Abstract
Purpose
To determine the applicability of central corneal thickness (CCT) based correction factor for non-contact tonometer (NCT) measured intraocular pressure (IOP) readings.
Method
A prospective, non-randomized study involved 346 eyes of 173 consecutive patients with age ⩾21 years undergoing laser-assisted in situ keratomileusis (LASIK) for myopia and/or myopic astigmatism. The CCT and IOP were measured before and after the LASIK procedure. The IOP pre and post-LASIK was compared after applying the correction factor for CCT. Patients not completing the 3 month postoperative follow-up were excluded.
Results
The median spherical equivalent before undergoing LASIK was −4.25D (inter-quartile range, −3.25D). The mean preoperative CCT was 536.82 ± 33.71 μm which reduced to 477.55 ± 39.3 μm (p < 0.01) post-LASIK. The mean IOP reduced from a preoperative value of 14.6 ± 2.32 mmHg to 10.64 ± 2.45 mmHg postoperatively (p < 0.01). On applying correction for the corneal thickness, the pre and postoperative IOP was 15.14 ± 2.8 mmHg and 15.37 ± 2.65 mmHg (p = 0.06) respectively with a strong positive correlation (r = 0.7, p < 0.01). Three hundred eyes (86.7%) had an absolute difference in IOP of less than 3.0 mmHg post-CCT correction which is within the retest variability of NCT. Only 46 eyes (13.3%) had an absolute difference of more than 3.0 mmHg.
Conclusion
The modified Ehler’s correction algorithm used in this study can be effectively applied in the normal IOP range in a majority of patients.
Keywords: NCT, CCT, LASIK, Intraocular pressure, Correction
Introduction
Laser-assisted in situ keratomileusis (LASIK) is a commonly employed procedure for the correction of myopia.1 Myopia is an independent risk factor for glaucoma progression.2 The intraocular pressure (IOP) measurements following LASIK are known to be inaccurate.3 With the IOP being the only modifiable risk factor, obtaining accurate IOP readings is essential in diagnosing and managing glaucoma.4 It is now possible to measure corneal biomechanics in the form of corneal hysteresis. Patients with glaucoma have been repeatedly shown to have a significantly lower corneal hysteresis and central corneal thickness (CCT).5, 6 The main source of error for measuring IOP post-LASIK is the change in CCT.7 The non-contact tonometer (NCT) is widely used as a screening tool for glaucoma because of its advantage of not requiring direct corneal contact and corneal anesthesia. Previous studies have shown that the NCT can produce accurate IOP assessment comparable to Goldmann applanation tonometer (GAT).8, 9, 10 The CCT readings affect the NCT measurements.11 The test–retest variability of NCT has already been reported.12 There is little information regarding the accuracy of correction factor application on the IOP readings of NCT. The aim of our study was to apply the CCT based correction factor before and after the LASIK procedure and to ascertain whether the difference lies within the retest variability of the NCT.
Materials and methods
This was a prospective, non-randomized, interventional study done at a tertiary care center and its collaborating center where the LASIK procedure was performed. A written consent was obtained from all patients and the study was approved by the local ethics committee. The study included 180 consecutive patients undergoing LASIK for myopia and/or myopic astigmatism from February 2011 to January 2012. Patients were excluded from surgery if they were younger than 21 years or had a history of uveitis, ocular trauma, severe dry-eye syndrome, collagen disease, drug allergy, glaucoma and diabetes mellitus. Those not willing to participate or not completing the 3 month postoperative follow-up were also excluded from the study. Seven patients did not complete the last follow-up. Three hundred and forty-six eyes of 173 patients were finally included in this study.
Preoperative examination
Baseline ocular examination included anterior segment and anterior vitreous evaluation by slit lamp biomicroscopy; posterior vitreous, disk, and macula evaluation by slit lamp biomicroscopy with a 90 diopter (D) lens and peripheral retina evaluation by indirect ophthalmoscopy. Intraocular pressure measurement was done by NIDEK NT-2000 NCT (Nidek CO., LTD., Hiroishi Gamagori, Aichi, Japan). An average of four daytime IOP readings (9 am, 11 am, 1 pm and 3 pm) on the same day was taken as the preoperative IOP. The delay in the time schedule was never more than 30 min. Central corneal thickness measurements were done by an ultrasonic pachymeter (SP-3000, TOMEY Corporation, Nagoya, Japan). The pre and postoperative assessment including the IOP measurement was done by one of the authors (JJ).
