Abstract
Although the prevalence of chronic fatigue is approximately 20% in healthy individuals, there are no studies of brain structure that elucidate the neural correlates of fatigue outside of clinical subjects. We hypothesized that fatigue without evidence of disease might be related to changes in the basal ganglia and prefrontal cortex and be implicated in fatigue with disease. We aimed to identify the white matter structures of fatigue in young subjects without disease using magnetic resonance imaging (MRI). Healthy young adults (n = 883; 489 males and 394 females) were recruited. As expected, the degrees of fatigue and motivation were associated with larger mean diffusivity (MD) in the right putamen, pallidus and caudate. Furthermore, the degree of physical activity was associated with a larger MD only in the right putamen. Accordingly, motivation was the best candidate for widespread basal ganglia, whereas physical activity might be the best candidate for the putamen. A plausible mechanism of fatigue may involve abnormal function of the motor system, as well as areas of the dopaminergic system in the basal ganglia that are associated with motivation and reward.
The prevalence of chronic fatigue is approximately 20% in some developed countries1 and more than 33% in Japan2. Chronic fatigue is sometimes irreversible, and the compensation mechanisms that are useful in reducing acute fatigue are not effective3. Chronic fatigue has been associated with an increase in traffic accidents due to inattention, and contributes to mental health issues, such as depression, burnout syndrome4, and karoshoi (death due to overworking)5. A younger age was related to fatigue levels in non-clinical samples that included Australian patients aged 18 to 70 years6. A high prevalence of fatigue was also demonstrated among graduate students in Taiwan (46.6%)7. Fatigue can have numerous implications on an individual’s health and well-being; thus, it is important to elucidate the mechanisms of fatigue in young adults.
Fatigue may be defined as the failure to initiate and sustain attention-oriented tasks and physical activities requiring self-motivation8. A loss of motivational influence from striato–thalamic inputs to the frontal lobe is integral to the development of fatigue8,9. Moreover, the evaluation of predicted rewards and energy costs might be central to the phenomenon of mental fatigue9. When tasks must be performed for a prolonged period, the amount of energy that must be invested in performing the task increases compared to the potential rewards, resulting in a decrease in motivation9. Dopamine is involved in the control of motivational processes10 and reward-seeking behaviour11. Thus, a disruption within the dopamine system has been proposed as a common mechanism underlying fatigue12.
Previous functional imaging studies of fatigue with healthy individuals have predominantly addressed the relationship between certain brain regions and the subjective feeling of acute fatigue13,14,15,16. In those studies, neural activity during attention-demanding tasks decreased in the ventrolateral prefrontal cortex (PFC)14 and the posterior parietal cortex15 but increased in the cerebellar, temporal, cingulate and frontal regions15 and in the medial orbitofrontal cortex16. Functional imaging studies in patients with chronic fatigue syndrome and multiple sclerosis have suggested the basal ganglia and frontal lobes play a role in chronic fatigue17. As far as we know, all prior studies of brain structure and fatigue are clinical studies that examined patients with chronic fatigue syndrome18,19, multiple sclerosis20,21, Parkinson’s disease22 or fibromyalgia23. These studies identified a direct relationship between brain structure and the degree of fatigue. It was demonstrated that the degree of fatigue showed a relationship with a decline in total grey matter volume linked to a reduction in physical activity (an element of fatigue)18, white matter decreases in the midbrain19, decreases in tissue perfusion in the deep grey matter20, cortical atrophy of the parietal lobe21, and lower grey matter density in the left supplementary motor area23. However, no significant correlation was identified between brain structure and the severity of apathy (a symptom similar to fatigue) in patients with Parkinson’s disease22. Interestingly, the combination of multimodal magnetic resonance imaging (MRI), such as mean diffusivity (MD) and fractional anisotropy (FA), has been shown to be the best discriminator between patients with Parkinson’s disease and healthy controls24.
Although fatigue is common during adolescence25, there are few studies that have examined fatigue in younger populations8,26. However, to our knowledge, there are no studies that have attempted to identify the anatomical correlates of fatigue in individuals outside of clinical subjects. Structural imaging studies are suitable measures for investigating the neural correlates of fatigue because the results of such studies are not limited to the regions engaged in a specified task.
