Abstract
Both the inflammatory potential and cognitive function decline during aging. The association between the repertoire of inflammatory biomarkers and cognitive decline is unclear. Inflammatory cytokines have been reported to be increased, decreased, or unchanged in the cerebrospinal fluid and sera of subjects with dementia. We assessed 112 postmortem brains from subjects diagnosed with poststroke dementia (PSD), vascular dementia, mixed dementia, and Alzheimer's disease (AD), comparing those to poststroke nondemented (PSND) subjects and age-matched controls. We analyzed 5 brain regions including the gray and white matter from the frontal and temporal lobes for a panel of cytokine and/or chemokine analytes using multiplex-array assays. Of the 37 analytes, 14 were under or near the detection limits, 7 were close to the lowest detection level, and 16 cytokines were within the linear range of the assay. We observed widely variable concentrations of C-reactive protein (CRP) and serum amyloid A at the high end (1–150 ng/mg protein), whereas several of the interleukins (IL, interferon-gamma and tumor necrosis factor) at the low end (1–10 pg/mg). There were also regional variations; most notable being high concentrations of some cytokines (e.g., CRP and angiogenesis panel) in the frontal white matter. Overall, we found decreased concentrations of several cytokines, including IL-1 beta (p = 0.000), IL-6 (p = 0.000), IL-7 (p = 0.000), IL-8 (p = 0.000), IL-16 (p = 0.001), interferon-inducible protein–10 (0.044), serum amyloid A (p = 0.011), and a trend in IL-1 alpha (p = 0.084) across all dementia groups compared to nondemented controls. IL-6 and IL-8 were significantly lower in dementia subjects than in nondemented subjects in every region. In particular, lower levels of IL-6 and IL-8 were notable in the PSD compared to PSND subjects. Because these 2 stroke groups had comparable degree of vascular pathology, the lower production of IL-6 and IL-8 in PSD reaffirms a possible specific involvement of immunosenescence in dementia pathogenesis. In contrast, CRP was not altered between dementia and nondementia subjects or between PSD and PSND. Our study provides evidence not only for the feasibility of tracking cytokines in postmortem brain tissue but also suggests differentially impaired inflammatory mechanisms underlying dementia including AD. There was a diminished inflammatory response, possibly reflecting immunosenescence and cerebral atrophy, in all dementias. Strategies to enhance anti-inflammatory cytokines and boost the immune system of the brain may be beneficial for preventing cognitive dysfunction, especially after stroke.
Keywords: Aging, Cognitive impairment, Immunosenescence, Inflammation, Poststroke dementia, Stroke, Vascular dementia, White matter
1. Introduction
During the last decade, there has been a surge of interest in whether inflammatory and immune responses contribute to aging-related dementias. Both the inflammatory potential and the immune system may decline in tandem with cognitive function during aging. However, it has been difficult to draw conclusions whether inflammation is a cause, a promoter, or simply a secondary phenomenon in dementing illness. A large number of studies focused on Alzheimer's disease (AD), as the most common form of dementia, indicate a direct role for inflammatory and immune processes in its pathogenesis (Rafnsson et al., 2007, Schmidt et al., 2002, Schram et al., 2007, Sudduth et al., 2013, Weaver et al., 2002, Whiteley et al., 2009). However, much less is known on the modulation of inflammatory responses in other dementias including vascular or stroke-related dementias.
The inflammatory response of the body is a dynamic process, and the profile of cytokine responses may differ with the duration and severity of an illness (Huberman et al., 1994, Huberman et al., 1995, Jabbari Azad et al., 2014). Cytokines in blood and cerebrospinal fluid (CSF) have been reported to be increased, decreased, or unaltered in AD, vascular dementia (VaD), and ischemic stroke survivors (Alvarez et al., 1996, Beridze et al., 2011, Narasimhalu et al., 2015, Schmidt et al., 2002, Singh and Guthikonda, 1997, Sun et al., 2009, Whiteley et al., 2012). Few population-based studies have shown that high levels of interleukin (IL) IL-6, IL-8, and C-reactive protein (CRP) are associated with poor cognitive status, including poor performance in memory and processing speed, as well as cognitive decline (Baune et al., 2008, Gimeno et al., 2008, Weaver et al., 2002, Wright et al., 2006). In ischemic stroke, higher serum ILs were associated with baseline cognitive impairment (IL-8) and subsequent cognitive decline (IL-12) (Narasimhalu et al., 2015). Clinical trials have generally failed to demonstrate a robust beneficial effect of anti-inflammatory drugs in the progression of AD (Hoozemans et al., 2011), implicating a complicated role of inflammation in the pathogenesis. In contrast, although most trials were negative in ischemic stroke injury, phase 2 trials with IL-1 receptor antagonist and the board spectrum anti-inflammatory agent minocycline demonstrated improved outcomes (Smith et al., 2015).