LASIK procedure
An 8 to 8.5-mm diameter corneal flap, approximately 130 μm in thickness, was created using the Wave Light FS 200 femtosecond laser and Allegretto Wave Eye-Q 400 Hz (Alcon, Fort Worth, Texas, USA) excimer laser was used to ablate the corneal stromal bed. All procedures were performed by one of the authors (YD).
Postoperative regimen
The postoperative regimen included nepafenac 0.03% eyedrops 3 times a day, moxifloxacin 0.5% eyedrops 4 times a day and polyethylene glycol 0.4% + propylene glycol 0.3% eyedrops 6 times a day. These drops were continued for 6 weeks after the LASIK procedure. Repeat IOP (Average of 4 daytime readings as before) and pachymetry measurements were again taken at 3 months postoperatively.
Correction factor
The CCT based correction algorithm was based on the Ehler’s correction13 for GAT and is shown in Table 1. This correction algorithm is distributed with most pachymetry instruments and has been used and validated in previous studies.14 The correction factor was applied depending on the closeness of the CCT values to the described category. If the CCT values were equidistant from both categories, the next category was chosen to apply the correction factor. For example, if the CCT was 480 μm, a correction factor of +4 was applied.
Table 1.
The modified Ehler’s correction factor algorithm.
| Central corneal thickness (μm) | Correction value (mmHg) |
|---|---|
| 410 | 10 |
| 415 | 10 |
| 420 | 9 |
| 425 | 9 |
| 430 | 8 |
| 435 | 8 |
| 440 | 7 |
| 445 | 7 |
| 455 | 6 |
| 465 | 6 |
| 475 | 5 |
| 485 | 4 |
| 495 | 4 |
| 505 | 3 |
| 515 | 2 |
| 525 | 1 |
| 535 | 1 |
| 545 | 0 |
| 555 | −1 |
| 565 | −1 |
| 575 | −2 |
| 585 | −3 |
| 595 | −4 |
| 605 | −4 |
| 615 | −5 |
| 625 | −6 |
| 635 | −6 |
| 645 | −7 |
Abbreviations: μm = microns, mmHg = millimeter mercury.
Statistical analysis
Descriptive and inferential statistics were performed using STATA version 12 for Windows (StataCorp LP, Texas). Normality of variables was tested by the Shapiro–Wilk test. Continuous normally distributed data were represented as mean and standard deviation. Data with non normal distribution were represented as median and quartiles. Paired t-test was used to compare the CCT and IOP values before and after the LASIK procedure. Multiple linear regression analysis was used to find the factors affecting the difference in IOP after LASIK. Pearson’s correlation coefficients were used to calculate correlations between corrected pre and postoperative IOP, preoperative IOP and CCT as well between the IOP change and CCT change post-LASIK. Scatter plots with regression line were made.
Results
