Abstract
Research on the relationship between aberrant long non-coding RNA (lncRNA) and cancer stem cell (CSC) biology in cancer patients has been recently gaining attention. The goal of this study was to investigate whether the decreasing lncRNA HOTAIR expression would inhibit human colorectal cancer (CRC) stem cells. CD133+CSCs were isolated from human CRC LoVo cell line by using a magnetic-activated cell sorting system, and were transfected with the expression vector-based small hairpin RNA targeting HOTAIR (shHOTAIR). The ability of cellular proliferation, migration, invasion, colony-forming, and the epithelial-mesenchymal transition (EMT)-associated molecule expression as well as the tumorigenicity of CD133+-shHOTAIR were evaluated by the MTT, wound-healing, cellular invasion, colony formation and Western blot assays, respectively. This study found that, when compared with control cells in vitro, CD133+-shHOTAIR exhibited the decreased HOTAIR expression, suppressed cellular proliferation, migration, invasion, colony-forming, and inhibited the Vimentin expression with increased E-cadherin expression. In particular, the down-regulation of the HOTAIR expression in CD133+CSCs markedly attenuated the tumor growth and lung metastasis in xenograft nude mice. Taken together, this study found that down-regulating the HOTAIR expression in CD133+CSCs could serve as a potential anti-cancer regimen to inhibit the invasiveness and metastasis of CRC CSCs.
Keywords: Human colorectal cancer, cancer stem cells, lincRNA HOTAIR, RNA interference, epithelial-mesenchymal transition
Introduction
Human colorectal cancer (CRC) is one of most common malignant tumors and it accounts for a large proportion of cancer-related deaths worldwide. This high mortality diease may be a subset of cells with stem/progenitorcell features known as cancer stem cells (CSCs) that lead to advanced tumors and a poor prognosis [1,2]. CSCs are responsible for tumor-initiating potential, invasion, metastasis, resistance to traditional therapies and eventual relapse [2,3]. The CSC model can comprehensibly explain essential clinical events that were insufficiently understood, such as therapy resistance, minimal residual disease, and tumor recurrence. A lot of effort has been made in the last decade to advance the research in this field, however, the complex biology of CRC CSCs and the underlying pathogenic mechanisms have remained largely unknown [4,5]. More recent studies have focused on the molecular mechanisms of the underlying CRC CSCs progression, the genes responsible for CSC chemoresistance, and the new therapies against CRC CSCs [6-9].
Long non-coding RNAs (lncRNAs) are defined as transcripts having longer than 200 nucleotides of which are 50 capped and 30 are polyadenylated, However, this class of transcripts has limited coding potential. LncRNAs can regulate gene expression at the epigenetic level, play an important role in differentiation, proliferation, apoptosis and invasiveness of tumor cells, and contribute to the metastatic capacity of cancers [9-12]. HOX transcript antisense RNA (HOTAIR) is one of lncRNAs; it interacts with polycomb repressive complex 2 and is responsible for histone H3 lysine-27 trimethylation of the HOD locus. Furthermore, aberrant HOTAIR expression has been markedly associated with metastasis and poor clinical outcomes in different tumor types including breast cancer [13,14], colorectal cancer [8,15], pancreatic carcinoma [16], hepatocellular carcinoma [17,18], gastrointestinal stromal tumor [19], and human epithelial ovarian cancer [20,21], etc. However, how the HOTAIR complex functions in CRC CSCs remains unclear [14,20,22]. To this end, we sought to investigate whether the knockdown of the endogenous HOTAIR expression by siRNA would attenuate the human CRC LoVo CD133+CSC’s invasion and metastasis in vitro and if the tumorigenicity as well as metastasis in xenograft nude mice were involoved in decreasing an epithelial-mesenchymal transition (EMT). We found that epigenetic silencing of HOTAIR in human CRC LoVo CD133+CSCs decreased cellular stemness, self-renewal, EMT, and tumorigenicity in vitro and in the nude mouse model.
Materials and methods
Cell line
Human CRC LoVo cell line was acquired from CRC patients as a generous gift from Professor Peiling Huang, Department of Pathology, School of Medicine, Southeast University, Nanjing, China. Cells were cultured in complete media consisting of RPMI 1640, 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, and 10% fetal bovine serum. The medium was refreshed every 3 days to maintain adherent cells. When LoVo cells reached 90% confluence, cells were harvested with 0.25% trypsin-1 mM EDTA (Sigma-Aldrich, St. Louis, MO, USA) treatment for 2 min.
The short hairpin RNA sequence and pSUPER-EGFP1-HOTAIR-shRNA recombinant
A short hairpin RNA sequence of lncRNA HOTAIR (Gene ID: 100124700) was designed as previously describled [13,20]. The shRNA sequences were as follows: pSUPER-EGFP1 (enhanced green fluorescent protein 1)-HOTAIR-shRNA (pSUPER-EGFP1-shHOTAIR), Forward 5’-GATCCCCGAACGGGAGTACAGAGAGATTCAAGAGATCTCTCTGTACTC C C GTTCTTTTTGGAAA-3’; antisense, 5’-AGCTT TTCCAAAAAGAACGGGAGTACAG AGAGATCTCTTGAATCTCTCTGTACTCCCGTTCGGG-3’; scramble-siRNA: sense, 5’-G ATCCCCTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGATTT TTGGAAA-3’; antisense, 5’-AGCTTTTCCAAAATTCTCCGAACGTGTCACGTTCTCTT GAAACGTGACACGTTCGGAGAAGGG-3’. All the primers were synthesized by Gene and Technology of China in Shanghai. A pSUPER-EGFP1 vector was used to construct the recombinant. The recombinant pSUPER-EGFP1-HOTAIR-shRNA (ShHOTAIR) was developed as previously describled [13,20] . A pSUPER-EGFP1-HOTAIR-scramble (Scramble) was used as a negative control. These recombinants were verified by the analysis of endonuclease digestion and sequencing.
