Abstract
Purpose
To study appropriateness of our modified screening criteria for detection of all cases of Retinopathy of Prematurity (ROP) among preterm babies.
Method
Retrospective observational cohort study among preterm neonates who underwent ROP screening as per set protocol for 11 years at Nizwa Hospital, Al Dhakilya Governorate, Oman. We screened all babies with gestational age ⩽32 weeks or BW ⩽ 1500 g. Preterm babies >32 weeks of GA or BW > 1500 g with unstable clinical course believed to be at high risk by the attending neonatologist also were screened.
Results
During the study period 528 babies were screened for ROP of which 76 babies were excluded due to death, associated congenital ocular malformation and loss for follow-up either due to transfer to other institution or defaulting. Thus 452 babies were included in the final analysis. Incidence of ROP was 46.4% of which 27.9% had mild ROP, 11.3% had severe ROP which regressed and 7.3% had severe ROP who were treated. The incidence of ROP among infants with GA < 26 wks, 26–28 wks, 29–30 wks, 31–32 wks and above 32 weeks was 100.0%, 80.0%, 59.3%, 34.4% and 19.4% respectively.
56 babies of this cohort belonged to Extended (modified) criteria group. Among these 12 babies had ROP out of which 9 had mild ROP and 3 had severe ROP. Among cases with severe ROP, two cases regressed spontaneously and one case needed treatment.
Multivariate analysis using stepwise regression model showed statistically significant association of GA and BW to development of ROP.
We would have missed few babies with ROP if we had followed other criteria.
Conclusion
Our modified screening criteria seem to be appropriate as no infant with severe ROP was missed during the study period. Incidence of severe ROP among babies in the extended criteria group (5.4%) is low but significant compared to lower gestational age. We plan to formulate a scoring system following all risk factor analysis to enable us to optimize the number of infants screened. Detection of all babies with ROP is important as they need long-term follow-up for the timely detection and management of associated ocular comorbidities.
Keywords: Retinopathy of Prematurity, Gestational age, Birth weight, Premature infant, Screening criteria, Risk factors, Oman
Introduction
Retinopathy of prematurity (ROP) is a vasoproliferative disorder affecting preterm infants that can potentially lead to blindness if not treated in time. With recent improvement in neonatal care, extremely low birth weight babies and extreme preterm babies are increasingly surviving and hence the incidence of ROP has been frequently reported from developing countries.
Retinopathy of prematurity was established in developed countries at the end of 1980s after the American CRYO-ROP study report was published.1 Some low and middle income countries introduced ROP screening in the 1990s2 and some still do not have programmes or their screening coverage is either low or only selectively implemented in some urban centres.3 The aim of the screening should be to target those who are most at risk, but the gold standard remains to detect and treat every possible case of ROP. The profile of babies developing ROP in countries with moderate and low human development index differs from countries with high human development index as bigger and more mature babies are also found to develop this disorder in these settings.2, 4, 5
The United Kingdom (UK) screening guidelines by the Royal College of Ophthalmologists, UK and British Association of Perinatal Medicine (RCOS-BAPM) recommend screening of infants with gestational age (GA) less than 32 weeks (up to 31 weeks and 6 days) or birth weight (BW) less than 1501 g.6 The American Academy of Paediatrics (AAP) updated the recommendations and has proposed screening for infants with BW ⩽ 1500 g or GA ⩽ 30 weeks with a caveat to screen heavy and older babies with unstable clinical course.7, 8 These criteria seem to have worked well in countries with high human development index.9 In view of the inconsistency in the GA and BW of babies with severe ROP in the literature, we cannot generalize the screening criteria to all neonatal units, regions and populations alike.5, 10, 11
The low incidence of ROP and related blindness in developed nations are due to reasons including population and ethnic variations, neonatal care and organized screening and timely intervention.9 There is a great variation in the standards of neonatal care and neonatal outcomes in different settings worldwide and hence population/institution based criteria are necessary to achieve optimal detection rates.12 Moreover, the incidence of ROP and the need for treatment vary due to the difference in the screening criteria, observer difference as well as ethnic variation in susceptibility. To prevent adverse effects from ROP, it is mandatory to assess the population at risk, to identify the risk factors and to adopt appropriate screening criteria. This is the first study undertaken in Oman till date to validate the appropriateness of our modified ROP screening criteria. Previous studies on ROP in Oman focused on risk factors and aetiology of ROP.13, 14
Materials and methods
This is a retrospective cohort study conducted in the Special Care Baby Unit (SCBU) and Medical Retina Clinic of Nizwa hospital, a governorate referral hospital in Al Dakhaliya governorate in Oman. This study was approved by the local research and ethical committee. It was conducted for a period of 11 years from January 1, 2003 to December 31, 2013.
