New therapies with the potential to eradicate HCV are available. Engagement of infected individuals with care is the major barrier to realising this vision. We describe an enhanced care pathway leading to a sustained increase in indices of engagement.
Keywords: care pathways, HCV, public health
Abstract
Background. Engagement of individuals infected with hepatitis C virus (HCV) with care pathways remains a major barrier to realizing the benefits of new and more effective antiviral therapies. After an exploratory study, we have undertaken an evidence-based redesign of care pathways for HCV, including the following: (1) reflex testing of anti-HCV-positive samples for HCV RNA; (2) annotation of laboratory results to recommend referral of actively infected patients to specialist clinics; (3) educational programs for primary care physicians and nurses; and (4) the establishment of needs-driven community clinics in substance misuse services.
Methods. In this study, we conducted a retrospective cohort study of progression through care pathways of individuals with a new diagnosis of HCV infection made between January 2010 and January 2012. We also analyzed patient flow through new care pathways and compared this with our baseline study of identical design.
Results. A total of 28 980 samples were tested for anti-HCV antibody during the study period and yielded 273 unique patients with a new diagnosis of HCV infection. Of these, 38% were tested in general practice, 21% were tested in substance misuse services, 23% were tested in secondary care, and 18% were tested in local prisons. Overall, 80% of patients were referred to specialist clinics, 70% attended for assessment, and 38% commenced treatment, in comparison to 49%, 27%, and 10%, respectively, in the baseline study. Referral rates from all testing sources improved.
Conclusions. This study provides timely evidence that progression through care pathways can be enhanced, and it demonstrates reduction of key barriers to eradication of HCV.
It is estimated that between 130 and 170 million individuals worldwide are chronically infected with hepatitis C virus, representing 2%–3% of the world population [1]. Hepatitis C virus (HCV) is estimated to cause more than 350 000 deaths per annum, and up to 27% of cases of cirrhosis of the liver and 25% of hepatocellular carcinomas (HCCs) worldwide are attributable to HCV [2]. In most developed countries, including those in Western Europe and North America, the burden of HCV is <2%, and transmission is strongly associated with injecting drug use [1, 3–6]. In England, approximately 215 000 individuals are living with HCV [7], and half of all those who inject drugs are infected [8]. Hospital admissions and deaths from HCV-related end-stage liver disease and HCC in the United Kingdom have risen year-on-year and will continue to do so exponentially until the burden of HCV infection is reduced [7].
The recent introduction of directly acting antiviral agents has demonstrated the potential to transform outcomes for individuals infected with HCV [9–13]. New treatment schedules are typically less toxic, more effective, and of shorter duration than previous interferon-based therapies [9–13] and have raised the possibility of eradicating HCV in populations where resources are not constrained. However, these improvements in the efficacy and tolerability of antiviral therapies have also brought into sharp focus the major challenges of accessing the HCV-infected population. Public Health England has estimated that between 2006 and 2012, only 3% of chronically infected patients in the United Kingdom were treated each year [7], and low rates of engagement and treatment of HCV-infected populations are widely reported in other developed countries [14–17].
In order to realize the vision of eradication of HCV infection, it will be mandatory to increase both the rates of diagnosis of HCV infection and the subsequent engagement of infected individuals with treatment pathways. We have previously evaluated outcomes for patients newly diagnosed as HCV antibody positive in a representative healthcare region in England during the period from November 2000 to October 2002. This study found that of the 256 individuals newly identified as infected with HCV during the study period, <30% attended specialist clinics only 26 (<10%) received treatment [18]. We accordingly introduced a comprehensive set of changes to local care pathways, and we report the results of a follow-up study interrogating outcomes for a cohort of patients from the same health region recently diagnosed with active HCV infection.
