Abstract
Consumption of cruciferous vegetables is associated with a decreased risk of developing prostate cancer. Antineoplastic effects of cruciferous vegetables are attributable to bioactive indoles, most prominently, 3, 3’-diindolylmethane (DIM). In addition to effects on proliferation and apoptosis, DIM acts as an antiandrogen in prostate cancer cell lines. This study characterized the effects of prostatic DIM on the androgen receptor (AR) in patients with prostate cancer. Men with localized prostate cancer were treated with a specially formulated DIM capsule designed for enhanced bioavailability (BR-DIM) at a dose of 225 mg orally twice daily for a minimum of 14 days. DIM levels and AR activity were assessed at the time of prostatectomy. Out of 28 evaluable patients, 26 (93%) had detectable prostatic DIM levels, with a mean concentration of 14.2 ng/gm. The mean DIM plasma level on BR-DIM therapy was 9.0 ng/mL; levels were undetectable at baseline and in follow-up samples. AR localization in the prostate was assessed with immunohistochemistry. After BR-DIM therapy, 96% of patients exhibited exclusion of the AR from the cell nucleus. In contrast, in prostate biopsy samples obtained prior to BR-DIM therapy, no patient exhibited AR nuclear exclusion. Declines in PSA were observed in a majority of patients (71%). Compliance was excellent and toxicity was minimal. In summary, BR-DIM treatment resulted in reliable prostatic DIM levels and anti-androgenic biologic effects at well tolerated doses. These results support further investigation of BR-DIM as a chemopreventive and therapeutic agent in prostate cancer.
Keywords: 3, 3’-Diindolylmethane, BR-DIM, prostate cancer, cruciferous vegetables, anti-androgen
Introduction
Prostate cancer is the most common cancer diagnosis and the second leading cause of cancer death for men in the United States [1]. Dietary factors have emerged as one potential modifiable prostate cancer risk factor [2]. In particular, consumption of cruciferous vegetables, such as broccoli, Brussels sprouts, and cauliflower, is associated with decreased cancer incidence [3,4]. High dietary intake of cruciferous vegetables has been associated with reduced prostate cancer risk [5] and less aggressive disease at presentation [6]. Two major bioactive compounds in cruciferous vegetables are indole-3-carbinol (I3C) and 3, 3’-diindolylmethane (DIM). I3C is converted to its dimeric derivative, DIM, during cooking. When added in vitro to cell culture media, I3C spontaneously dimerizes, forming DIM in a quantitative and time-dependent fashion [7]. These two indoles have multiple potentially therapeutic effects when studied in vitro. I3C and DIM administration have anti-proliferative and pro-apoptotic effects in several tumor cell lines [8-10]. I3C and DIM inhibit cell growth, induce G1 cell-cycle arrest, and promote apoptosis in androgen-independent and androgen-dependent prostate cancer cells [11-16].
Diverse molecular mechanisms appear to be responsible for these biological effects, but an intriguing finding in prostate cancer is that DIM functions as an androgen receptor (AR) antagonist [11]. DIM down-regulates expression of the AR [17] and prevents AR translocation to the nucleus [11]. As a consequence, DIM treatment leads to decreased transcription of androgen receptor target genes such as PSA. Furthermore, DIM exhibits no AR agonist activity. When evaluated in pre-clinical models, DIM has been shown to have therapeutic effect in flank and metastatic murine prostate cancer models [14,17].
Nutritional grade absorption-enhanced DIM (BR-DIM) is a dietary supplement that contains pharmaceutically pure DIM, microdispersed in spray-dried starch particles. Previous human studies with BR-DIM have reported minimal toxicity [18-20]. We have previously completed a phase I clinical trial of BR-DIM in patients with non-metastatic, hormone-refractory prostate cancer with rising serum PSA [19]. The recommended phase II dose based on toxicity and pharmacokinetic data was 225 mg orally twice daily. Armed with the appropriate dose and schedule, we proceeded with a phase II clinical trial in patients with prostate cancer. BR-DIM was administered to patients prior to radical prostatectomy (RP) with the primary objective of measuring DIM levels in prostate tissue and in plasma. We hypothesized that BR-DIM administration in prostate cancer patients would lead to the accumulation of DIM in the prostate tissue. Further, this study allowed us to test whether prostatic DIM would act as an anti-androgen in a clinical setting.
Material and methods
Study design and conduct
The review boards of both participating institutions (Wayne State University and Henry Ford Health System) approved the study. The study was conducted according to the provisions of the Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Conference on Harmonization. All patients provided written informed consent prior to participation in the study.
The study was designed and the protocol was written by the senior academic authors. The first draft of the manuscript was written by the first author, and the manuscript was then completed and approved by all the authors. All the authors were responsible for writing the manuscript and for the decision to submit the manuscript for publication, and all the authors assume responsibility for the completeness and integrity of the data and the fidelity of the study to the protocol and analysis plan (see supplemental information for full protocol). All the authors or authors’ institutions had agreements with the lead investigational site regarding confidentiality of the data. No one who is not an author contributed to the writing of the manuscript. The protocol was registered on the NIH clinical trials database prior to the enrollment of any patients (http://clinicaltrials.gov/show/NCT00888654).