In this study, we used voxel-based morphometry (VBM) to assess regional grey matter density (rGMD) and regional white matter density (rWMD) to identify the neural correlates of fatigue. We also used voxel-based FA for diffusion tensor imaging (DTI)27 to assess whether WM structural integrity is associated with fatigue. FA is interpreted as an indicator of WM pathway strength or integrity27. In addition, we used MD to examine white matter in healthy subjects to determine the neural correlates of fatigue. There are three diffusivities, i.e., the diffusion coefficient along the direction of maximal diffusion (axial diffusivity; λ1) and the diffusion coefficients along two orthogonal directions embedded in the plane perpendicular to the main diffusion direction (λ2 and λ3)28. The average diffusivity of λ1, λ2 and λ3, known as MD, can be inferred from the overall dimensions of the diffusion ellipsoid28. MD, which is another measure of DTI, is the rate of diffusivity and a direction-independent measure of the average diffusivity reflecting water motility in a voxel. Reduction of MD is considered to reflect tissue changes caused by neural plasticity, which include astrocyte swelling, synaptic changes, dendritic spine changes, and angiogenesis29,30. Accordingly, we hypothesized that the degree of fatigue without disease may be related to basal ganglia and prefrontal cortex (PFC) function, and this relationship can be demonstrated using rGMD, rWMD, FA and especially MD in clinical cases with fatigue8. The purpose of this study was to identify the anatomical correlates of fatigue in young people without mental or physical disease.
Results
Behavioural data
Table 1 shows the mean and standard deviation (SD) for age, Raven’s advanced progressive matrix scores and Checklist Individual Strength Questionnaire (CIS) scores for study participants. Figure 1 shows the distributions of CIS scores in men and women. Between men and women, there was a significant difference in Raven’s Advanced Progressive Matrix (RAPM) scores (p < 0.05, one-way analysis of variance [ANOVA]), but not in CIS scores (p = 0.089). As the data in presented in Table 2 indicate, the scores for all four elements of fatigue (subjective feeling of fatigue, concentration, motivation and physical activity) were significantly and positively correlated to one another (p < 0.05, two-tailed corrected using the Bonferroni method).
Table 1. Sex differences in age and scores on the RAPM and CIS; and one-way ANOVA results.
| Measure | Male |
Female |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age | 20.79 | 1.94 | 20.56 | 1.63 |
| RAPM | 28.89 | 3.66 | 28.06 | 3.78 |
| CIS | 70.37 | 17.52 | 68.34 | 11.77 |
Abbreviations: CIS, the Checklist Individual Strength Questionnaire; RAPM, Raven’s Advanced Progressive Matrix; SD, standard deviation.
Figure 1. Distribution of CIS scores for men and women (n = 883).
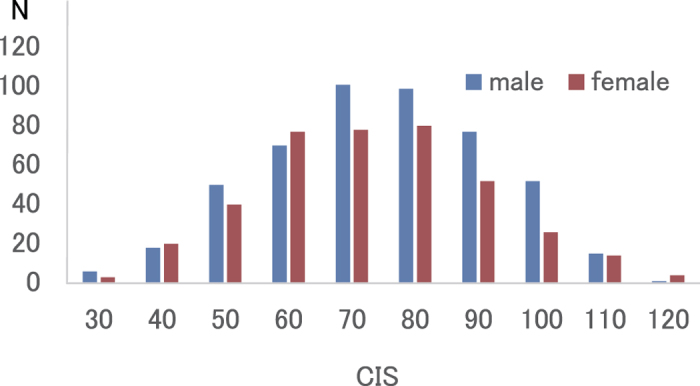
Histograms show the distributions of CIS scores for men and women. Abbreviations: CIS, Checklist Individual Strength Questionnaire.
Table 2. Pearson’s correlation coefficients among the four fatigue elements.
| Feeling | Concentration | Motivation | Physical activity | VIF | |
|---|---|---|---|---|---|
| Feeling | – | 1.204 | |||
| Concentration | 0.391* | – | 1.654 | ||
| Motivation | 0.273* | 0.445* | – | 1.498 | |
| Physical activity | 0.310* | 0.573* | 0.551* | – | 1.786 |
Abbreviations: feeling, subjective feeling of fatigue; VIF, variance inflation factor.
*p < 0.001 (two-tailed correction using the Bonferroni method).
MRI data
Analysis of VBM
We found no significant correlations between CIS scores and rGMD or rWMD.
Analysis of FA
We found no significant correlations between CIS scores and FA.
