Abstract
TDRP (Testis Development-Related Protein), a nuclear factor, might play an important role in spermatogenesis. However, the molecular mechanisms of TDRP underlying these fundamental processes remain elusive. In this study, a Tdrp-deficient mouse model was generated. Fertility tests and semen analysis were performed. Tdrp-deficient mice were not significantly different from wild-type littermates in development of testes, genitourinary tract, or sperm count. Morphologically, spermatozoa of the Tdrp-deficient mice was not significantly different from the wild type. Several sperm motility indexes, i.e. the average path velocity (VAP), the straight line velocity (VSL) and the curvilinear velocity (VCL) were significantly decreased in Tdrp-deficient mice (p<0.05). The proportion of slow velocity sperm also increased significantly in the mutant mice (p<0.05). However, fertility tests showed that no significant difference inaverage offspring amount (AOA), frequency of copulatory plug (FCP), and frequency of conception (FC). Furthermore, TDRP1 could interact with PRM2, which might be the molecular mechanism of its nuclear function in spermatozoa. In conclusion, these data collectively demonstrated that Tdrp deficiency impaired the sperm motility, but Tdrp deficiency alone was not sufficient to cause male infertility in mice. Additionally, TDRP1 might participate in spermatogenes is through interaction with PRM2.
Keywords: TDRP (Tdrp1 ), PRM2, sperm, gene knockout mouse model
Introduction
Reproduction and development is one of the most active topics in the field of biomedical research in recent years. The prevalence of current infertility is approximately 12% [1], withmales and female scontributingal most equally.This rateis even higher in low-income countries (range, 9%-30%) [2]. Although the prevalence of infertility in most regionshas remained stable, the absolute number of couples affected by infertility has increased [3]. Infertility leads to significant public health consequences, including psychological distress [4], financial and emotional burdens [5], and social stigmatization [6]. Infertility is a worldwide, major, multifaceted factor affecting human health.
Despite its importance in human health, the pathogenesis of male infertility remainspoorly understood. A multitude of conditions can cause male infertility, such as congenital malformations, exposure to polluted environment, genetic and endocrine disorders, and infectious, inflammatory and immunologic conditions [7]. The gene knock out mouse model is a powerful tool for investigation of male infertility. For example, recent studies showed deficiencies of various genes such as Flot2, Arl3, Mns1, and Paip2a led to male infertility in mice [8-11].
Previously, our group has cloned a novel full-length cDNA named Tdrp (encoding testis development-related protein) from a cDNA library of human testis tissue [12]. Of Tdrp’s two distinct transcripts, Tdrp1 and Tdrp2, the former was predominantly expressed in spermatogenic cells with particularly high expression in spermatocytes, and was also identified as a nuclear factor [12]. We generated rabbit polyclonal antibodies specific against human TDRP1 and found both Tdrp1 mRNA and protein increased along with sexual maturation in testis tissues in rats. It was also found that TDRP1 expression was significantly lower in testis tissues of azoospermic men compared with healthy controls [12].
In this study, we have established Tdrp knockout mouse model and observed its effects on male fertility from the aspects of sperm morphology, sperm motility, as well as fertile function. In addition, PRM2 was discovered as an interacting protein with TDRP1, suggesting the mechanism of disordered spermatogenesis caused by Tdrp deficiency.
Materials and methods
Animals
C57BL/6 and 129/SV mice obtained from Shanghai Research Center for Model Organisms were used for this study. All experimental procedures were done in accordance with the national experimental animal science guide for the care and use of laboratory animals and were approved by the Shanghai Animal Care and Use Committee on Animals.
Semi-quantitative RT-PCR analysis of expression profile
One microgram of total RNA extracted from testis was reverse-transcribed with oligo (dT) using the Omniscript Reverse Transcription kit (Qiagen). To normalize the expression level of Tdrp1 among different tissues, actin was used as reference. One microliter of RT product was amplified with primer pairs specific for mouse Tdrp1 by using HotStarTaq PCR kit (Qiagen). PCR conditions and primer sequences were available on request. Each RT-PCR product was loaded on 1.5% agarose gel and stained with 0.5 µg/ml ethidium bromide. Gel images were analyzed and quantified by Image J.
Construction of the Tdrp targeting vector and generation of Tdrp-deficient mice
DNA fragments for 5’ and 3’ homology arms were amplified from mouse (129/SvEv) genomic DNA by PCR to generate the Tdrp targeting vector. The upstream arm consists of a 2903 bp 5’ coding region and the downstream arm is a 2653 bp 3’ coding region. Both arms were cloned into the vector with phosphoglycerate kinase-neomycin (PGK-neo) and phosphoglycerate kinase-thymidine kinase (PGK-TK) sequences as positive or negative selection cassettes. They were confirmed by sequencing.
The Tdrp targeting vector was electroporated into 2610019F03Rik (129Sv derived) embryonic stem (ES) cells. Through drug selection of G418(+) and ganciclovir(-), 96 resistant ES cell clones survived and eight targeted ES cell clones that had undergone homologous recombination with the targeting vector were subsequently identified from among these. Primers for the 5’arm were P1 (5’-CCACTCTGCTTTCAGACTGTTAGG-3’) + P2 (5’-GGCCTACCCGCTTCCATTGCTC-3’) and for the 3’arm P3(5’-CCGTGCCTTCCTTGACCCTGG-3’) + P4 (5’-GGCTGCTTCCTCTATGGCTAAG-3’) with 3329 bp and 3011 bp product lengths respectively. All eight positive ES cell clones were re-verified by DNA sequencing. These ES cell clones were injected into blastula and then transferred into pseudopregnant foster mother mice. The chimeras were bred with C57BL/6 mice to obtain mutant mice on a 129Sv×C57BL/6 mixed background. Homozygotes were obtained by multiple intercrosses between heterozygotes. Mouse genotypes were identified by southern blotting analysis.