Isolation of CD133+CSCs and production of stably transfected clones
CD133+CSCs were sorted from the LoVo cell line by using the magnetic-activated cell sorting (MACS, Miltenyi Biotec., Bergisch Gladbach, Germany). To obtain the ShHOTAIR-expressing cells, the ShHOTAIR and Scramble were respectively transfected into CD133+CSCs, and the stably transfected clones were selected with G418 (Clontech, CA). The ShHOTAIR and Sc-HOTAIR-expressing clones were labeled ‘CD133+CSCs ShHOTAIR’ (CD133+-shHOTAIR) and ‘CD133+CSCs Scramble’ (CD133+-Scramble ), respectively [23,24]. CD133+CSCs without any transfectiion were labeled ‘CD133+ wild type (WT)’. The isolated cells were placed in the stem cell culture medium by resuspension in serum-free DMEM/F12 supplemented with 5 μg/mL insulin (Sigma-Aldrich, Missouri, USA), 20 ng/mL human recombinant epidermal growth factor (Invitrogen, CA, USA), 10 ng/mL basic fibroblast growth factor (Invitrogen, CA, USA) and 0.5% bovine serum albumin (Sigma-Aldrich, Missouri, USA) [24,25].
Quantitative RT-PCR
To evaluate the expression of HOTAIR, total RNA were used for the reverse transcription (RT) reactions, and qRT-PCR was performed on a Step One Plus real-time system (AB Applied Biosystems) [26]. The cDNAs were amplified by PCR with primers as follows: HOTAIR: sense, 5’-GGTAGAAAAAGCAACCACGAAGC-3’; antisense, 5’-TTGGGGAAGCATTTTCTGA C-3’; β-actin (sense, 5’-GGACTTCGAGCAAGAGATGG-3’; antisense, 5’-AGCACTGTGTT GGCGTACAG3’). The mRNA-level of the genes of interest were expressed as the ratio of a gene of interest to β-actin mRNA for each sample. The comparative Ct (ΔΔCt) method was used to determine the expression fold change [27].
Proliferative assay
2×103 CD133+-ShHOTAIR or CD133+-Scramble or CD133+-WT cell suspensions were seeded into 96-well plates and were assayed for proliferative activity in triplicate wells. To assay cell viability, a cell suspension was mixed with 0.4% Trypan blue (Sigma) in 1, 2, 3, 4, 5, and 6 days, respectively, after the incubation; the mean values of the viable counts were obtained by a Neubauer hemocytometer chamber [28].
Colony forming assay
The colony formation ability of the LoVo CD133+-ShHOTAIR or CD133+-Scramble or CD133+WT cells was investigated. A colony with a diameter larger than 75 µm or having more than 50 cells was counted for 1 positive colony according to our previous report [28,29]. The clone formation rate of common dish and soft agar was calculated as (number of colony/number of cells inoculated) ×100%.
Cell migration assay
To determine the effect of down-regulated HOTAIR expression on migration, the CD133+- ShHOTAIR cells were used in the wound healing assay. Briefly, the cells of CD133+ShHOTAIR, CD133+-Scramble, and CD133+-WT cells were plated, respectively, in 6-well plates (5×105 cells per well) to form a monolayer one day before the assay; non-adherent cells were removed by PBS washing. On the following day, a uniform scratch was made down in the center of the well using a sterile micropipette tip. The distance travelled by the cells was respectively measured between the two boundaries of the acellular area at 0, 12, and 24 hours, respectively, after the incubation. Each experiment was performed in triplicate [30].
Cell invasion assay
The invasion ability of the CD133+-ShHOTAIR, CD133+-Scramble, and CD133+-WT cells was evaluated by using the transwell invasion assay. Briefly, the transwell inserts with 8 μm pores were coated with Matrigel (20 μg/well; Becton Dickinson, Waltham, MA, USA); the cells were seeded in the upper chamber in the RPMI1640 medium supplemented with 10% fetal bovine serum. After incubation at 37°C, the cells that invaded to the lower surface of the Matrigel-coated membranes were fixed with 70% ethanol and stained with trypan blue, and counted under a light microscope as described previously [20,31].
Western blot
1×106 CD133+-ShHOTAIR, CD133+-Scramble, and CD133+-WT cells were respectively collected and lyzed in a protein extraction buffer (Novagen, Madison, WI, USA). A 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed, and proteins (10 μg/lane) were loaded in the way reported in the pulished papers [20,32]. The rabbit antibody specific to human E-cadherin (code number: 31955) or Vimentin (code number: 57415) was used in the assay (Bioworld Technology, Dublin, OH, USA).
Tumorigenicity of CD133+CSCs-ShHOTAIR in xenograft mice
Balb/c nude mice (female, age between 5 and 6 weeks, and weighting 16-18 g) were ordered from the Animal Center of Yang Zhou University of China and were raised under sterile conditions at the Experimental Animal Center, School of Medicine, Southeast University. The experiments were performed in compliance with the guidelines of the Animal Research Ethics Board of Southeast University, China. Eighteen nude mice were randomly divided into three groups of equal size (six per group): the CRC CD133+-ShHOTAIR group, the CRC CD133+-Scramble group, and the CD133+-WT group. The three groups of the nude mice were injected in the back subcutaneously with the 5×104 CRC CD133+-ShHOTAIR, CRC CD133+-Scramble, and CD133+-WT cells, respectively. Tumor formation in each mouse was monitored every three days by taking 2-dimensional measurements of a tumor [33].
Lung histopathology
Lung tissues were removed from the xenograft mice, fixed in 10% formalin, and then embedded in paraffin. Sections of 5 μm tissue in size were cut and mounted on SuperFrost Plus glass slides, and were fixed in methanol and stained in hematoxylin and eosin (HE). The slides were examined under a Zeiss Axioplan light microscope at the magnification of ×200 [34].