We screened all the preterm babies with GA ⩽ 32 weeks or BW ⩽ 1500 g. Besides, preterm babies >32 weeks (up to 36 weeks) of GA or BW 1501–2000 g with any of additional risk factors such as prolonged ventilatory support more than 10 days, prolonged oxygen therapy beyond 36 weeks of postconceptual age, life threatening recurrent apnoeas, anaemias needing more than 4 blood transfusions and gram negative or fungal neonatal sepsis were also screened at the discretion of neonatologist. (Extended criteria group).
The data of all babies who underwent screening for ROP during the study period were retrospectively reviewed. Data collected for each neonate included gender, plurality, GA, BW, postmenstrual age, age of onset of ROP and age at which treatment was carried out and nature of treatment. In many cases it was found that both eyes were affected except in 20 cases. Most severely affected eye was included in ROP grading, when ROP involved both the eyes. Initial screening was done at 31 weeks of postmenstrual age in babies with GA < 27 weeks and at 4 weeks of postnatal age in babies born with GA > 27 Weeks.7 Gestational age was calculated from the history given by the pregnant mothers about their last menstrual period (LMP). When there was a discrepancy, the mean of GA by LMP and GA by ultrasound was computed. Screening was done exclusively by two of the senior ophthalmologists with expertise in ROP.
Pupillary dilatation was done with 1% Phenylephrine and 0.5% Tropicamide starting two hours before the examination. Fundoscopy was done with binocular indirect ophthalmoscope using +28 Diopter Volk lens. Paediatric speculum and scleral depressor were used whenever needed. ROP was classified based on the international classification of ROP.15 In cases of Immature retina, follow-up examinations were made every two weeks till the vessels reached retinal periphery. On detection of ROP, babies were reviewed weekly or more frequently as per the discretion of the ophthalmologist. ROP stages 1 and 2 were labelled as mild ROP, and Stages 3–5 and aggressive posterior ROP were labelled as severe ROP.
Decision for treatment was taken as described in “Early Treatment of ROP randomized Trial” (ET-ROP).16 Infants with severe ROP needing treatment were also reviewed by vitreoretinal surgeons at tertiary care ophthalmic service and treatment was carried out after their ratification. Treatment was by Laser photocoagulation till year 2009 and either Laser treatment and/or intravitreal injection of Anti Vascular Endothelial Growth Factor agent during period after year 2009. Treatment was carried out within 72 h of decision to treat.
Statistical analysis was carried out using commercially available statistical software package (SPSS version 16). We considered the p value to be statistically significant when it was less than 0.05. Chi square test and logistic regression were used to find the odds ratio in univariate and multivariate analysis respectively.
Results
Among the 528 babies screened for ROP during the study period, seven babies expired before completing the ROP evaluation and two were excluded due to associated congenital ocular malformation. 67 babies were lost to follow-up due to either transfer to other institutions or defaulting. Thus 76/528 (14.4%) excluded for reasons cited earlier and 452 babies included for final analysis. 396 babies (87.6%) belonged to standard criteria group and 56 babies (12.4%) of this cohort belonged to the extended criteria group. 58.6% of the babies were male and 41.4% were female. 79.4% of the babies had GA up to 32 weeks and 20.6% of the babies had GA > 32 weeks. 66.1% babies had BW ⩽ 1.5 kg and 33.8% babies had birth weight >1.5 kg. 77.6% of the babies were born out of singleton pregnancy and 22.3% were born out of multiple pregnancy. Total incidence of ROP in our cohort was 46.4% of which 27.9% had mild ROP, 11.3% had severe ROP which regressed and 7.3% had severe ROP who were treated.
