Abstract
The perioperative stress response is one of the factors leading to postoperative cognitive dysfunction (POCD). Dexmedetomidine (Dex) can reduce the stress response and hippocampus neuroapoptosis, but its mechanism of action on POCD remains unknown. This study investigated the protective effect and possible mechanism of Dex on POCD in aged rats. Ninety-six aged male rats were randomly divided into four groups (n = 24 rats per group): a non-surgical control group, a surgical (model) group, a surgical group receiving a high dose of Dex (12 μg/kg), and a surgical group receiving a low dose of Dex (3 μg/kg). Cognitive function and neuronal apoptosis were evaluated after splenectomy. Compared with the control group, the model group had significantly longer escape latencies and fewer platform crossings in the Morris water-maze test. Immunohistochemistry showed that relaxin-3 and c-fos positive neurons in the hippocampus increased on postoperative days 1 and 3. Greater downregulation of the Bcl-2 protein and upregulation of Fas, caspase-8, and caspase-9 significantly increased neuroapoptosis in the model group. Compared with the model group, rats given Dex had (1) shorter escape latencies, (2) more platform crossings, (3) fewer relaxin-3 and c-fos positive neurons in the hippocampal CA1 area, (4) upregulation of Bcl-2, (5) downregulation of Fas, caspase-8, and caspase-9 proteins, and (6) decreased neuroapoptosis in the hippocampus. Thus, our data suggest that Dex may improve cognitive functioning in aged rats by inhibiting neural over-excitability. The mechanism may operate by restraining relaxin-3 and c-fos expression.
Keywords: Dexmedetomidine (Dex), aging, rats, postoperative cognitive dysfunction, hippocampus, neuroapoptosis
Introduction
Postoperative cognitive dysfunction (POCD) is a severe complication of anesthesia and surgery that is characterized by cognitive decline in patients. Previous studies have found that POCD is a common complication of surgery with anesthesia, particularly in elderly patients, 14% of whom have been found to experience cognitive decline and confusion for up to three months after surgery [1]. Although, the pathogenic mechanisms of postoperative cognitive dysfunction are unclear, the risk factors for this disorder include drugs, perioperative stress in advanced age, trauma surgery, and postoperative pain [2,3]. Excessive excitement of the neurons of the hippocampus in response to perioperative stress, which lead to neuroapoptosis, is thought to be a particularly important cause of POCD [4,5]. Yet, the pathogenic mechanisms of neuronal apoptosis caused by the perioperative stress remain unclear. Recent research has discovered that the over-expression of relaxin-3 in reaction to trauma and stress may be responsible for the neurovirulence that causes neuronal apoptosis and brain damage [6-8]. Recent studies also have shown that Dexmedetomidine (Dex) can alleviate the hippocampus neuronal apoptosis of young rats after isoflurane anesthesia, through the amelioration of the stress reaction and the release of cytokine to ameliorate the POCD in elderly patients [9,10]. Yet, the means by which this reduces the incidence of POCD has not been reported yet. The present study was designed to examine the effect of Dex on apoptosis and the expression of relaxin-3 in the hippocampus of aged rats with cognitive dysfunction and to explore its underlying mechanism.
Materials and methods
Reagents
The Dex injection was 200 μg/2 ml, formula: C13H16N2·HCL; molecular weight 236.7 (Batch No. 130621, Hengrui Medicine Co., Ltd, Lianyungang, China). Pentobarbital sodium was used for anesthesia (Batch No. WS20050411, Sinopharm Group Chemical Reagent Co., Ltd, Shanghai, China). Rabbit-origin primary antibodies against relaxin-3 were used to assess relaxin-3 (Batch No: bs-11412R, Bo’Aosen Bio-Tech Co., Ltd, Beijing, China). Rabbit-origin primary antibodies against c-fos were used to assess c-fos (1:150 dilution); biotinylation IgG/AP (1:200, second antibody), biotinylation HRP-IgG (1:100 dilution, second antibody), SABC/DAB Color-substrate solution, confining liquid (Bo-Shi-De Bio-Engineering Co., Ltd, Wuhan, China). Apoptosis was assessed using a TUNEL cell apoptosis in situ test detection kit (Batch No: KGA7042, Kai Ji Biology Co., Ltd, Tianjing, China). Other materials included: a BCA protein concentration determination kit; HRP-anti-rabbit lgG second antibody; HRP-anti-mice second antibody; Tubulin antibody (Bi Yun Tian Biology Co., Ltd, Nantong, China); Fas, Bcl-2, and caspase-9 antibodies (Santa Cruz Co., Ltd, Dallas, USA); and anti-caspase-8 antibody (Abcam Company, Cambridge, UK).
Animal selection, grouping, dosing and model preparation
All of the animal experiments were approved by the Animal Care and Use Committee of Fudan University, and were performed in according to the guidelines established by the Chinese Association for Laboratory Animal Sciences. A total of 96, 18 month-old (clean-grade) male Sprague-Dawley rats, weighing 500 g to 600 g were provided by the Chengdu Jianyang Dashuo Animal Science Co., Ltd (Qualifying No: SCXK 013-74).
First, the rats were trained on the Morris water-maze test. The rats were placed in a pool without a platform and allowed to swim freely for 2 min to let them get familiar with water-maze environment. Then, pre-training began by placing the platform in the pool; rats that failed to find the platform were guided to it and allowed to rest on the platform for 30 min. Four rats that could not swim were excluded from the study. The remainder of the rats received formal training until they could swim three consecutive times from the start point to the platform within 60 seconds. Those with fewer than two errors were considered to have “learned” the task.