Immunohistochemistry
The 5 μm-thin formalin fixed and paraffin-embedded samples on the slides were incubated with the rabbit antibody specific to human Vimentin or E-cadherin overnight at 4°C. The samples were then labeled with HRP-conjugated streptavidin (Invitrogen company, CA, USA), and the chromogenic reaction was developed using Liquid DAB Substrate Pack according to the manufacturer’s instructions. E-cadherin/Vimentin-stained cells from random and non- overlapping fields were counted under a miscroscope at the magnification of ×200 as described previously [33,35].
Statistical analysis
Values of interest were presented in plots the mean plus and minus two standard deviation. The data were analyzed for difference by using a two-tailed paired Student’s t test; p values less than 0.05 were considered statistically significant.
Results
HOTAIR expression in CRC CD133+CSCs and collection of stably transfected CD133+-shHOTAIR clones
Recent evidence has shown that the CD133 molecule is a specific marker of CRC CSCs [4,9,36-38]. To search for a novel therapeutic target to treat CRC, we first sorted the CD133+ cells, presumptively CRC CSCs, from the Human CRC LoVo cell line to find out if the HOTAIR expression was higher in CRC CD133+CSCs than in the wild type of CRC. Figure 1A shows the high expression of HOTAIR in CD133+CSCs in contrast with the wild type of CRC LoVo cells (p<0.0474). The HOTAIR expression was significantly decreased in CRC CD133+-ShHOTAIR compared with CD133+-Scramble (p < 0.0065) as is shown in Figure 1B. The CD133+-ShHOTAIR clones were isolated through the single-clone isolation assay after the antibiotic selection with 800 µg/ml G418 for 14 days. A representative set of data from the selected clones were expanded into the cells that were visible under a fluorescence microscope, which is shown at the photos of the bottom-panel in Figure 1C. The same clones (at the top panel in Figure 1C) were observed under a light microscope. Morphology of the CD133+-ShHOTAIR appeared round or elliptical (epithelium-like features at the right-panel in Figure 1D), while the CD133+-Scramble were spindle-shaped (mesenchyma-like features at the left-panel in Figure 1D).
Figure 1.
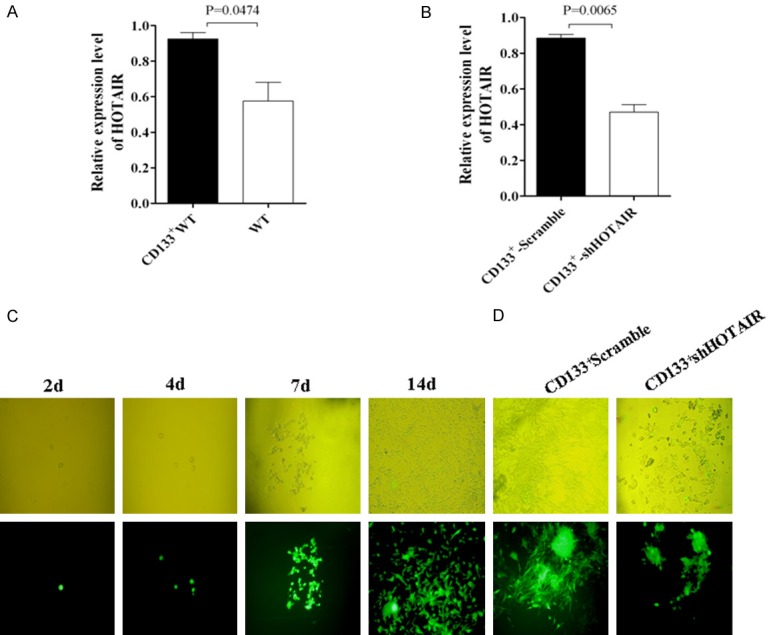
Detection of HOTAIR expression in LoVo CD133+CSCs and single-CD133+-ShHOTAIR clone isolation. A. HOTAIR expression in CD133+CSCs and wild type of LoVo cells identified by qRT-PCR. B. HOTAIR expression in CD133+CSCs-ShHOTAIR and CD133+CSCs-Scramble cells identified by qRT-PCR. C. A single-CD133+-ShHOTAIR clone isolated from the ShHOTAIR transfected CD133+ clones with 800 μg/ml G418 on Day 2, Day 4, Day 7, and Day 14, respectively, viewed under a light microscope (top) or a fluorescence microscope (bottom). D. The selected CD133+-ShHOTAIR or CD133+-Scramble clones were viewed under a light microscope (top) or fluorescence microscope (bottom). The p values were from the Student’s t test and indicated if the differences were statistically significant (p < 0.05).
CD133+-ShHOTAIR reduce its ability of migration, invasion, proliferation, clone and EMT-associated molecule expression
Since HOTAIR plays an important regulatory role in the malignant tumor progression by regulating cell cycle, apoptosis, invasion and metastasis, the HOTAIR expression correlates highly with some epithelial tumor metastasis and invasion [11,12]. Therefore, we investigated the effect of down-regulated HOTAIR expression by RNA interference in CD133+CSCs on the ability of proliferation, migration, invasion, clone and EMT-associated molecule expression. We found that CD133+-ShHOTAIR significantly reduced its ability of invasion, migration, proliferation, and clone formation compared with CD133+-Scramble or with CD133+CSCs (CD133+WT) after the HOTAIR expression was down-regulated in CD133+CSCs that had been stably transfected with the recombinant shHOTAIR. The results are shown in Figure 2A-E, and Figure 3A and 3B. Additionally, the EMT-associated molecule expression was remarkably changed in CD133+-ShHOTAIR (Figure 3C), indicating the increase of the E-cadherin expression and the reduction of the Vimentin/N-cadherin expression in comparision with CD133+-Scramble and CD133+WT, respectively; all the differences were statistically significant (p<0.05) (see Figure 3D). The elliptical epithelium features (right-panel in Figure 1D) of CD133+-ShHOTAIR and the spindle-shaped mesenchymal features (left-panel in Figure 1D) of CD133+-Scramble were markedly presented.
