Abstract
Objective: The effect of anticancer drugs Trichostation A (TSA) and GSK2126458 (GSK) on genetic recombination of sperm meiosis in mice was investigated, and their clinical feasibility of fertility preservation in cancer patients was also assessed. Methods: Eighteen Kunming mice were randomly given TSA or GSK at the concentrations of 0, 0.1 and 0.2 umol/L for three months. Immunofluorescence was used to evaluate the genetic recombination of homologous chromosomes and fidelity of chromosome synapsis. Sperm density, motility and viability were also examined to investigate the spermatogenic function. Results: The average number of MLH1 foci in each spermatocyte was greatly higher in TSA (0.1) group than that in control (P<0.05), but no difference with the TSA (0.2) group (P>0.05). The frequency of SC with no MLH1 foci was lower while the frequency of SC with one MLH1 foci was higher in spermatocyte of mice with different doses of TSA compared with controls (P<0.05). The weight of left testis in TSA (0.1) group was significant decreased compared with that in control (P<0.05). However, no significant differences were observed in average number of MLH1, frequency of SC with 0-3 MLH1 foci, spermatocyte percentage of XY chromosomes containing MLH1 foci and percentages of cells containing gaps and splits among groups with or without the treatment of GSK. Furthermore, there were no statistical differences in body weight, testicular weight, sperm density, sperm motility and sperm viability among the three groups. Conclusion: TSA increased genetic recombination frequency of spermatocyte meiosis. GSK had no significant effect on genetic recombination frequency of spermatocyte meiosis and spermatogenic function.
Keywords: Trichostation A, GSK, meiosis, genetic recombination, fertility preservation
Introduction
Meiosis, a way drives the differentiation of spermatogonium into sperm, is an important process of spermatogenesis in male [1]. Confederation, exchange, and separation of homologous chromosomes in prophase I greatly influence the success of meiosis, which is closely related to the genetic recombination [2]. Researches have demonstrated that the abnormality of meiotic prophase I in spermatocyte will lead to meiosis disorder, stagnation, and even azoospermia [3,4]. Therefore, the analysis in frequency and synapsis of meiotic recombination is conducive to better understand the mechanisms of spermatogenetic malfunction, and reveal the pathophysiology of male infertility.
With the rapid development of therapeutic strategy in cancer, the survival rate of patients are improved continuously. The needs of keeping male fertility preservation are increasing [5,6]. Therefore, it is an urgent challenge for reproductive medicine and oncology to securely obtain fertility of male patients with cancer. In order to prolong the life as well as fertility preservation in cancer patients, the choices of anti-tumor therapeutic strategy should be considered to keep safe. It has been indicated that spermatogonia is most active and sensitive to cytotoxic drugs in the process of cell differentiation [7]. There are many different kinds of anticancer drugs, and fractional ones have been studied to illustrate their toxicity for germ cells [8]. Although some progress is made, many issues have not yet been resolved. To choose the drugs with minimum toxicity on germ cells is very significant in the anti-tumor strategy.
Traditional anticancer drug Trichostatin A (TSA) and new anticancer drug GSK2126458 are two representative drugs among the various antitumor drugs [9,10]. Currently, the studies on TSA is focus on its efficacy in clinical trials. There have been few reports to investigate the toxic effect on ovocyte in animal, but not on spermatogenesis function. GSK2126458, a highly potent inhibitor of PI3K and mTOR, participates in inhibiting proliferation of tumor cells and angiopoiesis [11]. The clinical research of GSK2126458 for anti-cancer has been initiated, but its role in toxicity for germ cell is not seen before. In the present study, the effects of TSA and GSK2126458 on the spermatogenic function, sites of meiosis genetic recombination and fidelity of synapsis were investigated. This finding aims to evaluate the safety of these two anti-tumor drugs for reproductive function and provide theoretical foundation for clinical application.
Materials and methods
Animals and experimental design
Male Kunming mice aged 8 weeks (20 g~25 g), purchased from the Laboratory Animal Center of Henan Province (Zhengzhou, China), were housed and treated with the Animal Care and Use Committee of the First Affiliated Hospital of Zhengzhou University. TSA was diluted with dimethyl sulfoxide (DMSO) and GSK2126458 was diluted with dimethylformamide (DMF) to the concentrations of 0.1 umol/L and 0.2 umol/L, respectively. Eighteen mice were randomly grouped into control (n=6) and four experimental groups (n=3). All drugs were given by intraperitoneal injection. Group 1 (Control 1) received the DMSO. Group 2 (Control 2) received the DMF. Group 3 (TSA 0.1) was given the 0.1 umol/L TSA. Group 4 (TSA 0.2) was given the 0.2 umol/L TSA. Group 5 (GSK 0.1) received the 0.1 umol/L GSK2126458. Group 6 (GSK 0.2) was given the 0.2 umol/L GSK2126458. The drugs (1 ml/kg) were given to mice every other day according to the body weight, with a continuous medication for 3 months. After weighing, all mice were anesthetized and sacrificed by cervical dislocation. The testis was isolated and then performed histologic analysis and immunofluorescence observation.