The immune system is also a key player in central nervous system repair and maintenance that undergoes a profound remodeling process over the lifetime and has a major impact on individual's health and survival (Boraschi and Italiani, 2014, Fulop et al., 2010, Grubeck-Loebenstein et al., 2009). Immunosenescence, or the aging immune system, is a constellation of age-related changes to the immune system, resulting in greater susceptibility to infection and reduced responses to infectious pathogen(s). The characteristics of immunosenescence include age-induced thymic atrophy, bone marrow decreased hematopoietic compartment, and increased peripheral suppressor cell activity. It affects both innate and adaptive immune systems (Grubeck-Loebenstein et al., 2009).
Our aim was to investigate inflammatory cytokine profiles in extracts of postmortem brain tissues from subjects with different dementias using multiplex immunoarrays. In addition to poststroke demented (PSD) and poststroke nondemented (PSND) subjects, we assessed brains from prospectively assessed dementia subjects diagnosed with VaD, mixed dementia, and AD and from age-matched controls. With emphasis on PSD, we tested the hypothesis that different dementias have distinct profiles of cytokines compared to those without dementia.
2. Methods
2.1. Subjects
Brain tissues from a total of 112 subjects, including 21 PSND and 20 PSD, 17 VaD, 18 mixed dementia, and 16 AD, were obtained from the Newcastle Brain Tissue Resource, Newcastle University. In addition, we analyzed brain tissue from similar age controls (Table 1). For the poststroke subjects, final Mini–Mental State Examination (MMSE) and the highest and last revised Cambridge Cognition Examination (CAMCOG) battery scores of the relevant subjects were used to determine the cognitive profile of analyzed subjects. Thus, stroke survivors who did not meet DSM-IIIR or IV criteria for dementia and had MMSE scores >25 and CAMCOG scores >85 were designated as poststroke survivors with no dementia. In most cases, bronchopneumonia was recorded as the cause of death.
Table 1.
Demographic details and pathological features of the subjects
| Group | N | Age | Gender (M%:F%) | Braak stageb | MMSE | CAMCOG |
|---|---|---|---|---|---|---|
| Control | 20 | 79.2 ± 3.3 | 35:65 | 1.72 ± 0.32 | 28.8 ± 0.8 | na |
| PSNDa | 21 | 85.0 ± 1.0 | 57:43 | 2.48 ± 0.25 | 27.2 ± 0.4 | 89.7 ± 1.3 |
| PSDa | 20 | 87.3 ± 1.3 | 30:70 | 2.73 ± 0.24 | 15.9 ± 1.1 | 66.2 ± 2.5 |
| VaD | 17 | 83.9 ± 1.6 | 41:59 | 1.93 ± 0.25 | 13.4 ± 3.7 | na |
| Mixed | 18 | 84.5 ± 1.2 | 44:56 | 5.13 ± 0.22 | 10.6 ± 2.36 | na |
| AD | 16 | 83.9 ± 1.9 | 56:44 | 5.31 ± 0.17 | 7.4 ± 1.9 | 39.1 ± 6.8 |
Numbers represent mean values (±SEM) and for the given number (N) of subjects. The causes of death included bronchopneumonia (95%), cardiac arrest, and carcinoma with no particular distribution in any group. The time period of postmortem interval between death and tissue retrieval ranged 39–47 hours for all the cases. There were no differences in the length of postmortem delay between groups.
Key: AD, Alzheimer's disease; CAMCOG, Cambridge cognition examination; F, female; M, male; MMSE, Mini–Mental State Examination; N, number; na, not available; NPD, no pathological diagnosis; PSD, poststroke demented; PSND, poststroke nondemented; SEM, standard error of the mean; VaD, vascular dementia.
