Abstract
Purpose
To compare the choroidal thickness (CT) of subjects with Retinitis Pigmentosa (RP) with age-matched healthy subjects and to correlate the visual acuity with retinal parameters including central macular thickness (CMT), inner segment/outer segment junction (IS/OS junction) integrity, external limiting membrane (ELM) integrity and choroidal thickness in subjects with RP.
Methods
Eighty-eight eyes (69 patients) with typical RP and 188 eyes of 104 healthy subjects were enrolled between September 2012 and January 2013.
All subjects underwent a comprehensive ocular examination including choroidal imaging using enhanced depth imaging with spectral domain optical coherence tomography. Outcome measures were CT difference between RP and age-matched healthy subjects; and correlation of various factors such CMT, IS/OS junction integrity, ELM integrity, and CT with visual acuity.
Results
Among RP subjects, mean age was 31.39 ± 13.4 years with a mean BCVA of 0.99 ± 0.94 logMAR. Mean spherical equivalent was −0.6 ± 1.6D. Mean CMT was 148.48 ± 119 μm. Mean subfoveal CT was 296.9 ± 72 μm. Mean IS/OS and ELM integrity was 42.2 ± 46.6% and 43.75 ± 45.7%, respectively. The mean age was 40.0 ± 13.5 years with a mean spherical equivalent of 0.18 ± 0.6D for the normal age-matched healthy group. Mean subfoveal CT was 283.1 ± 47.8 μm.
CT at various locations in patients of various ages in the RP group did not show any statistical significant difference (P = ≫0.05) in comparison with age-matched healthy subjects. On multivariate regression, ELM percentage integrity had the strongest association with best corrected visual acuity, followed by IS/OS junction percentage integrity. Subfoveal choroidal thickness had very weak correlation with visual acuity as well other retinal parameters.
There was a significant difference in the outer retinal structure integrity (p = 0.002) and CMT (p = 0.02) between the eyes with good (⩾20/200) and poor vision (<20/200), but not in subfoveal choroidal thickness (p = 0.3).
Conclusions
Our study results did not show any significant difference in choroidal thickness between subjects with RP and age-matched healthy subjects. Choroidal thickness correlated better with the age but not with the vision or outer retinal structures in eyes with RP. Outer retinal structure integrity and CMT had a better correlation with visual acuity.
Keywords: Choroidal thickness, Retinitis Pigmentosa, RP, IS/OS junction, ELM junction
Introduction
Retinitis Pigmentosa (RP) is the most common inherited retinal dystrophy. It starts with nyctalopia and progressively leads to profound visual field loss. The prevalence of RP is 1 in 3000–5000 individuals.1, 2 Sen et al. reported very high prevalence in India including approximately 1 in 930 persons in urban populations and 1 in 372 in rural areas.3
RP is characterized by degeneration of retinal pigment epithelium (RPE), attenuation of retinal vessels, loss of photoreceptors, sclerosis, and atrophy of the choriocapillaris, leading to localized areas of clinically visible chorioretinal atrophy and, consequently, a dramatic reduction in visual function.4, 5
It has been hypothesized and partly proven that there is a primary vascular dysfunction and increased endothelin (ET-1) level in RP patients which ultimately leads to reduced choroidal as well as retinal blood flow.6 Relative choroidal ischemia is increased in severely visually impaired eyes with RP.7 Falsini et al. reported a relationship between the choroidal blood flow and reduced focal electroretinography responses.8 Compromised choroidal circulation leads to choriocapillaris atrophy and eventually damage to photoreceptors.