Testis weighing and sperm collection
Both sides of testiculus were weighted and recorded. The epididymal luminal fluid was extracted and incubated at 37°C for 15 min. The sperm density and sperm viability were counted three times per mice using microscope. The counting in all mice was performed by the same person.
Histological processing
The testicular tissue was fixed with 4% paraformaldehyde for 24 h, and dehydrated with 30% sucrose solution over 48 h. Samples were embedded in freezing microtome at -25°C, and frozen sections were prepared. The sections were stained with hematoxylin-eosin and observed with microscope. Statistical analysis of cell number: the amounts of sustentacular cells in seminiferous tubule, spermatogonium and multiple stage spermatocytes were calculated; the percentage of spermatogonium was analyzed.
Immunofluorescence
The fresh testicular tissue was incubated in a hypotonic buffer (HB; 30 mM Tris, pH 8.2, 50 mM sucrose, 17 mM dihydrate sodium citrate, 5 mM EDTA, 2.5 mM DTT, 1 mM PMSF) at room temperature for 30 min. The obtained seminiferous tubules were completely tore in 0.1 mol/L sucrose for preparing single cell suspension of spermatocyte. Then spermatocytes suspension was spread onto a glass slide covered with 1% poly formaldehyde (pH 9.2) containing 0.2% TritonX-100, and rested for 6-10 h in wet box at room temperature. After drying, the glass slide was washed with 0.04% Photo-Flo for 4 min, and incubated with antibody dilution buffer (ADB; 1% normal donkey serum, 0.3% BSA, 30 mM Tris-HCl buffer containing 0.005% Triton X-100) for 30 min in wet box. The mixed ADB solution containing primary antibodies anti-SCP3 and anti-MLH1 as well as human CREST serum were loaded on glass slide, which was then covered with coverslip to incubated at 37°C overnight. After rinsing with PBS for three times, the second antibodies anti-rabbit IgG (red, 555 nm), anti-rabbit IgG labeled (blue, AMCA) and anti-rat IgG (green, 488 nm) were then incubated with samples at 37°C for 90 min. The fluorescent images were observed under the fluorescence microscope.
Fluorescence microscopy
Fluorescence microscopy was used to observe and identify the various meiotic prophase stages of spermatocyte. Pictures were taken on spermatocytes at the pachytene stage and the analysis of meiosis was performed using Image Pro-Plus version 5.1 software. The features of pachytene spermatocytes were as follows (Figure 1). Autosomes appear in pairs and chromosomes begin pairing with a clear signal. The numbers of bivalents are correct. Synaptonemal complex (SC) has no obvious stretch, twist and/or overlap. Spermatocytes stained for SCP3 (red), MLH1 (green) and centromere (blue). Merged image of SCP3, MLH1 and centromere was shown and reflected a spermatocyte SC in prophase stage and genetic recombination map.
Figure 1.
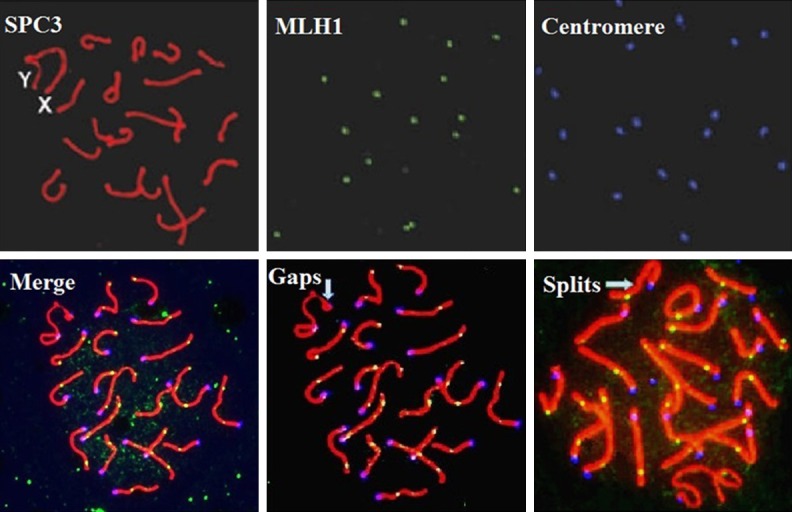
Spermatocytes meiosis in male mice. Spermatocytes stained for SCP3 (red), MLH1 (green) and centromere (blue).