Figure 2.
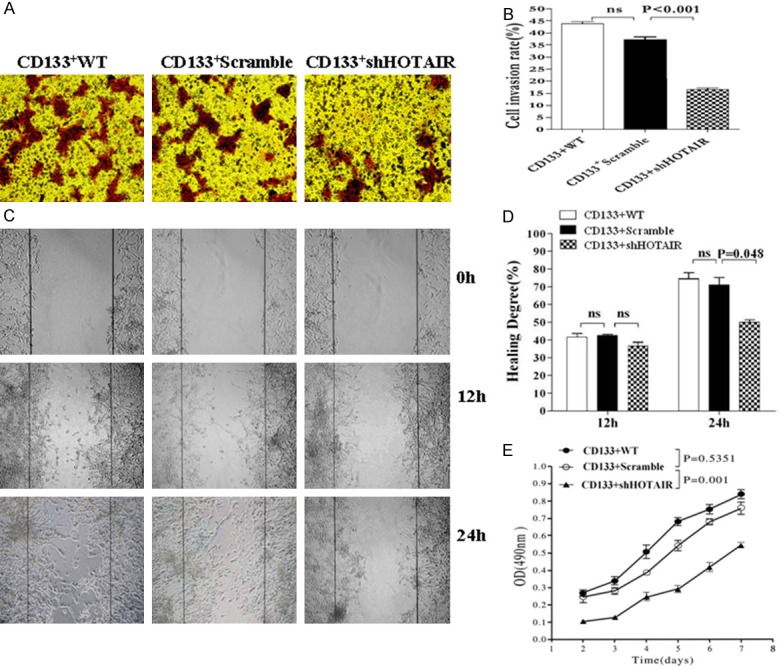
Decrease in the ability of invasion, migration and proliferation of CD133+CSCs-ShHOTAIR in vitro. (A) Invasive assay: 5×105 CD133+WT, CD133+-Scramble and CD133+-ShHOTAIR cells were respectively seeded in the upper chamber in RPMI1640 with serum-free. Cells that invaded to the lower surface of the matrigel-coated membranes were fixed with 70% ethanol, and stained with trypan blue. (B) Quantification of the invasive assay results: Cells from five randomly selected fields were counted under a light microscope (magnification ×100). (C) Wound healing assay: 5×105 cells of each of the three types in (A) were respectively plated in 6-well plates to form a monolayer, and a uniform scratch was made down the center of the wells. (D) Quantification of the wound healing assay results: Wound closure is presented as the percentage reduction of the freshly wounded area (viewed under a light microscopy, ×200). (E) The different cell proliferation was performed in triple wells and a mean value of the viable cells was counted in triple wells every day by MTT assay. The p values were from the Student’s t test and indicated if the differences were statistically significant (p < 0.05).
Figure 3.
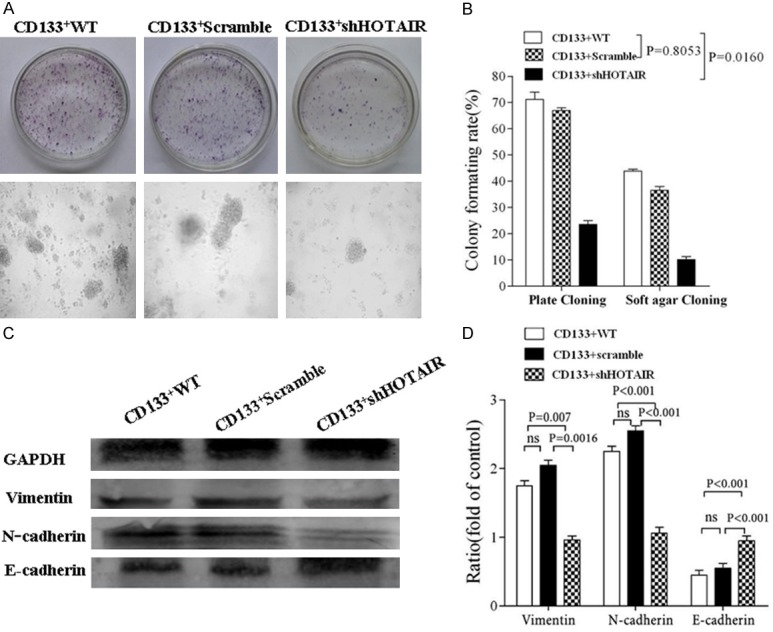
Detection of clone ability and EMT-associated molecule expression. A. Images of different clones on 11 day culture in the culture plate (top) and in the soft agar media (bottom) after 5×105 CD133+WT, CD133+-Scramble and CD133+-ShHOTAIR cells were respectively seeded in the different media (viewed under a light microscopy, ×100). B. Comparisons of the colony formative rate among the three types of cells. C. Western blot assay for detection of the expression of E-cadherin, N-cadherin and Vimentin in the CD133+WT, CD133+-Scramble and CD133+-ShHOTAIR cells, respectivley. D. Quantification of the Western blot results. The p values were from the Student’s t test and indicated if the differences were statistically significant (p < 0.05).