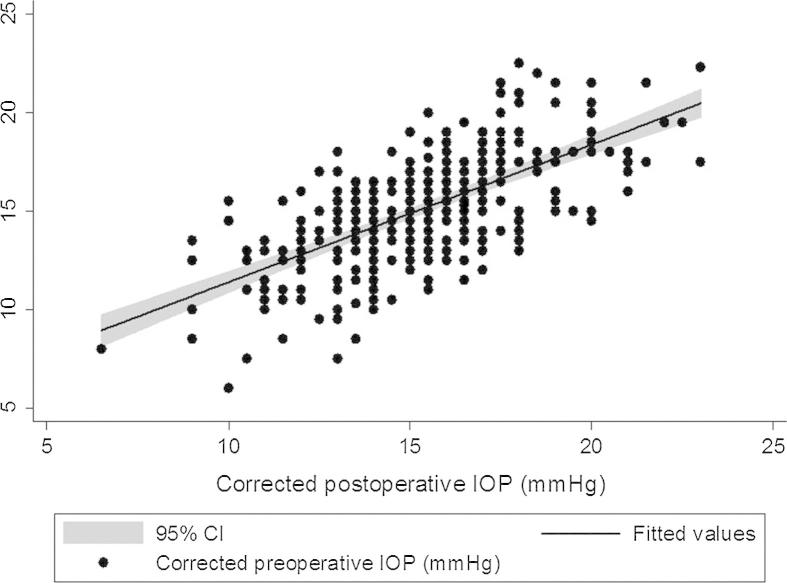
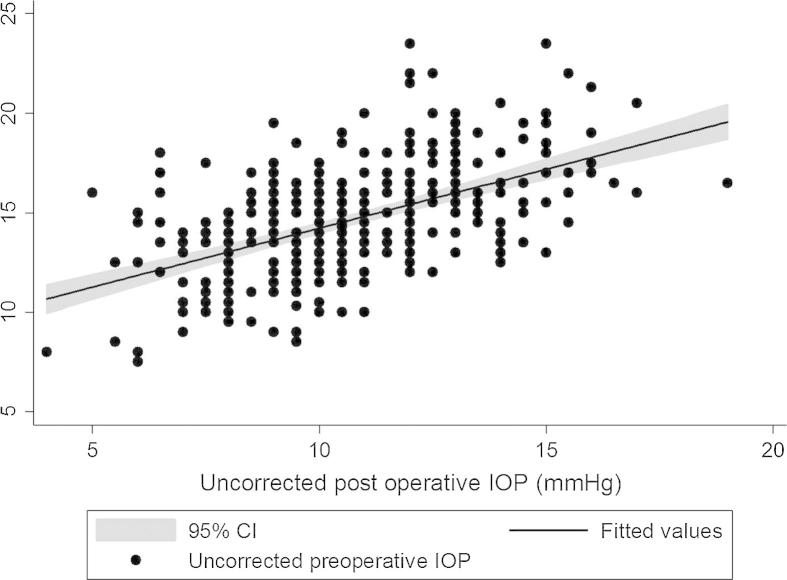
We analyzed 346 eyes of 173 patients for the study. The patient demographics are outlined in Table 2. The mean age of the patients was 27 ± 2.3 years which included 100 female and 73 male patients. The median spherical equivalent before undergoing LASIK was −4.25D (inter-quartile range, −3.25D). The mean preoperative CCT was 536.82 ± 33.71 μm which reduced to 477.55 ± 39.3 μm (p < 0.01) post-LASIK. The mean ablated corneal depth was 59.2 ± 24 μm. The mean IOP reduced from a preoperative value of 14.6 ± 2.32 mmHg to 10.64 ± 2.45 mmHg postoperatively (p < 0.01) with a moderately strong positive correlation between them (r = 0.54, p < 0.05). However, on applying correction for the corneal thickness, the pre and postoperative IOP was 15.14 ± 2.8 mmHg and 15.37 ± 2.65 mmHg (p = 0.06) respectively with a strong positive correlation (r = 0.7, p < 0.01). A positive moderately strong correlation was seen between preoperative IOP and CCT (r = 0.51, p < 0.05) as well as between the IOP change and CCT change (r = 0.53, p < 0.05) after LASIK. The median absolute difference between the pre and post-LASIK IOP was 1.5 mmHg (inter-quartile range, 2 mmHg). Three hundred eyes (86.7%) had an absolute difference of less than 3.0 mmHg. Only 46 eyes (13.3%) had an absolute difference of greater than or equal to 3.0 mmHg. The mean IOP difference for these eyes was 3.2 ± 0.14 mmHg (range 3.0–3.5 mmHg). The multiple linear regression analysis showed that age (p = 0.1) did not predict the IOP difference post-LASIK. The preoperative CCT (p < 0.01), preoperative IOP (p < 0.01), the spherical equivalent (p < 0.01) and the ablated corneal depth (p < 0.01) significantly predicted the IOP difference. The greater the preoperative CCT, amount of myopia and ablated corneal depth, the greater the IOP difference. The scatter plot with the regression line was plotted between the corrected pre and postoperative IOP as well as between the uncorrected pre and postoperative IOP (Figure 1, Figure 2).
Table 2.
Patient demographics.
| Mean age (years) | 27 ± 2.3 | |
| Gender (M:F) | 73:100 | |
| Pre-op SE (Diopter) | −4.6 ± 2.8 | |
| Pre-op CCT (microns) | 536.82 ± 33.71 | |
| Postop CCT (microns) | 477.55 ± 39.3 | p < 0.01 |
| Pre-op IOP (mmHg) | 14.6 ± 2.32 | |
| Postop IOP (mmHg) | 10.64 ± 2.45 | p < 0.01 |
| Corrected pre-op IOP (mmHg) | 15.14 ± 2.8 | |
| Corrected postop IOP (mmHg) | 15.37 ± 2.65 | p = 0.06 |
Abbreviations: M = male, F = female, Pre-op = preoperative, Postop = postoperative, SE = spherical equivalent, IOP = intraocular pressure, mmHg = millimeter mercury.