Mean vascular pathology scores (range) for PSND and PSD groups were 13.5 (13–14) and 13.3 (9–17) compared to 8.1 (8–10) for controls (p < 0.05). These scores were derived as described previously (Deramecourt et al., 2012).
Braak staging scores were different in mixed and AD cases compared to all other groups (p < 0.05).
Neuropathological assessment was carried out as described previously, using standardized protocols (Allan et al., 2011, Ihara et al., 2010, Kalaria et al., 2004). Macroscopical infarcts, detected by visual inspection while dissecting the brain, were subsequently confirmed by light microscopy. Hematoxylin-eosin staining was used for assessment of structural integrity and infarcts, Nissl and luxol fast blue staining for cellular patterns and myelin loss, Bielschowsky's silver impregnation and amyloid β for CERAD and/or Thal rating of neuritic plaques, Gallays for neuritic pathology, and tau immunohistochemistry for Braak staging of neurofibrillary tangles. Vascular pathology scores were determined in PSND and PSD cases and controls as described previously (Deramecourt et al., 2012).
Pathological diagnosis of AD was consistent with the National Institute on Aging–Alzheimer's Association guidelines for the neuropathological assessment of AD (Montine et al., 2012). VaD was diagnosed as described previously (Kalaria et al., 2004). In PSD cases, a definite diagnosis of VaD was made when there were multiple or cystic infarcts, lacunae, microinfarcts and small vessel disease, and Braak stage ≤IV in the presence of clinically overt cognitive impairment (Deramecourt et al., 2012, Kalaria et al., 2004). Mixed dementia was classified when there was significant vascular pathology and sufficient degree of pathology to reach Braak V–VI, in the presence of clinically verified dementia syndrome. Tissues from control subjects had occasional aging-related pathology and were classified having “no pathological diagnosis” (Table 1). However, they had no strokes, transient ischemic attacks (excluded because of hypertension or cardiovascular risk factors), or any type of dementing illness.
2.2. Preparation of samples
One hundred to 150 mg frozen brain tissues from 5 different brain regions namely, BA9 for frontal gray matter (FGM), BA21 for temporal gray matter (TGM), the underlying frontal white matter (FWM), and temporal white matter (TWM), as well as hippocampus (Hipp), were dissected from each case and control. Frozen brain tissues were homogenized in ice-cooled lysis buffer (3 μL per mg of tissue wet weight, 50 mM Tris-Buffer, 150 mM NaCl, 0.05% Tween-20, pH = 7.5) with 2× phosphostease inhibitor (78,420, Thermo Scientific) and Protease inhibitor cocktail (87,786, Thermo Scientific) using the Precellys 24 tissue homogenizer (Bertin Technologies) with 3 cycles of 20 seconds over 5 minutes interval). The homogenates were centrifuged at 10,000 rpm for 60 minutes at 4 °C. The supernatant was then collected and transferred immediately into 3 microtubes to avoid repeated freeze and thaw cycle: one 150 μL of sample was kept at −80 °C for multiplex array, one 20 μL of sample was kept at −20 °C for protein assay, and the rest of the sample was stored at −80 °C for future use. Protein concentrations were estimated with the DC kit (500-0112, Bio-Rad).
