Abstract
Purpose
To assess the safety and efficiency of combined phacoemulsification (PHACO) surgery and intravitreal triamcinolone (IVTA) injection with or without macular grid laser photocoagulation in patients with cataract and diabetic macular edema.
Material and methods
This prospective study included 41 eyes of 36 diabetic patients with cataract and coexisting clinically significant macular edema (CSME). After PHACO and IVTA injection eyes were divided into two groups: the laser and IVTA group (Group 1) and only IVTA group (Group 2). Preoperative and postoperative best corrected visual acuity (BCVA), central macular thickness (CMT), and intraocular pressure (IOP) were recorded. Paired sample t-test was used to compare data in the groups and C square test for qualitative variables.
Results
Postoperative BCVA was significantly higher than the initial BCVA during the follow-up period in both groups (p < 0.01). The BCVA 6 months after surgery was significantly higher in group 1 than in group 2 (p < 0.01). There was no statistically significant difference in IOP between two groups preoperatively and postoperatively during the follow-up period (p > 0.05). There was no statistically significant difference between both groups in mean CMT preoperatively and 2nd week, 2nd month and 3rd month after surgery (p > 0.05). The mean CMT 6 months after surgery was statistically significantly lower in group 1 than in group 2 (p < 0.01).
Conclusions
PHACO surgery combined with IVTA injection improves BCVA and provides a decrease in CMT in diabetic patients with CSME. Additional macular grid laser photocoagulation after surgery helps to preserve this improvement in BCVA and decrease in CMT.
Keywords: Phacoemulsification, Diabetic macular edema, Triamcinolone
Introduction
Diabetic macular edema is characterized by intraretinal and subretinal accumulations of fluid, resulting principally from retinal vascular leakage and is the main cause of visual impairment in diabetic patients.1, 2 Cataract is another ocular complication of diabetes and 20% of all cataract surgeries are performed on diabetics.3 Macular edema at the time of surgery has been suggested to be risk factor for poorer visual outcome. Royal College of Ophthalmologists’ guidelines for cataract surgery in diabetics state that if clinically significant macular edema (CSME) is identified before surgery, it should be treated at least 12 weeks prior to surgery.4, 5 But it is common to see patients with significant cataract that impedes the treatment or even diagnosis of CSME. Some studies have found an increased risk of retinopathy progression and in particular macular edema exacerbation with cataract surgery. However, some patients with diabetic CSME are refractory to conventional photocoagulation.6, 7, 8, 9, 10, 11
Triamcinolone acetonide is a corticosteroid with anti-inflammatory and antiangiogenic properties. Intravitreal injection of triamcinolone (IVTA) injection is an alternative for treating CSME especially in patients with advanced cataract.4, 5, 12, 13
In this study, we aimed to assess the safety and efficiency of combined phacoemulsification (PHACO) surgery and IVTA injection with or without macular grid laser photocoagulation in patients with cataract and diabetic macular edema.
Material and methods
This prospective study included 41 eyes of 36 multiethnic Turkish diabetic patients with cataract and coexisting clinically significant macular edema (CSME). All patients were diagnosed with CSME according to the ETDRS criteria. All patients filled up informed consent form. The study was carried out according to the tenets of the Declaration of Helsinki. Institutional Review Board approval has been obtained. The age of the patients ranged between 54 and 79 years (mean age 66.39). Twenty-two of them were female (53.7%) and 19 were (46.3%) male. HbA1c at the time of surgery differed from 6.2 to 7.3 (HbA1c 6.65). The duration of diabetes mellitus differed from 6 to 23 years (mean 14.05 years). Preoperative clinical characteristics of the groups are summarized in Table 1. As seen in Table 1 there is no statistically significant difference in patient characteristics between the two groups (p > 0.05).
Table 1.
Preoperative clinical characteristics of the groups.
| Group I |
Group II |
p | ||
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| aAge | 66.71 ± 5.14 | 66.05 ± 5.06 | 0.679 | |
| aHbA1c | 6.60 ± 0.34 | 6.70 ± 0.30 | 0.344 | |
| aDuration of DM | 14.19 ± 3.93 | 13.90 ± 4.71 | 0.831 | |
| Number of patients | n (%) | n (%) | ||
| bSex | Female | 11 (52.4%) | 11 (55.0%) | 0.867 |
| Male | 10 (47.6%) | 9 (45.0%) | ||
Student’s t test.
C square test.