Analysis of MD
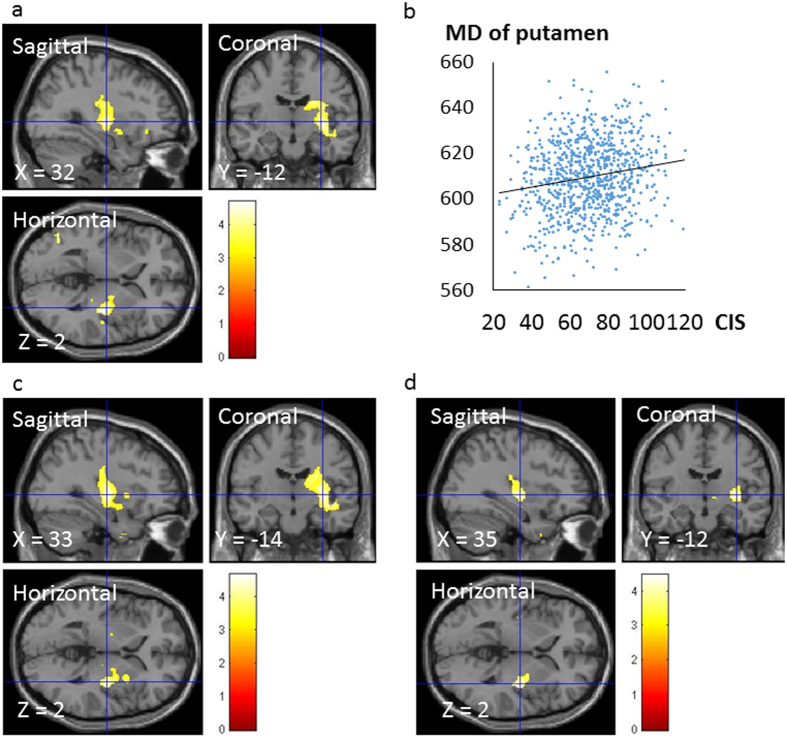
A whole-brain multiple regression analysis that controlled for sex, age, RAPM and both rGMD and regional cerebrospinal fluid density (rCSFD) at each voxel revealed a significant positive correlation between CIS scores and MD at areas corresponding to the right putamen that spanned from the palladium to the caudate (x, y, z = 32, −12, 2; t = 4.69; p < 0.001, k = 2449; corrected for multiple comparisons at the cluster with a cluster-determining threshold of p < 0.001, uncorrected) (Fig. 2a,b). Significant positive correlations were detected between the motivation subscores in areas corresponding to the right putamen that spanned from the palladium to the caudate (x, y, z = 33, −14, 2; t = 4.68; p < 0.001, k = 3503; corrected for multiple comparisons at the cluster with a cluster-determining threshold of p < 0.001, uncorrected) (Fig. 2c). Significant positive correlations were also observed between the physical activity subscores in areas corresponding to the right putamen (x, y, z = 35, −12, 2; t = 4.47; p < 0.001, k = 651; corrected for multiple comparisons at the cluster with a cluster-determining threshold of p < 0.001, uncorrected) (Fig. 2d).
Figure 2. Regions showing a correlation between MD and scores on CIS, motivation and physical activity subscores.
The red-to-yellow colour scale indicates the t-score for the positive correlation between MD and CIS scores (p < 0.001, uncorrected). Regions showing correlations were overlaid on a single T1 image in the SPM5 toolbox. Areas of significant correlations are shown in the right putamen (a). Scatter plots illustrating the relationship between mean MD and CIS scores (b). A cluster with significant correlations was seen in the right putamen and spanned from the palladium to the caudate. We set the statistical significance of analyses at p < 0.05, which was corrected for multiple comparisons at the adjusted cluster level with an underlying voxel level of p < 0.001, uncorrected. The areas of the significant correlations are shown in the right putamen when the red-to-yellow colour scale indicates the t-score for the positive correlation between MD and the motivation subscore (p < 0.001, uncorrected) (c). The areas of significant correlations are shown in the right putamen when the red-to-yellow colour scale indicates the t-score for the positive correlation between MD and physical activity subscore (p < 0.001, uncorrected) (d). Abbreviations: CIS, Checklist Individual Strength Questionnaire; MD, mean diffusivity.
Discussion
To our knowledge, this study is the first to investigate an association between fatigue and brain structures in healthy individuals at the whole-brain level. Consistent with our hypothesis, we found that fatigue scores were associated with larger MD values (but not for rGMD or rWMD) in the basal ganglia, which included the putamen, pallidus and body of the caudate. That is, the degree of fatigue without disease was associated with changes in the basal ganglia, thus implicating an altered brain structure as a cause of fatigue in clinical cases. Furthermore, motivation was the best candidate for widespread basal ganglia, whereas physical activity might be the best candidate for the putamen.