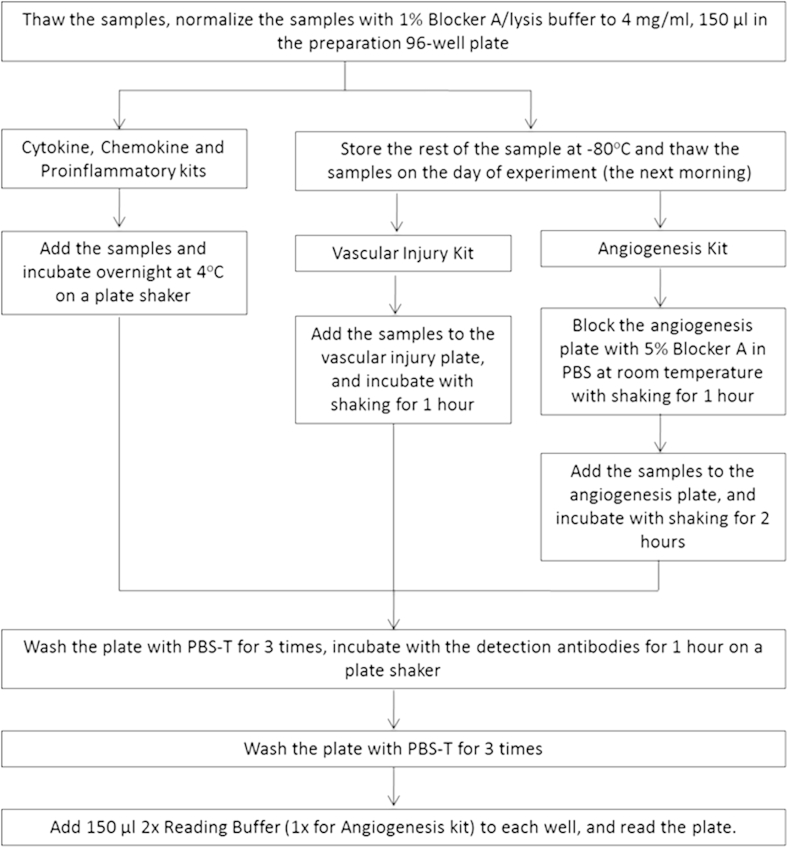
2.3. Multiplex arrays
On the day of assays, protein homogenates were thawed on ice. We assessed 37 cytokines in each sample with the Neuroinflammation Panel 1 (human) kit (Meso-Scale Discovery, K15210D). Duplicate aliquots of 150 μL of 4 mg/mL samples in lysis buffer containing 1% blocker A and 1× phosphatase and protease inhibitor were prepared in a 96-well preparation plate. There were 5 mini plates for each Neuroinflammation Panel 1 (human) kit, and each mini plate was designed for the measurement of each group of cytokines, up to 9 cytokines in each panel (Table 2). Calibrator dilutions and samples were prepared according to the manufacturer's recommendation (Table 2). The protocol for cytokine measurement is summarized in Fig. 1. Cytokine concentrations were read in the MESO QUICKPLEX SQ 120 from MSD with software DISCOVERY WORKBENCH 4.0. For those analytes below the lower limit of detection (LLOD, a calculated concentration corresponding to the signal 2.5 standard deviations above the background [zero calibrator]) and those with no-reading, the LLOD were used for individual samples and cytokines. The highest concentration at which the coefficient of variation (CV) of the calculated concentration (ULOQ) was <20%, and the recovery of each calibrator was within 80%–120% of the known value. The lowest concentration at which the CV of the calculated concentration (LLOQ) was <20%, and the recovery of each calibrator was within 80%–120% of the known value. The quantitative range of the assay lay between the lower limit (LLOQ) and upper limit (ULOQ) values.
Table 2.
Reagents and sample preparation of Neuroflammation Panel 1 (human) kit from MSD multispot assay system
| MSD panel kits | Analysts | Diluent for sample and calibrator | Dilution | Added volume for diluent (μL) | Sample (μL) | Diluent for detection antibody |
|---|---|---|---|---|---|---|
| Pronflammatory panel 1 | IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-13, TNF-α | Diluent 2 | 1:2 | 25 | 25 | Diluent 3 |
| Cytokine panel 1 | IL-1α, IL-5, IL-7, IL-12p40, IL-15, IL-16, IL-17A, TNF-β, VEGF | Diluent 43 | 1:2 | 25 | 25 | Diluent 3 |
| Chemokine panel 1 | Eotaxin, MIP-1β, Eotaxin-3, TARC, IP-10, MIP-1α, MCP-1, MDC, MCP-4 | Diluent 43 | 1:4 | 37 | 13 | Diluent 3 |
| Angiogenesis panel 1 | VEGF-C, VEGF-D, Tie-2, Flt-1, PIGF | Diluent 7 | 1:2 | 25 | 25 | Diluent 11 |
| Vascular injury panel 1 | SAA, CRP, VCAM-1, ICAM-1a | Diluent 101 | 1:5 | 20 | 5 | Diluent 101 |
| Human bFGF Kit V-PLEX | bFGFb | Diluent 7 | 1:2 | 25 | 25 | Diluent 11 |
Key: CRP, C-reactive protein; FGF, fibroblast growth factor; Flt-1, also known as VEGF-1 receptor; ICAM, intercellular adhesion molecule; IFN, interferon; IL, interleukin; IP, interferon-inducible protein; MCP, monocyte chemoattractant protein; MDC, macrophage-derived chemokine; MIP, macrophage inflammatory protein; PIGF, placenta growth factor; SAA, serum amyloid A; TARC, thymus and activation-regulated chemokine; TNF, tumor necrosis factor; VCAM, vascular cell adhesion molecule; VEGF, vascular endothelial growth factor.