Germ cell purification and western blotting analysis
Spermatogenic cells were isolated from adult mouse testes and discrete populations of germ cells were isolated using unit sedimentation velocity in 2-4% BSA gradient when required. The purity of the isolated germ cells was monitored by phase-contrast microscopy.
For western blotting, anti-TDRP1 and anti-GAPDH antibody were diluted in 2-4% BSA. Extracts were diluted in SDS-loading buffer and western blotting was performed as described previously [12].
Electron microscopy
Testis and epididymis from wild type and Tdrp-deficient mice were examined with both scanning and transmission electron microscopy (EM) as described [13]. In brief, testis and epididymis were fixed in 2.5% glutaraldehyde and 2% paraformaldehyde for 4 h, and postfixed in 1% osmium tetroxide for 1 h. The specimens were dehydrated in ethanol, transferred to propylene oxide, andembedded in EM-Bed 812 medium (Electron Microscopy Sciences). The specimens were polymerized at 68°C for 48 h. Ultrathin sections were cut with a diamond knife, mounted on single-hole grids, stained with bismuth solution, and examined with an electron microscope (TITAN Krios).
Fertility test
Adult male mice of three genotypes (homozygote Tdrp -/- heterozygote Tdrp +/-, wild type Tdrp +/+) were caged with wild type female mice of two different genetic backgrounds (B6 and C57BL/6). The reproductive capacity evaluation was performed as described [14]. Male mice were housed with two female mice. Female mice were replaced weekly. When plugged femalemice were found, theywere removed and replaced immediately. Pregnancy was defined as plugged mice that gave birth to offspring. The number of total males and females, plugged females, litters, and total offspring were counted to calculate frequency of copulatory plug (FCP), frequency of conception (FC) and average offspring amount (AOA). Student’s t-test and χ2 test were used to compare meansand frequencies among groups and p<0.05 was considered a significant difference.
Semen analysis
Sperm motility parameters were quantified by computer-aided semen analysis (CASA). Briefly, one side of the cauda epididymis was dissected from each mouse (8-12 weeks old) and each was opened in an identical manner to permit the release ofsperm. Afterwards, the cut cauda epididymis were separately put into 1.5-ml Eppendorf tubes containing 500 μl of human tube fluid (HTF) medium [15]. After incubation for 5 minutes, sperm from the upper layer of the medium were added to a counting chamber for analysis. Integrated visual optical system software (Hamilton Thorne Biosciences, IVOS) was used to generatethe results.
Immunofluorescence microscopy
Squashed samples were prepared and immunofluorescence microscopy was undergone as described [13]. Indirect immunofluorescence staining to study the localization of TDRP1 and PRM2 in testicular germ cells was carried out and images of stained cells were captured with a Leica TCS SP2 laser confocal microscope.
Yeast two-hybrid assay and co-immunoprecipitation (Co-IP)
To determine factors that interact with TDRP1, the ProQuest Two-Hybrid System with Gateway® Technology (Invitrogen) was used to ‘fish’ TDRP1 interacting proteins from a mouse testis cDNA library (GibcoBRL). Full-length mouse Tdrp1 was cloned into the plasmid pDBLeu (GibcoBRL) as ‘bait’. True positive cDNA clones that enabled yeast to grow in the presence of 25 mM 3-aminotriazole and the absence of histidine were further selected by uracil-independent growth and β-galactosidase-producing phenotype.
To confirm the interaction between TDRP1 and PRM2 which was identified by the yeast two-hybrid assay, ectopic and endogenousimmunoprecipitationwere performed. Forectopic overexpression, the Tdrp1 gene was cloned into p3XFLAG-myc-CMV-24 vector for fusion with a Myc tag at the C-terminal end, while the PRM2 gene was cloned into pCDNA3.1+ with a His tag at the C-terminal end. The Myc-tagged TDRP1 and His-tagged PRM2 fusion plasmids were cotransfected into HEK293T cell lines. Immunoprecipitation of Myc-TDRP1 and His-PRM2 was carried out followed by western blotting. The Myc-tagged fusion protein was detected with rabbit anti-Myc antibody (CST, #2278) and goat anti-rabbit IgG-HRP conjugate (Promega). The His-tagged fusion protein was detected with mouse anti-His antibody (CST, #9991) and goat anti-mouse IgG-HRP conjugate (Promega). For endogenous immunoprecipitation, the TDRP1 protein was detected with rabbit anti-TDRP1 antibody (Customized in Raygene Biotech Company, China) and goat anti-rabbit IgG-HRP conjugate (Promega). The PRM2 protein was detected with goat anti-PRM2 antibody (Santa Cruz, SC-23104) and rabbit anti-goat IgG-HRP conjugate (Promega).
Results
Testis specific expression pattern of Tdrp1 in adult mice
The relative expression levels of Tdrp1 in mouse tissues were assayed in cDNA samples prepared from the brain, thymus, heart, liver, spleen, lung, kidney, stomach, intestine, bladder, marrow, muscle, testis, epididymis, ovary and uterus of wild type mice. Semiquantitative RT-PCR assays detected a transcript of mouse Tdrp1 predominantly in testis tissue and slightly in epididymis, marrow and kidney tissues (Figure 1A). Since testis maturation is age-dependent, we have also investigated the expression pattern in different developmental stages at transcriptional and translational levels [12] and found notable increases at 3 weeks postpartum and a peak at 2 months postpartum.