METHODS
Identification of Patient Cohort and Data Collection
The starting point for this retrospective cohort study was a database of all serum samples tested for anti-HCV antibody and HCV RNA in the Microbiology Laboratory of the Nottingham University Hospitals NHS Trust (NUH) between January 2010 and January 2011. The Nottingham laboratory serves 2 large general hospitals, together with primary (community) care and specialist services in the city, conurbation and surrounding rural area, with a combined population of approximately 750 000. From this database, all samples with an anti-HCV-positive test result were extracted and filtered for unique records to identify the number of individuals with a positive anti-HCV result. For each anti-HCV-positive patient, pre-existing laboratory databases were searched for evidence of a previous diagnosis of HCV infection, and any patient previously found to have been infected was excluded. Patients were then assigned to 4 groups on the basis of the source of their diagnostic tests: general practice, substance misuse services, secondary care, and prisons. The study cohort was observed for an additional 12 months until January 2013 to ensure that all those with a new diagnosis of HCV had been given the opportunity to engage with specialist services. The only treatment available to our cohort throughout the study period was pegylated interferon and ribavirin. Therefore, our study results were not confounded by the availability of new antiviral regimens.
After our baseline study, we initiated 4 interventions designed to enhance engagement with the care pathway. These interventions were not introduced at the same time, because each needed to be developed and implemented separately. The changes made to our care pathway for HCV were as follows. (1) Reflex testing of any anti-HCV-positive sample for HCV RNA became standard practice within the NUH microbiology laboratory. This new practice unequivocally identified actively infected patients and eliminated the need to arrange repeat blood tests on all antibody-positive patients (introduced in 2008). (2) If the HCV-RNA test was positive, a note was added to the laboratory report requesting that the testing agency refer the patient to a hepatitis clinic, thus removing the need for primary care physicians to interpret the laboratory results and clearly setting out a recommended course of action (introduced in 2008). (3) An educational program was introduced regarding blood-borne viruses, comprising seminars and discussion groups delivered to local primary care physicians (general practitioners; GPs) and practice nurses, with the aim of improving their understanding of the natural history of HCV and its diagnosis and management (introduced in 2007). One us (B.J.T.) was then instrumental in establishing a national training resource for GPs, hosted by the UK Royal College of General Practitioners [19], and for ensuring high local uptake of this program. (4) We eased the local treatment selection criteria, which had previously excluded all patients who were active substance misusers, and established nurse-led treatment clinics in Nottingham's principal statutory drug treatment clinic in the city center, and in a large inner city primary care health center delivering shared care for intravenous drug users [20].
To assess outcomes, the hospital electronic patient information system was searched for evidence of referral to a hepatitis specialist services and subsequent assessment and treatment. We next interrogated the agency performing the diagnostic test if the patient was not referred to a specialist assessment. For GPs, this was done via a postal questionnaire; for drug services, contact was made by telephone, and the electronic patient information system was used to follow up outcomes of those diagnoses made in secondary care. For diagnoses made in large prisons, the electronic patient information system was searched for explanations for lack of referral. For several patients diagnosed at smaller prisons, who lacked evidence of referral for treatment, we sent follow-up questionnaires to the prison's healthcare department.
RESULTS
Of 28 980 samples tested for anti-HCV during the 2-year study period, 1083 were found to be positive. Of these samples, 1025 were patient identifiable and linked to 742 unique patients, 377 of whom met the criteria for a new diagnosis. Of these patients, 348 (94%) had an associated RNA HCV-RNA test result, and 237 of these patients were HCV-RNA positive and were adopted as the study cohort. The characteristics and source of referral for the study cohort are shown in Table 1.
Table 1.
Patient Characteristics
| Gender | General Practice | Substance Misuse Services | Secondary Care | Prisons | Total |
|---|---|---|---|---|---|
| Male | 58 | 36 | 32 | 43 | 169 |
| Female | 32 | 14 | 22 | 0 | 68 |
| Age range (years) | |||||
| <15 | 2% | 0 | 2% | 0 | 1% |
| 15–24 | 12% | 3% | 2% | 9% | 8% |
| 25–34 | 27% | 50% | 33% | 40% | 36% |
| 35–44 | 29% | 30% | 26% | 44% | 31% |
| 45–54 | 19% | 12% | 22% | 7% | 16% |
| 55+ | 11% | 2% | 15% | 0 | 8% |
| Past injecting drug use | |||||
| Yes | 43% | 88% | 46% | 60% | 57% |
| No | 33% | 0 | 24% | 5% | 19% |
| Unknown | 24% | 12% | 30% | 35% | 24% |
Of the 237 patients identified as newly diagnosed with active HCV infection, 90 (38%) had been tested within general practice. Fifty (21%) patients were diagnosed in substance misuse services. These include the city's principal drug treatment service, a specialist needle exchange, and an inpatient detoxification facility. We also note that 23 of the 90 patients assigned to general practice were diagnosed at practices operating “shared care” drug treatment clinics in association with a local substance misuse service. Fifty-four (23%) patients were diagnosed in a diverse range of specialties in secondary (hospital) care, including gastroenterology, hepatology, genitourinary medicine, antenatal clinics, and the admission wards. Forty-three (18%) patients were diagnosed in 3 local prisons.