Measurements of choroidal thickness in RP patients could be very useful for future therapies, such as suprachoroidal electrode arrays, to calculate the distance between the implant and the ganglion cell layer. Thus, a greater understanding of the choroid in diseased eyes is needed.9
Evaluation of the choroid is required to understand the pathogenesis of RP. With the help of enhanced depth imaging, changes in choroidal thickness have been reported in various retinal diseases. Previous studies on choroidal thickness have shown a decrease in choroidal thickness in eyes with RP and no correlation between the choroidal thickness and visual acuity in other inherited retinal dystrophies.10, 11 Correlation between visual acuity and photoreceptor damage has been shown in RP as well as various retinal dystrophies and retinal diseases.12, 13, 14, 15, 16
Dhoot et al. hypothesized that the poor choroidal blood flow leads to thinning of the choroid, which leads to photoreceptor damage.10 Therefore, correlation between the photoreceptor status, visual acuity and choroidal thickness should be analyzed. Available literature on choroidal thickness in RP includes small case series with poor distribution of subjects in various age groups. None of the previous studies have correlated outer retinal structures status with choroidal thickness.
The aim of this study is to compare choroidal thickness between subjects with RP and age-matched healthy subjects and to correlate visual acuity with retinal parameters including central macular thickness (CMT), inner segment/outer segment junction (IS/OS junction) integrity, external limiting membrane (ELM) integrity and choroidal thickness in subjects with RP.
Methods
This prospective observational study was performed at the L.V. Prasad Eye Institute in India from September 2012 to January 2013. Prior approval from the Institutional Review Board was obtained and informed consent was obtained from each study subject. This study was conducted in accordance with the tenets of the Declaration of Helsinki.
Eighty-eight eyes (69 patients) with a diagnosis of typical RP were included in this study. All participants underwent a comprehensive ophthalmic examination including best-corrected visual acuity (BCVA) testing using early treatment diabetic retinopathy study (ETDRS) charts, slit-lamp biomicroscopy, intraocular pressure (IOP) measurement using Goldmann applanation tonometer and a dilated fundoscopic examination. All patients underwent electroretinography and Humphery visual field testing to confirm the diagnosis of RP. Typical RP was diagnosed as the presence of clinical findings such as arteriolar attenuation, midperipheral retinal hyperpigmentation (bone spicules), and atrophy of the RPE in the mid periphery of the retina.
Exclusion criteria included optic atrophy associated with RP, visually significant cataract, high myopia or hyperopia (greater than −6 or +3 diopters of refractive error), poor image quality, any other associated retinal pathology, or history of any intraocular surgery. Control group included healthy subjects with no ocular disease and without high refractive power (more than −6D or +3D).
Choroidal imaging
The spectral domain optical coherence tomography (SD-OCT) scans were obtained by using Cirrus HD-OCT (Carl Zeiss Meditec, Inc., Dublin, CA.) after dilatation of the pupil with 1% Tropicamide and 10% Phenylephrine eye drops. The scan used for imaging in this study is HD 1-line raster with enhanced depth imaging which is a 6-mm line consisting of 4096 A-scans, with an imaging speed of 27,000 A-scans per second, an axial resolution of 5 μm, a transverse resolution of 15 μm in tissue and an average of 20 frames (B-scans). Enhanced depth imaging, which automatically sets the choroid closer to the zero-delay line and thus theoretically provides better visualization of the choroidal/scleral interface, was used for all scans. Scans with a signal strength of greater or equal to 6 were used for analysis.
Image analysis
Choroidal thickness measurement: Using the Cirrus linear measurement tool, a single observer measured choroidal thickness perpendicularly from the outer portion of the hyperreflective line, corresponding to the retinal pigment epithelium, to the inner surface of the sclera at 500 μm intervals temporal and nasal from the fovea, up to 2500 μm as published in the literature.17 Intraclass correlation coefficient for intra-observer reproducibility was 0.97.
Outer retinal structure analysis: For IS/OS and ELM integrity calculation, a previously described technique was used.13 IS/OS and ELM integrity was calculated as the percentage integrity for the central 1000 μm (500 μm on both sides of the fovea) for both horizontal and vertical scans.13 The average of both scans was considered for analysis.