Study participants
Patients were eligible for enrollment if they had a histologically or cytologically confirmed diagnosis of treatment-naïve prostate cancer, with clinical T1 or T2 disease, and suitable for prostatectomy according to the clinical judgment of their surgeon. Patients were required to have adequate hematologic, hepatic, and renal function, with an ECOG performance status of ≤ 2. Patients were not permitted to take any other antineoplastic agents, micronutrient supplements or dietary soy products while enrolled on study. Concomitant treatment with known P450 inducers and inhibitors (e.g. carbamazepine, clarithromycin, fluconazole, fosphenytoin, itraconazole, ketoconazole, phenobarbital, phenytoin, rifabutin, and rifampin) with potential to interfere with the metabolism of BR-DIM was not allowed. In addition, no androgen deprivation therapy, including the use of 5-alpha-reductase inhibitors, was allowed on study. Patients with serious and uncontrolled acute or chronic medical or psychiatric conditions were not eligible. A complete list of inclusion and exclusion criteria is provided in the protocol (Supplementary Information).
Study treatment and evaluation
Prior to planned prostatectomy, eligible and consenting subjects were prescribed BR-DIM (BioResponse, LLC, Boulder, CO) at a dose of 225 mg orally twice daily for a minimum of 14 days and up to 72 days (depending on surgical scheduling). Each 75 mg BR-DIM capsule was produced following Good Manufacturing Practice (GMP) regulations, which include in-process testing for DIM and post-production assay for DIM content using an HPLC method performed by independent analytic laboratory. Patients were instructed to take 3 tablets twice daily with 8 ounces of water, with or without food. The last dose of study drug was to be taken on the evening before surgery. If surgery was delayed beyond 72 days from the BR-DIM treatment start date, BR-DIM was discontinued. Otherwise, BR-DIM treatment continued until either surgery or one of the following events: disease progression, intercurrent illness which precluded further treatment, unacceptable toxicity (defined as CTCAE version 3.0 grade 3 or higher toxicity), or patient or investigator request. No dose modification was allowed. Eligible patients were evaluable if they had at least 14 days of BR-DIM treatment. Eligible, evaluable patients were compliant if they took at least 80% of the first 14 days of intended BR-DIM treatment based on pill count.
Pharmacokinetic and pharmacodynamic measures
Blood samples for DIM levels were collected before starting BR-DIM treatment, immediately prior to surgery, and during routine follow-up within the first 24 months after prostatectomy. Within 1 hour of collection in heparin anticoagulant tubes, blood samples were centrifuged at 25°C at 3000 rpm for 10 minutes. Plasma was immediately transferred to cryogenic tubes in two aliquots and kept at -80°C until analysis. DIM concentration in human plasma was measured using a validated high-performance liquid chromatographic method with tandem mass spectrometric (LC-MS/MS) detection as previously described [19]. DIM levels were also measured in prostate tissue. Approximately 250-500 mg of fresh tissue specimen from the prostatectomy (benign prostate) was collected and stored at -80°C until analysis. DIM concentration in the prostate was determined using a validated LC-MS/MS method. Serum PSA and testosterone levels were taken at the same time as DIM samples (pre-treatment, pre-surgery, and post-surgery) and processed in the clinical laboratory according to usual procedures.
Immunohistochemistry
Formalin-fixed paraffin-embedded tissues were processed for immunohistochemical analysis as follows. Deparaffinized sections were stained manually using the avidin-biotin-complex technique. Four micron tissue sections were cut from the selected tumor blocks on charged slides and stained for immunohistochemical analysis using specific antibodies for Androgen Receptor (Monoclonal Mouse Anti- Human Clone AR441, Dako Carpinteria, CA; Code # M3562) using the polymer based detection system. Standard staining protocols according to the laboratory manual and the manufacturer’s suggested specimen preparation and staining conditions were used in the Autostainer (Dako Link 48). All reagents used in the staining process on the autostainer were supplied in EnVision FLEX+, Mouse, High pH, (Link) kit (Dako code #K8002). The protocol was then optimized for antigen retrieval, antibody dilution and incubation conditions. A known positive tissue was stained with each investigative case study. Briefly, after deparaffinizing and hydrating to phosphate-buffered saline buffer (pH 7.4), the sections were pretreated with hydrogen peroxide (3%) for 5 minutes to remove endogenous peroxidase. Primary antibody was then applied, AR 1:75 for 20 minutes, followed by washing and incubation with FLEX+ Mouse (Linker) for 15 minutes. After buffer rinse it was incubated with FLEX/HRP for 20 minutes. Subsequent buffer rinse was followed by FLEX+ DAB+ Substrate- Chromogen for 10 minutes and counterstained with Mayer hematoxylin followed by dehydration and mounting. Immunostained samples were reviewed by a genitourinary pathologist who evaluated staining intensity and percent positively-stained cells. The AR staining score was the product of these two numbers.