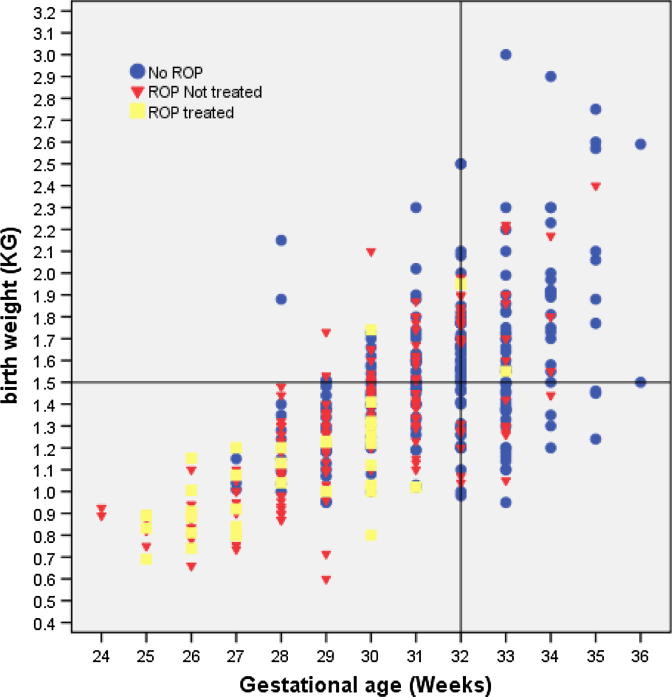
Fig. 1 is the scatter plot showing the relation of screening criteria of GA and BW with respect to ROP.
Figure 1.
Scatter plot showing the relation of gestational age and birth weight to ROP screening results.
The incidence of ROP among infants with GA < 26 wks, 26–28 wks, 29–30 wks, 31–32 wks and >32 weeks was 100.0%, 80.0%, 59.3%, 34.4% and 19.4% respectively. The odds ratio shows that as GA increased, the risk of ROP decreased and the association between ROP and GA was statistically significant (p < 0.001).
The incidence of ROP among males (48.3%) was slightly higher than that of female babies (44.1%), however the association with gender was not statistically significant (p = 0.616). The incidence of ROP among singleton babies (47%) almost matched with incidence among babies born out of multiple pregnancies (46.4%) and was statistically insignificant (p = 0.913).
Table 1 shows different stages of ROP and its relation to GA and BW.
Table 1.
ROP staging in relation to gestational age and birth weight.
| NO (%) | Mean gestational age weeks (range) | Mean birth weight (range) | |
|---|---|---|---|
| No ROP | 242 (53.5%) | 31.59 (27–36) | 1.55 (1.0–3.0) |
| ROP stage 1 | 35 (7.7%) | 30.74 (26–35) | 1.41 (0.7–2.2) |
| ROP stage 2 | 91 (20.1%) | 29.92 (25–34) | 1.37 (0.7–2.2) |
| ROP stage 3 not treated | 51 (11.3%) | 28.8 (24–33) | 1.16 (0.6–1.8) |
| Advanced ROP treated | 33 (7.3%) | 28.09 (25–33) | 1.08 (0.7–2.0) |
56 babies of this cohort belonged to extended criteria group. Among this 12 (21.4%) babies had ROP out of which nine (16.1%) were mild ROP and three (5.3%) were severe ROP. Among severe ROP two regressed spontaneously and one underwent treatment. Their mean BW was 1.97 Kg and GA was 33.68 weeks which was higher than babies with ROP in standard criteria group.