The genetic recombination of homologous chromosomes was evaluated with following indicators: the average number of MLH1 sites in each cell, the frequency of SC containing different number of MLH1 foci and the cell percentage of XY chromosomes containing MLH1 sites. The fidelity of homologous chromosomes synapsis was evaluated by the gaps and splits of SC. The cell percentage of XY chromosomes containing MLH1 sits was calculated using the formula: (cells with chromosomes containing MLH1 sits/total cell counts for analysis) × 100%. The percentage of cells containing gaps/splits was calculated with the formula: (cells containing gaps/splits/total cell counts for analysis) × 100%. The percentage of spermatogonium seminiferous tubule was calculated with the formula: (spermatogonium number/total cell counts).
statistical analysis
All data was analyzed with the SPSS 17.0 software. The comparison of ratios or percentages in different groups was performed with Chi-square (χ2) test. The measurement data was expressed as mean ± standard deviation (SD), and compared with Kruskal-Wallis test and Bonferroni method. The acceptance standard was α=0.05. P<0.05 was considered to be statistical difference.
Results
The effects of TSA on testis weight and sperm viability in mice
The mice were treated with different doses of TSA. As shown in Table 1, there were no statistical differences in body weight, testis weight at the right, sperm density, sperm motility and sperm viability among the three groups with or without TSA (P>0.05). However, the weight of left testis in mice with the administration of 0.1 umol/L TSA was significant decreased compared with that in control (P<0.05).
Table 1.
The testicular weights and sperm viability in male mice with the treatment of TSA (mean ± SD)
| Groups | Age (weeks) | Body weight (g) | Weight of left testis (g) | Weight of right testis (g) | Sperm density (× 106/ml)) | Sperm motility (%) | Sperm viability (%) |
|---|---|---|---|---|---|---|---|
| TSA (0.1) | 8 | 24.00±0.95 | 0.09±0.01* | 0.09±0.01 | 24.33±3.84 | 20.67±3.84 | 45.33±2.91 |
| TSA (0.2) | 8 | 25.80±1.00 | 0.10±0.01 | 0.11±0.01 | 26.00±1.00 | 18.00±7.00 | 49.50±0.50 |
| Control | 8 | 30.75±1.89 | 0.14±0.01 | 0.14±0.02 | 43.25±8.88 | 28.00±5.70 | 48.75±3.45 |
Versus with control group, P<0.05.
The effect of TSA on testis histology
The HE staining showed the regular arrangement, clear layers and normal structure in spermatogenic cells of contorted seminiferous in control mice (Figure 2). Leydig cells distributed between seminiferous tubule. Spermatogonium, spermatocyte of multiple stages and abundant sperm were observed in lumen section, with seminoma cells accounting for 10%. However, in mice with treatment of TSA, the lumen of contorted seminiferous tended to thin compared with that in controls. It was also observed that the spermatogenic cells partially fell off and their layers were reduced. The count of spermatogonium accounted for 43%, and the quantity of spermatocyte of multiple stages and sperm were decreased in lumen.
Figure 2.
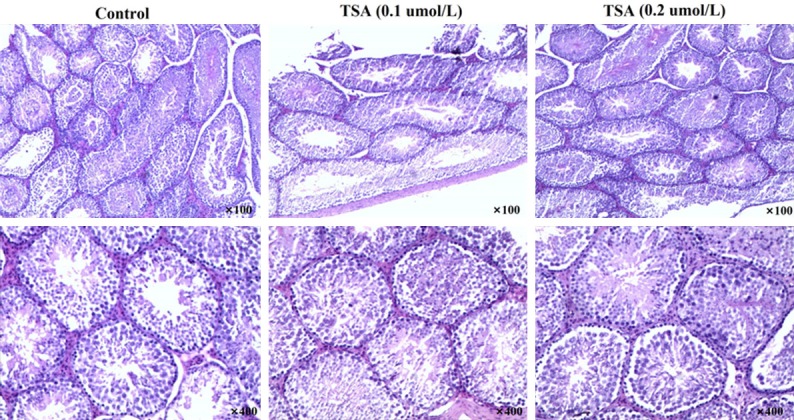
Testicular histology in male mice treated with differences concentrations of TSA.
The effect of TSA on meiosis genetic recombination of spermatocyte in mice
As shown in Table 2, the analysis of recombination sites demonstrated that the MLH1 number of each spermatocyte in TSA (0.1) group was greatly higher than that in control (P<0.05), while no difference was observed between TSA (0.2) and control groups (P>0.05). The frequency of SC with no MLH1 foci was lower while the frequency of SC with one MLH1 foci was higher in spermatocyte of mice with different doses of TSA compared with controls (P<0.05). There are no statistical differences in SC frequency with MLH1 foci (2-3) and the cell percentage of XY chromosomes containing MLH1 sites among controls and two TSA groups (P>0.05). The analysis of synapsis fidelity also showed no significant differences in the percentage of cells with gaps or splits among three groups (P>0.05).