Statistical methods
The study design was a 1-stage, 1-sample Phase 2 clinical trial, enrolling patients from 2 institutions. The primary objective was to measure the level of DIM in prostate tissue after treatment of prostate cancer patients with oral BR-DIM at 225 mg twice daily for a minimum of 14 days. The primary statistical endpoint was the mean level of DIM in prostate tissue after treatment with BR-DIM. It was desired to estimate the mean level of DIM in prostate tissue after treatment with BR-DIM to within 1/3 of a standard deviation (SD) unit, with 90% confidence. This level of precision in the estimation of the mean DIM level in post-treatment prostate tissue required N=27 patients (as determined via PASS 2011 software). These 27 patients were to be eligible, evaluable, and compliant (as defined above).
Standard descriptive statistics were used to summarize demographic and clinical variables, as well as the plasma and prostate tissue DIM levels. Point and 90% confidence interval (CI) estimates of the mean plasma and prostate tissue DIM levels were calculated. Due to substantial non-normality in the distributions of prostate tissue DIM level, plasma DIM level, and length of BR-DIM treatment, nonparametric methods of statistical analysis were used, including Spearman rank correlation coefficients. To explore the relationship of prostate tissue DIM level to plasma DIM level we used a nonparametric regression approach. We fit a locally estimated scatterplot smoother (LOESS) curve to that bivariate relationship using the LOESS procedure in SAS 9.3 software. A LOESS curve was also fit to the bivariate relationship of prostate tissue DIM level and length of BR-DIM treatment. The default smoothing parameter (percent of the total observations used in each smoothing neighborhood) was used in the LOESS procedure. These nonparametric LOESS curves better described the nonlinear character of those two relationships.
Results
Patient characteristics
A total of 41 prostate cancer patients with plans for prostatectomy were recruited from two institutions. One patient was deemed ineligible due to a disallowed concomitant medication yielding a total of 40 eligible patients. Patient recruitment was comparable between the two institutions (23 vs. 17 eligible patients). Baseline characteristics of the 40 eligible patients are presented in Table 1. The median age was 58 years (range, 40-76). The majority of patients were Caucasian (55%) and 40% of patients were African American. The significant African American population reflects the demographics of prostate cancer patients at the two institutions. All enrolled patients had excellent performance status (ECOG 0). The median pre-treatment PSA was 6.4 ng/mL (range, 2.1-29.4 ng/mL). The median baseline testosterone level was 301 ng/mL (range, 77-608). Nearly all patients (95%) had Gleason sum of 6 or 7 on pre-prostatectomy biopsy. Seventy-five percent of patients had clinical stage T1c prostate cancer; no patients had clinically apparent lymph node metastases.
Table 1.
Patient characteristics
| Characteristic | Eligible patients N=40 | Evaluable patients N=28 | |
|---|---|---|---|
| Age in years-Median (range) | 58 (40-76) | 59 (50-73) | |
| Race-N (%) | European American | 22 (55%) | 16 (57%) |
| African American | 16 (40%) | 11 (39%) | |
| Middle Eastern | 1 (3%) | 0 (0%) | |
| Other | 1 (3%) | 1 (4%) | |
| Performance status-N (%) | ECOG 0 | 40 (100%) | 28 (100%) |
| Pre-treatment PSA (ng/mL)-Median (range) | 6.4 (2.1-29.4) | 6.4 (2.1-20.5) | |
| Testosterone (ng/mL)-Median (range) | 301 (77-608) | 292 (77-557) | |
| Gleason sum from biopsy-N (%) | 6 | 17 (44%) | 11 (39%) |
| 7 | 21 (53%) | 16 (57%) | |
| ≥ 8 | 2 (5%) | 1 (4%) | |
| Clinical T stage-N (%) | T1c | 30 (75%) | 20 (71%) |
| T2a | 5 (13%) | 3 (11%) | |
| T2b | 3 (8%) | 3 (11%) | |
| T2c | 2 (5%) | 2 (7%) | |
| Clinical N stage-N (%) | N0 | 11 (28%) | 8 (29%) |
| NX | 29 (73%) | 20 (71%) | |
| Gleason sum from surgical specimen-N (%) | 6 | 11 (28%) | 7 (25%) |
| 7 | 26 (65%) | 20 (71 %) | |
| ≥ 8 | 2 (5%) | 1 (4%) | |
| unknown | 1 (3%) | 0 (0%) | |
| Pathologic T stage-N (%) | T2a | 2 (5%) | 1 (4%) |
| T2c | 23 (58%) | 16 (57%) | |
| T3a | 10 (25%) | 8 (29%) | |
| T3b | 4 (10%) | 3 (11%) | |
| TX | 1 (3%) | 0 (0%) | |
| Pathologic N stage-N (%) | N0 | 33 (85%) | 24 (86%) |
| NX | 7 (18%) | 4 (14%) |
Surgical findings are reported for the 39 patients for whom data was available (Table 1). At the time of resection, most patients were found to have Gleason 6 or 7 disease (28% and 67%, respectively). Disease was confined to the prostate (pT2) in 64% of patients. Extraprostatic extension or seminal vesicle invasion (pT3) was seen in 14 patients (36%).