We used stepwise logistic regression model to carry out multivariate analysis only of demographic variables. We included GA, BW, sex and plurality as independent risk factors. We found that GA was the most important risk factor for ROP (P < 0.001). The other risk factors were not significantly associated with ROP on multivariate analysis. The association between clinical risk factors (maternal, infant and interventional) and severity of ROP is not part of this study and will be reported elsewhere.
Discussion
In spite of recent developments in diagnosis and management, ROP represents a leading cause of preventable blindness in childhood. Retinopathy of prematurity is targeted in “Vision 2020” which is a global initiative with a mission to eliminate the cause of all preventable and treatable blindness by year 2020.17
Our study shows an incidence of ROP of 46.4% which is higher than an incidence of 34%13 and 25.4%14 respectively as reported from tertiary neonatal units in Oman. These earlier studies were over a shorter period of time and due to referral bias of tertiary centre cohort, cannot be applied to general population cohort as in our study.
Table 2 shows the incidence of ROP reported from some of the Middle Eastern countries in the recent past.
Table 2.
Incidence of ROP from Middle Eastern Countries.
| S. no | Study, Country, Reference | Year of publication | Duration of study | Incidence of ROP (%) |
|---|---|---|---|---|
| 1 | Amer et al., Saudi Arabia, 9 | 2012 | 3 yrs | 23.3 |
| 2 | Al Amro et al., Saudi Arabia, 18 | 2003 | 3.5 yrs | 37.4 |
| 3 | Binkhathlan et al., Saudi Arabia, 19 | 2008 | 1 yr | 56 |
| 4 | Karkhaneh et al., Iran, 20 | 2008 | 4 yrs | 34.5 |
| 5 | Sarikabadayi et al., Turkey, 21 | 2011 | 1 yr | 32.7 |
| 6 | Current study, Nizwa Hospital, Oman | 2015 | 11 yrs | 46.4 |
An analysis of incidence of ROP in Middle Eastern countries indicates that although total incidence of ROP shows wide range between 23.31% and 56%, the rates of threshold and severe ROP and severe ROP needing treatment are comparable. To illustrate further the percentage of severe ROP needing treatment of 6.48% in Amer et al. study in Saudi Arabia9 (Total ROP incidence 23.31%) is almost comparable to severe ROP needing treatment of 7.3% in our study (Total ROP incidence 46.4%).
No gender difference was seen in the incidence of ROP which is consistent with CRYO-ROP study and the New York Cohort.22, 23, 24 Gender and plurality were not associated with severity of ROP, a finding similar to other studies.12, 25 Wagner reported a higher incidence of ROP in older and more mature infants in his special report on the “ROP epidemiology in developing countries.” 26 Several reports of ROP in heavier and older babies from Indian subcontinent, China and rest of Asia, have emphasized the occurence of ROP in babies above BAPM or AAP criteria.27 Dogra reported 15.3% of threshold ROP28 and Deshpande reported an incidence of 21.7% of threshold ROP29 in babies with BW > 1500 g in India. Vinekar et al. from a tertiary neonatal intensive care unit in India reported that 62/138 of their babies (45%) with a mean BW of 1533 g (range 1251–2750) had threshold ROP.11 Similarly Chen et al. reported among cases with severe ROP in China, 27.2% babies were more than 1500 g at birth.30 Thus widening scope of ROP screening is desirable in several parts of the world so as to not to miss any cases with ROP. However, more detailed studies are required to further narrow down the basis for screening in this group. Screening five or six extra preterms yearly (56 babies in 11 years) did not cause excessive burden on our ROP screening services.