For vascular injury, prepare 50 μL (40 μL of diluent 101 and 10 μL of normalized sample) in the transfer plate and add 25 μL into the kit plate.
The concentration of bFGF was extremely high in the all brain tissue. A single human bFGF kit had to be used for this specific cytokine, and samples were diluted 1:100 with lysis buffer and then diluted 1:2 with Diluent 7.
Fig. 1.
Flowchart of brain tissue preparation and analysis.
2.4. Statistical analysis
Statistical analysis was carried out using SPSS, version 21, with the level of significance set at p < 0.05. The demographics of the samples including age, postmortem delay (PMD), were compared with the Mann-Whitney U test. There were no statistical differences between the groups. Because these cytokine measurements are from different patients and different areas with multiple factors involved, it is inadequate to analyze them with common nonparametric tests (e.g., Mann-Whitney U test), as they are unable to examine interaction effects. As such, raw data from multiplex array were ranked with aligned ranking transfer (Higgins and Tashtoush, 1994) (ARTool, http://depts.washington.edu/aimgroup/proj/art/), and the ranking was analyzed with one-way analysis of variance and Fisher's LSD post hoc tests (>3 groups) or independent sample t test (2 groups) for samples from all 5 areas and individual area. The data were analyzed for the differences between the different patient groups from all 5 areas and from individual area. They were compared between different areas as well to see the area effects. Homogenate measurements were presented as pg/mg of total protein.
3. Results
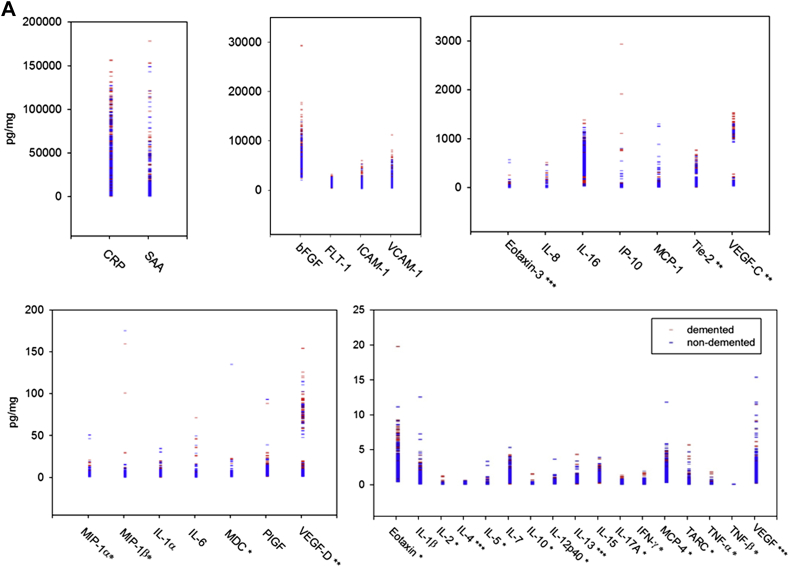
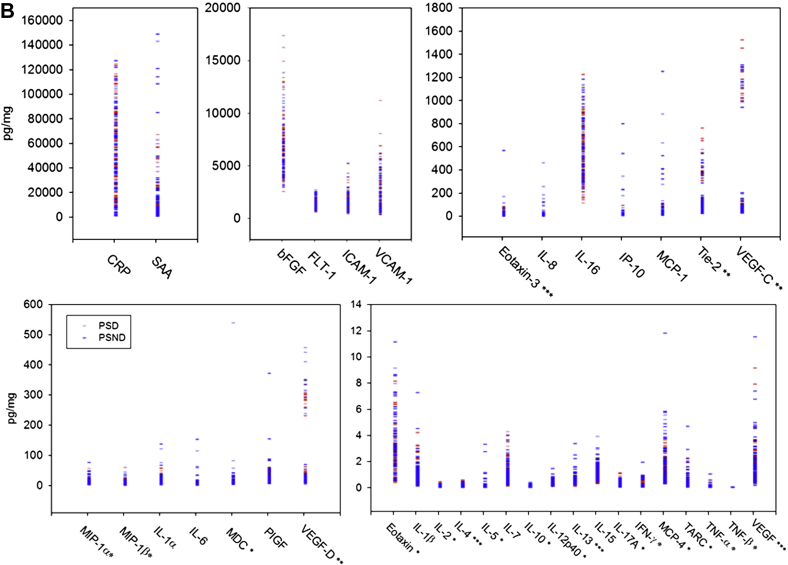
The LLODs from our experiment were within the LLOD range in the data sheet for almost all individual cytokines, except macrophage-derived chemokine, macrophage inflammatory protein (MIP)-1β, and vascular endothelial growth factor (VEGF)-C (Table 2 for abbreviations). Most recovery for each calibrator was within 80%–120% of the known value (data not shown). We noted predominantly similar concentration profiles of various cytokines when plotted as demented and nondemented groups or as PSD and PSND groups (Fig. 2A and B). The highest concentrations of the analytes were of CRP and SAA (1–150 ng/mg), whereas several of the key ILs were in low concentrations (1–10 pg/mg).