Silence of HOTAIR in CRC CD133+CSCs inhibites the tumor growth and lung metastasis in the xenograft mice
Having found that the effects of the down-regulated HOTAIR expression on the proliferation, migration, invasion, clone formation of CRC LoVo CD133+-ShHOTAIR in vitro, we wanted to know whether this effect would alter the cellular tumorigenicity and metastatic potential in the Balb/c nude mice. Figure 4A shows the representative images of the tumor bearing nude mice on day 30 after implantation. All 6 mice developed visible tumors in 9 days after being injected with 5×105 differently-treated CRC LoVo CD133+-WT (two on Day 6, (two on Day 9, one on Day 12, and one on Day 15, respectively). The six mice injected with 5×105 CD133+-Scramble developed the visible tumors on Day 9, Day 12, Day 12, Day 12, Day 15, and Day 15, respectively. In contrast, only one of the six mice injected with 5×105 CD133+-ShHOTAIR developed a visible tumor on Day 21, and the remaining 5 mice did not develop any tumor throughout the 45-day observation period. Figure 4B presents the photos of the tumor size and quantity. The percentages of the tumor volume and the tumor-free mice in the three groups are shown in Figure 4C and 4D. The tumor growth was significantly inhibited in the mice injected with CD133+-ShHOTAIR in comparison with the mice injected with CD133+-WT (*p < 0.001) or with CD133+-Scramble (*p < 0.0064).
Figure 4.
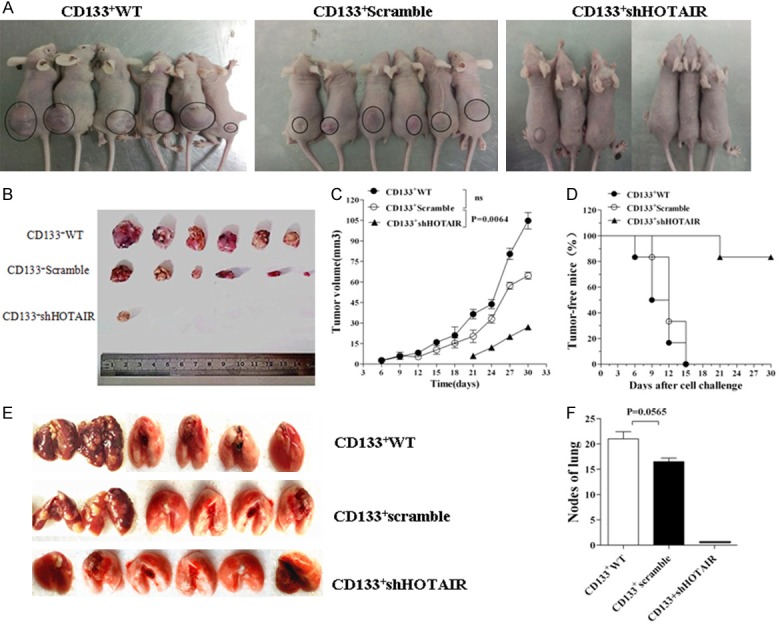
CD133+-ShHOTAIR reduce its tumorigenicity and metastatic potential in mice. A. Images represent the tumor growth in the nude mice 30 days after they were injected with 5×105 LoVo CD133+WT or CD133+-Scramble or CD133+-shHOTAIR. B. Images of tumor tissue sizes and quantity in 30 days. C, D. Tumor volume and the tumor-free mice injected with the CD133+WT or CD133+-Scramble or CD133+-shHOTAIR. E. Images of lung tissues taken from the mice 30 days after they were injected with the CD133+WT or CD133+-Scramble, and lung tissue images from the mice 45 days after they were injected with the CD133+-shHOTAIR. Presence of metastatic tumor nodes in lungs is visible in the mice injected with the CD133+WT or the CD133+-Scramble. F. Quantification of the lung metastatic tumor nodes, referring to the differences as indicated, which was analyzed by using the Student’s t test method.
To evalue the effect of the down-regulation of the HOTAIR expression in CD133+CSCs on tumor metastasis, the lung tissues of the mice were assessed by examining tumor nodes. The silence of HOTAIR inhibited the tumor cell lung metastasis and no tumor nodes were found in the lung tissues of the nude mice 45 days after they were injected with CD133+-ShHOTAIR. On the other hand, in the mice injected with CD133+-WT or CD133+-Scramble, the tumor nodes were found in their lung tissues (Figure 4E). The difference between the CD133+-ShHOTAIR group and the other two groups was statistically significant; the difference between the CD133+-WT and the CD133+-Scramble groups was not statistically significant (Figure 4F). Based on these findings, we concluded that the tumor cell growth and metastasis was remarkablely inhibited in the mice injected with CD133+-ShHOTAIR.
Decrease of the EMT-associated molecular expression in tumor tissues of mice injected with the CD133-ShHOTAIR
We next extended these studies to determine whether the EMT-associated molecular expression was changed in the tumor tissues of the mice injected with CD133-ShHOTAIR. We evaluated the expression of E-cadherin and Vimentin by using the immunohistochemistry assay. Figure 5A shows that the E-cadherin expression (brown cells pointed to by the white arrows) in the xenograft tumor tissues was significantly increased in the CD133+-ShHOTAIR group compared with the CD133+-WT group and the CD133+-Scramble group; the difference was statistically significant. The expression of Vimentin was notably reduced in the CD133+-ShHOTAIR group compared with the CD133+-WT group and the CD133+-Scramble group; the difference was statistically significant (Figure 5B). Similar findings were obtained from the Western blot assay (Figure 5C), and the difference between the CD133+-ShHOTAIR group and the other two groups was statistically significant (Figure 5D).
Figure 5.