Moreover, why then in the western countries with high human development index, ROP especially blinding ROP is not reported in heavier or more mature preterm babies? It could be due to population characteristics such as ethnicity or genetic predisposition, optimal standards of neonatal care allowing careful control of risk factors, optimal ROP screening and its timely intervention. Besides it could be that there are not many studies which have explored the ROP in preterm babies in GA between 32 and 36 weeks and BW bands of 1500–2000 g in their settings. Chiang et al. did report in their study on neonates from New York state in USA that 17 of their infants with ROP were more than 2000 g in BW although none required treatment.31 However on the other side of the spectrum especially from the Indian subcontinent high incidence of blinding ROP is reported in heavier and more mature babies.11, 32 This may possibly be due to unregulated neonatal care practices especially administration of high concentration of unblended oxygen without proper monitoring, suboptimal ROP screening programme due to non-availability of trained ophthalmologist and timely intervention facilities.32
Although we used “sickness criteria” for recommending babies for ROP screening beyond the GA cut-off of 32 weeks and or BW cut-off of 1500 g, such a proposition is difficult to implement. This is because besides GA and oxygen exposure, association between sickness criteria and ROP has shown wide variations among several studies in the published literature. Several of risk factors such as necrotizing enterocolitis, symptomatic patent ductus arteriosus, intra-ventricular haemorrhage, and sepsis are co-morbidities observed in preterm infants during the same period as when ROP is observed and may purely be epiphenomena rather than having causal relationship.
Our data show that we would have missed few babies with ROP if we had followed exclusively RCOS-BAPM criteria (Table 3). Detection of ROP, even though they may regress without intervention is important as these babies need long-term follow-up for the timely detection and management of ocular comorbidities such as refractive error, strabismus and amblyopia.33
Table 3.
Number of infants with ROP who would have missed if we have used different screening criteria.
| Screening criteria | No of Infants fulfilling criteria | Number of Infants that would have missed |
|
|---|---|---|---|
| ROP positive cases | Severe ROP treated | ||
| Present criteria in the study | 452 | 0 | 0 |
| If we haven’t screened babies with GA > 32 wks and BW > 1.5 kg | 56 | 12 | 1 |
In conclusion, we suggest that in our set-up in Oman with moderate human development index of 56 in 2015,34 we should also screen all babies with additional risk factors especially in GA range of 32–34 weeks and BW band 1500–1800 g for risk of ROP. Beyond this cut-off, severe ROP is exceptional in our set-up. The observation of not detecting any other children with low vision who had preterm birth in our governorate indirectly suggests that possibly no preterm babies other than in our cohort had ROP related blindness.
Our study has few limitations. The study being retrospective in nature has its own inherent limitations. Besides our study sample covered only one governorate and cannot be generalized to whole of Oman. We also lost 67 (12.7%) babies to follow-up whose outcome is not known which could have skewed the result. Since neonatal survival and care differ in different centres, it is also advisable to evaluate the effectiveness of the proposed modified screening system in other governorates and hospitals. This will help to formulate an appropriate national screening programme in Oman and other Middle Eastern countries with Arab lineage, in the future.
Conflict of interest
The authors declared that there is no conflict of interest.
Acknowledgements
The contribution of staff of Special Care Baby Unit and Medical Retina Clinic of Nizwa Hospital, Oman, in the management of infants with ROP is gratefully acknowledged.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
Contributor Information
Mary K. Jacob, Email: maryjacob07@gmail.com.
Kiran P. Sawardekar, Email: sawar.kiran@gmail.com.
Hani Gameel Ayoub, Email: hayob20@yahoo.com.
Ibrahim Al Busaidi, Email: albusaidi80@hotmail.com.