Treatment and compliance
Eligible patients were instructed to take 225 mg (3 × 75 mg capsules) of BR-DIM twice daily until the evening before planned prostatectomy. The median length of treatment on BR-DIM was 19 days (range 4-104). The planned duration of treatment was specified to be between 14 and 72 days, depending on the timing of surgery. Patients were deemed not evaluable for the primary endpoint if the length of BR-DIM treatment duration was outside the protocol-defined 14-72 day pre-prostatectomy window. A change in surgery date caused the duration of BR-DIM treatment to fall outside of the 14-72 day window for five patients. Prostatectomy was cancelled for one patient because of adhesions found at the time of surgery. Treatment duration was less than 14 days for two patients who withdrew from the study, and for one patient who discontinued therapy because of toxicity. Three patients did not have evaluable clinical samples for biomarker analyses on the day of prostatectomy. In summary, a total of 28 patients were evaluable for the primary endpoint of DIM levels in the prostate (Figure 1).
Figure 1.
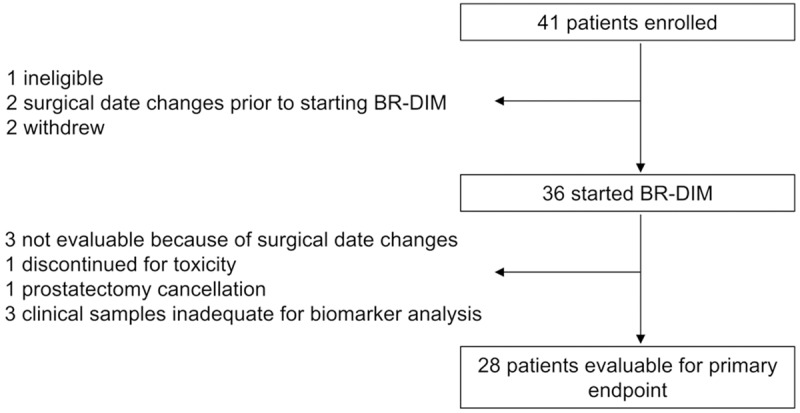
Patient disposition. Out of 41 patients who enrolled, 36 patients started protocol therapy. A total of 28 patients were evaluable for the primary endpoint of DIM levels in the prostate.
In addition to duration of treatment with BR-DIM, compliance was assessed to ensure adequate BR-DIM exposure. Compliance was measured with pill diaries and by comparing actual to expected returned pill counts. Median compliance by patient diary was 100% of intended BR-DIM treatment for all registered patients (range 0-100%). In the subset of 28 evaluable patients, compliance by patient diary ranged from 96-100% of intended treatment, rendering all evaluable patients compliant with therapy. Median compliance as measured by pill count was 95% for all 37 patients who were dispensed BR-DIM tablets (range 0-217%). In the 28 evaluable patients, compliance by pill count ranged from 80-217% of intended treatment, with a median value of 96%.
Pharmacokinetic and pharmacodynamic measures
Pre- and post-treatment pharmacokinetic and pharmacodynamic measures are summarized in Table 2. Plasma DIM levels were measured for each patient prior to starting to BR-DIM therapy. No patient had detectable DIM levels in the pre-treatment sample. Post-treatment DIM levels were taken on the morning of surgery, approximately twelve hours after the last dose of BR-DIM. Post-treatment DIM levels were available for all evaluable patients. Plasma DIM levels were detectable after BR-DIM therapy in all but one patient. For evaluable patients, the median plasma DIM level in the post-treatment sample was 7.5 ng/mL (range 0-24.7 ng/mL). The mean plasma DIM level on BR-DIM therapy was 9.0 ng/mL (90% CI: 6.8-11.3 ng/mL). Plasma DIM levels were again measured during follow-up, typically at least one month after surgery. Levels of DIM in the plasma were undetectable at this timepoint for the subset of patients with available data (n=8).
Table 2.
Pre- and post-treatment measures among the 28 evaluable patients
| Characteristic | N | Median | Mean | 90% confidence limits | SDa | Minimum | Maximum | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Lower | Upper | |||||||
| Plasma PSA pre-treatment (ng/mL) | 28 | 6.4 | 7.2 | 5.8 | 8.6 | 4.3 | 50.0 | 73.0 |
| Plasma PSA post-treatment (ng/mL) | 23 | 5.9 | 6.8 | 5.5 | 8.1 | 3.6 | 2.6 | 18.0 |
| Plasma PSA difference (ng/mL) | 23 | -0.6 | -1.0 | -2.0 | 0.0 | 2.7 | -8.1 | 5.3 |
| Plasma DIM pre-treatment (ng/mL) | 27 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Plasma DIM post-treatment (ng/mL) | 28 | 7.5 | 9.0 | 6.8 | 11.3 | 6.9 | 0.0 | 24.7 |
| Prostate tissue DIM post-treatment (ng/gm) | 28 | 11.6 | 14.2 | 10.6 | 17.7 | 11.1 | 0.0 | 50.1 |
Standard deviation.