Figure 1.
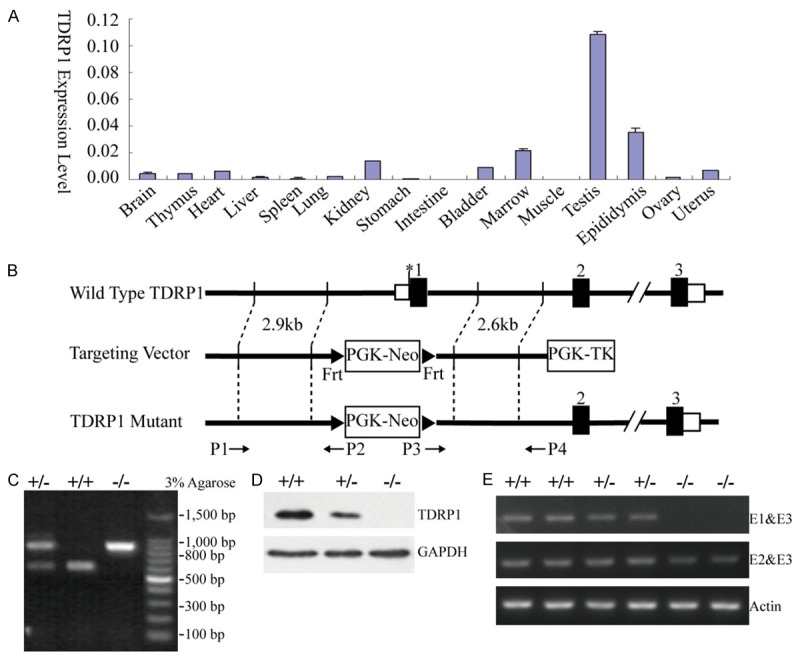
Construction of Tdrp-targeting vector and generation of Tdrp-deficientmice. A. RT-PCR analysis of Tdrp1 expression in adult mouse tissues. The expression level of Tdrp1 in each tissue was normalized by actin. B. Wild-type allele, targeting construct, and targeted allele of Tdrp gene with the location of primers. C. Identification of the specific transcription products through PCR. The transcription product of the mutant is 772 bp; of the wild type is 539 bp. The heterozygote expresses both 772 bp and 539 bp products. D. Western blotting analysis of the protein extracted from testes of 8 week old Tdrp-deficient and wild type mice. Anti-TDRP1 antibody was used. E. RT-PCR analysis of genotype. Upstream primer E1 and E2 were located at exon 1 and exon 2 of Tdrp gene, respectively, while E3 is downstream primer at exon 3. The product of E1&E3 transcription was full length Tdrp; the product transcribed by E2&E3 was truncated Tdrp.
Establishment of Tdrp-deficient mouse model
To investigate the role of TDRP during spermatogenesis, gene targeting was used to disrupt its function in mice. In the targeted allele, the exon 1 sequence encoding Tdrp was replaced by a cassette of the PGK-neomycin resistance gene (Figure 1B). The targeted disruption of the Tdrp gene and generation of knockout mice were confirmed by PCR of genomic DNA (Figure 1C, 1E) and western blotting analysis (Figure 1D). Considering the two isoforms of Tdrp, Tdrp1 and Tdrp2, share the common exon 1 and exon 2 [12], the knockout mouse model is deficient of both isoforms of Tdrp. We didn’t check the TDRP2 expression in the knockout mice due to unavailability of TDRP2 specific antibody.
Interbreeding of heterozygous mice produced 33 Tdrp +/+, 36 Tdrp +/- and 18 Tdrp -/- mice in F2 offspring on B6 genetic background. Among 256 mice from F1 to F8 generation, only 3 mice died, suggesting that Tdrp deficiency didn’t lead to embryonic lethality. The Tdrp-deficient mice grew to adulthood with no gross abnormalities and no increased lethality, and the adult TDRP null mice were normal in appearance. The body weight of adult mice with Tdrp deficiency was not significantly different from that of wild type mice. No obvious anatomical or behavioral abnormalities were observed.
Consequently, targeted disruption of the Tdrp gene in mice was successful. The following morphological study of testis and spermatozoa, and functional study including sperm count and fertility rate investigated mechanisms affectingfertility in Tdrp-deficient homozygous mice.
Morphology of testis and mature epididymal sperm from Tdrp-deficient mice
The morphology of testis and mature epididymal sperm in Tdrp-deficient mice was normal. The appearance of testis, seminal vesicles, and caudal epididymis in deficient mice was similar towild typemice in size, texture, shape, and weight with no differences present by Hematoxylin&Eosin (H.E.) staining (Figure 2A, 2B). Histology of seminiferous tubules did not reveal any gross abnormality in deficient mice.
Figure 2.