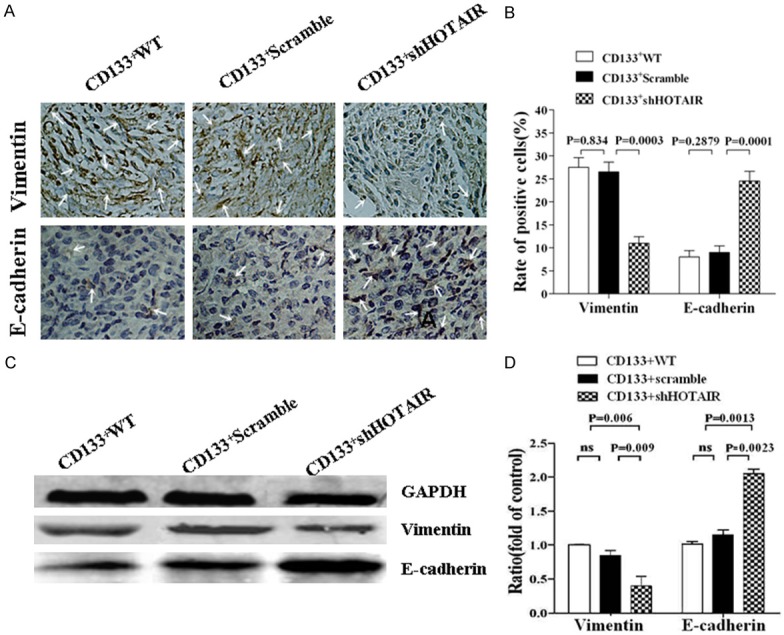
Analysis of EMT-associated molecular expression in the tumor tissues of nude mice. A. The expression of E-cadherin and Vimentin (pointed to by white as arrows) in the tumor tissues detected by immunohistochemistry assay; B. Quantification of the expression of E-cadherin and Vimentin in the tumor tissues; C. The expression of E-cadherin and Vimentin in the tumor tissues analyzed by Western blot; D. Relative intensity of protein expression. The p values were from the Student’s t test and indicated if the differences were statistically significant (p < 0.05).
Discussion
CRC is considered one of the main causes of death from neoplasia, and is characterized by a high rate of recurrence and heterogeneity. CRC CSCs may well contribute to both of these pathological properties, but the mechanisms underlying their self-renewal and stem characteristic maintenance is insufficiently understood [38]. Although HOTAIR has been associated with metastasis and poor prognosis in different tumor types, an in-depth characterization of its functions in CRC CSCs is still needed. In this study, we focused on the relationship between the downregulated HOTAIR expression and the tumorigenicity and metastasis in human CRC CSCs.
We found significant differences in the ability of proliferation and cloning between CD133+-ShHOTAIR and both of CD133+-Scramble and CD133+-WT from the assessement by using MTT and colony forming assays. In addition, the migration and invasion ability of CD133+-ShHOTAIR in vitro was also reduced, suggesting that the decreased HOTAIR expression in CD133+-WT led to the inhibition of a highly self-renewal, metastasis, and infiltration ability, which are the properties harbored by CSCs. Similar efficiency was observated in vivo, where the mice injected with CD133+-ShHOTAIR developed fewer tumors than that of the mice injected with CD133+-WT or CD133+-Scramble. CSCs are known to be responsible for propagating cancer in a highly efficient manner [36,39]. We observed that, in both the CD133+-WT and CD133+-Scramble groups, all the six mice developed tumors in 9 to 15 days, and had a distant lung metastasis. In contrast, in the CD133+-ShHOTAIR group of six mice, only one developed one tumor on Day 21, and without any evidence of metastasis. This disparity in tumorigenicity and lung metastasis from this in vivo animal experiment suggested that the downregulated HOTAIR expression may be closely associated with the CRC CSCs’ tumorigenicity and metastasis. However, further validation in independent studies along this line of research is highly recommended.
The mechanisms of CD133+-ShHOTAIR for decreasing the propagating of tumor and for inhibition of tumor’s distant metastasis have remained unknown. We investigated the EMT characteristics of the tumor tissue cells from the xenograft mice. Because the cellular epithelial (epithelium) and interstitial (mesenchyma) state conversions indicate the typical phenotype changes of EMT in the process of tumor cell growth, [38] the CRC epithelial cells can gain CSC characteristics through the EMT program to initiate a distant metastasis. As expected, the findings from the Western blot and Immunohistochemistry analyses showed the marked decrease in the Vimentin expression accompanied by notable increase in the E-cadherin expression in the tumor tissues of the mice injected with CD133+-ShHOTAIR. In contrast, only small changes were found in the mice injected with CD133+-WT or CD133+-Scramble. The results suggested the decrease in the HOTAIR expression in CRC CD133+CSCs was associated with the alterations to some specific EMT markers and, concurrently with reduced migratory potential [12,19]. Nevertheless, the mechanism for the downregulated HOTAIR expression to reduce the tumorgeniesis and to inhibit the metastasis of CRC CD133+CSCs warrants further study.
In summary, the fingings from our study demonstrated that the down-regulation of the HOTAIR expression in CRC CD133+CSCs decreased its tumorgeniesis and metastasis potential, which means the inhibition of CRC CD133+CSC EMT. Therefore, manipulating the downregulated HOTAIR expression may be a promising alternative therapeutic strategy for targeting treatment that targets CRC CD133+CSC mediated metastasis through inhibiting the EMT program.
Acknowledgements
This work was supported in part by the Collaborative Innovation Center of Suzhou Nano-Science and Technology, and part by the National Natural Science Foundation of China (No. 81572887, 81202372), and the Scientific Research Foundation of Graduated School of Southeast University (No. KYLX_0195), and the Jiangsu Planned Projects for Postdoctoral Research Funds (1301099C), and the China Postdoctoral Science Foundation (2013M530227).
Disclosure of conflict of interest
None.