To assess our primary endpoint, DIM levels were measured in the prostate using a freshly frozen prostate sample taken at the time of surgery. Prostatic DIM levels were available for all 28 evaluable patients. DIM levels were detectable in the prostate for 26 of 28 patients (92.9%). The median DIM level in the prostate was 11.6 ng/g and the mean DIM concentration was 14.2 ng/g (90% CI: 10.6-17.7 ng/g). Prostatic DIM levels did not correlate strongly with plasma DIM levels (Spearman rank correlation coefficient 0.53; 90% CI: 0.24-0.72). The nonparametric regression LOESS curve of prostatic DIM levels was increasing through about 12 ng/mL of plasma DIM, but flat thereafter (Figure 2A). There was virtually no relationship between prostatic DIM levels and length of therapy (Spearman rank correlation coefficient -0.08; 90% CI: -0.39-0.24), consistent with our previous demonstration that systemic steady state levels are achieved after one week of BR-DIM therapy [19]. The nonparametric regression LOESS curve of prostatic DIM levels was essentially flat through about 30 days of BR-DIM treatment, and slightly decreasing thereafter (Figure 2B). The mean (median) prostatic DIM levels for the six patients who took only the minimum amount of BR-DIM therapy (14 days) was 14.0 (10.1) ng/gm, compared to a mean level of 14.2 (13.6) ng/gm for the 22 patients who took BR-DIM therapy for > 14 days (see also Figure 2B).
Figure 2.
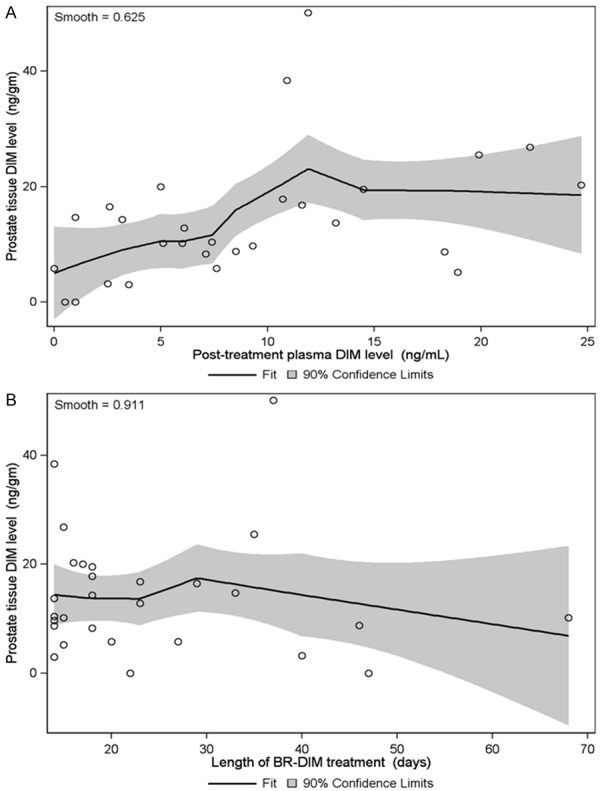
Prostatic DIM measurements. A. After a minimum of 14 days of BR-DIM therapy, DIM levels were measured in the plasma and at the time of prostatectomy. Prostate DIM levels are plotted against plasma DIM levels for each evaluable patient. The nonparametric regression LOESS curve is plotted, with the shaded area indicating the 90% confidence limits for the predicted mean prostate tissue DIM level. B. Prostate DIM levels are plotted against length of BR-DIM treatment for each evaluable patient. The nonparametric regression LOESS curve is plotted, with the shaded area indicating the 90% confidence limits for the predicted mean prostate tissue DIM level.
BR-DIM effects on in vivo androgen receptor signaling
Given the effects of DIM on the androgen receptor in pre-clinical models, we assessed androgen receptor staining in prostate samples before and after BR-DIM treatment. Pre-BR-DIM AR staining was performed on pre-therapy biopsy specimens while post-BR-DIM AR staining was performed on specimens taken at the time of prostatectomy. Androgen receptor status was unknown for 2 patients, yielding 26 patients evaluable for these analyses. A score was assigned to each prostate sample equal to the product of staining intensity and percent positively-stained cells. The median AR staining score in the 26 pre-BR-DIM specimens was 300 while the median AR staining score in the same 26 post-BR-DIM prostatectomy samples was 255. The mean AR staining in the pre-BR-DIM specimens was 278 (90% CI: 263-293) compared to a mean value of 245 (90% CI: 227-263) in the post-BR-DIM specimens. Staining scores suggested decreased protein expression of the androgen receptor in response to therapy.
As a nuclear receptor and transcriptional coactivator, translocation of the androgen receptor to the nucleus after ligand binding is an important measure of its biologic activity. Immunohistochemical staining for androgen receptor revealed nuclear exclusion in 25 of 26 prostatectomy specimens (Figure 3). One prostatectomy sample (4%; 90% CI: 1%-16%) showed nuclear androgen receptor staining. Androgen receptor staining was also performed on archived prostate biopsy samples obtained from the patient prior to starting BR-DIM therapy. These pre-BR-DIM prostate biopsy samples did not demonstrate nuclear exclusion of androgen receptor for any of the 26 evaluable patients, hence a nuclear androgen receptor staining rate of 100% (90% CI: 91%-100%) (Figure 3). Compared to pre-BR-DIM samples, post-BR-DIM prostate cancer samples demonstrated a striking effect on AR nuclear translocation.