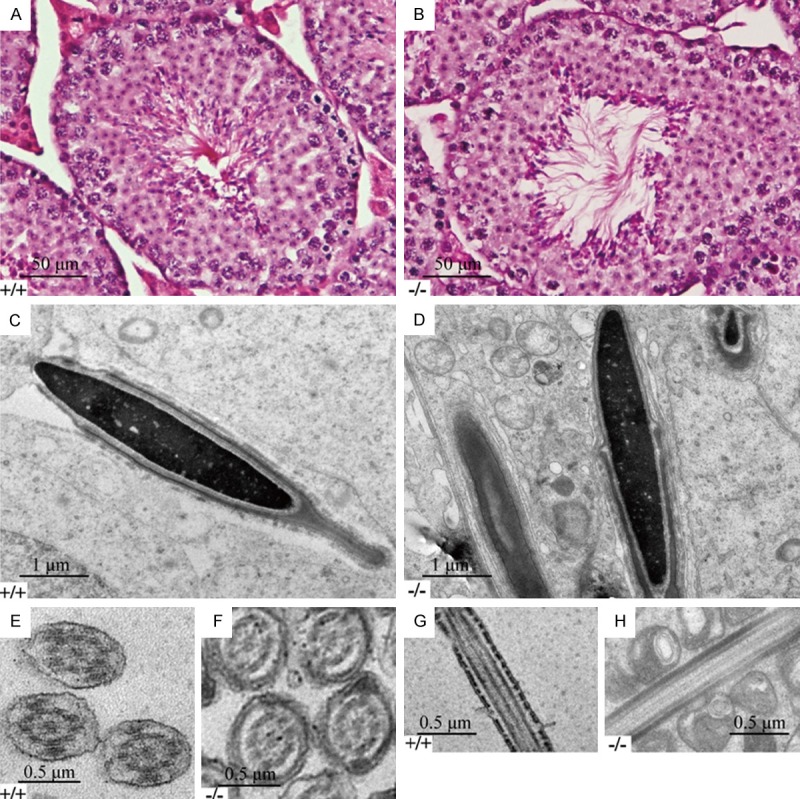
Morphology analysis of Tdrp-deficientand wild type mice. (A&B) Histological analysis of testes from wild-type (A) and Tdrp-deficient (B) mice undergoing H&E staining. (C&D) Ultrastructural analysis of longitudinal sections of the head of spermatozoa from wild-type (C) and Tdrp-deficient (D) mice. (E&F) Ultrastructural analysis of cross sections of the sperm flagellum from wild-type (E) and Tdrp-deficient (F) mice. (G&H) Ultrastructural analysis of longitudinal sections of the head of the sperm flagellumfrom wild-type (G) and Tdrp-deficient (H) mice.
Sperm were obtained from the caudal epididymis and observed by both scanning and transmission electron microscopy. The head of Tdrp-deficient sperm appeared normal in morphology, except for a slightly looser than the wild type in nuclear content (Figure 2C, 2D). The characteristic “9+2” microtubular axoneme and outer dense fibers (ODFs) of the Tdrp-deficient mice was not disorganized when comparing with the wild type in cross section (Figure 2E, 2F), as well as in longitudinal section (Figure 2G, 2H). These results implied that TDRP is not essential for the assembly ofsperm flagella in mice, but might affect the nuclear function of sperm.
Normal sperm countsbut low sperm motility in Tdrp-deficient mice
To determine the influence of quantity and quality of sperm on spermiogenesis, we comparedsperm counts in testis and epididymis between the Tdrp-deficient group and the wild type (Table 1). The analysis revealed no significant difference of total sperm number in epididymis. The average path velocity (VAP), the straight line velocity (VSL) and the curvilinear velocity (VCL) were significantly decreased in Tdrp-deficient mice (p<0.05). The percentage of progressive mobile sperm (level a and level b) was slightly reduced in the deficient group (p=0.14) and there was a significant increase in the percentage of sperm with slow velocity (level c) (p<0.05). According to the semen analysis, sperm motility was impaired with deficiency of Tdrp.
Table 1.
Comparable motility of cauda epididymal sperm from wild type (WT) and Tdrp-deficient mice
| CASA parameter | WT# | Tdrp deficiency# | p-value |
|---|---|---|---|
| Total sperm (M) | 6.8±2.7 | 7.0±3.2 | 0.869 |
| Motile sperm (%) | 70.3±8.3 | 60.8±15.5 | 0.116 |
| Progressive sperm (%) | 57.4±8.3 | 49.7±14.1 | 0.167 |
| Path velocity (VAP) (μm/sec) | 148.1±10.0 | 131.8±9.5 | 0.003* |
| Prog. velocity (VSL) (μm/sec) | 113.0±4.8 | 103.7±6.2 | 0.002* |
| Track speed (VCL) (μm/sec) | 246.5±22.4 | 212.4±17.6 | 0.003* |
| Lateral amplitude (ALH) (μm) | 12.1±0.8 | 11.2±1.6 | 0.149 |
| Beat frequency (BCF) (Hz) | 19.2±1.8 | 20.2±1.3 | 0.212 |
| Straightness (STR) (%) | 75.9±2.8 | 78.4±2.5 | 0.065 |
| Linearity (LIN) (%) | 48.0±2.8 | 50.9±3.0 | 0.052 |
| Elongation (%) | 93.3±1.3 | 92.9±1.6 | 0.541 |
| Rapid Velocity (level a) (%) | 65.7±8.5 | 56.0±15.8 | 0.115 |
| Medium Velocity (level b) (%) | 3.7±1.2 | 4.5±1.1 | 0.152 |
| Slow Velocity (level c) (%) | 22.8±4.5 | 29.8±7.7 | 0.029* |
| Static Velocity (level d) (%) | 7.1±5.1 | 9.6±8.1 | 0.432 |
| a+b (%) | 69.4±8.6 | 60.5±15.4 | 0.140 |
| c+d (%) | 29.9±8.7 | 39.4±15.5 | 0.120 |
Cauda epididymal sperm were incubated in HTF medium then subjected to CASA. At least 1000 sperm were examined for each sperm sample (n=5 for each genotype).