Statistical analysis
Descriptive statistics included mean and standard deviation for continuous variables. As both eyes of most subjects were included for analysis, the correlation between the two eyes of the same subject was adjusted using generalized estimating equations (GEE) during the calculation of summary descriptive parameters. Multivariate models adjusted using GEE methods were fit to assess the effects of age, gender, spherical equivalent and macular thickness on the CT measurements. Statistical analyses were performed using MedCalc for Windows, version 12.5 (MedCalc Software, Ostend, Belgium). The alpha level (type I error) was set at 0.05. All the graphs were made using GraphPad Prism (GraphPad Software, version 6.00 for Windows, La Jolla California, USA, www.graphpad.com).
Results
This study included 88 eyes of 69 subjects with typical RP with a mean age of 31.39 ± 13.4 years (range 13–65 years). Total number of females was 2 (4.3%). Mean BCVA in eyes was 0.99 ± 0.94 logMAR. Mean spherical equivalent was −0.6 ± 1.6D. Mean CMT was 148.48 ± 119 μm. Mean subfoveal choroidal thickness was 296.9 ± 72 μm. Mean IS/OS and ELM integrity was 42.2 ± 46.6% and 43.75 ± 45.7%, respectively.
Among the normal age-matched healthy group, there were 188 eyes of 104 healthy subjects. The mean age was 40.0 ± 13.5 years (range 14–66 years), with 65 (62.5%) females. Mean spherical equivalent in eyes was 0.18 ± 0.6 D. Mean CMT was 217.9 ± 28.8 μm and mean subfoveal CT was 283.1 ± 47.8 μm.
When the choroidal thickness at various locations was compared with choroidal thickness of age-matched healthy subjects, there was no statistical significant difference (P = ≫0.05) (Table 1). The variability in choroidal thickness at various locations in eyes with RP follows a similar pattern as that of age matched controls. There was no significant difference found in subfoveal choroidal thickness between the age-matched healthy subjects and subjects with RP (Table 2 & Fig. 1).
Table 1.
Choroidal thickness (μm) profile at various locations from fovea in subjects with retinitis pigmentosa and age-matched healthy subjects.
| Location | Age-matched healthy subjects | Subjects with retinitis pigmentosa | P value |
|---|---|---|---|
| Nasal_2500 μm | 200 ± 52.1 | 206.7 ± 74.2 | 0.35 |
| Nasal_2000 μm | 207.5 ± 43.4 | 223.6 ± 76.7 | 0.09 |
| Nasal_1500 μm | 216.5 ± 42.6 | 244.6 ± 76.6 | 0.07 |
| Nasal_1000 μm | 223.7 ± 47.1 | 262.4 ± 75.1 | 0.09 |
| Nasal_500 μm | 227.9 ± 56.5 | 275 ± 73.8 | 0.06 |
| Subfoveal | 283.1 ± 47.8 | 296.9 ± 72.8 | 0.06 |
| Temporal_500 μm | 252 ± 46.6 | 281.4 ± 71.3 | 0.1 |
| Temporal_1000 μm | 237.1 ± 44.9 | 269.3 ± 69.7 | 0.09 |
| Temporal_1500 μm | 222.7 ± 45.2 | 256.8 ± 68.4 | 0.1 |
| Temporal_2000 μm | 206.8 ± 44.3 | 241.8 ± 71.7 | 0.07 |
| Temporal_2500 μm | 188.3 ± 42.9 | 225 ± 73.1 | 0.07 |
Table 2.
Subfoveal choroidal thickness between the age-matched healthy subjects and Retinitis Pigmentosa (RP) subjects in various age groups.