Figure 3.
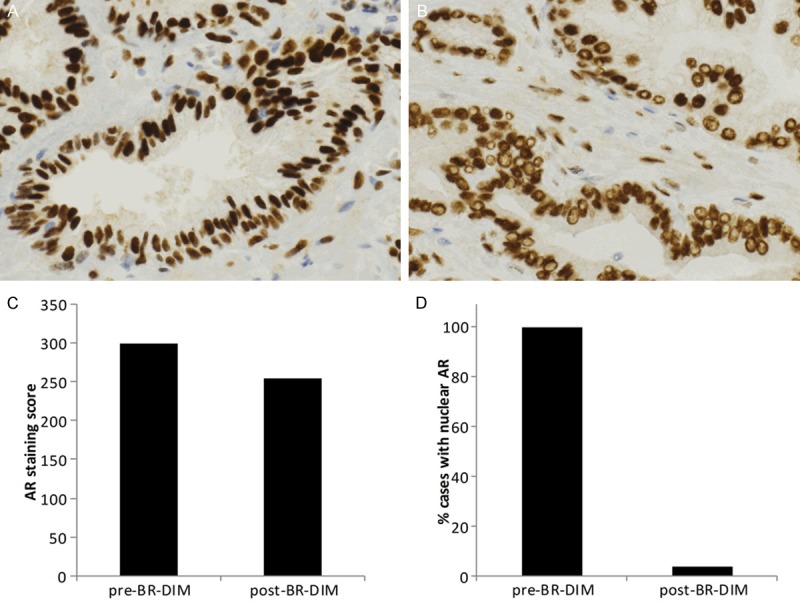
BR-DIM therapy results in nuclear exclusion of androgen receptor. Prostate specimens were stained for androgen receptor. Representative images from samples taken before (A) and after (B) BR-DIM treatment are shown at 40X magnification. Vacuoles in the post-BR-DIM treatment group are indicative of nuclear exclusion of the androgen receptor. AR staining score and the percentage of cases with nuclear staining of androgen receptor before and after BR-DIM therapy are plotted in (C) and (D), respectively.
Nuclear translocation of androgen receptor is expected in the presence of its ligand, testosterone. We therefore evaluated testosterone levels before and after BR-DIM treatment. Nuclear exclusion of androgen receptor was not dependent upon reduced circulating levels of testosterone. The median plasma testosterone level after BR-DIM treatment was 387 ng/mL (range 118-686). In comparison, the median plasma testosterone level prior to BR-DIM treatment was 280 ng/mL (range 77-557 ng/mL). In the 26 patients evaluable for androgen receptor staining, plasma testosterone levels increased post-BR-DIM therapy in 15 patients, decreased in six patients and were unknown in five patients. The mean difference between pre- and post-BR-DIM testosterone levels was an increase of 76 ng/mL (range -107 ng/mL to 359 ng/mL).
In addition to its clinical utility as a tumor marker, prostate-specific antigen (PSA) is an important target of AR-dependent transcription. PSA levels were assessed both before and after therapy with BR-DIM (Table 2). PSA levels declined in 16 of 23 patients (70%). In 28 patients, the median PSA level prior to therapy was 6.4 ng/mL (range 2.1 ng/mL to 20.5 ng/mL). In 23 patients, the median PSA level after BR-DIM treatment was 5.9 ng/mL (range 2.6 ng/mL to 18.0 ng/mL). The median change in absolute PSA levels of 23 patients was a 0.6 ng/mL decline (range 8.1 ng/mL decline to 5.3 ng/mL increase). PSA changes after BR-DIM therapy are presented in waterfall plots (Figure 4).
Figure 4.
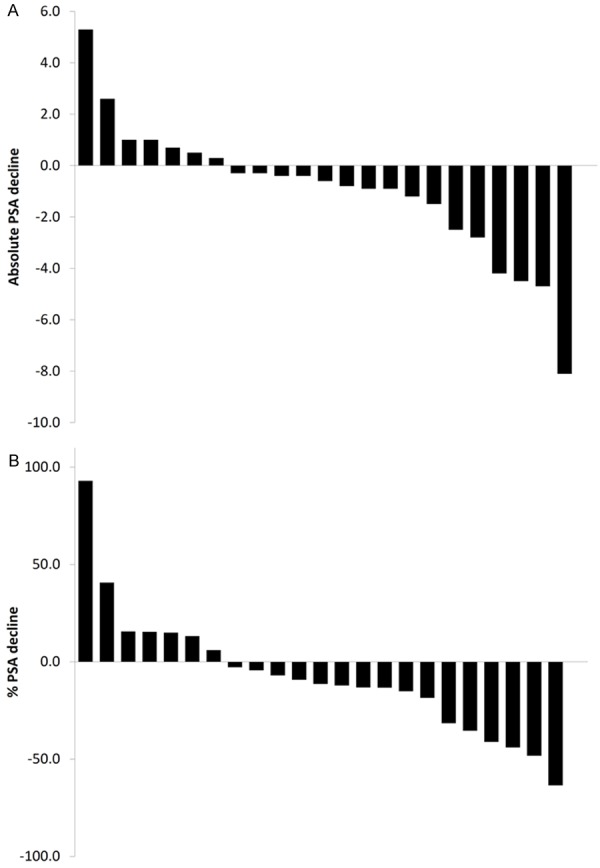
Waterfall plots of PSA declines. PSA declines are presented in waterfall format for both absolute (A) and % PSA declines (B).