The 96 trained rats were randomly divided into four groups (n = 24 rats per group): a non-surgical control group that received normal saline (NS, 2 ml/kg) (“control”); a surgical group that received NS (2 ml/kg) (“model”); a surgical group that received a high dose of Dex (12 μg/kg); and a surgical group that received a low dose of Dex (3 μg/kg,). The rats were anesthetized with 1% pentobarbital sodium (30 mg/kg, i.p). After the righting reflex disappeared, using a prescribed dose (volume: 2 ml/kg) to complete the caudal vein injection within 10 min. After 10 min, the 72 rats that were not in the non-surgery NS control group, were fixed in a supine position for surgery.
The surgery began with a 4-cm long medial abdominal incision, and an abbreviated laparotomy was performed, which started at the ileocecal junction, extended past the entire portion of intestines and up to stomach, and then extended downward to the rectum; the intestines were placed back into the enterocoelia. The spleen was removed at the root of the far end of spleen pedicle, using 3.0 silk thread to dissociate and ligate the spleen artery and vein in a step-by-step manner. The completely removed spleen was examined to ensure that no residual spleen was left. Then the incision was closed, covering it with sterile activated-iodine gauze, and securing it with adhesive tape. The operation time was about 90 min.
After the operation, each rat was given an intraperitoneal injection of 400 μg of penicillin once each day, for 3 days, to prevent infection. Five rats died after the splenectomy; based on gross anatomy and visual observation, two died of bleeding caused by ligature loosening and three died of post-operation ileus. The success rate of the splenectomy was 91.7%.
Morris water-maze measures of postoperative memory
The Morris water-maze was used to conduct two tests. The first test was the positional swimming test. The transparent platform was placed within a 1/2 radius of any of the four quadrants, and located of 1/2 the distance between the pool’s center and the pool’s wall. The water surface was 1 cm higher than the top of the platform. One series of tests was conducted each day, which included four tests in each of the four quadrants of the pool; the interval between each test was 30 s. The test duration was 60 s and the animal was allowed to stay on the platform for 5 s. The duration of each test was timed until the rat found and climbed onto the platform; an observer recorded the time, i.e., the escape latency. If a rat failed to find the platform, the escape latency was recorded as 60 s. The average escape latency for the four quadrants was used as the measure of performance for each day. Testing was conducted four times per day for 3 days.
The second test was the space exploitation test. The platform was removed from the pool on the 4th day and the rats were placed in water in the opposite quadrant of the former test. An observer recorded the swimming track or path of each rat for 60 s, and recorded the number of times the rat crossed over the original platform position. Pre-operative training was conducted for 5 days, with four tests each day, and the average value recorded. Six to eight rats from each group were selected for testing on postoperative days 1, 3, and 7, and their escape latencies and number of platform crossings recorded.
TUNEL method to detect hippocampus structure neuronal apoptosis
The left brain was fixed with 4% triformol, dehydrated, and embedded in paraffin; we referred to the “Old Rat Brain Section Note and Figure”. The brain tissue was sliced into 5 μm continuous sections, and fixed on slides using polyamino acid. We used 4% triformol solution to fix the sections for 20 min, and followed the other steps in the Manual of the TUNEL cell apoptosis detection kit. We observed the results microscopically, and took photographs by magnifying the images 400 times. Under the light microscope, the apoptosis body had a color of claybank or toffee inside the cell nucleus. From each section, we selected five non-overlapping images with 400 magnification of the hippocampus, recorded the total total number of cells and the number of apoptotic cells, and calculated the percentage of positive cells: Neuronal apoptosis rate (%) = apoptosis cell total number/cell total number.
Western blot method for detecting the expression of Fas, caspase-8, caspase-9, Bcl-2 proteins
40 mg portion of the hippocampus of the right brain was placed in a 400 ul 1% PMSF RIPA lysis solution to extract protein, using the BCA method to detect protein concentration; samples of 40 µg protein were made for each well, using a 5-μl protein pre-dyeing Maker liquid for each sample (10%SDS-PAGE separation gel and 5% spacer gel). We used 80 V for 30 min and 120 V for 60 min for electrophoresis; caspase-9, Fas, and tubulin proteins were processed using the wet trans-membrane method (USA Bio-Rad)-CC 250 mA, 2 h trans-membrane; Bcl-2 and caspase-8 proteins were processed using the half-dry trans-membrane-CC150 mA, 20 min trans-membrane. Membranes were blocked in 5% skim milk powder in TBST for 2 h at room temperature. The blots of Bcl-2 (1:200), caspase-9 (1:200), Fas (1:200), caspase-8 (1:1000), and tubulin (1:1000) were incubated with their primary antibodies at 4°C for 16-18 h, and then washed with TBST. Next, the Bcl-2, Fas, and caspase-8 blots were put into a HRP-anti-rabbit IgG 1:2000, and the caspase-9 and tubulin blots were put into TBSTHRP-anti-mice IgG 1:2000) for incubation with their secondary antibodies at 37°C for 2 h, and washed again in TBST. We used the enhanced chemical lighting (ECL) method for image development, and Image J 1.43 (National Institutes of Health) software for analysis. The expression intensity of the selected protein was expressed by: (integration density value of the selected protein/integration density value of tubulin) x100%.