| Age groups | Age-matched healthy subjects | RP subjects | P value |
|---|---|---|---|
| 11–20 | 335.7 ± 61.4 | 294.9 ± 43.7 | 0.06 |
| 21–30 | 300.4 ± 70.9 | 295.3 ± 47.8 | 0.72 |
| 31–40 | 281.3 ± 89.4 | 298.3 ± 39.2 | 0.32 |
| 41–50 | 289.8 ± 65.8 | 288.6 ± 44.1 | 0.9 |
| 51–60 | 234 ± 33.1 | 258.1 ± 33.3 | 0.08 |
| 61–70 | 240 ± 33.9 | 231.4 ± 66.9 | 0.86 |
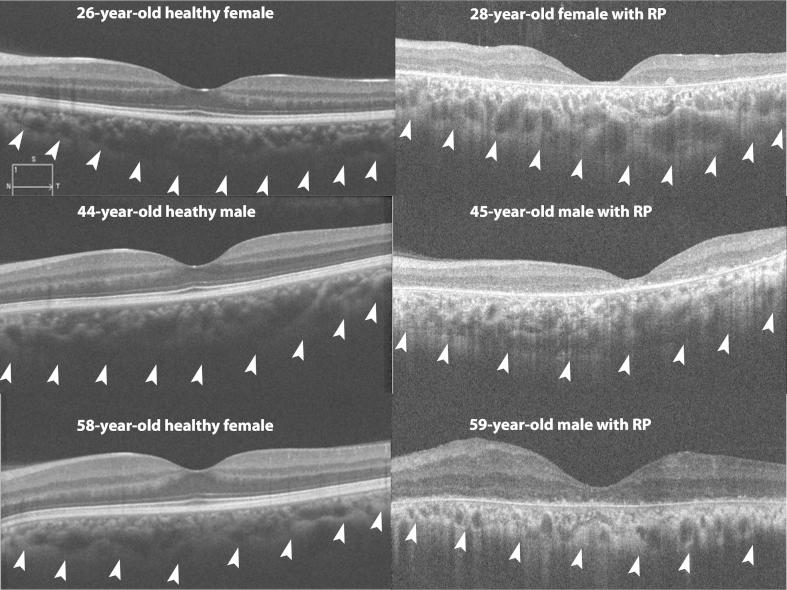
Figure 1.
Composite figure shows choroidal scans of subjects with Retinitis Pigmentosa (RP) and age-matched healthy subjects. Top left image shows choroidal scan of a 26-year-old healthy female with subfoveal choroidal thickness (SFCT) of 280 μm, which is comparable to choroidal thickness of 320 μm in a 28-year-old female with Retinitis Pigmentosa (RP) (top right image). Middle left image shows choroidal scan of a 44-year-old healthy male with SFCT of 340 μm, which is comparable to choroidal thickness of 380 μm in a 28-year-old male with RP (middle right image). Bottom left image shows choroidal scan of a 58-year-old healthy female with SFCT of 278 μm, which is comparable to choroidal thickness of 290 μm in a 59-year-old male with RP (bottom right image).
Using multivariate regression, we assessed the correlation between visual acuity and various factors such as CMT, subfoveal choroidal thickness, IS/OS and ELM damage. We found that IS/OS (r = 0.52, p = 0.0001) and ELM (r = 0.54, p = 0.001) damage and CMT (r = 0.38, p = 0.0001) had a good correlation with visual acuity, however, subfoveal choroidal thickness (r = 0.13, p = 0.0019) had a very weak correlation with visual acuity. Among these factors ELM percentage integrity had the strongest association with BCVA, followed by IS/OS junction percentage integrity.
The relationship between subfoveal choroidal thickness and variables such as CMT (r = 0.14, p = 0.17), IS/OS damage (r = 0.14, p = 0.21) and ELM damage (r = 0.14, p = 0.20) was not significant. Among these factors, age (r = 0.36, p = 0.001) had the strongest association with subfoveal choroidal thickness (negative correlation).