Adverse events
Patients were deemed evaluable for toxicity from their first dose of BR-DIM. Laboratory monitoring included glucose, creatinine, sodium, liver function testing and coagulation parameters given previous reports of asymptomatic laboratory abnormalities in patients treated with BR-DIM [19]. Overall, BR-DIM was very well tolerated. Two patients reported headaches (maximal CTCAE grade 3) which were deemed by the investigator to be possibly related to BR-DIM therapy. No other toxicity was reported (Table 3).
Table 3.
Adverse Event rates among all 36 treated patients
| Adverse Event | N (%) | 90% confidence limits | Grade | |
|---|---|---|---|---|
|
| ||||
| Lower | Upper | |||
| Headache | 2 (6%) | 2% | 15% | 3 |
| Gastrointestinal toxicity | 0 (0%) | 0% | 7% | N/A |
| Hematologic toxicity | 0 (0%) | 0% | 7% | N/A |
| Hyperglycemia | 0 (0%) | 0% | 7% | N/A |
| Hyponatremia | 0 (0%) | 0% | 7% | N/A |
| Increased creatinine | 0 (0%) | 0% | 7% | N/A |
| Increase in coagulation parameters | 0 (0%) | 0% | 7% | N/A |
N/A = Not applicable.
Discussion
Overall, our results indicate that treatment with BR-DIM is associated with biologically significant DIM concentrations in the human prostate. After BR-DIM treatment (225 mg orally twice daily), we found that prostatic DIM levels are reliably detected by fourteen days, and levels do not increase with longer treatment. These findings are consistent with our previous reports that steady state levels in the plasma are achieved by one week [19]. DIM was not detectable in the plasma in any of 8 follow-up samples. Toxicity from BR-DIM appears to be minimal, with headache the only reported side effect.
Prostatic DIM may result in decreased expression of AR as assessed by immunohistochemical staining, but more prominent was the near universal presence of nuclear vacuoles indicating AR nuclear exclusion. Inhibition of nuclear translocation has also been reported for other novel anti-androgens such as enzalutamide and may contribute to the anti-neoplastic activity of this class of drug. AR nuclear exclusion was observed even in the presence of ligand, as serum testosterone levels generally were higher after BR-DIM therapy. The inability of AR to translocate to the nucleus would be expected to impact its ability to direct transcription in response to ligand signaling. We observed decreases in serum PSA in a majority of patients, consistent with a potential effect on AR signaling. Although observed decreases were small, treatment duration was quite short (median of 19 days) and the study was not designed to assess changes in PSA as its primary endpoint. Clinical guidelines suggest continuing treatment for at least 12 weeks to ensure adequate drug exposure when evaluating changes in PSA [21].
Attrition rate on this study was higher than anticipated from our previous experience in the setting of castrate-resistant biochemical recurrence, potentially related to the studied surgical patient population. In contrast to patients experiencing cancer recurrence, prostatectomy patients being treated with curative intent may be less tolerant of toxicity or inconvenience from an experimental therapy, even if mild. Furthermore, several patients were not evaluable because patients chose to change surgical dates rather than continue on study. Although attrition is a study limitation, adequate numbers of patients were enrolled to evaluate our primary endpoint according to the original statistical plan.
In summary, treatment with BR-DIM was successful in maintaining biologically active levels of DIM in the prostate. BR-DIM clearly impacts nuclear translocation of the androgen receptor, which plays a key role in prostate tumor pathogenesis. Confirming the anti-androgenic activity of BR-DIM in humans supports the further study of this nutraceutical as both a therapeutic and chemopreventive agent.