Streptavidin-biotin complex (SABC) to detect the expression of hippocampal relaxin-3 and c-fos positive neurons
Two hours after observing the rat’s behaviors, each rat was anesthetized with 1% pentobarbital sodium (30 mg/kg, i.p), its head cut off, and its brain extracted, using icy normal saline to wash it. The brain tissue was split into two parts along the sagittal plane. The left part of the brain was put in 4% triformol to be fixed overnight, using conventional dehydration, and embedded in transparent paraffin. Continuous corona-sections were taken from portions of the hippocampus, with each sliced section having a thickness of 5 μm. The sections were divided into two sets; one set was used for color generation of relaxin-3, and the other set was used for dyeing c-fos. Sections were processed by normal dewaxing and dehydration, using 3% H2O2 inactivated endogenous peroxidase, citric acid buffer solution (0.01 mol/L, pH 6.0), and microwave antigen retrieval. The sections were placed in rabbit serum confining liquid for 1 h, respectively, adding relaxin-3 (1:150, primary antibody) and c-fos (1:150, primary antibody), and then put in a 4°C refrigerator overnight. Biotinylation IgG/AP (1:200, second antibody) and biotinylation HRP-IgG (1:100, second antibody) were added, respectively, and the sections were incubated for 2 h. The sections were then placed in streptavidin-biotin-peroxidase complex liquid (SABC, 1:100) and incubated for 1 h. Diaminobenzidine (DAB) coloration was performed using a DAB kit. After the above steps were completed we used 0.02 mol/L phosphate buffer solution (PBS), pH 7.4, to fully wash and clean the sections (5 min/ 3 times). The slides were dehydrated in a graded alcohol and xylene series, and sealed with neutral gum. In order to secure comparability, the enzyme reaction solution, reaction time and temperature for each group during the experiment were all the same. In this test, PBS was used to replace the primary antibody as a negative control; the negative control group had no expression.
The assessment of positive neurons was performed as follows: the relaxin-3 and c-fos immuno-reactions produced a color of claybank or sepia; coloring occurred mainly on the cell body and the protuberant intracytoplasm; the cell nucleus was not colored or only slightly dyed. For each rat, 7 sections from same position of the hippocampus CA1 were taken for observation, using an Olympus BX51 microscope (Olympus Company, Japan). Image J picture analysis software was used to detect the gray value of each unit of area expressing relaxin-3 positive and c-fos positive cells. We used the average value for semi-quantitative analysis. Smaller gray values indicate stronger expression levels and larger gray values indicate weaker expression levels.
Statistical analysis
We used the SPSS 16.0 software package to perform the statistical analyses. Quantitative data were expressed as the mean ± the standard error of mean (x̅ ± SEM), and one-way analysis of variance was used for multi-group comparisons of the means. Pairwise comparisons were conducted to compare the groups, using the t-test when the variance was homogeneous; when heterogeneity of variance occurred we used t’ to make pairwise comparisons. The significance level was P < 0.05.
Results
The effects of Dex on cognitive dysfunction in old rats on the 1st, 3rd, and 7th days after the operation
Compared to the rats in the normal saline control group, the rats in the model group had significantly longer escape latencies (P < 0.01) and significantly fewer platform crossings (P < 0.01) on postoperative days 1, 3, and 7. Compared to rats in the model group, the rats in the two Dex groups had significantly shorter escape latencies (P < 0.01) and significantly more platform crossings (P < 0.01 or P < 0.05). These results imply that Dex can alleviate the POCD of old rats (Figure 1).
Figure 1.
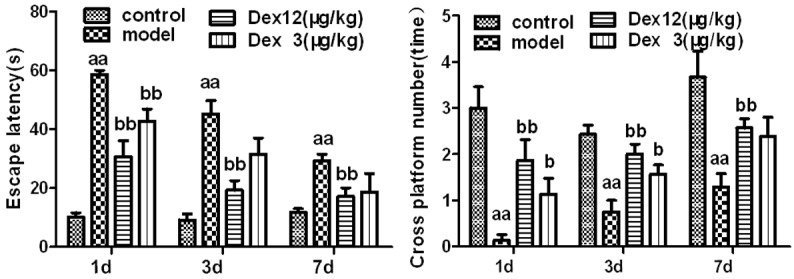
The effects of Dex on cognitive dysfunction in old rats on postoperative days 1, 3, and 7 (n = 6-8, x̅ ± SEM); aP < 0.05, aaP < 0.01 vs. control group; bP< 0.05, bbP < 0.01 vs. model group.
Compared to the rats in the control group, the rats in the model group exhibited a significantly higher rate of postoperative neural apoptosis in hippocampal areas CA1, CA2, and CA3 and the dentate gyrus (DG) (P < 0.01). Compared to rats in the model group, the rats of in the two Dex groups had significantly lower rates of the neural apoptosis at CA1 (P < 0.05), CA2 (P < 0.01 or P < 0.05), CA3 (P < 0.05) and the DG (P < 0.01). These findings imply that Dex can reduce postoperative neural apoptosis in the hippocampus of old rats (Figures 2 and 3).
Figure 2.