Relationship Between the Source of the Positive Test and Progress Along Care Pathway
A summary of the progress along the care pathway for each of the 4 patient groups is shown in Table 2, with the following commentary.
Table 2.
Progress of Patients Diagnosed With Active Hepatitis C Virus (HCV) Infection Along the Care Pathwaya
| Source of Diagnosis | No. of Patients Diagnosed With Active HCV Infection | No. of Patients Referred to Specialist Care (%) | No. of Patients Attended Assessment (%) | No. of Patients Commencing Antiviral Treatment (%) |
|---|---|---|---|---|
| General Practice | 90 | 83 (92) | 74 (82) | 41 (46) |
| Substance Misuse | 50 | 42 (84) | 35 (70) | 18 (36) |
| Secondary Care | 54 | 41 (76) | 38 (70) | 21 (39) |
| Prisons | 43 | 24 (56) | 18 (42) | 11 (25) |
| Total | 237 | 190 (80) | 165 (70) | 91 (38) |
a Figures at each point are expressed as absolute numbers and as percentage of the total number of patients diagnosed for each source.
General Practice
By the end of the study, of the 90 patients diagnosed in general practice, there was evidence to indicate that 83 (92%) had been referred for assessment at a hepatitis clinic. In 3 cases, the patients had been referred for treatment in a neighboring city. Of the 7 patients who were not referred, the GP sent feedback in 6 cases. One patient had not returned for their results, 4 had been given their results but had since left the practice, and 1 had declined the offer of referral. There was evidence that 74 of the 83 referred patients (89%) attended the specialist clinic.
Substance Misuse Services
Of the 50 patients diagnosed in substance misuse services, 42 (84%) had been referred to a specialist for treatment in a hepatitis clinic. Thirty-six of these patients were seen in one of the community hepatitis clinics recently established in Nottingham. Of the 8 patients for whom no evidence of referral could be found, 4 had been diagnosed at the inpatient detoxification facility, which routinely informs the drug treatment provider of results rather than refer directly. Only 3 patients had been assessed as too chaotic to refer by their substance misuse service. There was evidence that 35 (83%) of the referred patients attended an assessment with a hepatitis specialist, and the majority of these patients attended specialist HCV services within community clinics. Thirty-four of these assessments took place in specialist HCV services placed within community clinics.
Secondary Care
Of the 54 patients diagnosed by secondary care, 41 (76%) were subsequently referred for assessment by a specialist. Although it was difficult to determine reasons for nonreferrals, we found that 2 patients died shortly after diagnosis, 1 from HCC and 1 due to human immunodeficiency virus-related illness. We also found that 2 patients were from outside the Nottingham area and a third had left the area since diagnosis. One patient tested in the emergency department had no known GP. Thirty-eight patients (93%) attended initial assessment.
