproterenol-induced Acute Myocardial Infarction in Rat. Iran J Pharm Res. 2015;14:279–289. [PMC free article] [PubMed] [Google Scholar]
- 8.Saad SY, Najjar TA, Al-Rikabi AC. The preventive role of deferoxamine against acute doxorubicin-induced cardiac, renal and hepatic toxicity in rats. Pharmacol Res. 2001;43:211–218. doi: 10.1006/phrs.2000.0769. [DOI] [PubMed] [Google Scholar]
- 9.Gustafson DL, Swanson JD, Pritsos CA. Role of xanthine oxidase in the potentiation of doxorubicin-induced cardiotoxicity by mitomycin C. Cancer Commun. 1991;3:299–304. doi: 10.3727/095535491820873038. [DOI] [PubMed] [Google Scholar]
- 10.Bains OS, Karkling MJ, Grigliatti TA, Reid RE, Riggs KW. Two nonsynonymous single nucleotide polymorphisms of human carbonyl reductase 1 demonstrate reduced in vitro metabolism of daunorubicin and doxorubicin. Drug Metab Dispos. 2009;37:1107–1114. doi: 10.1124/dmd.108.024711. [DOI] [PubMed] [Google Scholar]
- 11.Cassidy SC, Chan DP, Rowland DG, Allen HD. Effects of doxorubicin on diastolic function, contractile reserve, and ventricular-vascular coupling in piglets. Pediatr Cardiol. 1998;19:450–457. doi: 10.1007/s002469900355. [DOI] [PubMed] [Google Scholar]
- 12.Olson RD, Mushlin PS. Doxorubicin cardiotoxicity: analysis of prevailing hypotheses. FASEB J. 1990;4:3076–3086. [PubMed] [Google Scholar]
- 13.Rajagopalan S, Politi PM, Sinha BK, Myers CE. Adriamycin-induced free radical formation in the perfused rat heart: implications for cardiotoxicity. Cancer Res. 1988;48:4766–4769. [PubMed] [Google Scholar]
- 14.Lee V, Randhawa AK, Singal PK. Adriamycin-induced myocardial dysfunction in vitro is mediated by free radicals. Am J Physiol. 1991;261:H989–95. doi: 10.1152/ajpheart.1991.261.4.H989. [DOI] [PubMed] [Google Scholar]
- 15.Mansat-de Mas V, Bezombes C, Quillet-Mary A, Bettaieb A, D’Orgeix AD, Laurent G, Jaffrezou JP. Implication of radical oxygen species in ceramide generation, c-Jun N-terminal kinase activation and apoptosis induced by daunorubicin. Mol Pharmacol. 1999;56:867–874. doi: 10.1124/mol.56.5.867. [DOI] [PubMed] [Google Scholar]
- 16.Panaretakis T, Laane E, Pokrovskaja K, Bjorklund AC, Moustakas A, Zhivotovsky B, Heyman M, Shoshan MC, Grander D. Doxorubicin requires the sequential activation of caspase-2, protein kinase Cdelta, and c-Jun NH2-terminal kinase to induce apoptosis. Mol Biol Cell. 2005;16:3821–3831. doi: 10.1091/mbc.E04-10-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto Y, Hoshino Y, Ito T, Nariai T, Mohri T, Obana M, Hayata N, Uozumi Y, Maeda M, Fujio Y, Azuma J. Atrogin-1 ubiquitin ligase is upregulated by doxorubicin via p38-MAP kinase in cardiac myocytes. Cardiovasc Res. 2008;79:89–96. doi: 10.1093/cvr/cvn076. [DOI] [PubMed] [Google Scholar]
- 18.Aroui S, Mili D, Brahim S, De Waard M, Kenani A. Doxorubicin coupled to penetratin promotes apoptosis in CHO cells by a mechanism involving c-Jun NH2-terminal kinase. Biochem Biophys Res Commun. 2010;396:908–914. doi: 10.1016/j.bbrc.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Yu TW, Anderson D. Reactive oxygen species-induced DNA damage and its modification: a chemical investigation. Mutat Res. 1997;379:201–210. doi: 10.1016/s0027-5107(97)00141-3. [DOI] [PubMed] [Google Scholar]
- 20.Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 21.Balanehru S, Nagarajan B. Protective effect of oleanolic acid and ursolic acid against lipid peroxidation. Biochem Int. 1991;24:981–990. [PubMed] [Google Scholar]
- 22.Price KR, Johnson IT, Fenwick GR. The chemistry and biological significance of saponins in foods and feedingstuffs. Crit Rev Food Sci Nutr. 1987;26:27–135. doi: 10.1080/10408398709527461. [DOI] [PubMed] [Google Scholar]
- 23.Nishino H, Nishino A, Takayasu J, Hasegawa T, Iwashima A, Hirabayashi K, Iwata S, Shibata S. Inhibition of the tumor-promoting action of 12-O-tetradecanoylphorbol-13-acetate by some oleanane-type triterpenoid compounds. Cancer Res. 1988;48:5210–5215. [PubMed] [Google Scholar]
- 24.Kashiwada Y, Wang HK, Nagao T, Kitanaka S, Yasuda I, Fujioka T, Yamagishi T, Cosentino LM, Kozuka M, Okabe H, Ikeshiro Y, Hu CQ, Yeh E, Lee KH. Anti-AIDS Agents. 30. Anti-HIV Activity of Oleanolic Acid, Pomolic Acid, and Structurally Related Triterpenoids. J Nat Prod. 1998;61:1090–1095. doi: 10.1021/np9800710. [DOI] [PubMed] [Google Scholar]