References
- 1.Larsson E., Holsmstrom G. Screening for retinopathy of prematurity: evaluation and modification of guidelines. Br J Ophthalmol. 2002;86:1399–1402. doi: 10.1136/bjo.86.12.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weerakoon I.K., Fonselea C. Retinopathy of prematurity in Sri Lanka Ceylon. Med J. 1998;43:1945. [PubMed] [Google Scholar]
- 3.Gilbert C., Foster A. Causes of blindness in children attending four schools for the blind in Thailand and Philippines. A comparison between urban and rural blind school population. Int Ophthalmol. 1993;17:229–234. doi: 10.1007/BF01007745. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert C., Field A., Gordillo l. Characteristics of infants with severe ROP in countries with low, moderate and high levels of development: implications for screening programs. Pediatrics. 2005;115(May):e518–e522. doi: 10.1542/peds.2004-1180. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert C., Rahi J., Eckstem M. Retinopathy of prematurity in middle income countries. Lancet. 1997;350:12–14. doi: 10.1016/S0140-6736(97)01107-0. [DOI] [PubMed] [Google Scholar]
- 6.Wilkinson A.R., Haines L., Head K. Guideline Development Group of the Royal College of Paediatrics and Child Health; Royal College of Ophthalmologists; British Association of Perinatal Medicine. UK retinopathy of prematurity guideline. Eye (Lond) 2009;23:2137–2139. doi: 10.1038/eye.2008.128. [DOI] [PubMed] [Google Scholar]
- 7.Policy statement. Screening examination of premature infants for Retinopathy of Prematurity. American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus, and American association of certified orthoptists. Pediatrics 2013;131:189–95. [DOI] [PubMed]
- 8.Sudha C., Vidyadhar P., Umesh V., Sandeep K., Aarti K. Retinopathy of prematurity in a tertiary care center – incidence, risk factors and outcome. Indian Paediatr. 2009;46:219–224. [PubMed] [Google Scholar]
- 9.Amer M., Jafri W.H., Nizam A.M., Shomrani A.I., Al Dabaan A.A., Rashid K. Retinopathy of prematurity: are we missing any infant with retinopathy of prematurity. Br J Ophthalmol. 2012;96:1052–1055. doi: 10.1136/bjophthalmol-2012-301570. [DOI] [PubMed] [Google Scholar]
- 10.Quinn G.E. What do you do about ROP screening in “big” babies. Br J Ophthalmol. 2002;86:1072–1073. doi: 10.1136/bjo.86.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinekar A., Dogra M.R., Sangtam T. Retinopathy of prematurity in Asian Indian babies weighing more than 1250 grams at birth: ten year data from a tertiary care centre in a developing country. Indian J Ophthalmol. 2007;55:331–336. doi: 10.4103/0301-4738.33817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.YusuffUnal S., Ozge A., Zuhal Tumay O., Cumhur A., Levent T., Serife S.O. Screening for retinopathy of prematurity in a large tertiary neonatal intensive care unit in Turkey: frequency and risk factors. Ophthal Epidemiol. 2011;18(6):269–274. doi: 10.3109/09286586.2011.615449. [DOI] [PubMed] [Google Scholar]
- 13.Bassiouny M.R. Risk factors associated with retinopathy of prematurity: a study from Oman. J Trop Pediatr. 1996;42(6):355–358. doi: 10.1093/tropej/42.6.355. [DOI] [PubMed] [Google Scholar]
- 14.Nair P.M., Ganesh A., Mitra S., Ganguly S.S. Retinopathy of prematurity in VLBW and extreme LBW babies. Indian J Pediatr. 2003;70(4):303–306. doi: 10.1007/BF02723585. [DOI] [PubMed] [Google Scholar]
- 15.International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol 2005;123(7):991–9. [DOI] [PubMed]
- 16.Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 2003;121:1684–94. [DOI] [PubMed]
- 17.Thylefors B. A global initiative for the elimination of avoidable blindness. Am J Ophthalmol. 1998;125:90–93. doi: 10.1016/s0002-9394(99)80239-6. [DOI] [PubMed] [Google Scholar]
- 18.Al-Amro S.A., Al Kharfi T.M., Thahit A.A. Retinopathy of prematurity at University Hospital in Riyadh, Saudi Arabia. Saudi Med J. 2003;24:720–724. [PubMed] [Google Scholar]