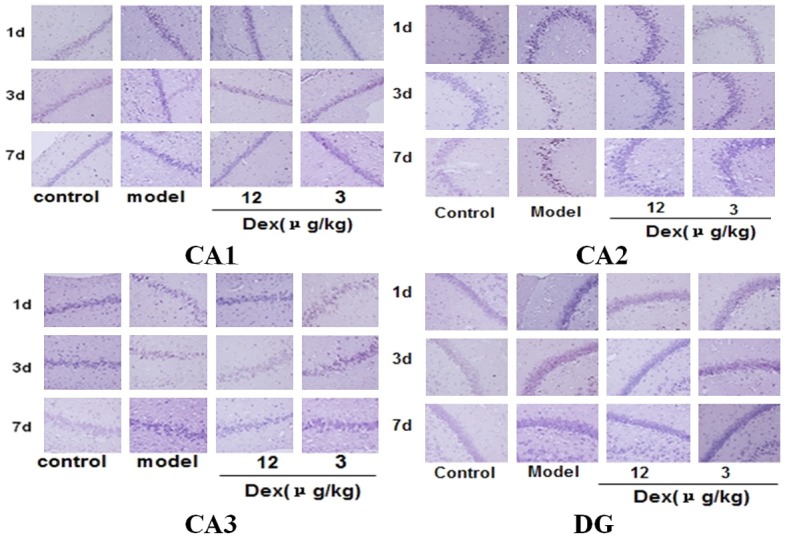
The effects of Dex on neural apoptosis in the hippocampus of old rats on postoperative days 1, 3, and 7 (x400).
Figure 3.
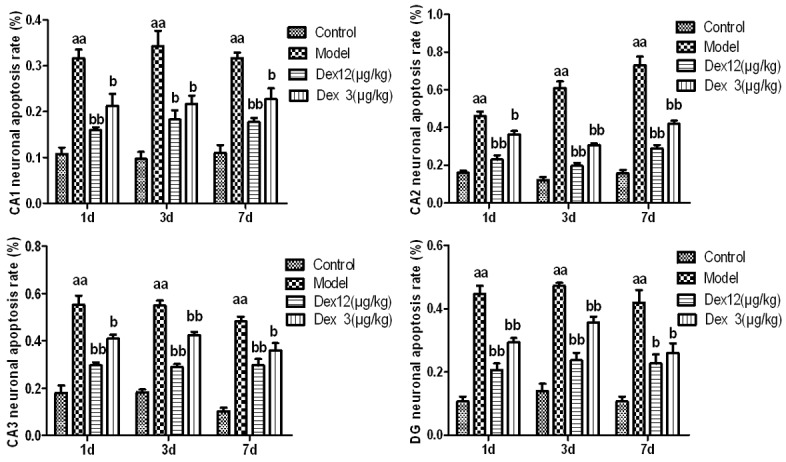
The effects of Dex on the apoptosis rate of hippocampal neurons in old rats on postoperative days 1, 3, and 7 (n = 3, x̅ ± SEM); aP < 0.05, aaP < 0.01 vs. control group; bP < 0.05, bbP < 0.01 vs. model group.
Effects of Dex on the expression of the Fas, caspase-8, caspase-9, and Bcl-2 proteins in the hippocampus on the 1st, 3rd, and 7th day after the operation
Compared with the control group, the expression of the caspase-9, fas, and caspase-8 proteins was significantly higher in the hippocampus of the model group, post-operatively (P < 0.01); the postoperative expression of the Bcl-2 protein was significantly lower in the model group (P < 0.01). Compared with the model group, the two Dex groups had a significantly lower postoperative expression of the Fas, caspase-8, and caspase-9 proteins (P < 0.01 or P < 0.05); Bcl-2 protein expression was significantly higher in the two Dex groups (P < 0.05). By postoperative day 7, the caspase-9 and caspase-8 levels of the high-dose Dex group and the caspase-8 level of the low-dose Dex group had dropped significantly (P < 0.01 or P < 0.05). These results suggest that Dex had the effect of downregulating Fas, caspase-8, and caspase-9, and upregulating Bcl-2 in the hippocampal neurons of the old rats, post-operatively (Figure 4).
Figure 4.