First, we should discuss the mechanism through which the basal ganglia, including the putamen, pallidus and body of the caudate, are closely related to fatigue. As explained in the introduction, motivation and reward are related to fatigue8,12,31. Humans and animals will quickly take action when they expect the action will lead to a reward, and this action reflects their motivation10. In the basal ganglia, the percentage of tonically active neurons that respond to an action is higher in the putamen than in the caudate nucleus, especially in anticipation of a reward32. The putamen has been implicated primarily in motor control and learning habits and skills33,34. Activation of the ventral pallidum can lead to reward and enhanced motivation via phasic bursts of excitation in response to an incentive or hedonic stimuli35. The vigour scale of the Profile of Mood States (POMS) appears to be the most widely used and accepted measure of the energy mood state, and it is also a valid measure for nutrition-related research, such as studies of caffeine intake36. Among the mood states included in the POMS, only factors related to vigour were negatively correlated with MD in widespread regions that included the putamen, pallidus and body of the caudate37. Hence, insufficient functioning of the putamen and ventral pallidum leads to a loss of motivation and physical activity.
Second, our aforementioned findings show two possibilities (i.e., cause and effect). One finding is that a naturally higher MD (lower neuronal density) in the basal ganglia reflects a dysfunction of the basal ganglia that might cause fatigue (cause). Furthermore, the putamen, ventral pallidum and body of the caudate are associated with a loss of reward and motivation and are therefore related to fatigue. If these automatic functions are disrupted, then additional energy might be required to execute complex motor programmes, and a subsequent loss of motivation could occur. Hence, dysfunctions of the basal ganglia might lead to fatigue. This idea is consistent with the theory that fatigue results from a failure to integrate limbic inputs and motor functions in the basal ganglia, which subsequently affects the striatal–thalamic–frontal cortical system8. The other possibility is that fatigue affects the structure of the putamen (effect); fatigue may increase MD (decreasing neuronal density) in the basal ganglia. This idea is consistent with the fact that prolonged stress produces opposing effects on structural plasticity, notably the growth of dendrites and spines in the amygdala38, because repetitive and prolonged stress seems to cause fatigue39.
We should explain the mechanism through which MD alone could detect the neural correlates of fatigue. In the basal ganglia, MD showed a positive relationship with fatigue. From the molecular point of view, an increased MD, which is an increased water diffusivity measured on MRI, is related to a decreased tortuosity and increased volume fraction of the fast diffusivity extracellular compartment. Interestingly, the combination of MD, FA and R2* (inverse of relaxation times, i.e., relaxation rates values) in the dopaminergic system has been shown to be the best discriminator between patients with Parkinson’s disease and healthy controls24. There was a significant negative correlation between dopamine synthesis capacity and MD in the posterior caudate and putamen using MD with positron emission tomography (PET)40. Assuming that MD reflects the density of widespread axonal terminals in the striatum, dopamine synthesis may be related to the density of dopaminergic neuronal fibres40. Hence, MD could detect neural plasticity, especially in the dopaminergic system. Further, we could speculate the neural mechanism of fatigue based on two important aspects of fatigue, i.e., motivation and reward8,9. When a reward is greater than expected, the firing rates of certain dopaminergic neurons increase, which consequently increases motivation for the reward11. Interestingly, Dobryakova et al. reported that dopamine may have an important role in fatigue and suggested that fatigue results from disruption of communication between the striatum and PFC41. Thus, MD seems to be more sensitive for identifying the neural correlates of fatigue than rGMD, rWMD or FA because the relationships among fatigue, motivation and reward are based on the dopaminergic system. However, in present report, we can only speculate on the relationships among fatigue, motivation, reward and dopamine functioning in the basal ganglia because dopamine was not measured in the present study. Future studies involving more direct measures of dopamine functioning, such as PET, should examine these relationships.
Finally, there are a few limitations of this study that should be mentioned. Because the present study used a cross-sectional design, the results cannot be used to determine the causality between fatigue and the basal ganglia. Thus, to overcome this limitation, a prospective study that confirms such causality is necessary. Furthermore, we used young healthy subjects who possessed high levels of education, and such individuals might be more likely to demonstrate a high degree of plasticity.
In conclusion, fatigue without disease might result from changes in the basal ganglia, which therefore implicates the basal ganglia in fatigue in clinical cases. A plausible mechanism of fatigue may involve motivation and physical activity for maintaining performance. The neural correlates of fatigue in non-clinical and clinical subjects might overlap.