At baseline examination, best corrected visual acuity (BCVA), central macular thickness, and intraocular pressure (IOP) were recorded. Patients who had visually significant cataract and either diffuse central macular edema of at least 250 μm demonstrated by optic coherence tomography (OCT) or persistent diabetic macular edema unresponsive to laser treatment were included in the study. None of the patients had received any previous intravitreal injection. Exclusion criteria were a history of either ocular hypertension or glaucoma, previous ocular trauma or surgery, and intraoperatively complicated PHACO surgery with vitreous loss.
After surgery the enrolled eyes were divided into two groups, the laser and IVTA group (Group 1) and the only IVTA group (Group 2). Patients in group 1 underwent IVTA, PHACO surgery and macular grid photocoagulation 4 weeks after the surgery. Patients in group 2 underwent only IVTA and PHACO surgery.
Phacoemulsification and in-the-bag intraocular lens implantation were performed under topical anesthesia using 0.5% proparacaine HCl drop with a self-sealing corneal tunnel. At the end of cataract surgery 4 mg in 0.1 ml of preservative free triamcinolone acetonide (Kenacort A, Bristol Myers Squibb) was injected via the inferotemporal pars plana (3.5 mm from limbus), using 27 G needle. Patients were instructed to semi-sitting position in the immediate postoperative to avoid macular staining by the Triamcinolone. Postoperatively, 1% prednisolone acetate eye drop and 0.5% ofloxacin eye drop were applied four times daily 1 week, and then tapered weekly over 3-week period.
Grid laser photocoagulation 4 weeks after surgery was applied only in group 1 under topical anesthesia with fundus contact lens. The laser spots were applied with argon green wavelength, duration of 100 msn, diameter of 100 μm, and the power increased from 75 mW to produce a mild gray burn on all areas of capillary nonperfusion and retinal thickening based on the findings of fundus fluorescein angiography and OCT.
Patients were examined 1 day, 1 week, 2 weeks, 2 months, 3 months and sixth months after surgery. The response to treatment was monitored functionally by BCVA assessment by Snellen chart and anatomically measuring the central macular thickness by OCT. Biomicroscopic examinations and IOP monitoring were performed on each visit. Topical antiglaucomatous treatment was initiated if IOP was more than 21 mmHg.
Statistical analysis was performed. The results were analyzed with NCSS 2007&PASS 2008 Statistical Software (Utah, USA). Student’s t test was used for comparing quantitative data and also for comparing the data of two groups. Paired sample t test was used to compare data in the group and C square test for qualitative variables. Results were 95% reliable and the value of p < 0.05 was considered as significant.
Results
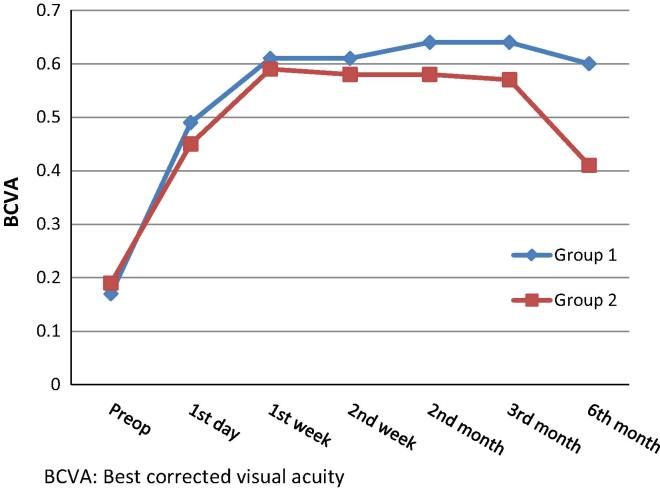
There was no statistically significant difference in BCVA between two groups preoperatively and 1 day, 1 week, 2 weeks, 2 months and 3 months postoperatively (p > 0.05) (Table 2). The BCVA at the 1st day, 1st week, 2nd week, 2nd month, 3rd month and 6th month visit after surgery was significantly higher than the initial BCVA in both groups (p < 0.01). On the other hand the BCVA 6 months after surgery was significantly higher in group 1 than in group 2 (p < 0.01) (Table 2 and Fig. 1).
Table 2.
Evaluation of best corrected visual acuity (BCVA).
| BCVA | Group I | Group II | p⁎ |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Preoperative | 0.17 ± 0.10 | 0.19 ± 0.10 | 0.561 |
| 1st day | 0.49 ± 0.15 | 0.45 ± 0.14 | 0.385 |
| 1st week | 0.61 ± 0.17 | 0.59 ± 0.13 | 0.695 |
| 2nd week | 0.61 ± 0.16 | 0.58 ± 0.15 | 0.512 |
| 2nd month | 0.64 ± 0.12 | 0.58 ± 0.15 | 0.208 |
| 3rd month | 0.64 ± 0.12 | 0.57 ± 0.15 | 0.140 |
| 6th month | 0.60 ± 0.14 | 0.41 ± 0.11 | 0.001⁎⁎ |
Student’s t test.
p < 0.01.