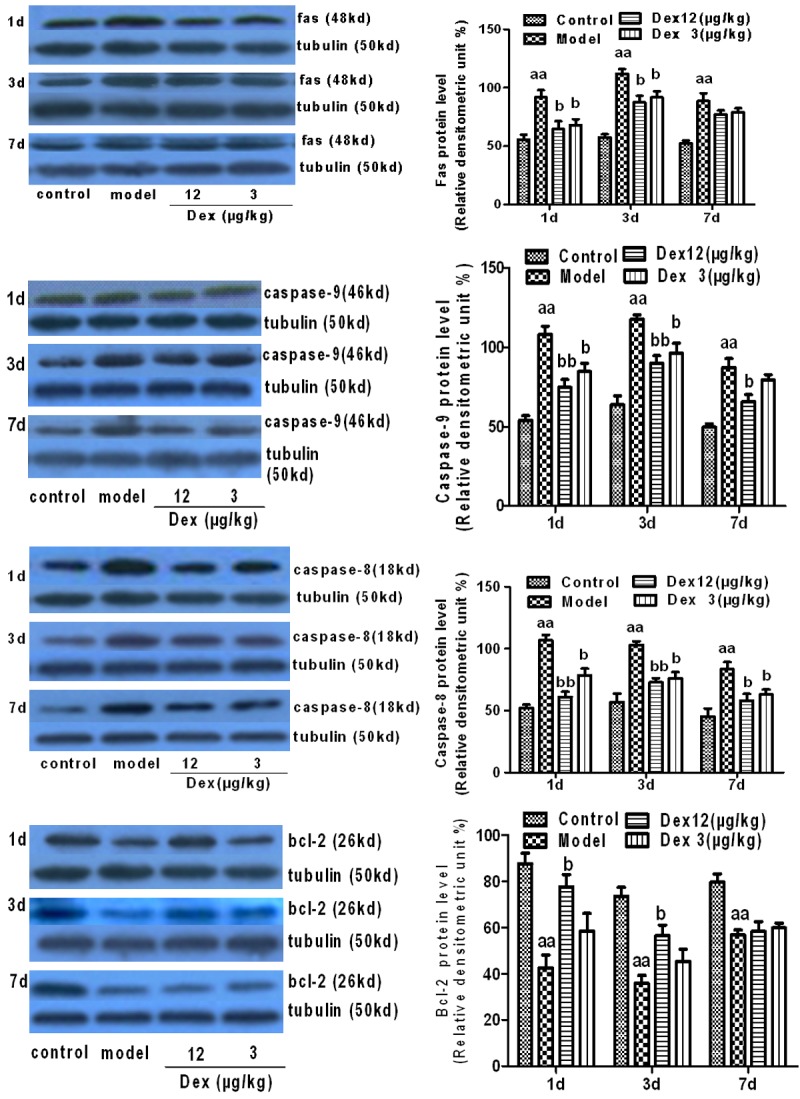
The effects of Dex on the expression of Fas, caspase-8, caspase-9, Bcl-2 proteins in the hippocampus of old rats, indicating the neural apoptosis rate on postoperative days 1, 3, and 7 (n = 3, x̅ ± SEM); aP < 0.05, aaP < 0.01 vs. control group; bP < 0.05, bbP < 0.01 vs. model group; cP < 0.05, ccP < 0.01 vs. Dex 12.50 μg/kg.
Effects of Dex on relaxin-3 positive neurons in hippocampal area CA1 on the 1st, 3rd, and 7th days after the operation
Compared to the control group, the model group’s expression of relaxin-3 positive neurons in hippocampal area CA1 was significantly higher on postoperative days 1 and 3 (P < 0.01), but there was no significant difference between the groups on postoperative day 7 (P > 0.05). Compared with the model group, the two Dex groups exhibited a significantly lower expression of relaxin-3 positive neurons in hippocampal area CA1 on postoperative days 1 and 3 day (P < 0.01 or P < 0.05), but not on postoperative day 7 (P > 0.05). These results suggest that Dex can restrain the postoperative expression of relaxin-3 positive neurons in hippocampal area CA1 of old rats (Figure 5).
Figure 5.
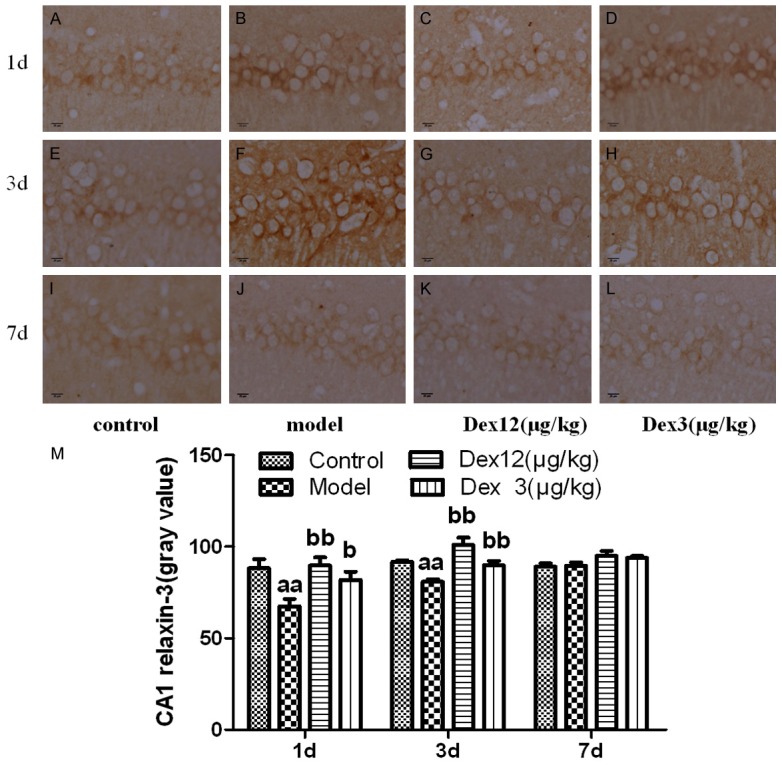
The effects of Dex on the expression of relaxin-3 positive neurons in the CA1 area of the hippocampus of old rats on postoperative days 1, 3, and 7 (x400) (A-L) and the gray value of relaxin-3 positive neurons in the CA1 area of the hippocampus of old rats on postoperative days 1, 3, and 7 (M) (n = 7 x̅ ± SEM); aP < 0.05, aaP < 0.01 vs. control group; bP < 0.05, bbP < 0.01 vs. model group (M).