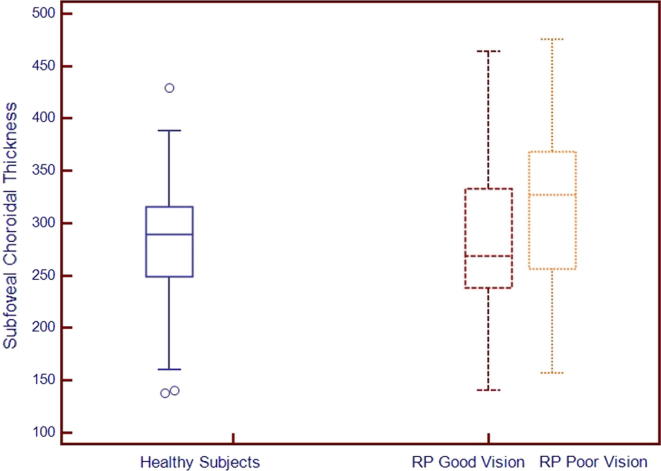
We divided subjects into two groups: group 1 (good visual acuity, equal or better than 20/200) and group 2 (poor visual acuity, worse than 20/200). Comparative analysis of groups is shown in Table 3. There was a significant difference in the outer retinal structure integrity (p = 0.002) and CMT (p = 0.02) between the groups. Regarding subfoveal thickness, there is no significant difference between the groups (p = 0.3). Fig. 2 shows subfoveal choroidal thickness variations among groups; age-matched healthy subjects, RP subjects with good vision and RP subjects with poor vision.
Table 3.
Comparative analysis of group 1 (BCVA ⩾ 20/200) and group 2 (BCVA < 20/200).
| Characteristics | Group 1 (BCVA ⩾ 20/200) | Group 2 (BCVA < 20/200) |
|---|---|---|
| Number of eyes (patients) | 58 (47) | 30 (22) |
| Male: female | 46:1 | 21:1 |
| Mean age (years) | 34.0 ± 13.8 years | 26.0 ± 11.1 (p = 0.07) |
| Mean BCVA (logMAR) | 0.43 ± 0.22 | 2.07 ± 0.8 (p = 0.01) |
| Mean spherical equivalent | −0.78 ± 1.54D | −0.38 ± 1.7D (p = 0.1) |
| Mean central macular thickness (μm) | 171.48 ± 139 | 103.6 ± 41 (p = 0.03) |
| Mean IS/OS junction percentage damage | 40.2 ± 54.5% | 92.3 ± 75.9 (p = 0.000) |
| Mean ELM percentage damage | 38.4 ± 56.7% | 91.3 ± 73.4 (p = 0.000) |
| Mean subfoveal thickness (μm) | 287.3 ± 72 | 315 ± 71.4 (p = 0.06) |
Figure 2.
Comparison of subfoveal choroidal thickness between age-matched healthy subjects and subjects with retinitis pigmentosa with good (⩾20/200) and poor vision (<20/200).
Discussion
We did not find any evidence of choroidal thinning in eyes with RP; the change was well correlated with age-matched healthy subjects. Subfoveal choroidal thickness did not correlate with visual acuity in eye with RP, a finding similar to previous reports.10, 11 Visual acuity did correlate with CMT and outer retinal structure integrity. However, when we looked at the correlation between the subfoveal choroidal thickness and outer retinal structure, we found that the correlation was very weak (0.14). Among the various factors including age, CMT, IS/OS junction integrity and ELM integrity, age was the only factor, which correlated with subfoveal choroidal thickness. Therefore, we believe that a change in choroidal thickness in RP subjects corresponds to age related change.
We analyzed RP subjects with poor and good visual acuity separately, to understand the reason for vision loss. This subgroup analysis clearly showed that CMT and outer retinal structure integrity were the only two factors, which were significantly different in the two groups. There was no difference in terms of subfoveal thickness among the groups. This finding also supports that the visual acuity has better correlation with outer retinal structure rather than choroidal thickness.
Dhoot et al.10 reported reduced choroidal thickness in 21 patients with RP compared to 25 healthy individuals. The major limitation in their study was a very small sample size with poor distribution of patients in various age groups. Adhi et al.18 reported decreased choroidal thickness in 14 RP subjects with a significance of p = 0.04. Our study results differ from previous reports, which could be due to a large sample size with even distribution of RP subjects in age groups compared to age-matched controls.