Table 2.
Meiosis genetic recombination of spermatocyte in mice with the treatment of TSA (%)
| Groups | Cell population | MLH1 numbera | Frequency of SC with MLH1 | MLH1 Percentageb | Gaps percentagec | Splits percentaged | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 0 | 1 | 2 | 3 | ||||||
| TSA (0.1) | 70 | 19.98±0.34* | 2.15±0.17* | 15.97±0.25* | 1.78±0.22 | 0.15±0.05 | 14.29 | 24.29 | 8.57 |
| TSA (0.2) | 56 | 18.68±0.53 | 3.88±0.29# | 13.91±0.24# | 2.05±0.22 | 0.23±0.08 | 12.96 | 26.79 | 10.71 |
| Control 1 | 81 | 17.46±0.42 | 4.53±0.33 | 13.48±0.32 | 1.94±0.15 | 0.11±0.05 | 20 | 20 | 6.25 |
Versus with control group, P<0.05;
Versus with TSA (0.1) group, P<0.05;
The average MLH1 number in each spermatocyte;
The cell percentage of spermatocyte containing MLH1 foci in XY chromosomes;
The percentage of spermatocyte containing gaps;
The percentage of spermatocyte containing splits.
The effects of GSK2126458 on testis weight and sperm viability in mice
We also investigated the effects of GSK2126458 on spermatogenesis in mice. As shown in Table 3, it was showed that there were no statistical differences in body weight, testicular weight, sperm density, sperm motility and sperm viability among the three groups with or without the treatment of GSK2126458 (P>0.05).
Table 3.
The testicular weights and sperm viability in male mice with the treatment of GSK2126458 (mean ± SD)
| Groups | Age (weeks) | Body weight (g) | Weight of left testis (g) | Weight of right testis (g) | Sperm density (× 106/ml) | Sperm motility (%) | Sperm viability (%) |
|---|---|---|---|---|---|---|---|
| GSK (0.1) | 8 | 32.65±0.65 | 0.12±0 | 0.13±0.01 | 19.00±2.00 | 30.50±9.50 | 45.50±5.50 |
| GSK (0.2) | 8 | 37.30±0.70 | 0.14±0.02 | 0.12±0.01 | 32.00±4.00 | 33.50±6.50 | 47.50±6.50 |
| Control 2 | 8 | 30.75±1.89 | 0.14±0.01 | 0.14 ±0.02 | 43.25±8.88 | 34.75±6.82 | 55.00±3.81 |
The effect of GSK2126458 on testis histology
Histological examination results demonstrated that spermatogenic cells of contorted seminiferous had regular arrangement, clear layers and normal structure, and leydig cells distributed between seminiferous tubule in control mice (Figure 3). It was observed abundant spermatid and sperm in lumen, and the percentage of seminoma cells was 11%. Administrated the mice with different doses of GSK2126458, the spermatogenic cells slightly fell off. However, large numbers of sperm could be observed in the lumen, with spermatogonium accounting for 16%. These results suggested that the side effect of GSK2126458 on spermatogenesis was mild.
Figure 3.
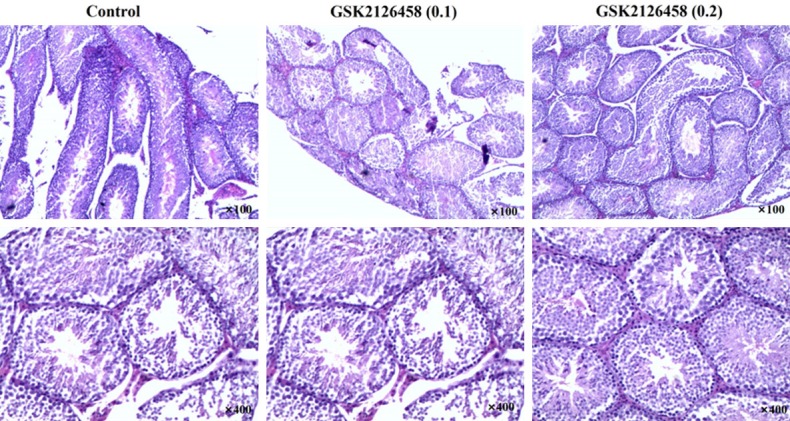
Testicular histology in male mice treated with differences concentrations of GSK2126458.
The effect of GSK2126458 on meiosis genetic recombination of spermatocyte in mice
The analysis of recombination sites and synapsis fidelity demonstrated that the MLH1 number of each spermatocyte, frequency of SC with MLH1 foci (0-3), percentage of spermatocyte with XY chromosomes containing MLH1 sites and cell percentages of gaps and splits were in the same levels among the spermatocyte of mice with or without GSK2126458 treatment (Table 4).
Table 4.