Methods
Subjects
Eight hundred and eighty-three healthy, right-handed individuals (489 males and 394 females) participated in this study. The present study was a part of our ongoing project to investigate associations among brain imaging data, cognitive function, aging, genetics and daily habits. The mean age of the subjects was 20.7 years (SD, 1.81). All of the subjects in our study were university or post-graduate students with normal vision, no history of neurological or psychiatric illness, and no report of recent psychoactive or antipsychotic drug use. Handedness was evaluated using the Edinburgh Handedness Inventory42. Written informed consent was obtained from each subject for the projects in which they participated. The procedures for all studies were approved by the Ethics Committee of Tohoku University. All experiments were performed in accordance with the approved guidelines. For more details regarding the study procedures, see the Supplemental Methods.
Psychological outcome measures
Fatigue assessment
The CIS, which was developed by Vercoulen et al.43, is the most frequently used fatigue questionnaire worldwide43,44. Further, the questionnaire has been used in patients other than those who suffer from chronic fatigue syndrome45,46 and in healthy populations that included graduate students26 and working individuals44,47. The CIS is divided into four dimensions: subjective feeling of fatigue, motivation, activity and concentration. The CIS consists of 20 statements. Examples of these statements are as follows: “I feel tired”, “I do quite a lot within a day”, “I feel very active” and “I can concentrate well”. The total score for the CIS is an index of fatigue44,47, with a higher score indicating a higher degree of fatigue. In the present study, participants were asked to rate any subjective symptoms that they perceived themselves as having during the previous 2 weeks using a rating scale that ranged from 1 to 7. Participants were administered the Japanese version of the CIS, which was translated into Japanese by Aratake et al.47. Using a cut-off score of 76, as suggested by a previous study48, 317 participants (35.9%; men: 190, 38.9%; women: 127, 32.2%) were regarded as possible chronic fatigue. For more details regarding the study procedures, see the Supplemental Methods.
Assessment of psychometric measures of general intelligence
The RAPM, which is the best measure of general intelligence49, was used and adjusted to examine the effect of general intelligence on brain structures50,51,52,53,54. This measure was also used to exclude the possibility that a significant correlation between MD and CIS scores was caused by (a) an association between CIS scores and general intelligence or (b) an association between MD and general intelligence.
Behavioural data analyses
Behavioural data were analysed with the IBM SPSS Statistics 22.0 software package (IBM Corp.; Armonk, NY, USA). Differences between men and women in age and the scores for cognitive measures (RAPM and CIS) were analysed with one-way ANOVA. A two-tailed p value <0.05 was considered to indicate statistical significance. We also used Pearson’s correlation coefficient to test for correlations among feelings of fatigue, concentration, motivation, physical activity scores and MD in the significant cluster in relation to the CIS scores. A two-tailed p value <0.05 that was corrected using the Bonferroni method was deemed statistically significant.
Image acquisition
MRI data were acquired using a 3T Philips Achieva scanner.
Scan for VBM
Three-dimensional, high-resolution, T1-weighted images (T1WI) were collected using a magnetisation-prepared rapid gradient-echo (MPRAGE) sequence. The parameters were as follows: 240 × 240 matrix, TR = 6.5 ms, TE = 3 ms, TI = 711 ms, FOV = 24 cm, 162 slices, in plane resolution = 1.0 × 1.0 mm, slice thickness = 1.0 mm and scan duration of 483 s.
Scan for FA and MD
Diffusion-weighted data were acquired using a spin-echo EPI sequence (TR = 10293 ms, TE = 55 ms, FOV = 22.4 cm, 2 × 2 × 2 mm3 voxels, 60 slices, SENSE reduction factor = 2, number of acquisitions =1). The diffusion weighting was isotropically distributed along 32 directions (b value = 1,000 s/mm2). For more details regarding these procedures, see the Supplemental Methods.
Pre-processing and analysis of structural data
VBM
Pre-processing of the MRI data was performed using Statistical Parametric Mapping software (SPM12; Wellcome Department of Cognitive Neurology, London, UK) and following the protocol described for VBM analysis in our previous report55. For more details regarding these procedures, see the Supplemental Methods.
FA and MD
Pre-processing and analysis of imaging data were performed using SPM8 implemented in MATLAB. FA and MD maps were calculated from the collected images using a commercially available diffusion tensor analysis package on the MR consol. These procedures involved correction for motion and distortion caused by eddy currents56. Calculations were performed according to a previously described method27. For more details regarding these procedures, see the Supplemental Methods.
Statistical group-level analysis of imaging and behavioural data
The whole-brain multiple regression analysis assessed the association between rGMD and CIS scores using SPM12. The whole-brain multiple regression analysis was performed using SPM12 and assessed the relationship between rWMD and CIS scores. The whole-brain multiple regression analysis was performed using SPM8 and assessed the relationship between FA and CIS scores. The covariates included sex, age, RAPM scores and total intra-cranial brain volume (TIV: total GM volume + total WM volume + total cerebrospinal fluid volume). For each covariate, the “overall mean” was used for mean centring.