Figure 1.
Progression of best corrected visual acuity (BCVA).
There was no statistically significant difference in IOP between two groups preoperatively and 1 day, 1 week, 2 weeks, 2 months, 3 months and 6 months postoperatively (p > 0.05). The differences between preoperative IOP and all postoperative intervals until 6 months were not statistically significant in both groups (p > 0.05) (Table 3, Table 4).
Table 3.
Evaluation of IOP in group 1 and group 2.
| IOP | Group I | Group II | p |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Preop | 16.04 ± 2.59 | 16.85 ± 2.53 | 0.324 |
| 1st day | 15.95 ± 2.59 | 15.90 ± 2.10 | 0.944 |
| 1st week | 16.76 ± 5.13 | 16.15 ± 2.71 | 0.639 |
| 2nd week | 16.19 ± 4.36 | 17.55 ± 5.79 | 0.400 |
| 2nd month | 16.00 ± 2.66 | 15.40 ± 2.56 | 0.467 |
| 3rd month | 16.09 ± 2.30 | 16.95 ± 2.74 | 0.285 |
| 6th month | 15.80 ± 2.76 | 16.45 ± 2.87 | 0.472 |
Student’s t test, IOP: intraocular pressure.
Table 4.
Evaluations of IOP inside the groups.
| IOP | Group I Mean ± SD | Group I p | Group II Mean ± SD | Group II p |
|---|---|---|---|---|
| Preoperative – 1st day | 0.09 ± 3.12 | 0.890 | 0.95 ± 3.73 | 0.269 |
| Preoperative – 1st week | −0.71 ± 5.61 | 0.566 | 0.70 ± 2.75 | 0.270 |
| Preoperative – 2nd week | −0.14 ± 4.57 | 0.888 | −0.70 ± 6.16 | 0.617 |
| Preoperative – 2nd month | 0.04 ± 2.78 | 0.938 | 1.45 ± 3.57 | 0.086 |
| Preoperative – 3rd month | −0.04 ± 3.30 | 0.948 | −0.10 ± 3.37 | 0.896 |
| Preoperative – 6th month | 0.23 ± 4.06 | 0.791 | 0.40 ± 4.25 | 0.679 |
Paired sample t test, IOP: intraocular pressure.
There was no statistically significant difference between both groups in mean CMT preoperatively and 2nd week, 2nd month and 3rd month after surgery (p > 0.05). The mean CMT 6 months after surgery was statistically significantly lower in group 1 than in group 2 (p < 0.01) (Table 5).
Table 5.
Evaluation of optic coherence tomography (OCT) between two groups.
| OCT | Group I | Group II | p |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Preoperative | 455.66 ± 57.09 | 456.85 ± 56.90 | 0.947 |
| 2nd week | 261.42 ± 52.06 | 258.15 ± 61.21 | 0.854 |
| 2nd month | 272.76 ± 62.81 | 276.50 ± 56.94 | 0.843 |
| 3rd month | 284.71 ± 56.96 | 280.05 ± 56.66 | 0.794 |
| 6th month | 291.76 ± 52.79 | 433.30 ± 77.29 | 0.001⁎⁎ |
Student’s t test.
p < 0.01.
In Group 1; the CMT decreased from preoperative values with statistically significant reduction at all postoperative intervals until 6 months (p < 0.01). There was no statistically significant difference in the mean CMT comparing the 2nd week to 2nd month, 3rd month, 6th months after surgery (p > 0.05), as well as comparing 2nd month to 3rd month, 6th month and comparing the 3rd month to 6th month after surgery (p > 0.05). There was an acceptable decline in the mean CMT with an average of 44.78% reduction at the 2nd week, 39.35% reduction at the 2nd month, 36.34% reduction at the 3rd month and 34.67% reduction at the 6th month. In Group 2; the CMT decreased from preop values with statistically significant reduction at all postoperative intervals until 3 months (p < 0.01); but the difference in the mean CMT between preoperative and postoperative 6th months was not statistically significant (p > 0.05). The decrease in the mean CMT in the 2nd week compared to 2nd month, 3rd month and 6th month was high statistically significant (p < 0.01). There was no statistically significant difference in the mean CMT between 2nd month and 3rd month (p > 0.05). Six months after surgery there was an increase in the mean CMT compared to 2nd month and 3rd month which was high statistically significant (p < 0.01). There was an average of 46.45% reduction in the mean CMT at the 2nd week, 41.87% reduction at the 2nd month, 41.21% reduction at the 3rd month and 2.24% at the 6th month (Table 6).