Meiosis genetic recombination of spermatocyte in mice with the treatment of GSK2126458 (%)
| Groups | Cell population | MLH1 numbera | Frequency of SC with MLH1 | MLH1 percentageb | Gaps percentagec | Splits percentaged | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 0 | 1 | 2 | 3 | ||||||
| GSK (0.1) | 60 | 17.78±0.33 | 4.33±0.22 | 13.80±0.22 | 1.77±0.12 | 0.15±0.05 | 15.00 | 28.33 | 16.67 |
| GSK (0.2) | 54 | 16.88±0.45 | 4.89±0.33 | 13.28±0.31 | 1.72±0.14 | 0.11±0.05 | 9.26 | 20.37 | 11.11 |
| Control 2 | 81 | 17.46±0.42 | 4.53±0.33 | 13.48±0.32 | 1.94±0.15 | 0.11±0.05 | 19.75 | 22.22 | 7.41 |
The average MLH1 number in each spermatocyte;
The cell percentage of spermatocyte containing MLH1 foci in XY chromosomes;
The percentage of spermatocyte containing gaps;
The percentage of spermatocyte containing splits.
Discussion
Epigenetics of malignant tumor has drawn extensive attention from tumor bilogists, as it retates to occurrence, development, diagnosis and treatment of cancer. Abnormal epigenetic modification will lead to dysregulation of gene expression, causing metabolic disorders and tumors [12,13]. The histone deacetylase inhibitor is commonly studied in the field of epigenetic modification. Inhibiting the activity of histone deacetylase is critical to regulate the expression of target proteins in transcriptional and post-translational levels, which activates the apoptotic signaling pathways, thereby inducing tumor cell apoptosis [14].
Traditional antineoplastic TSA belongs to hydroxamic acids, and it is the most effective known HDAC inhibitor. The effect of TSA is reversible noncompetitive. It has been demonstrated that TSA plays a role in inhibiting various tumor cells, including bladder cancer, oral squamous cell carcinoma, gastric cancer, lung cancer, nasopharyngeal carcinoma, salivary gland cancer, solid tumors in breast cancer cells and blood system diseases, etc [15-17]. Currently, the anti-tumor mechanism of TSA has been widely studied. The research showed that TSA has a high activity in the process of anticancer at nanomole level, and the effects are in time-dependent and dose-dependent manners [18]. However, the potential mechanisms remain unclear. Vigushin et al found that TSA at the concentration of 0.5 mg/kg~5 mg/kg had a dose-dependent role in inducing highly acetylation of H4 in breast cancer cell and with no obvious side effects [19]. The previous showed that the treatment of TSA contributes to the decrease of cell proliferation and telomerase activity in cancers [20].
Chemotherapeutics kill the tumor cells generally through the alterations of cellular DNA, RNA and proteins, which also results in the damage of normal cells. Spermatogenic cells are sensitive to the toxicant, thereby being greatly affected by chemotherapy drugs. Different chemotherapy drugs act on different stages of germ cells. Carboplatin can be inserted into the DNA strand directly kill spermatogonia, leading to the decrease of sperm production. Azithromycin acts on sperm, and it can reduce the sperm count in the first day of medicine [21]. Cyclophosphamides functions as reducing the activity of radical scavenge enzyme and elevating the accumulation of intracellular free radicals and lipid peroxidation, which leads to the damage of spermatogenic cells [22].
The possible mechanism of TSA on tumor suppression is the induction of histone acetylation, which is involved in the driving chromosomal untwisting, exposing the connection area of DNA and leading to the DNA breakage. Death receptor signaling in cell apoptosis pathway can also be activated by histone acetylation, contributing to stagnation in cell proliferation [23,24]. However, the related reports of TSA on toxic effects in clinical trials and animals are rarely available. The nuclear transfer shows that TSA could achieve synchronization of fibroblast cycle, and elevate the development level of reconstructed embryo with no side effects. Jun Iwasshita et al found that TSA had no effect on meiosis of oocytes but slowed down its maturation in XenoPus laevis [25]. However, more evidences in the toxic effect of TSA on germ cells are very limited.
In this study, the TSA was diluted into two concentrations at 0.1 umol/L and 0.2 umol/L and the volume for injection was dependent on the body weight of mice. The testicular weight, sperm motility, recombination sites of spermatocyte meiosis and synapsis fidelity were examined to evaluate the effects of TSA at different concentrations on the spermatogenic function in male mice. The results showed that TSA increased the frequency of genetic recombination. To further analyze the average number of SC containing different frequency of MLH1 foci, the data demonstrated that the number of SC with non MLH1 foci was lower and the number with 1 MLH1 foci was higher in mice with the treatment of TSA than controls. Additionally, there was no statistical difference in SC frequency with MLH1 foci (2-3), percentages of cells with XY chromosomes containing MLH1 sits and percentages of cells with gaps/splits among groups with or without TSA administration. These data suggested that TSA elevated frequency of genetic recombination, with the increase of SC number containing one MLH1 foci and the decrease of SC number containing non MLH1 foci. However, the synapsis fidelity was not altered by TSA.