For analyses involving MD, we used the biological parametric mapping (BPM) toolbox57, which is an extension software of SPM5, the latest available version for the BPM toolbox (Wellcome Department of Cognitive Neurology, London). Using the BPM toolbox, we performed multimodality voxel-wise multiple regression analyses adjusted for the effects of rGMD and rCSFD to investigate associations between MD and CIS scores. These values were adjusted to exclude the possibility that the extent of GM, WM or CSF itself affected the results rather than MD (in the areas analysed, tissues were either GM, WM or CSF; thus, regressing out the effects of the rGMD or rCSFD should address these issues). The BPM toolbox can perform multiple regression analyses using multimodal images. We performed a voxel-by-voxel regression analysis, and in this analysis, the dependent variable at each voxel was the MD value at that voxel. The independent variables included the rGMD value and the rCSFD map at that voxel, as well as age, sex, RAPM score and the CIS score. The analyses were limited to areas within the grey + white matter mask that was created using the procedures described above. Three of the Pearson’s correlation coefficients among the four subscales were >0.5. Accordingly, multicollinearity may be doubted among the four subscales based on the multiple regression analysis, which can have severe effects on parameter estimates. However, we performed voxel-by-voxel regression analyses using the same covariates and added the four CIS subscales simultaneously, rather than the total CIS score. No significant MD was related to any of the CIS subscores. Furthermore, we also performed four voxel-by-voxel regression analyses using the same covariates mentioned above and added each CIS subscore in turn, rather than the total CIS score, to determine which subscore was the best candidate for the region. We set the statistical significance for these analyses at p < 0.05, and corrected for multiple comparisons at the adjusted cluster level with an underlying voxel level of p < 0.001, uncorrected.
Additional Information
How to cite this article: Nakagawa, S. et al. Basal ganglia correlates of fatigue in young adults. Sci. Rep. 6, 21386; doi: 10.1038/srep21386 (2016).
Supplementary Material
Acknowledgments
Dr. S. N. was supported by Health Science Center Foundation (H25). Dr. H.T. was supported by JST/RISTEX, JST/CREST, a Grant-in-Aid for Young Scientists (B) (KAKENHI 23700306), a Grant-in-Aid for Young Scientists (A) (KAKENHI 25700012) from the Ministry of Education, Culture, Sports, Science, and Technology, and Health Science Center Foundation. We respectfully thank Yuki Yamada for operating the MRI scanner and Haruka Nouchi for administering the psychological tests. Furthermore, we would like to thank the participants in the study, the other individuals who administered psychological tests, and our colleagues in the Institute of Development, Ageing and Cancer at Tohoku University for their support.
Footnotes
Author Contributions S.N. H.T., Y.T. and R.K. designed the study. S.N., H.T., R.N., Y.K., T.S., T.M., A.S., K.I., R.Y., Y.Y., S.H., T.A., C.M.M., D.M., K.S., H.J., and Y.S. collected the data. S.N. and H.T. analyzed the data and prepared the manuscript. All authors reviewed the manuscript.