Fig. 2.
(A and B) Distribution of brain analytes in demented and control subjects. The analytes for all samples are grouped from the highest to the lowest concentrations. Red symbols represent demented samples, and blue symbols represent nondemented sample. *Most of the measurements are undetectable or close to LLOD. **All measurements from FWM are all in the quantitative range of the assay, and some of the measurements from all other areas are near to LLOD. ***Most of the measurements were close to LLOQ, and some of the measurements were marginally below the LLOQ. For interpretation of the analyte abbreviations, see Table 2. (B) Brain analytes in poststroke demented (PSD) and nondemented (PSND) subjects.
Because Extaxin, interferon (IFN)-gamma, IL-2, IL-5, IL-10, IL-12p40, IL-17A, monocyte chemoattractant protein–4, macrophage-derived chemokine, MIP-1 alpha, MIP-1 beta, TARC, TNF-alpha, and TNF-beta were undetectable or close to LLOD, and not in the quantitative range of the assay between LLOQ and ULOQ, we did not take these analyses further. We also found that IL-4, IL-13, Eotaxin-3, and VEGF needed to be interpreted carefully because most of the measurements were close to or just below LLOQ. For Tie 2, VEGF-C, and VEGF-D, the measurements from FWM were within the quantitative range of the assay, whereas some measurements from the other regions were near the LLOD. We therefore concentrated on the following predominantly proinflammatory cytokines: basic fibroblast growth factor (bFGF), CRP, fms-related tyrosine kinase 1, IL-1α, IL-1β, IL-6, IL-7, IL-8, IL-15, IL-16, ICAM-1, interferon-inducible protein–10, monocyte chemoattractant protein–1, placenta growth factor, SAA, and VCAM-1. Most of the assay values for these lay in the quantitative range of the assay and considered reliable. The median and 5%–95% range of concentrations of these measurable cytokines are provided in Table 3. In terms of brain regions, we observed wide variations, but the FWM often exhibited higher concentrations of some of the cytokines, particularly vascular growth related factors (not shown) and CRP.
Table 3.