The increased frequency of meiosis genetic recombination results in the high risk of chromosome non-disjunction, which closely related to the spermatogenetic malfunction [26]. The current study showed that TSA at the concentration of 0.1 mol/L significantly lowered the left testis weight compared with control group. The increased frequency of meiotic recombination leading to a decrease of spermatogenic function is the possible mechanism to explain the reduction of testicular weight induced by TSA. Furthermore, the sperm density, sperm motility and sperm viability were not influenced by the different doses of TSA. There was also no difference in the effects on frequency of meiotic recombination and synapsis between the groups with two concentrations of TSA. Two mechanisms are proposed to explain this phenomenon. One possible mechanism is medication time. Although the recombination frequency was increased and the testicular weight was decreased by the supplement of TSA for 3 months, the spermatogenic function was not affected. It is expected to observe a reduction of spermatogenic function in the mice with longer TSA duration. Another possible mechanism is genetic background and sample size. The previous study has confirmed that recombination frequency is regulated by genetic background and external factors, contributing to the differences within the group [27].
With the development of therapy in malignant tumor, updated new antitumor drugs are emerging. Many traditional antitumor drugs show a low selectivity and high toxicity, leading to the failure of cancer treatment and prognosis. It is urgent to explore the new drugs with the features of high selectivity and low toxicity. However, the safety of novel drugs still plagues the researchers. In recent years, molecular biology has been rapidly developed and signal transduction pathways in tumors have been well known. PI3K/ATK pathway is important in cells to activate various downstream effector molecules, thereby regulating the cell proliferation, apoptosis and the formation of tumor vessels [28,29]. The inhibitors of this pathway have a potential targeted therapeutic strategy for cancers.
The new anticancer drug GSK2126458, a dual inhibitor of PI3K/mTOR, inhibits the activity of PI3K/mTOR and affects the cellular energy metabolism, suppressing the proliferation of cancer cell and formation of tumor vessel [11]. GSK2126458 has been used in clinical anti-tumor therapy, including the treatment of solid tumors, lymphomas and leukemia [30]. The studies of GSK2126458 are currently focused on its clinical therapeutic effects. Pharmacokinetics demonstrated that the bioavailability of oral GSK2126458 in mice was 100% [31]. Administrated BT474 tumor mice with 3 mg/kg GSK2126458 for 7 days, a significant effect on inhibiting tumor growth was observed [32]. However, no study has been reported to evaluate its safety on germ cells.
In the present study, GSK2126458 was used to assess its effects on spermatogenic function in male mice. The results showed that both two doses of GSK2126458 had no significant effects on body weight, testicular weight, sperm density, sperm motility and sperm viability. Additionally, the average number of SC containing different frequency of MLH1 and the percentages of cells with gaps/splits were at the same levels among three groups. The findings suggested that the meiosis genetic recombination of spermatocytes and spermatogenic function were not affected greatly by GSK2126458, which could be proposed to act as a new choice for fertility preservation in male.
In conclusion, this study showed that TSA increased the frequency of meiotic recombination in spermatocytes and reduced testicular weight, while no similar effects were observed in groups with the treatment of GSK2126458. The abnormal genetic recombination may lead to the stagnation of meiosis or dyszoospermia via triggering one or more meiosis related checkpoints. Since the knowledge of meiotic genetic background remains largely unknown, the cytogenetic mechanisms of synapsis, recombination and crossover should be further studied in the process of spermatocyte meiosis after the drug withdrawal, and the relationship between withdrawal time and the recovery of genetic recombination also should be investigated.
Acknowledgements
This work was supported by two National Natural Science Foundation of China (NO. 31401274 and NO. 31271605) and one Municipal Science and Technology Bureau Project of Zhengzhou, China (NO. 340600531813).
Disclosure of conflict of interest
None.