References
- Pigeon W. R., Sateia M. J. & Ferguson R. J. Distinguishing between excessive daytime sleepiness and fatigue: toward improved detection and treatment. J Psychosom Res 54, 61–69 (2003). [DOI] [PubMed] [Google Scholar]
- Watanabe T., Evengård B., Natelson B. H., Jason L. A. & Kuratsune H. Preface and mini-review: fatigue science for human health. 5–11 (Springer, 2007). [Google Scholar]
- Mizuno K. et al. Mental fatigue caused by prolonged cognitive load associated with sympathetic hyperactivity. Behav Brain Funct 7, 17 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armon G., Melamed S., Toker S., Berliner S. & Shapira I. Joint effect of chronic medical illness and burnout on depressive symptoms among employed adults. Health Psychol 33, 264–272 (2014). [DOI] [PubMed] [Google Scholar]
- Hiyama T. & Yoshihara M. New occupational threats to Japanese physicians: karoshi (death due to overwork) and karojisatsu (suicide due to overwork). Occup Environ Med 65, 428–429 (2008). [DOI] [PubMed] [Google Scholar]
- Thorsteinsson E. B. & Brown R. F. Mediators and moderators of the stressor-fatigue relationship in nonclinical samples. J Psychosom Res. 66, 21–29 (2009). [DOI] [PubMed] [Google Scholar]
- Lee Y.-C., Chien K.-L. & Chen H.-H. Lifestyle risk factors associated with fatigue in graduate students. J Formos Med Assoc 106, 565–572 (2007). [DOI] [PubMed] [Google Scholar]
- Chaudhuri A. & Behan P. O. Fatigue and basal ganglia. J Neurol Sci 179, 34–42 (2000). [DOI] [PubMed] [Google Scholar]
- Boksem M. A. & Tops M. Mental fatigue: costs and benefits. Brain Res Rev 59, 125–139 (2008). [DOI] [PubMed] [Google Scholar]
- Morita K., Morishima M., Sakai K. & Kawaguchi Y. Dopaminergic control of motivation and reinforcement learning: a closed-circuit account for reward-oriented behavior. J Neurosci 33, 8866–8890 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Carrion O. & Poppel E. Dopamine, learning, and reward-seeking behavior. Acta Neurobiol Exp 67, 481–488 (2007). [DOI] [PubMed] [Google Scholar]
- Lorist M. M. et al. The influence of mental fatigue and motivation on neural network dynamics; an EEG coherence study. Brain Res 1270, 95–106 (2009). [DOI] [PubMed] [Google Scholar]
- Tanaka M. et al. Reduced responsiveness is an essential feature of chronic fatigue syndrome: a fMRI study. BMC Neurol 6, 9 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda M. et al. Subjective feeling of psychological fatigue is related to decreased reactivity in ventrolateral prefrontal cortex. Brain Res 1252, 152–160 (2009). [DOI] [PubMed] [Google Scholar]
- Cook D. B., O’Connor P. J., Lange G. & Steffener J. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. Neuroimage 36, 108–122 (2007). [DOI] [PubMed] [Google Scholar]
- Tajima S. et al. Medial orbitofrontal cortex is associated with fatigue sensation. Neurol Res Int 2010, 671421 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca J., Genova H. M., Capili E. J. & Wylie G. R. Functional neuroimaging of fatigue. Phys Med Rehabil Clin N Am 20, 325–337 (2009). [DOI] [PubMed] [Google Scholar]
- de Lange F. P. et al. Gray matter volume reduction in the chronic fatigue syndrome. Neuroimage 26, 777–781 (2005). [DOI] [PubMed] [Google Scholar]
- Barnden L. R. et al. A brain MRI study of chronic fatigue syndrome: evidence of brainstem dysfunction and altered homeostasis. NMR Biomed 24, 1302–1312 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglese M. et al. Deep gray matter perfusion in multiple sclerosis: dynamic susceptibility contrast perfusion magnetic resonance imaging at 3 T. Arch Neurol 64, 196–202 (2007). [DOI] [PubMed] [Google Scholar]
- Pellicano C. et al. Relationship of cortical atrophy to fatigue in patients with multiple sclerosis. Arch Neurol 67, 447–453 (2010). [DOI] [PubMed] [Google Scholar]
- Isella V. et al. Clinical, neuropsychological, and morphometric correlates of apathy in Parkinson’s disease. Mov Disord 17, 366–371 (2002). [DOI] [PubMed] [Google Scholar]
- Puri B. K. et al. Reduction in left supplementary motor area grey matter in adult female fibromyalgia sufferers with marked fatigue and without affective disorder: a pilot controlled 3-T magnetic resonance imaging voxel-based morphometry study. J Int Med Res 38, 1468–1472 (2010). [DOI] [PubMed] [Google Scholar]
- Peran P. et al. Magnetic resonance imaging markers of Parkinson’s disease nigrostriatal signature. Brain 133, 3423–3433 (2010). [DOI] [PubMed] [Google Scholar]
- Lewis G. & Wessely S. The epidemiology of fatigue: more questions than answers. J Epidemiol Community Health 46, 92–97 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. C., Chien K. L. & Chen H. H. Lifestyle risk factors associated with fatigue in graduate students. J Formos Med Assoc 106, 565–572 (2007). [DOI] [PubMed] [Google Scholar]
- Le Bihan D. et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13, 534–546 (2001). [DOI] [PubMed] [Google Scholar]
- Acosta-Cabronero J., Williams G. B., Pengas G. & Nestor P. J. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain 133, 529–539 (2010). [DOI] [PubMed] [Google Scholar]
- Sagi Y. et al. Learning in the fast lane: new insights into neuroplasticity. Neuron 73, 1195–1203 (2012). [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H., Baptista C. S. & Thomas A. G. Human structural plasticity at record speed. Neuron 73, 1058–1060 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranishi M. et al. Inactivation of the putamen selectively impairs reward history-based action selection. Exp Brain Res 209, 235–246 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Yamada H. & Matsumoto N. Tonically active neurons in the striatum encode motivational contexts of action. Brain Dev 25 Suppl 1, S20–23 (2003). [DOI] [PubMed] [Google Scholar]
- Balleine B. W. & O’Doherty J. P. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35, 48–69 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux P. F., Schiffmann S. N. & de Kerchove d’Exaerde A. Targeting neuronal populations of the striatum. Front Neuroanat 5, 40 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. S., Tindell A. J., Aldridge J. W. & Berridge K. C. Ventral pallidum roles in reward and motivation. Behav Brain Res 196, 155–167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor P. J. Mental energy: Assessing the mood dimension. Nutr Rev 64, S7–9 (2006). [DOI] [PubMed] [Google Scholar]
- Takeuchi H. & Kawashma R. Neural bases of individual differences of creativity measured by the divergent thinking test. Association for Psychological Science 25th Annual Convention, Washington DC, US symposium, invited (24th May, 2013).