Table 6.
Evaluation of optic coherence tomography (OCT) inside the groups.
| OCT | Group I Mean ± SD | Group I p | Group II Mean ± SD | Group II p |
|---|---|---|---|---|
| Preoperative – 2nd week | 194.23 ± 72.08 | 0.001⁎⁎ | 198.70 ± 77.95 | 0.001⁎⁎ |
| Preoperative – 2nd month | 182.90 ± 66.18 | 0.001⁎⁎ | 180.35 ± 73.53 | 0.001⁎⁎ |
| Preoperative – 3rd month | 170.95 ± 61.71 | 0.001⁎⁎ | 176.80 ± 75.77 | 0.001⁎⁎ |
| Preoperative – 6th month | 163.90 ± 61.33 | 0.001⁎⁎ | 23.55 ± 77.99 | 0.193⁎ |
Paired sample t test.
p < 0.05.
p < 0.01.
During the study period, 6 eyes had an increase in IOP. Increment is to 28 mmHg, 26 mmHg, and 31 mmHg in 3 eyes of Group 1 at 1st week and to 29 mmHg, 27 mmHg and 33 mmHg in 3 eyes of Group 2. Intraocular pressure was controlled with topical antiglaucoma medication in these eyes. No other complications including injection related complications, vitreous hemorrhage, endophthalmitis, and retinal detachment were encountered in this study.
Discussion
Diabetic macular edema is characterized by intraretinal and subretinal accumulations of fluid, resulting principally from retinal vascular leakage.1, 12 The development of macular edema is thought to involve breakdown of inner blood–retina barrier with release of endogenous permeability factors by an ischemic retina.4, 5, 14
In this study, we assessed visual outcome and CMT after the combination of phacoemulsification surgery and intravitreal triamcinolone and subsequent grid laser photocoagulation in patients with cataract and diabetic macular edema. Significant cataract may impede the treatment and even diagnosis of CSME. Diabetic CSMO refractory to conventional laser treatment methods is also a common problem.4, 5 Previous studies suggest that diabetic patients with macular edema at the time of cataract surgery have poorer visual outcomes because of progression in retinopathy and maculopathy.15, 16, 17, 18, 19, 20 However short-time phacoemulsification surgery with small self-sealed corneal incisions without iris trauma, and in-the-bag implantation of intraocular lenses, in general do not cause progression of diabetic retinopathy.19 In this study we performed phacoemulsification and in-the-bag intraocular lens implantation with a self-sealing corneal tunnel. Chung et al. studied the effect of phacoemulsification on the progression of diabetic retinopathy and reported that retinopathy progressed significantly more in the operated eye and related to preoperative CSME and poor renal function.7 Somaiya et al. reported that postoperative BCVA of 6/12 was five times less likely in nonproliferative diabetic retinopathy patients and 30 times less likely in proliferative diabetic retinopathy patients compared to diabetic patients without retinopathy.21 A number of other patient characteristics such as increasing age, female sex, duration of diabetes mellitus, poor glycemic control with Hemoglobin A 1C at the time of surgery and moderate to severe retinopathy have been found associated with poor prognosis after cataract surgery in diabetic patients.13, 22, 23 In our study 53.7% of our patients were female, the mean HbA1c level at the time of surgery was 6.65, and the mean duration of diabetes mellitus was 14.05 years.
Previous reports have demonstrated improvements in the visual acuity and macular edema after IVTA in diabetic patients.12, 13, 24, 25, 26 Triamcinolone acetonide, an intermediate acting corticosteroid suspension with a depot effect lasting up to 41 days in animal studies, inhibits or downregulates inflammatory mediators such as prostaglandins and vascular endothelial growth factor and reduces breakdown of the blood–retinal barrier.4, 5, 27, 28, 29, 30, 31 Giving triamcinolone before surgery as a separate procedure has the potential for progression of lens opacities which may impede laser photocoagulation in CSME. Combining cataract surgery with IVTA rather than giving triamcinolone before surgery as a separate procedure avoided the potential for progression of lens opacities and IOP elevation associated with intraocular steroids which could have further interfered with retinopathy assessment. Combining the two procedures reduces the patient’s potential risk of endophthalmitis from two separate intraocular episodes to one, while at the same time offering improved patient convenience.4, 5, 13 We had no cases of endophthalmitis. All the surgeries were performed in the operating room with full asepsis, topical povidone preoperatively. Studies investigating the efficacy of IVTA have reported the prevalence of IOP elevations to be between 9% and 77%. The wide range in prevalence may be because of varying definitions of IOP increase, triamcinolone concentration and considering time of increase in IOP.13, 25, 26, 31, 32, 33 Similar to other studies only six (14%) of 41 eyes developed an increased IOP of over 21 mmHg. The IOP was normalized by topical antiglaucomatous agents.