Data in sperm analysis are means ± SEM. P<0.05 was regarded as a significant difference in all parameters between wildtypeand Tdrp-deficient and is indicated with *.
Deficiency of Tdrp does not affect male fertility in mice
We studied fertility ability in Tdrp +/+, Tdrp +/- and Tdrp -/- mice by continuously mating them with 8-week-oldwild-type female mice at a 1:2 male-to-female sex ratio (Table 2). In order to reduce the impact of different genetic backgrounds, mating was completed with female mice of different genetic backgrounds (S129 and C57BL/6). No significant differences were found among both two groups in the ability of male mice of any genotype to plug wild type female mice or to make them pregnant, and the mean number of offspring was almost identical as well (Table 2), suggesting deficiency of TDRP does not affect the vaginal plug rate or pregnancy rate.
Table 2.
Targeted disruption of Tdrp did not affect male fertility in mice
| Genetic background | Male mice | Female mice | Plugged mice | Pregnant mice | Offspring (M/F)* | AOA# | FCP (%)$ | FC (%)& |
|---|---|---|---|---|---|---|---|---|
| C57BL/6 | Tdrp -/- (n=5) | WT (n=55) | 38 | 32 | 237 (131:106) | 7.4 | 69.1 | 58.2 |
| Tdrp +/- (n=5) | WT (n=54) | 43 | 37 | 297 (140:157) | 8.0 | 79.6 | 68.5 | |
| WT (n=5) | WT (n=39) | 23 | 22 | 164 (84:80) | 7.5 | 60.0 | 56.4 | |
| S129 | Tdrp -/- (n=2) | WT (n=14) | 7 | 6 | 33 (18:15) | 5.5 | 50.0 | 42.8 |
| Tdrp +/- (n=3) | WT (n=26) | 22 | 18 | 142 (68:74) | 7.9 | 84.6 | 69.2 | |
| WT (n=3) | WT (n=23) | 14 | 11 | 84 (37:47) | 7.6 | 60.9 | 47.8 |
M, male; F, female.
Average offspring amount (AOA) was calculated as the total number of offspring divided by the number of females that gave birth to offspring.
Frequency of copulatory plug (FCP) was calculated as the ratio of the number of plugged females to total number of females to which males with the same genotype had access.
Frequency of conception (FC) was calculated as the ratio of the number of females that gave birth to offspring to total number of females.
p>0.05 for all the comparison of FCP or FC.
The interaction between TDRP1 and PRM2
The yeast two-hybrid assay indicated that TDRP1 interacted with PRM2. Previous work has demonstrated that TDRP1 is dominantly localized in the nuclei of spermatogenic cells [12]. In this study, immunofluorescence staining was applied to confirm the localization of TDRP1 and its relation to PRM2 inmature spermatozoafrom human beings and from mice. Our data indicated that TDRP1 was also primarily localized in the nuclei of spermatozoa, in addition to its distribution along the sperm flagellum, especially the mid-piece, which means there was also protein in the cytoplasm (Figure 3A). Moreover, immunofluorescent staining revealed similar localization of PRM2 and TDRP1 in mature spermatozoa from both humans and mice, which means that TDRP1 mayfunction physiologically through interaction with PRM2 (Figure 3B). Immunoprecipitation of PRM2 and TDRP1 were confirmed both withectopic and endogenous proteins (Figure 4A, 4B). Collectively, these data demonstrate that TDRP1 mayact as a nuclear factor partially through interaction with PRM2 in spermatozoa.
Figure 3.
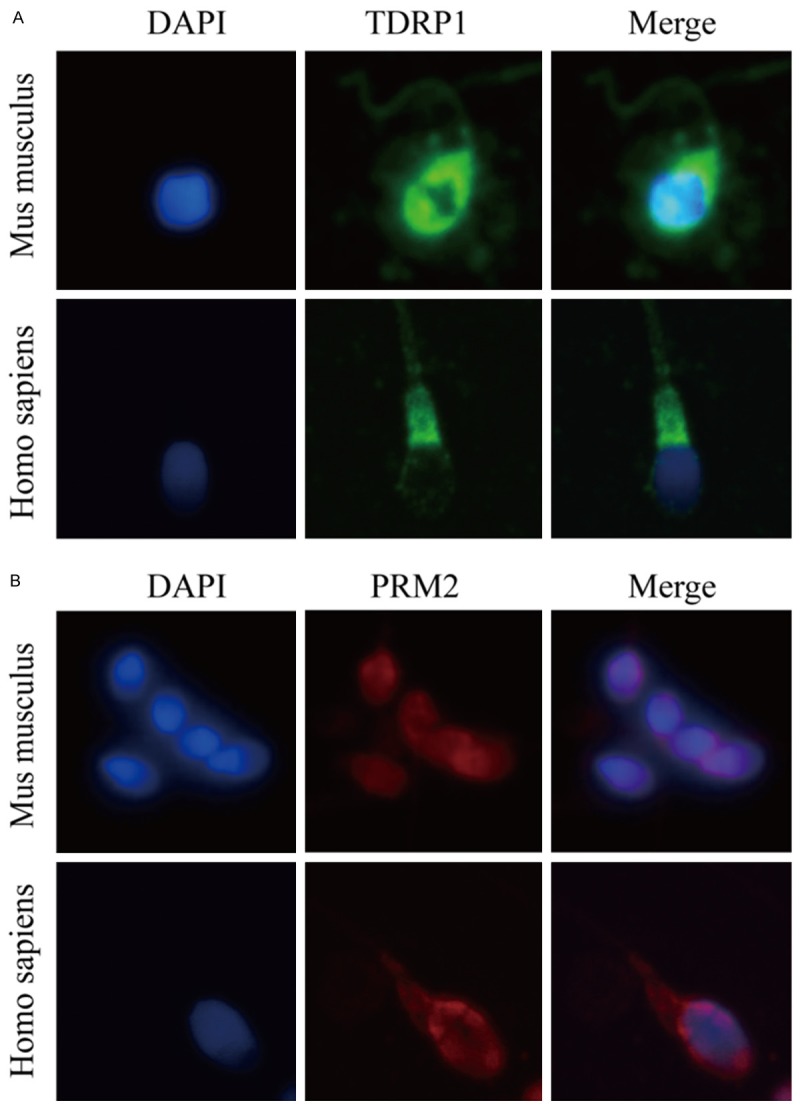
Cellular location of TDRP1 and PRM2. A. Localization of TDRP1 in spermatozoa from Homo sapiens and Mus musculus. TDRP1 was stained with green fluorescence and DAPI was blue. B. Localization of PRM2 in spermatozoa from Homo sapiens and Mus musculus. PRM2 was stained with red fluorescence and DAPI was blue.