Ayton et al.19 reported a decrease in choroidal thickness in 42 RP subjects compared to age-matched healthy individuals. They reported a significant correlation between choroidal thickness and visual acuity as well as duration of the disease. They reported thinner choroid in poor visual acuity and longer duration of the disease. However, no correlation of the disease duration was found with visual acuity. We did not include disease duration, as it is very difficult to assess accurate disease duration for RP. The patient, according to his/her symptoms, reports the duration of the disease.
Ocular blood flow in RP patients is reduced not only in the retina and choroid but also in retrobulbar vessels. RP has a clear genetic background; however, role of reduced blood flow leading to retinal atrophy has been established.20 An increase in the endothelin-1 level in the eye has been reported to reduce ocular blood flow in RP subjects especially in choroid.21 Reduced choroidal blood flow could be the cause for photoreceptor damage. In particular, choriocapillaris assessment may be beneficial to explain the damage to the photoreceptor layer; however, assessing a quantitative change in choriocapillaris with available imaging modalities may not be possible. The presence of choroidal thickening or thinning on SD-OCT may not accurately assess changes in the change of choriocapillaris thickness.
The limitations of our study include its cross-sectional nature, lack of follow-up data which may elucidate the role of choroidal thickness in RP. There were only two females in the RP group, which could have affected the results. We did not perform morphological evaluation of the choroid, which may improve understanding of choroidal changes in this disease profile. Strengths of our study include large sample size, age-matched controls, and multifactorial correlation including outer retinal structures.
In conclusion, there was no significant difference in subjects with RP and age-matched healthy subjects. Choroidal thickness correlated better with the age but not with the vision or outer retinal structures in eyes with RP. Outer retinal structure integrity and CMT had a better correlation with visual acuity. Choroidal thickness may be the crude measure to assess change in choroidal blood flow or damage to choriocapillaris. Morphological evaluation of choroidal structure and correlation with choroidal blood flow in future studies may provide the missing links in pathogenesis of this multifactorial blinding disorder.
Conflict of interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Haim M., Holm N.V., Rosenberg T. Prevalence of retinitis pigmentosa and allied disorders in Denmark. I Main results. Acta Ophthalmol (Copenh) 1992;70:178–186. doi: 10.1111/j.1755-3768.1992.tb04121.x. [DOI] [PubMed] [Google Scholar]
- 2.Berson E.L. Retinitis pigmentosa. The friedenwald lecture. Invest Ophthalmol Vis Sci. 1993;34:1659–1676. [PubMed] [Google Scholar]
- 3.Sen P., Bhargava A., George R. Prevalence of retinitis pigmentosa in South Indian population aged above 40 years. Ophthalmic Epidemiol. 2008;15:279–281. doi: 10.1080/09286580802105814. [DOI] [PubMed] [Google Scholar]
- 4.Heckenlively J.R., Yoser S.L., Friedman L.H., Oversier J.J. Clinical findings and common symptoms in retinitis pigmentosa. Am J Ophthalmol. 1988;105:504–511. doi: 10.1016/0002-9394(88)90242-5. [DOI] [PubMed] [Google Scholar]
- 5.Shintani K., Shechtman D.L., Gurwood A.S. Review and update: current treatment trends for patients with retinitis pigmentosa. Optometry. 2009;80:384–401. doi: 10.1016/j.optm.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Cellini M., Strobbe E., Gizzi C., Campos E.C. ET-1 plasma levels and ocular blood flow in retinitis pigmentosa. Can J Physiol Pharmacol. 2010;88:630–635. doi: 10.1139/Y10-036. [DOI] [PubMed] [Google Scholar]