Prisons
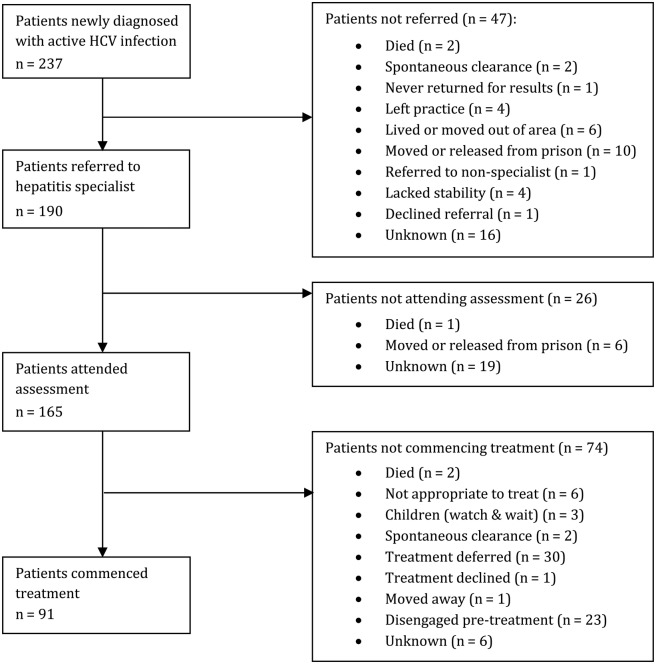
Forty-three people were diagnosed after testing in the 3 local prisons. Of these, there is evidence that 24 (56%) were referred for assessment. Of the 19 patients who may not have been referred, 10 had been moved to another prison or released shortly after diagnosis, 2 had spontaneously cleared the virus, and 1 had not been referred due to mental health issues. Reasons for nonreferral of the remaining 6 prisoners could not be determined. Eighteen of the 24 patients (75%) had attended an assessment in a hepatitis clinic, and we found that the 6 who did not attend were either released or moved after referral. The attrition along the care pathway is shown for all clients in Figure 1.
Figure 1.
A summary of progress of the patient cohort along the care pathway with the reasons for loss of patients at each point identified.
DISCUSSION
The principle findings of this study are the striking improvement in engagement with care pathways as assessed by rates of referral to specialist care and attendance for assessment, together with commencement of treatment, for patients with newly diagnosed HCV infection. These results are in contrast to our previous identical study [18].
Overall, 80% of the patients were referred to a hepatitis specialist, 70% attended assessment, and 38% commenced treatment, compared with 49%, 27%, and 10%, respectively, in the earlier study (Table 3). Patients diagnosed in primary care remain the most likely to be referred to specialist care, with 92% of patients referred, compared with 66% of patients in the earlier study. Referral rates for patients diagnosed in substance misuse services have also improved dramatically, with 84% of patients being referred, compared with 42% of patients in the earlier study. Taking into account the 23 patients who were diagnosed in shared care clinics in general practice, the percentage of patients identified as HCV positive in substance misuse services and referred to specialist clinics rises to 88%, with the majority attending for assessment. Attendance at the community-based clinics among patients with a history of injecting drugs was particularly excellent. The proportion of patients diagnosed in secondary care, who were then referred for assessment, increased from 55% to 76%. Although the prisons showed the highest increase in the referral rate, from 18% of patients to 56%, they remain the setting in which referral to specialist care is least likely to occur.
Table 3.
Comparison of Outcomes With Earlier Study
| Study Cohort | No. of Patients in Cohort | No. of Patients Referred to Specialist Care (%) | No. of Patients Who Attended Assessment (%) | No. of Patients Commenced on Treatment (%) |
|---|---|---|---|---|
| 2010–2011 cohort | 237 | 190 (80) | 165 (70) | 91 (38) |
| 2000–2002 cohort | 256 | 125 (49) | 68 (27) | 26 (10) |
United Kingdom national policies were initiated during the study period and may have contributed to increased engagement observed in our study. The Hepatitis C Strategy and Action Plan for England in 2004 [21, 22] set out to increase public and professional awareness of HCV and provide an operational framework for more effective intervention, and there is some evidence to support a positive effect of these policies on rates of testing. The proportion of the participants in the UK Health Protection Authority's Unlinked Anonymous Monitoring Survey of People Who Inject Drugs who reported having ever been tested for hepatitis C rose from 54% in 2001 to 83% in 2011 [8]. Overall, however, the introduction of national guidelines has not led to major sustained improvement in rates of HCV testing over the study period, and there is no evidence of widespread increases in engagement with HCV pathways [23].