- Radley J. J. & Morrison J. H. Repeated stress and structural plasticity in the brain. Ageing Res Rev 4, 271–287 (2005). [DOI] [PubMed] [Google Scholar]
- Tanaka M., Ishii A. & Watanabe Y. Neural mechanisms underlying chronic fatigue. Rev Neurosci 24, 617–628 (2013). [DOI] [PubMed] [Google Scholar]
- Kawaguchi H. et al. Relation between dopamine synthesis capacity and cell-level structure in human striatum: a multi-modal study with positron emission tomography and diffusion tensor imaging. PloS one 9, e87886 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobryakova E., Genova H. M., DeLuca J. & Wylie G. R. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Front Neurol 6, 52 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R. C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113 (1971). [DOI] [PubMed] [Google Scholar]
- Vercoulen J. H. et al. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res 38, 383–392 (1994). [DOI] [PubMed] [Google Scholar]
- Beurskens A. J. et al. Fatigue among working people: validity of a questionnaire measure. Occup Environ Med 57, 353–357 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins J. B. et al. Cognitive behaviour therapy for chronic fatigue syndrome: a multicentre randomised controlled trial. Lancet 357, 841–847 (2001). [DOI] [PubMed] [Google Scholar]
- The G. K., Bleijenberg G. & van der Meer J. W. The effect of acclydine in chronic fatigue syndrome: a randomized controlled trial. PLoS Clin Trials 2, e19 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aratake Y. et al. Development of Japanese version of the checklist individual strength questionnaire in a working population. J Occup Health 49, 453–460 (2007). [DOI] [PubMed] [Google Scholar]
- Bultmann U. et al. Measurement of prolonged fatigue in the working population: determination of a cutoff point for the checklist individual strength. J Occup Health Psychol 5, 411–416 (2000). [DOI] [PubMed] [Google Scholar]
- Raven J. Manual for Raven’s progressive matrices and vocabulary scales (Oxford Psychologists Press, Oxford, 1998). [Google Scholar]
- Colom R., Jung R. E. & Haier R. J. Distributed brain sites for the g-factor of intelligence. Neuroimage 31, 1359–1365 (2006). [DOI] [PubMed] [Google Scholar]
- Haier R. J., Jung R. E., Yeo R. A., Head K. & Alkire M. T. Structural brain variation and general intelligence. Neuroimage 23, 425–433 (2004). [DOI] [PubMed] [Google Scholar]
- Jung R. E. & Haier R. J. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: Converging neuroimaging evidence. Behav Brain Sci 30, 135–154 (2007). [DOI] [PubMed] [Google Scholar]
- Narr K. L. et al. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex 17, 2163–2171 (2007). [DOI] [PubMed] [Google Scholar]
- Toga A. W. & Thompson P. M. Genetics of brain structure and intelligence. Annu Rev Neurosci 28, 1–23 (2005). [DOI] [PubMed] [Google Scholar]
- Takeuchi H. et al. The Impact of Television Viewing on Brain Structures: Cross-Sectional and Longitudinal Analyses. Cereb Cortex 25, 1188–1197 (2013). [DOI] [PubMed] [Google Scholar]
- Netsch T. & Van Muiswinkel A. Quantitative evaluation of image-based distortion correction in diffusion tensor imaging. IEEE Trans Med Imaging 23, 789–798 (2004). [DOI] [PubMed] [Google Scholar]
- Casanova R. et al. Biological parametric mapping: A statistical toolbox for multimodality brain image analysis. Neuroimage 34, 137–143 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.