References
- 1.Parvinen M, Wright WW, Phillips DM, Mather JP, Musto NA, Bardin CW. Spermatogenesis in vitro: completion of meiosis and early spermiogenesis. Endocrinology. 1983;112:1150–1152. doi: 10.1210/endo-112-3-1150. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Cheng Z, Ma H. Meiosis: Interactions Between Homologous Chromosomes. In: Assmann S, Liu B, editors. Cell Biology. Springer New York; 2014. pp. 1–34. [Google Scholar]
- 3.Hann MC, Lau PE, Tempest HG. Meiotic recombination and male infertility: from basic science to clinical reality? Asian J Androl. 2011;13:212–218. doi: 10.1038/aja.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Handel MA, Schimenti JC. Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet. 2010;11:124–136. doi: 10.1038/nrg2723. [DOI] [PubMed] [Google Scholar]
- 5.Quinn GP, Knapp CA, Malo TL, McIntyre J, Jacobsen PB, Vadaparampil ST. Physicians’ undecided attitudes toward posthumous reproduction: fertility preservation in cancer patients with a poor prognosis. J Support Oncol. 2012;10:160–165. doi: 10.1016/j.suponc.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ethics Committee of the American Society for Reproductive M. Fertility preservation and reproduction in cancer patients. Fertil Steril. 2005;83:1622–1628. doi: 10.1016/j.fertnstert.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Tournaye H, Dohle GR, Barratt CLR. Fertility preservation in men with cancer. Lancet. 2014;384:1295–1301. doi: 10.1016/S0140-6736(14)60495-5. [DOI] [PubMed] [Google Scholar]
- 8.Meistrich ML. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril. 2013;100:1180–1186. doi: 10.1016/j.fertnstert.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong H, Du W, Zhang YJ, Hong J, Su WY, Tang JT, Wang YC, Lu R, Fang JY. Trichostatin A, a histone deacetylase inhibitor, suppresses JAK2/STAT3 signaling via inducing the promoter-associated histone acetylation of SOCS1 and SOCS3 in human colorectal cancer cells. Mol Carcinog. 2012;51:174–184. doi: 10.1002/mc.20777. [DOI] [PubMed] [Google Scholar]
- 10.Wang M, Gao M, Miller KD, Sledge GW, Zheng QH. [11 C] GSK2126458 and [18 F] GSK2126458, the first radiosynthesis of new potential PET agents for imaging of PI3K and mTOR in cancers. Bioorg Med Chem Lett. 2012;22:1569–1574. doi: 10.1016/j.bmcl.2011.12.136. [DOI] [PubMed] [Google Scholar]
- 11.Liu T, Sun Q, Li Q, Yang H, Zhang Y, Wang R, Lin X, Xiao D, Yuan Y, Chen L. Dual PI3K/mTOR inhibitors, GSK2126458 and PKI-587, suppress tumor progression and increase radiosensitivity in nasopharyngeal carcinoma. Mol Cancer Ther. 2015;14:429–439. doi: 10.1158/1535-7163.MCT-14-0548. [DOI] [PubMed] [Google Scholar]
- 12.Valdés-Mora F, Song JZ, Statham AL, Strbenac D, Robinson MD, Nair SS, Patterson KI, Tremethick DJ, Stirzaker C, Clark SJ. Acetylation of H2A. Z is a key epigenetic modification associated with gene deregulation and epigenetic remodeling in cancer. Genome Res. 2012;22:307–321. doi: 10.1101/gr.118919.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nature Rev Drug Discov. 2014;13:673–691. doi: 10.1038/nrd4360. [DOI] [PubMed] [Google Scholar]
- 15.Sun DF, Zhang YJ, Tian XQ, Chen YX, Fang JY. Inhibition of mTOR signalling potentiates the effects of trichostatin A in human gastric cancer cell lines by promoting histone acetylation. Cell Biol Int. 2014;38:50–63. doi: 10.1002/cbin.10179. [DOI] [PubMed] [Google Scholar]
- 16.Sun S, Han Y, Liu J, Fang Y, Tian Y, Zhou J, Ma D, Wu P. Trichostatin A targets the mitochondrial respiratory chain, increasing mitochondrial reactive oxygen species production to trigger apoptosis in human breast cancer cells. PLoS One. 2014;9:e91610. doi: 10.1371/journal.pone.0091610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wisnieski F, Calcagno DQ, Leal MF, Chen ES, Gigek CO, Santos LC, Pontes TB, Rasmussen LT, Payão SLM, Assumpção PP. Differential expression of histone deacetylase and acetyltransferase genes in gastric cancer and their modulation by trichostatin A. Tumour Biol. 2014;35:6373–6381. doi: 10.1007/s13277-014-1841-0. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, Yang Y, Liu B, Wang B, Sun M, Zhang L, You H, Zhou M. Promotion of metastasisassociated gene expression in survived PANC-1 cells following trichostatin A treatment. Anticancer Agents in Med Chem. 2015;15:1317–1325. doi: 10.2174/1871520615666150520093040. [DOI] [PubMed] [Google Scholar]