The availability of new and less toxic treatments for HCV will unquestionably increase the proportion of individuals seen in clinic who will progress to antiviral treatment. Better available treatments may also increase levels of engagement with care pathways. However, fear of side effects is only one factor in a complex and interlinked set of barriers to engagement with treatment services among people who currently or previously injected drugs. These include the following: a lack of knowledge and understanding about HCV and its consequences; competing priorities with current drug use, housing, health, or finances; stigma associated with HCV and injecting drug use; the asymptomatic nature of HCV infection; and fear of medical investigations, together with concerns over the efficacy and side effects of treatment [24–31]. The barriers to engagement with testing and treatment for HCV in disadvantaged populations are located in society, rather than solely pharmacological [17, 32], and continue to be perceived as a major challenge to reducing the burden of HCV disease in the United Kingdom [23]. Our solution included the creation of facilities designed to meet the needs of our vulnerable populations, such as the delivery of antiviral therapy as part of drug treatment programs [20], but it included simple measures to engage with healthcare professionals that also led to increased attendance at our hospital-based clinics.
CONCLUSIONS
This is the first study to provide unequivocal evidence that engagement with care pathways for patients with HCV infection in England can be improved. These improvements are likely to be a direct result of an intervention set designed to integrate and enhance the continuum of HCV care. The patient group studied and the medical facilities in which the diagnosis and referral occur are highly heterogeneous and a particularly challenging area for research. It is likely that the efficacy of any single intervention will differ between patient groups, and therefore we designed this study to determine the impact of the interventions as a composite set of public health measures. Our work also identifies points where loss of patients from the care pathway continues to occur and provides an analysis of the reasons for this attrition (Figure 1). We note that improvements in the outcomes of the care pathway after a number of interventions, including the provision of outreach clinics in drug services and prison settings, have been reported in Scottish Specialist HCV setting [33]. It is also interesting to note that an analysis of the care continuum in a major US urban center [16] found results remarkably similar to those of our previous study in 2006 [18]. The study by Viner et al [16] found that of those individuals with a new diagnosis of HCV infection, 46% were subsequently confirmed to be RNA positive, 13% entered care pathways, and only 7% received treatment. This contemporary study confirms that patient engagement is a prerequisite for realizing the benefits of new directly acting antiviral therapies, and it emphasizes the importance of our central message that targeted public health measures can increase engagement of the HCV-infected population with care pathways.
Acknowledgments
We are grateful to all of the health professionals who have contributed to our care pathways for HCV.
N. H. is now Commissioning Manager for Substance Misuse, Public Health (Derbyshire County Council, County Hall, Matlock, Derbyshire, United Kingdom).
Financial support. This work was funded by a grant from Nottingham City Primary Care Trust.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Global Burden of Hepatitis C Working Group. Global burden of disease (GBD) for hepatitis C. J Clin Pharmacol 2004; 44:20–9. [DOI] [PubMed] [Google Scholar]
- 2.Perez JF, Armstrong GL, Farrington LA et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006; 45:529–38. [DOI] [PubMed] [Google Scholar]
- 3.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol 2007; 13:2436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong GL, Wasley A, Simard E et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006; 144:705–14. [DOI] [PubMed] [Google Scholar]
- 5.Cornberg M, Razavi HA, Alberti A et al. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int 2011; 31:30–60. [DOI] [PubMed] [Google Scholar]
- 6.Shepard CW, Finelli L, Alter M. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 2005; 5:558–67. [DOI] [PubMed] [Google Scholar]
- 7.Public Health England. Hepatitis C in the UK, 2014 Report. Available at: http://www.hpa.org.uk Accessed 4 January 2015.
- 8.www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1202115519183.
- 9.Butt AA, Kanwal F. Boceprevir and telaprevir in the management of hepatitis C virus-infected patients. Clin Infect Dis 2012; 54:96–104. [DOI] [PubMed] [Google Scholar]
- 10.Lawitz E, Mangia A, Wyles D et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013; 368:1878–87. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson IM, Dore GJ, Foster GR et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naïve patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 2014; 384:403–13. [DOI] [PubMed] [Google Scholar]
- 12.Afdhal N, Zeuzem S, Kwo P et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370:1889–98. [DOI] [PubMed] [Google Scholar]
- 13.Bourlière M, Bronowicki JP, de Ledinghen V et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS). Lancet Infect Dis 2015; 15:397–404. [DOI] [PubMed] [Google Scholar]
- 14.Grebely J, Raffa JD, Lai C et al. Low uptake of treatment for hepatitis C virus infection in a large community-based study of inner city residents. J Viral Hepat 2009; 16:352–8. [DOI] [PubMed] [Google Scholar]
- 15.Mehta SH, Genberg BL, Astemborski J et al. Limited uptake of hepatitis C treatment among injection drug users. J Commmunity Health 2008; 33:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viner K, Kuncio D, Newbern EC, Johnson CC. The continuum of hepatitis C testing and care. Hepatology 2015; 61:783–9. [DOI] [PubMed] [Google Scholar]
- 17.Zeremski M, Zibbell JE, Martinez AD et al. Hepatitis C virus control among persons who inject drugs requires overcoming barriers to care. World J Gastroenterol 2013; 19:7846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irving WL, Smith S, Cater R et al. Clinical pathways for patients with newly diagnosed hepatitis C – What actually happens. J Viral Hepat 2006; 13:264–71. [DOI] [PubMed] [Google Scholar]
- 19.Royal College of General Practitioners. Certificate in the detection, diagnosis and management of hepatitis B and C in primary care arts 1 and 2. Available at: http://www.rcgp.org.uk/courses-and-events/online-learning/ole/hepatitis-b-and-c.aspx Accessed 6 November 2015.