Acknowledgements
We thank our patients and the many research staff, including Susan Bolton, Constance Harkness, and Kannagi Chinnakannu, who made this work possible. We also acknowledge with gratitude the contributions of Dr. Jing Li, Director of the Karmanos Cancer Institute Pharmacology Core. This work was funded by a grant from the National Cancer Institute, National Institutes of Health (5R01CA108535-09 and 5R01CA164318-01A1) awarded to FHS. This work was also partially supported by NIH Cancer Center Support Grant CA-22453 (LKH) and a grant from the Vattikuti Urology Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Chan JM, Gann PH, Giovannucci EL. Role of diet in prostate cancer development and progression. J. Clin. Oncol. 2005;23:8152–8160. doi: 10.1200/JCO.2005.03.1492. [DOI] [PubMed] [Google Scholar]
- 3.Kristal AR, Lampe JW. Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2002;42:1–9. doi: 10.1207/S15327914NC421_1. [DOI] [PubMed] [Google Scholar]
- 4.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733–748. [PubMed] [Google Scholar]
- 5.Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92:61–68. doi: 10.1093/jnci/92.1.61. [DOI] [PubMed] [Google Scholar]
- 6.Kirsh VA, Peters U, Mayne ST, Subar AF, Chatterjee N, Johnson CC, Hayes RB. Prospective study of fruit and vegetable intake and risk of prostate cancer. J Natl Cancer Inst. 2007;99:1200–1209. doi: 10.1093/jnci/djm065. [DOI] [PubMed] [Google Scholar]
- 7.Bradlow HL, Zeligs MA. Diindolylmethane (DIM) spontaneously forms from indole-3-carbinol (I3C) during cell culture experiments. In Vivo. 2010;24:387–391. [PubMed] [Google Scholar]
- 8.Bonnesen C, Eggleston IM, Hayes JD. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001;61:6120–6130. [PubMed] [Google Scholar]
- 9.Abdelrahim M, Newman K, Vanderlaag K, Samudio I, Safe S. 3,3’-diindolylmethane (DIM) and its derivatives induce apoptosis in pancreatic cancer cells through endoplasmic reticulum stress-dependent upregulation of DR5. Carcinogenesis. 2006;27:717–728. doi: 10.1093/carcin/bgi270. [DOI] [PubMed] [Google Scholar]
- 10.Cover CM, Hsieh SJ, Tran SH, Hallden G, Kim GS, Bjeldanes LF, Firestone GL. Indole-3-carbinol inhibits the expression of cyclin-dependent kinase-6 and induces a G1 cell cycle arrest of human breast cancer cells independent of estrogen receptor signaling. J Biol Chem. 1998;273:3838–3847. doi: 10.1074/jbc.273.7.3838. [DOI] [PubMed] [Google Scholar]
- 11.Le HT, Schaldach CM, Firestone GL, Bjeldanes LF. Plant-derived 3,3’-Diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J Biol Chem. 2003;278:21136–21145. doi: 10.1074/jbc.M300588200. [DOI] [PubMed] [Google Scholar]
- 12.Leibelt DA, Hedstrom OR, Fischer KA, Pereira CB, Williams DE. Evaluation of chronic dietary exposure to indole-3-carbinol and absorption-enhanced 3,3’-diindolylmethane in sprague-dawley rats. Toxicol Sci. 2003;74:10–21. doi: 10.1093/toxsci/kfg103. [DOI] [PubMed] [Google Scholar]
- 13.Nachshon-Kedmi M, Yannai S, Fares FA. Induction of apoptosis in human prostate cancer cell line, PC3, by 3,3’-diindolylmethane through the mitochondrial pathway. Br J Cancer. 2004;91:1358–1363. doi: 10.1038/sj.bjc.6602145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nachshon-Kedmi M, Fares FA, Yannai S. Therapeutic activity of 3,3’-diindolylmethane on prostate cancer in an in vivo model. Prostate. 2004;61:153–160. doi: 10.1002/pros.20092. [DOI] [PubMed] [Google Scholar]
- 15.Garikapaty VP, Ashok BT, Tadi K, Mittelman A, Tiwari RK. 3,3’-Diindolylmethane downregulates pro-survival pathway in hormone independent prostate cancer. Biochem Biophys Res Commun. 2006;340:718–725. doi: 10.1016/j.bbrc.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 16.Vivar OI, Lin CL, Firestone GL, Bjeldanes LF. 3,3’-Diindolylmethane induces a G(1) arrest in human prostate cancer cells irrespective of androgen receptor and p53 status. Biochem Pharmacol. 2009;78:469–476. doi: 10.1016/j.bcp.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahman KM, Banerjee S, Ali S, Ahmad A, Wang Z, Kong D, Sakr WA. 3,3’-Diindolylmethane enhances taxotere-induced apoptosis in hormone-refractory prostate cancer cells through survivin down-regulation. Cancer Res. 2009;69:4468–4475. doi: 10.1158/0008-5472.CAN-08-4423. [DOI] [PubMed] [Google Scholar]
- 18.Reed GA, Sunega JM, Sullivan DK, Gray JC, Mayo MS, Crowell JA, Hurwitz A. Singledose pharmacokinetics and tolerability of absorption-enhanced 3,3’-diindolylmethane in healthy subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:2619–2624. doi: 10.1158/1055-9965.EPI-08-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heath EI, Heilbrun LK, Li J, Vaishampayan U, Harper F, Pemberton P, Sarkar FH. A phase I dose-escalation study of oral BR-DIM (BioResponse 3,3’-Diindolylmethane) in castrate-resistant, non-metastatic prostate cancer. Am J Transl Res. 2010;2:402–411. [PMC free article] [PubMed] [Google Scholar]
- 20.Dalessandri KM, Firestone GL, Fitch MD, Bradlow HL, Bjeldanes LF. Pilot study: effect of 3,3’-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer. 2004;50:161–167. doi: 10.1207/s15327914nc5002_5. [DOI] [PubMed] [Google Scholar]
- 21.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]