Figure 1.
Scatter plot between the corrected pre and postoperative intraocular pressure with the regression line.
Figure 2.
Scatter plot between the uncorrected pre and postoperative intraocular pressure with the regression line.
Discussion
After excimer laser surgery for the correction of myopia and myopic astigmatism, false low IOP readings with NCT have become a well-known phenomenon with the reduction in CCT. It has also been reported that NCT readings are unaffected by changes in the corneal curvature.15 There is very little information on the applicability of any CCT based correction formula on the IOP taken by NCT. On applying correction for the corneal thickness, the pre and postoperative IOP was similar at 15.14 ± 2.8 mmHg and 15.37 ± 2.65 mmHg (p = 0.06) respectively with a strong positive correlation (r = 0.7, p < 0.01). Tonnu and associates12 have reported a retest variability of ±3.2 mmHg for NCT. Three hundred eyes (86.7%) in our study had an absolute difference between the corrected preoperative and postoperative IOP of less than 3.0 mmHg. This was within the retest variability of NCT. We employed a very stringent protocol wherein the pre and postoperative IOP was measured at the same time of the day. Diurnal variations affecting the IOP measurements were negated by the fact that an average of 4 day time readings was taken by the same examiner (JJ). This may have contributed to the increased accuracy of the IOP measurements. We did not use steroids in our postoperative regimen which ruled out a steroid response. We have already published that nevanac eyedrops do not increase the IOP.16 Our study is limited by the fact that we did not compare the IOP with GAT which is the current gold standard. However, we are not suggesting that NCT is better than GAT. Our data just shows that the modified Ehler’s correction used in the study can be applied accurately in a majority of our patients (86.7%). Changes in the IOP measurements using different tonometers after LASIK have been studied before.17 However, there are no reports on the applicability of the correction factor for CCT which was the purpose of our study. IOP measurements with newer tonometers such as the dynamic contour tonometer have been shown to be little affected by corneal properties such as CCT.18 The instrument though ideal for post-LASIK patients, is expensive and not commonly used by general ophthalmologists. It is for this reason that the study was done on the NCT which is more widely used. Flap thickness has been shown to affect the measured IOP after LASIK.19 The strength of our study lies in the fact that a single surgeon (YD) performed the procedure with a uniform flap thickness of 130 μm thereby negating the effect of flap thickness in our study. A study by El Maghraby et al.20 has shown that the corneal stabilization in terms of vision is complete before 3 months post-LASIK. It is for this reason that the 3 month time point was chosen to measure the post-LASIK IOP in our study. Seven patients did not complete the 3 month follow-up. This small number is unlikely to have a major impact on our study results. None of our patients had a pre-operative IOP of >21 mmHg. Hence, the correction algorithm might not be applicable to this subset of patients having an IOP > 21 mmHg.
To conclude, the modified Ehler’s correction algorithm used in this study can effectively give the CCT corrected IOP within the normal intraocular pressure range in majority of patients.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Yuen L.H., Chan W.K., Koh J., Mehta J.S., Tan D.T., SingLasik Research Group A 10-year prospective audit of LASIK outcomes for myopia in 37,932 eyes at a single institution in Asia. Ophthalmology. 2010;117(1236–44):e1. doi: 10.1016/j.ophtha.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 2.Rivera J.L., Bell N.P., Feldman R.M. Risk factors for primary open angle glaucoma progression: what we know and what we need to know. Curr Opin Ophthalmol. 2008;19:102–106. doi: 10.1097/ICU.0b013e3282f493b3. [DOI] [PubMed] [Google Scholar]
- 3.Faucher A., Grégoire J., Blondeau P. Accuracy of Goldmann tonometry after refractive surgery. J Cataract Refract Surg. 1997;23:832–838. doi: 10.1016/s0886-3350(97)80239-8. [DOI] [PubMed] [Google Scholar]