- 25.Somova LI, Shode FO, Mipando M. Cardiotonic and antidysrhythmic effects of oleanolic and ursolic acids, methyl maslinate and uvaol. Phytomedicine. 2004;11:121–129. doi: 10.1078/0944-7113-00329. [DOI] [PubMed] [Google Scholar]
- 26.Mnafgui K, Khlif I, Hajji R, Derbali F, Kraiem F, Ellefi H, Michel T, Halabalaki M, Skaltsounis AL, Elfeki A, Gharsallah N, Allouche N. Preventive effects of oleuropein against cardiac remodeling after myocardial infarction in Wistar rat through inhibiting angiotensin-converting enzyme activity. Toxicol Mech Methods. 2015;25:538–46. doi: 10.3109/15376516.2015.1053648. [DOI] [PubMed] [Google Scholar]
- 27.Martin R, Cordova C, San Roman JA, Gutierrez B, Cachofeiro V, Nieto ML. Oleanolic acid modulates the immune-inflammatory response in mice with experimental autoimmune myocarditis and protects from cardiac injury. Therapeutic implications for the human disease. J Mol Cell Cardiol. 2014;72:250–262. doi: 10.1016/j.yjmcc.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Mohan M, Patankar P, Ghadi P, Kasture S. Cardioprotective potential of Punica granatum extract in isoproterenol-induced myocardial infarction in Wistar rats. J Pharmacol Pharmacother. 2010;1:32–37. doi: 10.4103/0976-500X.64533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 30.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 31.Cabaud PG, Wroblewski F. Colorimetric measurement of lactic dehydrogenase activity of body fluids. Am J Clin Pathol. 1958;30:234–236. doi: 10.1093/ajcp/30.3.234. [DOI] [PubMed] [Google Scholar]
- 32.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 33.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 34.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 35.Bergmeyer H. Methods of enzymatic analysis. 1974;3:1125–1624. [Google Scholar]
- 36.Iqbal M, Dubey K, Anwer T, Ashish A, Pillai KK. Protective effects of telmisartan against acute doxorubicin-induced cardiotoxicity in rats. Pharmacol Rep. 2008;60:382–90. [PubMed] [Google Scholar]
- 37.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 38.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 39.Petit T. [Anthracycline-induced cardiotoxicity] . Bull Cancer. 2004;91:159–165. [PubMed] [Google Scholar]
- 40.Yagmurca M, Fadillioglu E, Erdogan H, Ucar M, Sogut S, Irmak MK. Erdosteine prevents doxorubicin-induced cardiotoxicity in rats. Pharmacol Res. 2003;48:377–382. doi: 10.1016/s1043-6618(03)00185-3. [DOI] [PubMed] [Google Scholar]
- 41.Du Y, Ko K. Effects of pharmacological preconditioning by emodin/oleanolic acid treatment and/or ischemic preconditioning on mitochondrial antioxidant components as well as the susceptibility to ischemia-reperfusion injury in rat hearts. Mol Cell Biochem. 2006;288:135–142. doi: 10.1007/s11010-006-9129-3. [DOI] [PubMed] [Google Scholar]
- 42.Golikov AP, Polumiskov V, Davydov BV, Karev VA, Bashkatova VG, Belozerov GE, Golikov PP, Berestov AA. [Lipid peroxidation and the major factors of its activation in patients with myocardial infarction] . Kardiologiia. 1989;29:53–59. [PubMed] [Google Scholar]
- 43.Dianita R, Jantan I, Amran AZ, Jalil J. Protective effects of Labisia pumila var. alata on biochemical and histopathological alterations of cardiac muscle cells in isoproterenol-induced myocardial infarction rats. Molecules. 2015;20:4746–4763. doi: 10.3390/molecules20034746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Momin FN, Kalai BR, Shikalgar TS, Naikwade NS. Cardioprotective effect of methanolic extract of Ixora coccinea Linn. leaves on doxorubicin-induced cardiac toxicity in rats. Indian J Pharmacol. 2012;44:178–183. doi: 10.4103/0253-7613.93844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu H, Luo P, Fu Y, Wang J, Dai J, Shao J, Yang X, Chang L, Weng Q, Yang B. Dihydromyricetin prevents cardiotoxicity and enhances anticancer activity induced by adriamycin. Oncotarget. 2015;6:3254–3267. doi: 10.18632/oncotarget.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]