Figure 4.
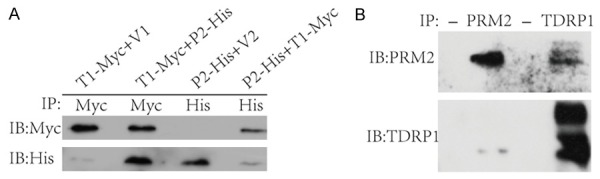
TDRP1 interact with PRM2. A. In vitro co-immunoprecipitation of TDRP1 and PRM2. The recombined TDRP1 was tagged with Myc, PRM2 with His. Both were expressed in 293T cells. T1:TDRP1; P2: PRM2; V1 and V2 are vectors. B. In vivo co-immunoprecipitation of TDRP1 and PRM2. The first and third lane served as controls with agarose beads alone.
Discussion
Our previous work has demonstrated that TDRP is associated with spermatogenesis in both mice and humans [12]. In the present study, we further investigated its role in Tdrp-deficient mouse models. Here, wereported for the first time that Tdrp deficiency alonewas not sufficient for male infertilityin mice, but causeda significant decrease in sperm motility.
It was hypothesized that Tdrp deficiency might contribute to the pathogenesis of male infertility. Therefore, we successfully generated the Tdrp-deficient mice with targeting vectors. Tdrp-deficient mice had morphologically normal spermatozoa. Fertility tests showed no significant differences in the ability of male mice of any genotype to plug or impregnate wild type females. However, the key parameters for sperm motility, including VAP, VSL and VCL were significantly decreased in Tdrp-deficient mice.
In the present study, low sperm motility caused by Tdrp deficiency did notaffect fertility rate in mice. In contrast, human male infertility patients frequently have low sperm motility, and it is well known that sperm motility is a critical parameter that definesnormal semen. Men with impaired sperm motility have reduced fertilization possibility which may result in infertility. There are several possible explanations for the discrepancy between human clinical results and the results of the present investigation. In the present Tdrp-deficient mouse model, the effects of the single mutation of Tdrp could be compensated by other motility genes, such as Catspers, especially in mice, which haveextraordinary reproductive ability [16]. It is important to note that the genetic background for the reproductive system is different between human being and mus musculus. The mouse is not a perfect animal model of human reproduction, since its reproductive ability is much stronger than men [17]. Human beings with TDRP deficiency would be more vulnerable to pathological factors and lifestyles, including other genetic disorders, genital tract infections, environmental contamination and cigarette smoking [18,19]. It is acknowledged that accumulation of these mild defectscould cause male infertility. In addition to multiple pathological factors, such as radiation and reactive oxygen species, genetic disorders canplay a key role in sperm damage [20,21]. Here we report that Tdrp is one of the genes likely involved inmale infertility.
Our team has proved that TDRP1 is primarily located inspermatogenic cells of testis, which is the precursor of spermatozoa [12]. However, the morphology of testis in Tdrp-deficient mice is not different fromthe wild type. Tdrp deficiency is not lethal for development of spermatozoa within the epithelium of the seminiferous tubules in mice. The spermatozoa of this stage is morphologically complete but is immotile and unable to fertilize an oocyte. This fertilization ability is the result of discrete post-gonadaldifferentiation stages during transit along theepididymal tubule. Immunofluorescence staining indicated that TDRP1 was not restricted to nuclei in mature spermatozoa, but wasalso located in the cytoplasm of the head and flagella. Simultaneously, CASA revealed differences in sperm motility between Tdrp-deficient mice and wild type mice. Given that TDRP1 is a novel protein that has been cloned recently, its biological function remains unclear [22]. It is proposed that TDRP1 is exported from nuclei to the cytoplasm during maturation in the epididymis and capacitation in vitro, and then plays a role in the capacitation process, including the efflux of cholesterol from the plasma membrane [23]. The mechanism of its cytoplasmic distribution might be post-translational modification during epididymal maturation and capacitation, such as phosphorylation, acetylation, or methylation, since spermatozoa are transcriptionally and translationally silent [24,25].