Effects of Dex on c-fos positive neurons in hippocampal area CA1 on the 1st, 3rd, and 7th days after the operation
Compared with the control group, the model group’s expression of c-fos positive neurons in hippocampal area CA1 on postoperative days 1 and 3 was significantly higher (P < 0.01); there was no obvious group difference on postoperative day 7 (P > 0.05). Compared with the model group, the two Dex groups exhibited a significantly lower expression of c-fox positive neurons in hippocampal area CA1 on postoperative days 1 and 3 (P < 0.01 or P < 0.05), but there was no obvious difference on postoperative day 7 (P > 0.05). These results imply that Dex can restrain the postoperative expression of c-fos positive neurons in hippocampal area CA1 of old rats (Figure 6).
Figure 6.
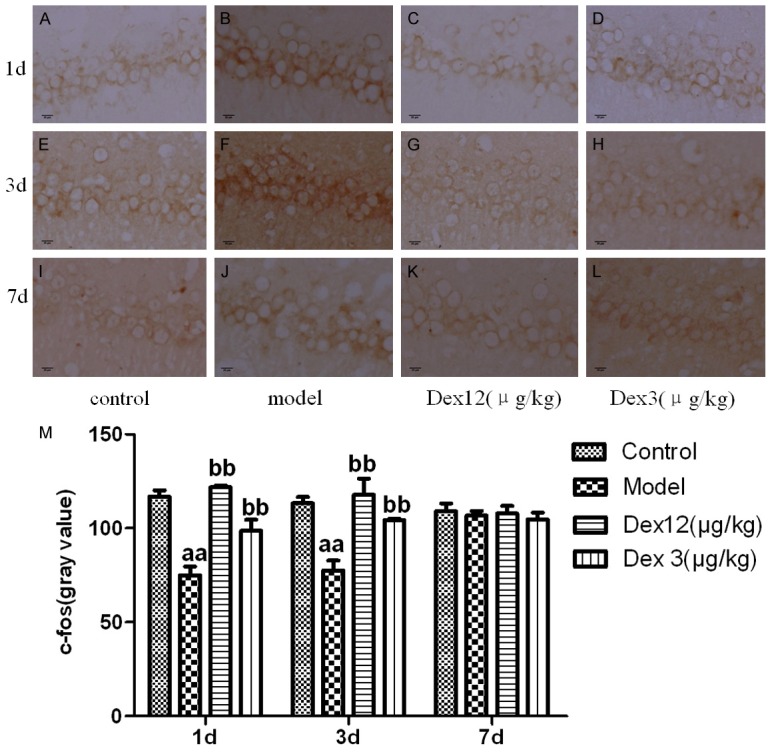
The effects of Dex on the expression of c-fos positive neurons in the CA1 area of the hippocampus of old rats on postoperative days 1, 3, and 7 (x400) (A-L) and the gray value of c-fos positive neurons in the CA1 area of the hippocampus of old rats on postoperative days 1, 3, and 7 (M) (n = 7, x̅ ± SEM); aP < 0.05, aaP < 0.01 vs. control group; bP < 0.05, bbP < 0.01 vs. model group.
Discussion
Our study found that Dex can alleviate neurotoxicity by reducing the release of the excitatory neurotransmitter, relaxin-3, thus, preventing the apoptosis of hippocampal neurons. This may explain the mechanism of the effect of Dex and provide a way for preventing and/or treating POCD.
The 18 month-old rats in our study underwent abdominal laparotomy and splenectomy to replicate the POCD model. The Morris water-maze test showed that the escape latency of the rats in the model group was significantly higher than that of control rats on the 1st, 3rd, and 7th day after the operation, and that their number of platform crossings was significantly lower. These results indicate that cognitive dysfunction had been produced successfully in the rats in the model group (Figure 1).
Observations of the morphological changes related to neural apoptosis in the hippocampus, using the TUNEL method, revealed changes in the model group indicative of apoptosis, including cell shrinkage, chromatin concentration, and the formation of an apoptosis body in the neurons of the hippocampus. Other measures provided evidence of a high rate of apoptosis (Figures 2 and 3). According to the existing research, there are mainly two apoptotic cellular pathways: the exogenous apoptotic pathway and the endogenous apoptotic pathway. The Fas, caspase-8, and caspase-9 proteins are important molecules in both of these pathways. In contrast, Bcl-2 can inhibit the activity of caspase; thus, it plays an important role in the mechanism of anti-apoptosis. Immunoblotting tests for apoptotic proteins in the hippocampus found that the anti-apoptosis protein Bcl-2 decreased considerably, and the pro-apoptotic proteins, Fas, caspase-8, and caspase-9, increased considerably (Figure 4). All these results suggest that the apoptosis of hippocampal neurons is, indeed, related to POCD.
Relaxin-3 is mainly concentrated in the nucleus incertus of the pons, and has nerve fibers and receptors that are widely distributed in parts of the forebrain, including the hypothalamus and hippocampus; it is mainly involved in stress reactions, ingestive behaviors, wakefulness, and cognition [11]. Under stress, relaxin-3 is expressed in large quantities as a type of excitability neurotransmitter [7], and studies have reported that relaxin-3 can restrain the myocardial apoptosis induced by hyperglycemia [12], and that the over-expression of relaxin-3 can produce over-excitement of neurons, and thus induce excitotoxicity. It also has been reported that the cellular pathways of ERK1/2 and JNK are activated after a combination of relaxin-3 and its receptor RXFP3, increasing the activity of the downstream transcription factor AP-1. However, applying the inhibitor of ERK1/2 (PTX) and the inhibitor of JNK (SP600125) can reverse the activity of these signal pathways [13-15].