References
- 1.Shin HK, Kim MS, Lee JK, Lee SS, Ji YH, Kim JI, Jeong JH. Combination effect of cetuximab with radiation in colorectal cancer cells. Tumori. 2010;96:713–720. doi: 10.1177/030089161009600513. [DOI] [PubMed] [Google Scholar]
- 2.Svoboda M, Slyskova J, Schneiderova M, Makovicky P, Bielik L, Levy M, Lipska L, Hemmelova B, Kala Z, Protivankova M, Vycital O, Liska V, Schwarzova L, Vodickova L, Vodicka P. HOTAIR long non-coding RNA is a negative prognostic factor not only in primary tumors, but also in the blood of colorectal cancer patients. Carcinogenesis. 2014;357:1510–1515. doi: 10.1093/carcin/bgu055. [DOI] [PubMed] [Google Scholar]
- 3.Pan Q, Li Q, Liu S, Ning N, Zhang X, Xu Y, Chang AE, Wicha MS. Concise Reviews: Targeting Cancer Stem Cells Using Immunologic Approaches. Stem Cells. 2015;33:2085–92. doi: 10.1002/stem.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang ZL, Zheng Q, Yan J, Pan Y, Wang ZG. Upregulated CD133 expression in tumorigenesis of colon cancer cells. World J Gastroenterol. 2011;17:932–937. doi: 10.3748/wjg.v17.i7.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dou J, Gu N. Emerging strategies for the identification and targeting of cancer stem cells. Tumor Biol. 2010;31:243–253. doi: 10.1007/s13277-010-0023-y. [DOI] [PubMed] [Google Scholar]
- 6.Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, Clevers H. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 7.Garza-Treviño EN, Said-Fernández SL, Martínez-Rodríguez HG. Understanding the colon cancer stem cells and perspectives on treatment. Cancer Cell Int. 2015;15:2. doi: 10.1186/s12935-015-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pádua Alves C, Fonseca AS, Muys BR, de Barros E Lima Bueno R, Bürger MC, de Souza JE, Valente V, Zago MA, Silva WA Jr. The lincRNA Hotair is required for epithelial- to-mesenchymal transition and stemness maintenance of cancer cell lines. Stem Cells. 2013;31:2827–2832. doi: 10.1002/stem.1547. [DOI] [PubMed] [Google Scholar]
- 9.Lang J, Lan X, Liu Y, Jin X, Wu T, Sun X, Wen Q, An R. Targeting cancer stem cells with an 131Ilabeled anti-AC133 monoclonal antibody in human colorectal cancer xenografts. Nucl Med Biol. 2015;42:505–512. doi: 10.1016/j.nucmedbio.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, He L, Du Y, Huang G, Luo J, Yan X, Ye B, Li C, Xia P, Zhang G, Tian Y, Chen R, Fan Z. The Long Noncoding RNA lncTCF7 Promotes Self-Renewal of Human Liver Cancer Stem Cells through Activation of Wnt Signaling. Cell Stem Cell. 2015;16:413–425. doi: 10.1016/j.stem.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23:1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai B, Song XQ, Cai JP, Zhang S. H OTAIR: a cancer-related long non-coding RNA. Neoplasma. 2014;61:379–391. doi: 10.4149/neo_2014_075. [DOI] [PubMed] [Google Scholar]
- 13.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, Cai K, Wang J, Wang X, Cheng K, Shi F, Jiang L, Zhang Y, Dou J. MiR-7, inhibited by lincRNA HOTAIR, directly inhibites SETDB1 and reverses the EMT of breast cancer stem cells by downregulating the STAT3 pathway. Stem Cell. 2014;32:2858–2868. doi: 10.1002/stem.1795. [DOI] [PubMed] [Google Scholar]
- 15.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, Miyano S, Mori M. Long Noncoding RNA HOTAIR Regulates Polycomb-Dependent Chromatin Modification and Is Associated with Poor Prognosis in Colorectal Cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 16.Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, Kim S, Safe S. HOTAIR is a negative prognostic factor and exhibits prooncogenic activity in pancreatic cancer. Oncogene. 2013;32:1616–1625. doi: 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geng YJ, Xie SL, Li Q, Ma J, Wang GY. Large intervening non-coding RNA HOTAIR is associated with hepatocellular carcinoma progression. J Int Med Res. 2011;9:2119–2128. doi: 10.1177/147323001103900608. [DOI] [PubMed] [Google Scholar]
- 18.Ishibashi M, Kogo R, Shibata K, Sawada G, Takahashi Y, Kurashige J, Akiyoshi S, Sasaki S, Iwaya T, Sudo T, Sugimachi K, Mimori K, Wakabayashi G, Mori M. Clinical significance of the expression of long non-coding RNA HOTAIR in primary hepatocellular carcinoma. Oncol Rep. 2013;29:946–950. doi: 10.3892/or.2012.2219. [DOI] [PubMed] [Google Scholar]
- 19.Niinuma T, Suzuki H, Nojima M, Nosho K, Yamamoto H, Takamaru H, Yamamoto E, Maruyama R, Nobuoka T, Miyazaki Y, Nishida T, Bamba T, Kanda T, Ajioka Y, Taguchi T, Okahara S, Takahashi H, Nishida Y, Hosokawa M, Hasegawa T, Tokino T, Hirata K, Imai K, Toyota M, Shinomura Y. Upregulation of miR-196a and HOTAIR drive malignant character in gastrointestinal stromal tumors. Cancer Res. 2012;72:1126–1136. doi: 10.1158/0008-5472.CAN-11-1803. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Chen D, He X, Zhang Y, Shi F, Wu D, Chen J, Zhang Y, Zhao F, Dou J. Downregulated lincRNA HOTAIR expression in ovarian cancer stem cells decreases its tumorgeniesis and metastasis by inhibiting epithelial-mesenchymal transition. Cancer Cell Int. 2015;15:24. doi: 10.1186/s12935-015-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu JJ, Lin YY, Ye LC, Ding JX, Feng WW, Jin HY, Zhang Y, Li Q, Hua KQ. Overexpression of long non-coding RNA HOTAIR predicts poor patient prognosis and promotes tumor metastasis in epithelial ovarian cancer. Gynecol Oncol. 2014;134:121–128. doi: 10.1016/j.ygyno.2014.03.556. [DOI] [PubMed] [Google Scholar]