Results from different studies indicate that cataract surgery with injection of IVTA may be performed safely with significant improvement in BCVA postoperatively.4, 5, 13, 20, 31 But Ahmadabadi et al. reported injection of triamcinolone after phacoemulsification had no effect on visual acuity.33 In our study the BCVA during the 6 months of follow-up period after a successful PHACO surgery was significantly higher than the initial BCVA in both groups (p < 0.01). On the other hand the mean BCVA 6 months after surgery was significantly higher in group 1 than in group 2. Previous studies have demonstrated that direct argon laser photocoagulation applied to focally leaking micro-aneurysms and/or grid treatment applied to areas of diffuse macular edema results in a substantial reduction of the risk of visual loss in eyes with diabetic macular edema.12, 14, 34, 35, 36, 37, 38 Although the exact mechanism underlying grid photocoagulation remains a matter of some controversy, it may be attributable to the effects on both endothelial cells of the retinal blood vessels and the retinal pigment epithelial cells.12 Laser-induced changes in the retinal pigment epithelium may also stimulate the repair of endothelial cells in the inner blood–retinal barrier with subsequent resolution of the macular edema.12, 36, 37, 38 Some authors have suggested that grid photocoagulation enhances the debridement of disordered retinal pigment epithelial cells and fosters their replacement by a health population of cells.36, 38 Grid laser photocoagulation may also work simply by destroying a certain population of photoreceptors, and eliminating high oxygen consumers may result in an increase in the level of inner retinal oxygen and a reduction in tissue vascular endothelial growth factor, which has been implicated in the development of macular edema.12, 38
Anatomically, in Group 1; the CMT decreased from preoperative values with statistically significant reduction at all postoperative intervals until 6 months (p < 0.01). There was an average of 44.78% reduction in the mean CMT at the 2nd week and 34.67% reduction at the 6th month. In Group 2; the CMT decreased from preoperative values with statistically significant reduction at all postoperative intervals until 3 months (p < 0.01); but the difference in the mean CMT between preoperative and postoperative 6th months was not statistically significant (p > 0.05). So we found combined IVTA and grid laser photocoagulation more successful in long term. Some other studies also reported a significant reduction in CMT in patients undergoing phacoemulsification surgery with intravitreal triamcinolone injection.4, 5, 20, 32 Kang et al. applied macular grid photocoagulation after intravitreal triamcinolone acetonide for diffuse diabetic macular edema and found the CMT less than the normal upper limit (206 μm) after 6 months in 15 eyes of 44 eyes in the laser group and 6 of 19 in the control group. Although the exact mechanism underlying the maintenance of improved vision and decreased central macular thickness due to grid laser photocoagulation after IVTA was not precisely identified, Kang et al., speculate that decreased foveal thickness after IVTA facilitates the delivery of the laser energy selectively to the photoreceptors and retinal pigment epithelia or steroids might act beneficially in the process of mature laser scar formation by suppressing inflammation caused by laser treatment.12 DRCR-net study indicated that intravitreal steroid or ranibizumab injections combined with laser treatment have a superior effect on VA improvement than laser treatment alone in diabetic macular edema (DME).39
There are other treatment options such as intravitreal injections of antivascular endothelial growth factor (anti-VEGF) drugs or pars plana vitrectomy for DME. Ranibizumab and bevacizumab are the two main anti-VEGF drugs used commonly. Although ranibizumab has been recently approved by the United States Food and Drug Administration for the treatment of DME, it is expensive. Bevacizumab, which costs much less than ranibizumab, is commonly used off-label in treating DME.40, 41, 42 The results of studies comparing IVTA and intravitreal bevacizumab (IVB) in DME are controversial. Some studies found IVTA more effective than IVB, some the same and some less effective. A meta analysis shows that the group receiving IVTA has a statistically significant improvement in BCVA than the group receiving IVB in the first 3 months. But the difference in BCVA was not observed at 6 months. Also the side effects of IVTA such as elevation of IOP, cataract formation and risk of endophthalmitis cause limitations in their use.40
The recent study by Cheema et al. revealed that after combined cataract surgery and intravitreal IVB, macular edema progressed only in 5.71% patients while 45.45% patients in control group.43 In the study by Wahab et al. it was reported that both grid laser and IVB in the management of cataract with macular edema due to diabetes mellitus and hypertension had a success rate of 60.5% in approaching visual acuity of 6/6.20 Ju Byung et al. compared the results of diabetic patients undergoing PHACO surgery receiving intraoperative IVB or not. They concluded that intravitreal ranibizumab injection at cataract surgery may prevent the postoperative worsening of macular edema and may improve the final visual outcome.44
Intravitreal steroid sustained release devices are being developed to achieve long-standing concentrations and less side effects. After a three year follow-up an 15 letter improvement of BCVA from baseline was detected in 22.2% of the patients receiving 0.7 mg Dexamethasone implant (Ozurdex) and 18.4% of the patients receiving 0.35 mg dexamethasone implant. The mean average reduction in CMT was −111.6 μm and 107.9 μm in the 0.7 mg and 0.35 mg dexamethasone implant receiving groups, respectively.45
In conclusion, phacoemulsification combined with IVTA injection improves the BCVA in diabetic patients with macular edema. This improvement is statistically significant. In addition to this therapy macular grid laser photocoagulation after surgery helps to preserve the improvement in BCVA and the decrease in OCT values.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
Contributor Information
Ozlen Rodop Ozgur, Email: ozlen74@yahoo.com.