C-fos belongs to the downstream proteins of ERK and JNK, and it is also an important component of the transcription factor AP-1. The c-fos of rats has a 94% homology with the c-fos of humans. Under normal conditions, the hippocampus expresses human-like c-fos, which participates in neural cell growth and the atomization process, and is closely related with learning and memory [6,16]. The proper expression of c-fos may have a protective function for brain tissues, but when organs are suffering from stress or injury, the expression of c-fos in large quantities may cause secondary lesions to brain tissue and interfere with the repair of brain tissue [8]. Research shows that traumatic events, such cerebral ischemia-reperfusion injury induce stronger expression of c-fos in hippocampal neurons, which show necrosis and apoptosis to different extents, with the neurons of the CA1 area of the hippocampus being the most seriously affected [17].
Our study also observed the expression level of relaxin-3 and c-fos in the neurons of the hippocampus CA1 area of old rats in different groups. The results showed that on postoperative days 1 and 3, relaxin-3 and c-fos positive neurons in area CA1 of the hippocampus had increased significantly in the model group, while no obvious difference was found on postoperative day 7, which is basically consistent with the results of the behavioral tests (Figures 5 and 6). It may be that the surgical operation caused the release of large quantities of many hormones and neurotransmitters, such as the over-release of hippocampal EAA. Glutamic acid then acted on postsynaptic NMDA and non-NMDA receptors, with high levels of adrenocortical hormones causing oxidative stress [18-19]. Therefore, we think that the stress of the surgery caused the over-expression of relaxin-3 and led to the over-expression of c-fos, thus, inducing neural over-excitation that caused excitotoxicity, which might be the possible mechanism promoting the apoptosis of hippocampal neurons.
Given the limitations of the experimental conditions, we did not find the specific inhibitor of relaxin-3 to determine whether the apoptosis of hippocampal neurons is related to the over-expression of relaxin-3. We chose Dex as the intervention because it can act as a type of adrenergic α2 receptor stimulant; its acts on the nucleus coeruleus, and can reduce the speed of depolarization of locus coeruleus neurons, reduce the release of nucleus ceruleus noradrenaline, and reduce the release of cortisol. Given its calming and analgesic functions in the central nervous system, it can also reduce stress reactions. Studies have shown that it can promote the survival rate of neurons in the CA1 area of the hippocampus and reduce pathological injury to brain tissue caused by ischemia [20].
In this experiment, we administered Dex to old rats before performing abdominal laparotomy and splenectomy, and observed that the escape latency of old rats on postoperative days 1, 3, and 7 were significantly shortened, whereas the number of platform crossings was significantly increased (Figure 1). These results imply that Dex can be effective in preventing POCD. In accordance with research on mechanisms, TUNEL testing and Western Blot testing showed that Dex can considerably up regulate the anti-apoptosis protein Bcl-2, and can downregulate the pro-apoptotic proteins Fas, caspase-8, and caspase-9 in the hippocampus, thus reducing the apoptosis rate of neurons (Figures 2, 3 and 4). The immunohistochemical results showed that the administration of Dex to old rats can substantially reduce the expression of relaxin 3 and c-fos positive neurons in hippocampus area CA1 on postoperative days 1 and 3 (Figures 5 and 6).
In conclusion, POCD in old rats mainly seems to be the result of changes in the expression of pro-apoptosis proteins and anti-apoptosis proteins, which increase the apoptosis rate of neurons. This may be due to a substantial expression of relaxin-3 that produces neurovirulence and stimulates the over-expression of c-fos, thus inducing the apoptosis of hippocampal neurons, which produces POCD. Dex might be able to limit the degree of expression of relaxin-3 and the over-expression of c-fos in the hippocampus, downregulating the pro-apoptosis proteins, Fas, caspase-8, caspase-9, and up-regulating the anti-apoptosis protein Bcl-2. This apparently reduces the occurrence of POCD. However, further research on the exact way that Dex regulates the release of relaxin-3 and on the target cerebral nuclei of Dex are needed.
Acknowledgements
This work was supported by the Shanghai Natural Science Foundation of China (Grant Number: 12ZR1405600).
Disclosure of conflict of interest
None.