- 22.Yang G, Zhang S, Gao F, Liu Z, Lu M, Peng S, Zhang T, Zhang F. Osteopontin enhances the expression of HOTAIR in cancer cells via IRF1. Biochim Biophys Acta. 2014;1839:837–848. doi: 10.1016/j.bbagrm.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Wang J, Chen DY, Yang J, Yang C, Zhang Y, Zhang H, Dou J. Evaluation of characteristics of CD117+CD44+ ovarian cancer stem cells in three dimensional basement membrane extract scaffold versus two dimensional monocultures. BMC Cell Biol. 2013;14:7. doi: 10.1186/1471-2121-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnhold S, Glüer S, Hartmann K, Raabe O, Addicks K, Wenisch S, Hoopmann M. Amniotic-Fluid Stem Cells: Growth Dynamics and Differentiation Potential after a CD-117-Based Selection Procedure. Stem Cells Int. 2011;23:715341. doi: 10.4061/2011/715341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo L, Zeng J, Liang B, Zhao Z, Sun L, Cao D, Yang J, Shen K. Ovarian cancer cells with the CD117 phenotype are highly tumorigenic and are related to chemotherapy outcome. Exp Mol Pathol. 2011;91:596–602. doi: 10.1016/j.yexmp.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Wang J, Cai K, Jiang L, Zhou D, Yang C, Chen J, Chen D, Dou J. Downregulation of gene MDR1 by shRNA to reverse multidrug-resistance of ovarian cancer A2780 cells. J Cancer Res Ther. 2012;8:226–231. doi: 10.4103/0973-1482.98975. [DOI] [PubMed] [Google Scholar]
- 27.He X, Wang J, Zhao F, Yu F, Chen D, Cai K, Yang C, Chen J, Dou J. Antitumor efficacy of viable tumor vaccine modified by heterogenetic ESAT-6 antigen and cytokine IL-21 in melanomatous mouse. Immunol Res. 2012;52:240–249. doi: 10.1007/s12026-012-8332-4. [DOI] [PubMed] [Google Scholar]
- 28.Dou J, Jiang CL, Wang J, Zhang X, Zhao F, Hu W, He X, Li X, Zou D, Gu N. Using ABCG2-Molecule-Expressing Side Population Cells to Identify Cancer Stem-Like Cells in a Human Ovarian Cell Line. Cell Biol Int. 2011;35:227–234. doi: 10.1042/CBI20100347. [DOI] [PubMed] [Google Scholar]
- 29.Dou J, Pan M, Wen P, Li Y, Tang Q, Chu L, Zhao F, Jiang C, Hu W, Hu K, Gu N. Isolation and identification of cancer stem-like cells from murine melanoma cell lines. Cell Mol Immunol. 2007;4:467–472. [PubMed] [Google Scholar]
- 30.Shen B, Chu ES, Zhao G, Man K, Wu CW, Cheng JT, Li G, Nie Y, Lo CM, Teoh N, Farrell GC, Sung JJ, Yu J. PPARgamma inhibits hepatocellular carcinoma metastases in vitro and in mice. Br J Cancer. 2012;106:1486–1494. doi: 10.1038/bjc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ngora H, Galli UM, Miyazaki K, Zöller M. Membrane-bound and exosomal metastasisassociated C4.4A promotes migration by associating with the α(6)β(4) integrin and MT1-MMP. Neoplasia. 2012;14:95–107. doi: 10.1593/neo.111450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen D, Zhang Y, Wang J, Chen J, Yang C, Cai K, Wang X, Shi F, Dou J. MicroRNA-200c overexpression inhibits tumorigenicity and metastasis of CD117+CD44+ ovarian cancer stem cells by regulating epithelial-mesenchymal transition. J Ovarian Res. 2013;6:50. doi: 10.1186/1757-2215-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu W, Wang J, Dou J, He X, Zhao F, Jiang C, Yu F, Hu K, Chu L, Li X, Gu N. Augmenting therapy of ovarian cancer efficacy by secreting IL-21 human umbilical cord blood stem cells in nude mice. Cell Transplant. 2011;20:669–680. doi: 10.3727/096368910X536509. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Wang J, Ren M, Li M, Chen D, Chen J, Shi F, Wang X, Dou J. Gene therapy of ovarian cancer using IL-21-secreting human umbilical cord mesenchymal stem cells in nude mice. J Ovarian Res. 2014;7:8. doi: 10.1186/1757-2215-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrandina G, Martinelli E, Petrillo M, Prisco MG, Zannoni G, Sioletic S, Scambia G. CD133 antigen expression in ovarian cancer. BMC Cancer. 2009;9:221. doi: 10.1186/1471-2407-9-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsurumi C, Esser N, Firat E, Gaedicke S, Follo M, Behe M, Elsässer-Beile U, Grosu AL, Graeser R, Niedermann G. Non-invasive in vivo imaging of tumor-associated CD133/prominin. PLoS One. 2010;5:e15605. doi: 10.1371/journal.pone.0015605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–728. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Ausiliatrice Puglisi M, Cenciarelli C, Tesori V, Cappellari M, Martini M, Di Francesco AM, Giorda E, Carsetti R, Ricci-Vitiani L, Gasbarrini A. High nitric oxide production, secondary to inducible-nitric oxide synthase expression, is essential for regulation of the tumour-initiating properties of colon cancer stem cells. J Pathol. 2015;236:479–490. doi: 10.1002/path.4545. [DOI] [PubMed] [Google Scholar]
- 39.Liu S, Tetzlaff MT, Liu A, Liegl-Atzwanger B, Guo J, Xu X. Loss of microRNA-205 expression is associated with melanoma progression. Lab Invest. 2012;92:1084–1096. doi: 10.1038/labinvest.2012.62. [DOI] [PubMed] [Google Scholar]