- 19.Binkhathlan A.A., Almahmoud L.A., Saleh M.J. Retinopathy of prematurity in Saudi Arabia. Incidence, risk factors, and the applicability of current screening criteria. Br J Ophthalmol. 2008;92:167–169. doi: 10.1136/bjo.2007.126508. [DOI] [PubMed] [Google Scholar]
- 20.Karkhaneh R., Mousavi S.Z., Riazi Esfchani M. Incidence and risk factors of retinopathy of prematurity in a tertiary eye hospital in Tehran. Br J Ophthalmol. 2008;92:1446–1449. doi: 10.1136/bjo.2008.145136. [DOI] [PubMed] [Google Scholar]
- 21.Sarikabadayi Y.U., Aydemir O., Ozen Z.T. Screening for retinopathy of prematurity in a large tertiary neonatal intensive care unit in Turkey: frequency and risk factors. Ophthal Epidemiol. 2011;18:269–274. doi: 10.3109/09286586.2011.615449. [DOI] [PubMed] [Google Scholar]
- 22.Palmer E.A., Flynn J.T., Hardy R.J. Incidence and early course of retinopathy of prematurity – the cryotherapy for retinopathy of prematurity co-operative group. Ophthalmology. 1991;98:1628–1640. doi: 10.1016/s0161-6420(91)32074-8. [DOI] [PubMed] [Google Scholar]
- 23.Lad E.M., Nguyen T.C., Morton J.M., Mosh D.M. Retinopathy of prematurity in the United States. Br J Ophthalmol. 2008;92:320–325. doi: 10.1136/bjo.2007.126201. [DOI] [PubMed] [Google Scholar]
- 24.Chiang M.F., Arnos R.R., Rynn J.T. Incidence of retinopathy of prematurity from 1996 to 2000: analysis of comprehensive New York State patient database. Ophthalmology. 2004;111:1317–1325. doi: 10.1016/j.ophtha.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 25.Darlow B.A., Hutchinson J.L., Henderson-Smart D.J. Australian and New Zealand neonatal network – prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand neonatal network. Pediatrics. 2005;115:990–996. doi: 10.1542/peds.2004-1309. [DOI] [PubMed] [Google Scholar]
- 26.Wagner R.S. Increase incidence and severity of retinopathy of prematurity in developing nations. J Paediatr Ophthamol Stabismus. 2003;40:193. doi: 10.3928/0191-3913-20030701-03. [DOI] [PubMed] [Google Scholar]
- 27.Phan M.H., Nguyen P.N., Reynolds J.D. Incidence and severity of retinopathy of prematurity in Vietnam, a developing middle-income country. J Paediatr Ophthalmol Stabismus. 2003;40:208–212. doi: 10.3928/0191-3913-20030701-07. [DOI] [PubMed] [Google Scholar]
- 28.Dogra M.R., Narang S., Biswas C., Gupta A., Narang A. Threshold retinopathy of prematurity. Ocular changes and sequelae following cryotherapy. Indian J Ophthalmol. 2001;49:97–101. [PubMed] [Google Scholar]
- 29.Deshpande D., Chaturvedi M., Gopal L., Ramachandran S., Shan Mugha Sundaram R. Treatment of threshold retinopathy of prematurity. Indian J Ophthalmol. 1998;46:159. [PubMed] [Google Scholar]
- 30.Chen Y., Li X. Characteristics of severe retinopathy of prematurity patterns in China. A report of the first epidemic. Br J Ophthalmol. 2000;90:268–271. doi: 10.1136/bjo.2005.078063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiang M.F., Arons R.R., Flynn J.T., Starron J.B. Incidence of retinopathy of prematurity from 1996–2000. Ophthalmology. 2004;111:1317–1325. doi: 10.1016/j.ophtha.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 32.Shah P.K., Narendran V., Kalpana N. Aggressive posterior retinopathy of prematurity in large preterm babies in South India. Arch Dis Child Fetal Neonatal Ed. 2012;97:F371–F375. doi: 10.1136/fetalneonatal-2011-301121. [DOI] [PubMed] [Google Scholar]
- 33.Schalij-Delfos N.E., de Graaf M.E., Trevers W.F., Engel J., Cats B.P. Long term follow up of premature infants: detection of strabismus, amblyopia, and refractive errors. Br J Ophthalmol. 2000;84:9637. doi: 10.1136/bjo.84.9.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.United Nations Development Program. Human development indicators: Oman, Country Profile [Internet]; 2015. <http://hdrstats.Undp.org/en/countries/profiles/SAU.html> [accessed 6 August 2015].