References
- 1.Norkiene I, Samalavicius R, Misiuriene I, Paulauskiene K, Budrys V, Ivaskevicius J. Incidence and risk factors for early postoperative cognitive decline after coronary artery bypass grafting. Medicina (Kaunas) 2010;46:460–4. [PubMed] [Google Scholar]
- 2.Cao XZ, Ma H, Wang JK, Liu F, Wu BY, Tian AY, Wang LL, Tan WF. Postoperative cognitive deficits and neuroinflammation in the hippocampus triggered by surgical trauma are exacerbated in aged rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1426–32. doi: 10.1016/j.pnpbp.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Wuri G, Wang DX, Zhou Y, Zhu SN. Effects of surgical stress on long-term memory function in mice of different ages. Acta Anaesthesiol Scand. 2011;55:474–85. doi: 10.1111/j.1399-6576.2011.02402.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Liu W, Ma C, Geng J, Li Y, Li S, Yu F, Zhang X, Cong B. Endoplasmic reticulum stress contributes to CRH-induced hippocampal neuron apoptosis. Exp Cell Res. 2012;318:732–40. doi: 10.1016/j.yexcr.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Veena J, Srikumar BN, Mahati K, Raju TR, Shankaranarayana Rao BS. Oxotremorine treatment restores hippocampal neurogenesis and ameliorates depression-like behaviour in chronically stressed rats. Psychopharmacology (Berl) 2011;217:239–53. doi: 10.1007/s00213-011-2279-3. [DOI] [PubMed] [Google Scholar]
- 6.Pfeilschifter J, Rob P, Mulsch A, Fandrey J, Vosbeck K, Busse R. Interleukin 1 beta and tumour necrosis factor alpha induce a macrophage-type of nitric oxide synthase in rat renal mesangial cells. Eur J Biochem. 1992;203:251–5. doi: 10.1111/j.1432-1033.1992.tb19854.x. [DOI] [PubMed] [Google Scholar]
- 7.Ma S, Olucha-Bordonau FE, Hossain MA, Lin F, Kuei C, Liu C, Wade JD, Sutton SW, Nunez A, Gundlach AL. Modulation of hippocampal theta oscillations and spatial memory by relaxin-3 neurons of the nucleus incertus. Learn Mem. 2009;16:730–42. doi: 10.1101/lm.1438109. [DOI] [PubMed] [Google Scholar]
- 8.Dragunow M, Beilharz E, Sirimanne E, Lawlor P, Williams C, Bravo R, Gluckman P. Immediateearly gene protein expression in neurons undergoing delayed death, but not necrosis, following hypoxic-ischaemic injury to the young rat brain. Brain Res Mol Brain Res. 1994;25:19–33. doi: 10.1016/0169-328x(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 9.Sanders RD, Xu J, Shu Y, Januszewski A, Halder S, Fidalgo A, Sun P, Hossain M, Ma D, Maze M. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110:1077–85. doi: 10.1097/ALN.0b013e31819daedd. [DOI] [PubMed] [Google Scholar]
- 10.Hofer S, Steppan J, Wagner T, Funke B, Lichtenstern C, Martin E, Graf BM, Bierhaus A, Weigand MA. Central sympatholytics prolong survival in experimental sepsis. Crit Care. 2009;13:R11. doi: 10.1186/cc7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka M. Relaxin-3/insulin-like peptide 7, a neuropeptide involved in the stress response and food intake. FEBS J. 2010;277:4990–7. doi: 10.1111/j.1742-4658.2010.07931.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Ma X, Zhao M, Zhang B, Chi J, Liu W, Chen W, Fu Y, Liu Y, Yin X. H2 and H3 relaxin inhibit high glucose-induced apoptosis in neonatal rat ventricular myocytes. Biochimie. 2015;108:59–67. doi: 10.1016/j.biochi.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 13.van der Westhuizen ET, Christopoulos A, Sexton PM, Wade JD, Summers RJ. H2 relaxin is a biased ligand relative to H3 relaxin at the relaxin family peptide receptor 3 (RXFP3) Mol Pharmacol. 2010;77:759–72. doi: 10.1124/mol.109.061432. [DOI] [PubMed] [Google Scholar]
- 14.van der Westhuizen ET, Werry TD, Sexton PM, Summers RJ. The relaxin family peptide receptor 3 activates extracellular signal-regulated kinase 1/2 through a protein kinase C-dependent mechanism. Mol Pharmacol. 2007;71:1618–29. doi: 10.1124/mol.106.032763. [DOI] [PubMed] [Google Scholar]
- 15.Shen CP, Tsimberg Y, Salvadore C, Meller E. Activation of Erk and JNK MAPK pathways by acute swim stress in rat brain regions. BMC Neurosci. 2004;5:36. doi: 10.1186/1471-2202-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wendel M, Petzold A, Koslowski R, Kasper M, Augstein A, Knels L, Bleyl JU, Koch T. Localization of endothelin receptors in bleomycininduced pulmonary fibrosis in the rat. Histochem Cell Biol. 2004;122:507–17. doi: 10.1007/s00418-004-0708-7. [DOI] [PubMed] [Google Scholar]
- 17.Wittner M, Sivenius J, Koistinaho J. Alpha2-adrenoreceptor agonist, dexmedetomidine, alters acute gene expression after global ischemia in gerbils. Neurosci Lett. 1997;232:75–8. doi: 10.1016/s0304-3940(97)00585-5. [DOI] [PubMed] [Google Scholar]
- 18.Allen CE, Worsley MA, King AE, Boissonade FM. Fos expression induced by activation of NMDA and neurokinin-1 receptors in the trigeminal subnucleus caudalis in vitro: role of protein kinases. Brain Res. 2011;1368:19–27. doi: 10.1016/j.brainres.2010.10.072. [DOI] [PubMed] [Google Scholar]
- 19.Reyes EP, Abarzua S, Martin A, Rodriguez J, Cortes PP, Fernandez R. LPS-induced c-Fos activation in NTS neurons and plasmatic cortisol increases in septic rats are suppressed by bilateral carotid chemodenervation. Adv Exp Med Biol. 2012;758:185–90. doi: 10.1007/978-94-007-4584-1_26. [DOI] [PubMed] [Google Scholar]
- 20.Sato K, Kimura T, Nishikawa T, Tobe Y, Masaki Y. Neuroprotective effects of a combination of dexmedetomidine and hypothermia after incomplete cerebral ischemia in rats. Acta Anaesthesiol Scand. 2010;54:377–82. doi: 10.1111/j.1399-6576.2009.02139.x. [DOI] [PubMed] [Google Scholar]