- 20.Jack K, Willott S, Manners J et al. Clinical trial: a primary-care-based model for the delivery of anti-viral treatment to injecting drug users infected with hepatitis C. Aliment Pharmacol Ther 2008; 29:38–45. [DOI] [PubMed] [Google Scholar]
- 21.Department of Health. Hepatitis C Strategy for England. London: Department of Health, 2002. [Google Scholar]
- 22.Department of Health. Hepatitis C Action Plan for England. London: Department of Health, 2004. [Google Scholar]
- 23.National Institute for Health and Clinical Excellence (NICE). Hepatitis B and C: Ways to Promote and Offer Testing to People at Increased Risk of Infection. Public Health Guidance 43. London: National Institute for Health and Clinical Excellence, 2012. [Google Scholar]
- 24.Doab A, Treloar C, Dore GJ. Knowledge and attitudes about treatment for hepatitis C virus infection and barriers to treatment among current injection drug users in Australia. Clin Infect Dis 2005; 40: S313–20. [DOI] [PubMed] [Google Scholar]
- 25.Grebely J, Genoway KA, Raffa JD et al. Barriers associated with treatment of hepatitis C virus infection among illicit drug users. Drug Alcohol Depend 2008; 93:141–7. [DOI] [PubMed] [Google Scholar]
- 26.Munoz-Plaza CE, Strauss S, Astone-Twerell J et al. Exploring drug user’ attitudes and decisions regarding hepatitis C (HCV) treatment in the U.S. Int J Drug Policy 2008; 19:71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNally S, Temple-Smith M, Sievert W, Pitts MK. Now, later or never? Challenges associated with hepatitis C treatment. Aust N Z J Public Health 2006; 30:422–7. [DOI] [PubMed] [Google Scholar]
- 28.Swan D, Long J, Carr O et al. Barriers to and facilitators of hepatitis C testing, management, and treatment among current and former injecting drug users: a qualitative exploration. AIDS Patient Care STDS 2010; 24:753–62. [DOI] [PubMed] [Google Scholar]
- 29.Treloar C, Hull P, Dore G, Grebely J. Knowledge and barriers associated with assessment and treatment for hepatitis C virus infection among people who inject drugs. Drug Alcohol Rev 2012; 31:918–24. [DOI] [PubMed] [Google Scholar]
- 30.Treloar C, Newland J, Rance J, Hopwood M. Uptake and delivery of hepatitis C treatment in opiate substitution treatment: perceptions of clients and health professionals. J Viral Hepat 2010; 17:839–44. [DOI] [PubMed] [Google Scholar]
- 31.Weaver T, Madden P, Charles V et al. Comorbidity of substance misuse and mental illness in community mental health and substance misuse services. Br J Psychiatry 2003; 183:304–13. [DOI] [PubMed] [Google Scholar]
- 32.Harris M, Rhodes T. Hepatitis C treatment access and uptake for people who inject drugs: a review mapping the role of social factors. Harm Reduct J 2013: 10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tait JM, McIntyre PG, McLeod S et al. The impact of a managed care network on attendance, follow-up and treatment at a hepatitis C specialist centre. J Viral Hepat 2010; 17:698–704. [DOI] [PubMed] [Google Scholar]