- 7.Langham M.E., Kramer T. Decreased choroidal blood flow associated with retinitis pigmentosa. Eye (London) 1990;4(Pt 2):374–381. doi: 10.1038/eye.1990.50. [DOI] [PubMed] [Google Scholar]
- 8.Falsini B., Anselmi G.M., Marangoni D. Subfoveal choroidal blood flow and central retinal function in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011;52:1064–1069. doi: 10.1167/iovs.10-5964. [DOI] [PubMed] [Google Scholar]
- 9.Wilke R., Gabel V.P., Sachs H. Spatial resolution and perception of patterns mediated by a subretinal 16-electrode array in patients blinded by hereditary retinal dystrophies. Invest Ophthalmol Vis Sci. 2011;52:5995–6003. doi: 10.1167/iovs.10-6946. [DOI] [PubMed] [Google Scholar]
- 10.Dhoot D.S., Huo S., Yuan A. Evaluation of choroidal thickness in retinitis pigmentosa using enhanced depth imaging optical coherence tomography. Br J Ophthalmol. 2013;97:66–69. doi: 10.1136/bjophthalmol-2012-301917. [DOI] [PubMed] [Google Scholar]
- 11.Yeoh J., Rahman W., Chen F. Choroidal imaging in inherited retinal disease using the technique of enhanced depth imaging optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2010;248:1719–1728. doi: 10.1007/s00417-010-1437-3. [DOI] [PubMed] [Google Scholar]
- 12.Chhablani J., Kim J.S., Freeman W.R. Predictors of visual outcome in eyes with choroidal neovascularization secondary to age related macular degeneration treated with intravitreal bevacizumab monotherapy. Int J Ophthalmol. 2013;6:62–66. doi: 10.3980/j.issn.2222-3959.2013.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chhablani J.K., Kim J.S., Cheng L. External limiting membrane as a predictor of visual improvement in diabetic macular edema after pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2012;250:1415–1420. doi: 10.1007/s00417-012-1968-x. [DOI] [PubMed] [Google Scholar]
- 14.Mitamura Y., Hirano K., Baba T., Yamamoto S. Correlation of visual recovery with presence of photoreceptor inner/outer segment junction in optical coherence images after epiretinal membrane surgery. Br J Ophthalmol. 2009;93:171–175. doi: 10.1136/bjo.2008.146381. [DOI] [PubMed] [Google Scholar]
- 15.Wakabayashi T., Sawa M., Gomi F., Tsujikawa M. Correlation of fundus autofluorescence with photoreceptor morphology and functional changes in eyes with retinitis pigmentosa. Acta Ophthalmol. 2010;88:e177–183. doi: 10.1111/j.1755-3768.2010.01926.x. [DOI] [PubMed] [Google Scholar]
- 16.Testa F., Rossi S., Sodi A. Correlation between photoreceptor layer integrity and visual function in patients with Stargardt disease: implications for gene therapy. Invest Ophthalmol Vis Sci. 2012;53:4409–4415. doi: 10.1167/iovs.11-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita T., Shirasawa M., Arimura N. Repeatability and reproducibility of subfoveal choroidal thickness in normal eyes of Japanese using different SD-OCT devices. Invest Ophthalmol Vis Sci. 2012;53:1102–1107. doi: 10.1167/iovs.11-8836. [DOI] [PubMed] [Google Scholar]
- 18.Adhi M., Regatieri C.V., Branchini L.A. Analysis of the morphology and vascular layers of the choroid in retinitis pigmentosa using spectral-domain OCT. Ophthalmic Surg Lasers Imag Retina. 2013;44:252–259. doi: 10.3928/23258160-20130503-08. [DOI] [PubMed] [Google Scholar]
- 19.Ayton L.N., Guymer R.H., Luu C.D. Choroidal thickness profiles in retinitis pigmentosa. Clin Exp Ophthalmol. 2013;41:396–403. doi: 10.1111/j.1442-9071.2012.02867.x. [DOI] [PubMed] [Google Scholar]
- 20.Konieczka K., Flammer A.J., Todorova M. Retinitis pigmentosa and ocular blood flow. EPMA J. 2012;3:17. doi: 10.1186/1878-5085-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polak K., Petternel V., Luksch A. Effect of endothelin and BQ123 on ocular blood flow parameters in healthy subjects. Invest Ophthalmol Vis Sci. 2001;42:2949–2956. [PubMed] [Google Scholar]