Yelda Ozkurt, Email: yeldaozkurt@yahoo.com.
Zeynep Kulekci, Email: zeynepklk@mynet.com.
Tufan Evciman, Email: tufanevciman@gmail.com.
References
- 1.Bresnick G.H. Diabetic macular edema: a review. Ophthalmology. 1986;93:989–997. doi: 10.1016/s0161-6420(86)33650-9. [DOI] [PubMed] [Google Scholar]
- 2.Gaudric A., Massin-Korobelnik P. Diabetic maculopathy: classification, epidemiology, spontaneous outcome, treatment. Diabetes Metab. 1993;19:422–429. [PubMed] [Google Scholar]
- 3.Hamilton A.M.P., Ulbig M.W., Polkinghorne P., editors. Epidemiology of diabetic retinopathy. Management of diabetic retinopathy. BMJ Publishing Group; London: 1996. pp. 1–15. [Google Scholar]
- 4.Lam D.S.C., Chan C.K.M., Mohamed S., Lai T.Y.Y., Lee V.Y.W., Lai W.W. Phacoemulsification with intravitreal triamcinolone in patients with cataract and coexisting diabetic macular edema: a 6-month prospective pilot study. Eye. 2005;19:885–890. doi: 10.1038/sj.eye.6701686. [DOI] [PubMed] [Google Scholar]
- 5.Sadiq S.A., Sleep T., Amoaku W.M. The visual results and changes in retinopathy in diabetic patients following cataract surgery. Eur J Ophthalmol. 1999;9:14–20. doi: 10.1177/112067219900900103. [DOI] [PubMed] [Google Scholar]
- 6.Chung J., Kim M.Y., Kim H.S., Yoo J.S., Lee Y.C. Effect of cataract surgery on the progression of diabetic retinopathy. J Cataract Refract Surg. 2002;28:626–630. doi: 10.1016/s0886-3350(01)01142-7. [DOI] [PubMed] [Google Scholar]
- 7.The royal college of ophthalmologists: guidelines for diabetic retinopathy. London: RCO; 2002. [DOI] [PMC free article] [PubMed]
- 8.Henricsson M., Heijl A., Janzon L. Diabetic retinopathy before and after cataract surgery. Br J Ophthalmol. 1996;80:789–793. doi: 10.1136/bjo.80.9.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollack A., Dotan S., Oliver M. Progression of diabetic retinopathy after cataract extraction. Br J Ophthalmol. 1991;75:547–551. doi: 10.1136/bjo.75.9.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunliffe I.A., Flanagan D.W., George N.D.L., Aggarwaal R.J., Moore A.T. Extra capsular cataract surgery with lens implantation in diabetics with and without proliferative retinopathy. Br J Ophthalmol. 1991;75:9–12. doi: 10.1136/bjo.75.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang S.W., Sa H.S., Cho H.Y., Kim J.I. Macular grid photocoagulation after intravitreal triamcinolone acetonide for diffuse diabetic macular edema. Arch Ophthalmol. 2006;124:653–658. doi: 10.1001/archopht.124.5.653. [DOI] [PubMed] [Google Scholar]
- 12.Habib M.S., Cannon P., Steel D.H.W. The combination of intravitreal triamcinolone and phacoemulsification surgery in patients with diabetic foveal oedema and cataract. BMC Ophthalmol. 2005;5:15. doi: 10.1186/1471-2415-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bresnick G.H. Diabetic maculopathy: a critical review highlighting diffuse macular edema. Ophthalmology. 1983;90:1301–1317. doi: 10.1016/s0161-6420(83)34388-8. [DOI] [PubMed] [Google Scholar]
- 14.Dowler J.G., Kulwant S.S., Hykin P.G., Hamilton P.A. The naturel history of macular edema after cataract surgery in diabetes. Ophthalmology. 1999;106:663–668. doi: 10.1016/S0161-6420(99)90148-3. [DOI] [PubMed] [Google Scholar]
- 15.Dowler J.F., Hykin P.G., Hamilton P.A. Phacoemulsification versus extracapsuler cataract extraction in patients with diabetes. Ophthalmology. 2000;107:457–462. doi: 10.1016/s0161-6420(99)00136-0. [DOI] [PubMed] [Google Scholar]
- 16.Antcliff R.J., Poulson A., Flanagan D.W. Phacoemulsification in diabetics. Eye. 1996;10:737–741. doi: 10.1038/eye.1996.171. [DOI] [PubMed] [Google Scholar]