TDRP1 is primarily located in nuclei of spermatogenic cells and spermatozoa, which makes it plausible that TDRP1 might possess nuclear functions. During sperm maturation and fertilization, sperm protamines (PRM1 and PRM2) sequentially replace somatic histones through a multi-step process [26]. Both protamines are essential, and haploinsufficiency caused by a deletion in one allele of PRM1 or PRM2 is sufficient to alter spermatogenesis and prevents genetic transmission [27]. PRM1 and PRM2 may also act as a checkpoint for abnormal protamine ratio leading to an increased level of apoptosis [28]. The substitution is completed in the elongated spermatids. Transition proteins, that replace histones and non-histone proteins in round spermatids, are replaced by protamines, which is the dominant nuclear protein of mature spermatozoa [26,29]. Finally, the sperm chromatin is highly condensed and transcriptionally silent, which is essential formaturation of spermatozoa and is unique to haploid cells [30]. Here we reported that TDRP1 and PRM2 were both localized in the head of mature spermatozoa, and TDRP1 and PRM2 could bind to each other. This indicates that the nuclear function of TDRP1 is partially mediated by PRM2. We hypothesized that TDRP1 might function asa non-histone protein, acting to complement or supplement protamines during spermatogenesis.
It is important to note that, in addition to mice and humans, TDRP1 is also associated with the reproduction traits of pigs, which indicates that TDRP1 has functions in reproduction that are conserved among species [39, 40]. However, the molecular and cellular mechanism of TDRP1 remains elusive and needs further investigation.
In conclusion, we successfully generateda mouse model with Tdrp deficiency. Although morphological examination and fertility test shows no significant differences, parameters of sperm motility, including VAP, VSL and VCL, were significantly decreased in Tdrp-deficient mice. Furthermore, we confirmed the subcellular localization and protein interaction of TDRP1 and PRM2 in spermatozoa. Although Tdrp deficiencyalone is not sufficient to cause male infertility in mice, it impairs sperm motility partially through the interaction with PRM2.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (grant number: 81070528&81370753 to Qiang Ding, 81070647&81370936 to XuanchunWang, 81270904 to Zhihong Yang) as well as grants from Science and Technology Commission of Shanghai Municipality (grant number: 09DJ1400405 to Qiang Ding, 12140903202&13140901600 to Xuanchun Wang). The work was also funded by a grant from Shanghai Municipal Education Commission (grant number: 11ZZ08) to Qiang Ding. We would like to thank David Pober at Harvard Medical School for his help in preparing the manuscript.
Disclosure of conflict of interest
There were no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Louis JF, Thoma ME, Sorensen DN, McLain AC, King RB, Sundaram R, Keiding N, Buck Louis GM. The prevalence of couple infertility in the United States from a male perspective: evidence from a nationally representative sample. Andrology. 2013;1:741–748. doi: 10.1111/j.2047-2927.2013.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ombelet W, Cooke I, Dyer S, Serour G, Devroey P. Infertility and the provision of infertility medical services in developing countries. Hum Reprod Update. 2008;14:605–621. doi: 10.1093/humupd/dmn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, Regional, and Global Trends in Infertility Prevalence Since 1990: A Systematic Analysis of 277 Health Surveys. PLoS Med. 2012;9:e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bak CW, Seok HH, Song SH, Kim ES, Her YS, Yoon TK. Hormonal Imbalances and Psychological Scars Left Behind in Infertile Men. J Androl. 2012;33:181–189. doi: 10.2164/jandrol.110.012351. [DOI] [PubMed] [Google Scholar]
- 5.Wu AK, Elliott P, Katz PP, Smith JF. Time costs of fertility care: the hidden hardship of building a family. Fertil Steril. 2013;99:2025–2030. doi: 10.1016/j.fertnstert.2013.01.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slade P, O’Neill C, Simpson AJ, Lashen H. The relationship between perceived stigma, disclosure patterns support and distress in new attendees at an infertility clinic. Hum Reprod. 2007;22:2309–2317. doi: 10.1093/humrep/dem115. [DOI] [PubMed] [Google Scholar]
- 7.Bachir BG, Jarvi K. Infectious, Inflammatory, and Immunologic Conditions Resulting in Male Infertility. Urol Clin North Am. 2014;41:67–81. doi: 10.1016/j.ucl.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Chen X, Wang S, Jiang M, Zheng B, Zhou Q, Bi Y, Zhou Z, Huang X, Sha J. Flotillin-2 is an acrosome-related protein involved in mouse spermiogenesis. J Biomed Res. 2012;26:278–287. doi: 10.7555/JBR.26.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi Y, Jiang M, Yuan Y, Bi Y, Zheng B, Guo X, Huang X, Zhou Z, Sha J. ADP-ribosylation factor-like 3, a manchette-associated protein, is essential for mouse spermiogenesis. Mol Hum Reprod. 2013;19:327–335. doi: 10.1093/molehr/gat001. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Yang F, Leu NA, Wang PJ. MNS1 is essential for spermiogenesis and motile ciliary functions in mice. PLoS Genet. 2012;8:e1002516. doi: 10.1371/journal.pgen.1002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delbes G, Yanagiya A, Sonenberg N, Robaire B. PABP interacting protein 2A (PAIP2A) regulates specific key proteins during spermiogenesis in the mouse. Biol Reprod. 2012;86:95. doi: 10.1095/biolreprod.111.092619. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Jiang H, Zhou W, Zhang Z, Yang Z, Lu Y, Lu B, Wang X, Ding Q, Hu R. Molecular cloning of a novel nuclear factor, TDRP1, in spermatogenic cells of testis and its relationship with spermatogenesis. Biochem Biophys Res Commun. 2010;394:29–35. doi: 10.1016/j.bbrc.2010.02.061. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Du YR, Qin WH, Hu YG, Huang YN, Bao L, Han D, Mansouri A, Xu GL. RIM-BP3 is a manchette-associated protein essential for spermiogenesis. Development. 2009;136:373–382. doi: 10.1242/dev.030858. [DOI] [PubMed] [Google Scholar]
- 14.Shen C, Kuang Y, Liu J, Feng J, Chen X, Wu W, Chi J, Tang L, Wang Y, Fei J, Wang Z. Prss37 is required for male fertility in the mouse. Biol Reprod. 2013;88:123. doi: 10.1095/biolreprod.112.107086. [DOI] [PubMed] [Google Scholar]
- 15.Quinn P, Kerin JF, Warnes GM. Improved pregnancy rate in human in vitro fertilization with the use of a medium based on the composition of human tubal fluid. Fertil Steril. 1985;44:493–498. doi: 10.1016/s0015-0282(16)48918-1. [DOI] [PubMed] [Google Scholar]
- 16.Chung JJ, Shim SH, Everley RA, Gygi SP, Zhuang X, Clapham DE. Structurally distinct Ca(2+) signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell. 2014;157:808–822. doi: 10.1016/j.cell.2014.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamsai D, O’Bryan MK. Mouse models in male fertility research. Asian J Androl. 2011;13:139–151. doi: 10.1038/aja.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Kretser DM. Male infertility. Lancet. 1997;349:787–790. doi: 10.1016/s0140-6736(96)08341-9. [DOI] [PubMed] [Google Scholar]
- 19.Skakkebaek NE, Jorgensen N, Main KM, Rajpert-De Meyts E, Leffers H, Andersson AM, Juul A, Carlsen E, Mortensen GK, Jensen TK, Toppari J. Is human fecundity declining? Int J Androl. 2006;29:2–11. doi: 10.1111/j.1365-2605.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 20.Shamsi MB, Kumar K, Dada R. Genetic and epigenetic factors: Role in male infertility. Indian J Urol. 2011;27:110–120. doi: 10.4103/0970-1591.78436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferlin A, Raicu F, Gatta V, Zuccarello D, Palka G, Foresta C. Male infertility: role of genetic background. Reprod Biomed Online. 2007;14:734–745. doi: 10.1016/s1472-6483(10)60677-3. [DOI] [PubMed] [Google Scholar]
- 22.Wang XC, Xu SY, Wu XY, Song HD, Mao YF, Fan HY, Yu F, Mou B, Gu YY, Xu LQ, Zhou XO, Chen Z, Chen JL, Hu RM. Gene expression profiling in human insulinoma tissue: genes involved in the insulin secretion pathway and cloning of novel full-length cDNAs. Endocr Relat Cancer. 2004;11:295–303. doi: 10.1677/erc.0.0110295. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Gonzalez EO, Sosnik J, Edwards J, Acevedo JJ, Mendoza-Lujambio I, Lopez-Gonzalez I, Demarco I, Wertheimer E, Darszon A, Visconti PE. Sodium and epithelial sodium channels participate in the regulation of the capacitation-associated hyperpolarization in mouse sperm. J Biol Chem. 2006;281:5623–5633. doi: 10.1074/jbc.M508172200. [DOI] [PubMed] [Google Scholar]
- 24.Baker MA, Nixon B, Naumovski N, Aitken RJ. Proteomic insights into the maturation and capacitation of mammalian spermatozoa. Syst Biol Reprod Med. 2012;58:211–217. doi: 10.3109/19396368.2011.639844. [DOI] [PubMed] [Google Scholar]
- 25.Wu F, Zhao ZH, Ding ST, Wu HH, Lu JJ. High Mobility Group Box 1 Protein Is Methylated and Transported to Cytoplasm in Clear Cell Renal Cell Carcinoma. Asian Pac J Cancer Prev. 2013;14:5789–5795. doi: 10.7314/apjcp.2013.14.10.5789. [DOI] [PubMed] [Google Scholar]
- 26.Oliva R, Dixon GH. Vertebrate protamine genes and the histone-to-protamine replacement reaction. Prog Nucleic Acid Res Mol Biol. 1991;40:25–94. doi: 10.1016/s0079-6603(08)60839-9. [DOI] [PubMed] [Google Scholar]
- 27.Cho C, Willis WD, Goulding EH, Jung-Ha H, Choi YC, Hecht NB, Eddy EM. Haploinsufficiency of protamine-1 or -2 causes infertility in mice. Nat Genet. 2001;28:82–86. doi: 10.1038/ng0501-82. [DOI] [PubMed] [Google Scholar]
- 28.Steger K, Wilhelm J, Konrad L, Stalf T, Greb R, Diemer T, Kliesch S, Bergmann M, Weidner W. Both protamine-1 to protamine-2 mRNA ratio and Bcl2 mRNA content in testicular spermatids and ejaculated spermatozoa discriminate between fertile and infertile men. Hum Reprod. 2008;23:11–16. doi: 10.1093/humrep/dem363. [DOI] [PubMed] [Google Scholar]
- 29.Hecht NB. Regulation of ‘haploid expressed genes’ in male germ cells. J Reprod Fertil. 1990;88:679–693. doi: 10.1530/jrf.0.0880679. [DOI] [PubMed] [Google Scholar]
- 30.Sassone-Corsi P. Unique chromatin remodeling and transcriptional regulation in spermatogenesis. Science. 2002;296:2176–2178. doi: 10.1126/science.1070963. [DOI] [PubMed] [Google Scholar]