- 4.Foster P.J., Buhrmann R., Quigley H.A., Johnson G.J. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–242. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bochmann F., Ang G.S., Azuara-Blanco A. Lower corneal hysteresis in glaucoma patients with acquired pit of the optic nerve (APON) Graefes Arch Clin Exp Ophthalmol. 2008;246:735–738. doi: 10.1007/s00417-007-0756-5. [DOI] [PubMed] [Google Scholar]
- 6.Abitbol O., Bouden J., Doan S., Hoang-Xuan T., Gatinel D. Corneal hysteresis measured with the ocular response analyzer in normal and glaucomatous eyes. Acta Ophthalmol. 2010;88:116–119. doi: 10.1111/j.1755-3768.2009.01554.x. [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann C., Bachmann L.M., Thiel M.A. Intraocular pressure measurements using dynamic contour tonometry after laser in situ keratomileusis. Invest Ophthalmol Vis Sci. 2003;44:3790–3794. doi: 10.1167/iovs.02-0946. [DOI] [PubMed] [Google Scholar]
- 8.Jorge J., Diaz-Rey J.A., Gonzalez-Meijome J.M., Almeida J.B., Parafita M.A. Clinical performance of the Reichert AT 550: a new non-contact tonometer. Ophthalmic Physiol Opt. 2002;22:560–564. doi: 10.1046/j.1475-1313.2002.00077.x. [DOI] [PubMed] [Google Scholar]
- 9.Cho P., Lui T. Comparison of the performance of the Nidek NT-2000 noncontact tonometer with the Keeler Pulsair 2000 and the Goldmann applanation tonometer. Optom Vis Sci. 1997;74:51–58. doi: 10.1097/00006324-199701000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Mackie S.W., Jay J.L., Ackerley R., Walsh G. Clinical comparison of the Keeler Pulsair 2000, American Optical MkII and Goldmann applanation tonometers. Ophthalmic Physiol Opt. 1996;16:171–177. [PubMed] [Google Scholar]
- 11.Tonnu P.A., Ho T., Newson T., Sheikh A.E., Sharma K., White E. The influence of central corneal thickness and age on intraocular pressure measured by pneumotonometry, noncontact tonometry, the Tono-Pen XL, and Goldmann applanation tonometry. Br J Ophthalmol. 2005;89:851–854. doi: 10.1136/bjo.2004.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tonnu P.A., Ho T., Sharma K., White E., Bunce C., Garway-Heath D. A comparison of four methods of tonometry: method agreement and interobserver variability. Br J Ophthalmol. 2005;89:847–850. doi: 10.1136/bjo.2004.056614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehlers N., Bramsen T., Sperling S. Applanation tonometry and central corneal thickness. Acta Ophthalmol. 1975;53:34–43. doi: 10.1111/j.1755-3768.1975.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 14.Patwardhan A., Khan M., Mollan S.P., Haigh P. The importance of central corneal thickness measurements and decision making in general ophthalmology clinics: a masked observational study. BMC Ophthalmol. 2008;8:1. doi: 10.1186/1471-2415-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harada Y., Hirose N., Kubota T., Tawara A. The influence of central corneal thickness and corneal curvature radius on the intraocular pressure as measured by different tonometers: noncontact and Goldmann applanation tonometers. J Glaucoma. 2008;17:619–625. doi: 10.1097/IJG.0b013e3181634f0f. [DOI] [PubMed] [Google Scholar]
- 16.Dave P., Shah K., Ramchandani B., Jain R. Effect of nepafenac eye drops on intraocular pressure: a randomized prospective study. Am J Ophthalmol. 2014;157:735–738. doi: 10.1016/j.ajo.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Francis B.A., Hsieh A., Lai M.Y. Effects of corneal thickness, corneal curvature, and intraocular pressure level on Goldmann applanation tonometry and dynamic contour tonometry. Ophthalmology. 2007;114:20–26. doi: 10.1016/j.ophtha.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 18.Hamed-Azzam S., Briscoe D., Tomkins O., Shehedeh-Mashor R., Garzozi H. Evaluation of intraocular pressure according to corneal thickness before and after excimer laser corneal ablation for myopia. Int Ophthalmol. 2013;33:349–354. doi: 10.1007/s10792-012-9701-7. [DOI] [PubMed] [Google Scholar]
- 19.Schallhorn J.M., Schallhorn S.C., Ou Y. Factors that influence intraocular pressure changes after myopic and hyperopic LASIK and photorefractive keratectomy: a large population study. Ophthalmology. 2015;122:471–479. doi: 10.1016/j.ophtha.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 20.El Maghraby A., Salah T., Waring G.O., 3rd, Klyce S., Ibrahim O. Randomized bilateral comparison of excimer laser in situ keratomileusis and photorefractive keratectomy for 2.50 to 8.00 diopters of myopia. Ophthalmology. 1999;106:447–457. doi: 10.1016/S0161-6420(99)90102-1. [DOI] [PubMed] [Google Scholar]