- 17.Gupta A., Gupta V. Diabetic maculopathy and cataract surgery. Ophthalmol Clin North Am. 2001;14:625–637. doi: 10.1016/s0896-1549(05)70262-5. [DOI] [PubMed] [Google Scholar]
- 18.Squirrell D., Bhola R., Bush J., Winder S., Talbot J.F. A prospective, case controlled study of the natural history of diabetic retinopathy and maculopathy after uncomplicated phacoemulsification cataract surgery in patients with type 2 diabetes. Br J Ophthalmol. 2002;86:565–571. doi: 10.1136/bjo.86.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mota H., Lara C., Vazquez H. Use of triamcinolone and bevacizumab in 25G phaco-vitrectomy surgery for the treatment of cataract and diabetic macular edema. Arch Soc Esp Oftalmol. 2008;83:293–300. doi: 10.4321/s0365-66912008000500004. [DOI] [PubMed] [Google Scholar]
- 20.Somaiya M.D., Burns J.D., Mintz R., Warren R.E., Uchida T., Godley B.F. Factors affecting visual outcomes after small-incision phacoemulsification in diabetic patients. J Cataract Refract Surg. 2002;28:1364–1371. doi: 10.1016/s0886-3350(02)01319-6. [DOI] [PubMed] [Google Scholar]
- 21.Chew E.Y., Benson W.E., Remaley N.A., Lindley A.A., Burton T.C., Csaky K. Results after lens extraction in patients with diabetic retinopathy: early treatment diabetic retinopathy treatment study report number 25. Arch Ophthalmol. 1999;117:1600–1606. doi: 10.1001/archopht.117.12.1600. [DOI] [PubMed] [Google Scholar]
- 22.Nelson M.L., Martidis A. Managing cystoids macular edema after cataract surgery. Curr Opin Ophthalmol. 2003;14:39–43. doi: 10.1097/00055735-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Massin P., Audren F., Hauchine B., Erginay A., Bergmann J.F., Benosman R. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema. Ophthalmology. 2004;111:218–225. doi: 10.1016/j.ophtha.2003.05.037. [DOI] [PubMed] [Google Scholar]
- 24.Wingate R.J., Beaumont P.E. Intrvitreal triamcinolone and elevated intraocular pressure. Aust NZJ Ophthalmol. 1999;27:431–432. doi: 10.1046/j.1440-1606.1999.00238.x. [DOI] [PubMed] [Google Scholar]
- 25.Jonas J.B., Kreissig I., Degenring R. Intraocular pressure after intravitreal injection of trimcinolone acetonide. Br J Ophtalmol. 2003;87:24–27. doi: 10.1136/bjo.87.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scholes G.N., O’Brein W.J., Abrams G.W., Kubicek M.F. Clearance of triamcinolone from vitreus. Arch Ophthalmol. 1985;103:1567–1569. doi: 10.1001/archopht.1985.01050100143037. [DOI] [PubMed] [Google Scholar]
- 27.Wilson C.A., Berkowita B.A., Sato Y., Ando N., Handa J.T., de Juan E. Treatment with intravitreal steroid reduces blood–retinal barrier breakdown due to retinal photocoagulation. Arch Ophthalmol. 1992;110:1155–1159. doi: 10.1001/archopht.1992.01080200135041. [DOI] [PubMed] [Google Scholar]
- 28.Jonas J.B., Kreissig I., Sofker A., Degenring R.V. Intravitreal injection of triamcinolone for diffuse diabetic macular oedema. Arch Ophthalmol. 2003;121:57–61. [PubMed] [Google Scholar]
- 29.Martidis A., Ducker J.S., Greenberg P.B., Rogers A.H., Puliafito C.A., Reichel E. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109:920–927. doi: 10.1016/s0161-6420(02)00975-2. [DOI] [PubMed] [Google Scholar]