- 19.Vigushin DM, Ali S, Pace PE, Mirsaidi N, Ito K, Adcock I, Coombes RC. Trichostatin A is a histone deacetylase inhibitor with potent antitumor activity against breast cancer in vivo. Clin Cancer Res. 2001;7:971–976. [PubMed] [Google Scholar]
- 20.Woo HJ, Lee SJ, Choi BT, Park YM, Choi YH. Induction of apoptosis and inhibition of telomerase activity by trichostatin A, a histone deacetylase inhibitor, in human leukemic U937 cells. Exp Mol Pathol. 2007;82:77–84. doi: 10.1016/j.yexmp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Gol’Dberg ED, Borovskaya TG. Gonadotoxic Effects of Antitumor Preparations. Bull Exp Biol Med. 2003;135:211–217. doi: 10.1023/a:1024192925038. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh D, Das UB, Misro M. Protective Role of alpha-tocopherol-succinate (Provitamin-E) in Cyclophosphamide Induced Testicular Gametogenic and Steroidogenic Disorders: A Correlative Approach to Oxidative Stress. Free Radic Res. 2002;36:1209–1218. doi: 10.1080/1071576021000016472. [DOI] [PubMed] [Google Scholar]
- 23.Xiong H, Du W, Zhang YJ, Hong J, Su WY, Tang JT, Wang YC, Lu R, Fang JY. Trichostatin A, a histone deacetylase inhibitor, suppresses JAK2/STAT3 signaling via inducing the promoter-associated histone acetylation of SOCS1 and SOCS3 in human colorectal cancer cells. Mol Carcinog. 2012;51:174–184. doi: 10.1002/mc.20777. [DOI] [PubMed] [Google Scholar]
- 24.Bartova E, Pachernik J, Harnicarova A, Kovarik A, Kovarikova M, Hofmanova J, Skalnikova M, Kozubek M, Kozubek S. Nuclear levels and patterns of histone H3 modification and HP1 proteins after inhibition of histone deacetylases. J Cell Sci. 2005;118:5035–5046. doi: 10.1242/jcs.02621. [DOI] [PubMed] [Google Scholar]
- 25.Iwashita J, Kodama A, Konno Y, Abe T, Murata J. Histone deacetylase induces accelerated maturation in Xenopus laevis oocytes. Dev Growth Differ. 2013;55:319–329. doi: 10.1111/dgd.12038. [DOI] [PubMed] [Google Scholar]
- 26.Koehler KE, Hawley RS, Sherman S, Hassold T. Recombination and nondisjunction in humans and flies. Hum Mol Genet. 1996;5 Spec No:1495–1504. doi: 10.1093/hmg/5.supplement_1.1495. [DOI] [PubMed] [Google Scholar]
- 27.Krappmann S, Sasse C, Braus GH. Gene targeting in Aspergillus fumigatus by homologous recombination is facilitated in a nonhomologous end-joining-deficient genetic background. Eukaryotic Cell. 2006;5:212–215. doi: 10.1128/EC.5.1.212-215.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lejeune FJ, Rimoldi D, Speiser D. New approaches in metastatic melanoma: biological and molecular targeted therapies. Expert Rev Anticancer Ther. 2007;7:701–713. doi: 10.1586/14737140.7.5.701. [DOI] [PubMed] [Google Scholar]
- 29.Hosford SR, Miller TW. Clinical potential of novel therapeutic targets in breast cancer: CDK4/6, Src, JAK/STAT, PARP, HDAC, and PI3K/AKT/mTOR pathways. Pharmgenomics Pers Med. 2014;7:203–215. doi: 10.2147/PGPM.S52762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bedard PL, Grilley-Olson JE, Cornfeld M, Cartee L, Warwick S, Razak AA, Stayner LA, Wu Y, Greenwood R, Viana-Gilmartin V. Abstract CT205: A phase I dose-escalation study of trametinib (T) in combination with continuous or intermittent GSK2126458 (GSK458) in patients (pts) with advanced solid tumors. Cancer Res. 2014;74:CT205–CT205. [Google Scholar]
- 31.Dolman MEM, Westerhout EM, Hamdi M, Schellens JH, Beijnen JH, Sparidans RW. Liquid chromatography-tandem mass spectrometric assay for the PI3K/mTOR inhibitor GSK2126458 in mouse plasma and tumor homogenate. J Pharm Biomed Anal. 2015;107:403–408. doi: 10.1016/j.jpba.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 32.Knight SD, Adams ND, Burgess JL, Chaudhari AM, Darcy MG, Donatelli CA, Luengo JI, Newlander KA, Parrish CA, Ridgers LH, Sarpong MA, Schmidt SJ, Van Aller GS, Carson JD, Diamond MA, Elkins PA, Gardiner CM, Garver E, Gilbert SA, Gontarek RR, Jackson JR, Kershner KL, Luo L, Raha K, Sherk CS, Sung CM, Sutton D, Tummino PJ, Wegrzyn RJ, Auger KR, Dhanak D. Discovery of GSK2126458, a Highly Potent Inhibitor of PI3K and the Mammalian Target of Rapamycin. ACS Med Chem Lett. 2010;1:39–43. doi: 10.1021/ml900028r. [DOI] [PMC free article] [PubMed] [Google Scholar]