- 30.Parke D.W., Sisk R.A., Murray T.G. Intraoperative intravitreal triamcinolone decreases macular edema after vitrectomy with phacoemulsification. Clin Ophthalmol. 2012;6:1347–1353. doi: 10.2147/OPTH.S34653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhee D.J., Peck R.E., Belmont J., Martidis A., Liu M., Chang J. Intraocular pressure alterations following intravitreal triamcinolone acetonide. Br J Ophthalmol. 2006;90:999–1003. doi: 10.1136/bjo.2006.090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmadabadi H.F., Mohammadi M., Beheshtnejad H., Mirshahi A. Effect of intravitreal triamcinolone acetonide injection on the central macular thickness in diabetic patients having phacoemulsification. J Cataract Refract Surg. 2010;36:917–922. doi: 10.1016/j.jcrs.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 33.Early Treatment Diabetic Retinopathy Study Research Group Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–1806. [PubMed] [Google Scholar]
- 34.Lee C.M., Olk R.J. Modified grid laser photocoagulation for diffuse diabetic macular edema: long term visual results. Ophthalmology. 1991;98:1594–1602. doi: 10.1016/s0161-6420(91)32082-7. [DOI] [PubMed] [Google Scholar]
- 35.Early Treatment Diabetic Retinopathy Study Research Group Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema: Early Treatment Diabetic Retinopathy Study report number 2. Ophthalmology. 1987;94:761–774. doi: 10.1016/s0161-6420(87)33527-4. [DOI] [PubMed] [Google Scholar]
- 36.Early Treatment Diabetic Retinopathy Study Research Group Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report number 4. Int Ophthalmol Clin. 1987;27:265–272. doi: 10.1097/00004397-198702740-00006. [DOI] [PubMed] [Google Scholar]
- 37.Clover G.M. The effects of argon and krypton photocoagulation on the retina: implications for the inner and outer blood retinal barriers. In: Gitter K.A., Schatz H., Yannuzzi L.A., McDonald H.R., editors. Laser photocoagulation of retinal disease. Pacific Medical Press; San Francisco, Calif: 1988. pp. 11–17. [Google Scholar]
- 38.Weiter J.J., Zuckerman R. The influence of photoreceptor–RPE complex on the inner retina: an explanation for beneficial effects of photocoagulations. Ophthalmology. 1980;87:1133–1139. doi: 10.1016/s0161-6420(80)35119-1. [DOI] [PubMed] [Google Scholar]
- 39.The Diabetic Retinopathy Clinical Research Network Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Xiao-Ling, Chen Jian, Zhang Ri-Jia, Wang Wen-Jie, Zhou Qing, Qin Xiao-Yan. Intravitreal triamcinolone versus intravitreal bevacizumab for diabetic macular edema: a meta-analysis. Int J Ophthalmol. 2013;6:546–552. doi: 10.3980/j.issn.2222-3959.2013.04.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ozkiris A. Intravitreal bevacizumab (Avastin) for primary treatment of diabetic oedema. Eye. 2009;23:616–620. doi: 10.1038/eye.2008.40. [DOI] [PubMed] [Google Scholar]
- 42.Arvello J.F., Guerra J.F., Mercado H.Q., Sanchez J.G., Wu L., Maia M. Primary intravitreal bevacizumab (Avastin) for diabetic macular oedema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007;114:743–750. doi: 10.1016/j.ophtha.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 43.Cheema R.A., Al-Mubarak M.M., Amin Y.M., Cheema M.A. Role of combined cataract surgery and intravitreal bevacimuzab injection in preventing progression of diabetic retinopathy. J Cat Refract Surg. 2009;35:18–25. doi: 10.1016/j.jcrs.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 44.Ju Byung C., Soo Geun J., Sung Jae Y., Joo Yong L., †Kyung Rim S., Jae Yong K. Effect of combined cataract surgery and ranibizumab injection in postoperative macular edema in nonproliferative diabetic retinopathy. Retina. 2014;34:149–156. doi: 10.1097/IAE.0b013e3182979b9e. [DOI] [PubMed] [Google Scholar]
- 45.Boyer D., Hee Yoon Y., Belfort R., Jr., Bandello F., Maturi R.K., Augustin A. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;212:1904–1914. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]