The median and 5%–95% range of measurements (pg/mg of total protein) for the detectable cytokines in PSND, PSD, nondemented (including control and PSND), and demented (including PSD, VaD, mixed dementia, and AD)
| Median (5%–95%) | Nondemented | Demented | PSND | PSD |
|---|---|---|---|---|
| Proinflammatory panel 1 | ||||
| IL-1β | 0.77 (0.25–2.82) | 0.63 (0.21–2.11) | 0.77 (0.29–2.39) | 0.73 (0.23–2.71) |
| IL-4 | 0.09 (0.03–0.41) | 0.08 (0.02–0.36) | 0.09 (0.02–0.33) | 0.09 (0.02–0.37) |
| IL-6 | 0.84 (0.13–8.75) | 0.42 (0.10–3.19) | 0.69 (0.09–7.68) | 0.39 (0.10–1.39) |
| IL-8 | 8.47 (1.79–81.58) | 4.92 (1.75–27.71) | 6.11 (1.51–116.09) | 4.67 (2.21–17.96) |
| IL-13 | 0.66 (0.27–1.24) | 0.60 (0.20–0.99) | 0.63 (0.25–1.37) | 0.62 (0.23–0.94) |
| Cytokine panel 1 | ||||
| IL-1α | 3.75 (1.29–9.50) | 3.32 (1.25–8.99) | 3.62 (1.39–9.47) | 4.21 (1.34–10.61) |
| IL-7 | 1.02 (0.40–2.90) | 0.85 (0.22–2.38) | 1.08 (0.27–2.48) | 0.88 (0.32–2.63) |
| IL-15 | 1.10 (0.51–2.14) | 1.13 (0.49–2.24) | 1.10 (0.54–2.28) | 1.14 (0.46–2.13) |
| IL-16 | 551.34 (268.54–1032.67) | 489.37 (157.19–939.47) | 558.50 (277.44–1032.67) | 512.03 (170.91–920.59) |
| VEGF | 1.84 (0.37–4.99) | 1.49 (0.26–3.52) | 1.59 (0.29–4.68) | 1.55 (0.42–3.54) |
| Chemokine panel 1 | ||||
| Eotaxin3 | 7.25 (0.54–63.90) | 5.91 (0.76–50.47) | 7.63 (1.70–56.63) | 6.89 (1.03–48.29) |
| IP-10 | 10.20 (3.48–71.07) | 8.88 (2.90–27.54) | 9.11 (3.65–156.22) | 8.49 (3.81–26.69) |
| MCP1 | 19.51 (6.63–312.18) | 19.82 (6.83–101.38) | 18.92 (8.21–397.51) | 18.25 (7.35–77.46) |
| Angiogenesis panel 1 | ||||
| VEGF-C | 60.68 (29.09–1144.00) | 62.30 (32.57–1242.13) | 64.12 (29.10–1163.87) | 58.42 (29.09–1187.55) |
| VEGF-D | 4.91 (2.07–78.97) | 6.60 (2.19–86.86) | 4.91 (2.46–86.14) | 6.01 (2.30–75.22) |
| Tie-2 | 84.83 (30.06–410.65) | 80.67 (26.35–420.77) | 87.81 (34.49–434.40) | 95.20 (36.54–420.15) |
| FLT-1 | 1327.51 (655.84–2475.34) | 1338.74 (726.02–2409.87) | 1369.08 (878.99–2475.82) | 1304.33 (684.08–2261.78) |
| PIGF | 4.89 (1.85–13.90) | 5.56 (2.29–14.37) | 5.36 (1.97–14.67) | 5.12 (2.52–11.72) |
| bFGF | 5737.0 (3165.4–10,483.7) | 6142.0 (3468.6–12,547.3) | 5960.3 (3417.2–10,889.5) | 6281.8 (3701.5–13,014.6) |
| Vascular injury panel 1 | ||||
| SAA | 4176.5 (628.7–58,673.4) | 3871.6 (608.0–56,675.8) | 5721.2 (1000.1–80,591.9) | 4934.4 (1067.1–48,981.4) |
| CRP | 39,603.0 (5883.4–96,862.0) | 33,381.4 (4486.4–106511) | 44,230.4 (3257.3–110510.6) | 44,845.5 (11,561.2–110865.6) |
| VCAM-1 | 1619.7 (642.3–4879.7) | 1694.3 (601.4–5555.9) | 1767.9 (648.5–5435.9) | 1442.3 (565.3–5720.9) |
| ICAM-1 | 1233.7 (468.0–2501.3) | 1310.4 (617.8–3307.6) | 1365.3 (670.0–2549.2) | 1179.6 (684.6–2747.0) |
Values in BOLD show significant differences between demented and non-demented or PSD and PSND subjects.
Key: AD, Alzheimer's disease; CRP, C-reactive protein; ICAM, intercellular adhesion molecule; IL, interleukin; IP, interferon-inducible protein; MCP, monocyte chemoattractant protein; PIGF, placenta growth factor; PSD, poststroke demented; PSND, poststroke nondemented; SAA, serum amyloid A; VaD, vascular dementia; VCAM, vascular cell adhesion molecule; VEGF, vascular endothelial growth factor.
3.1. Proinflammatory cytokines in dementia
When samples were designated into the demented group, which included VaD, mixed, AD and PSD, and the non-demented group, which included normal controls and PSND, we noted significantly lower concentrations of cytokines overall in demented subjects (Table 3): IL-1 beta (p = 0.000), IL-6 (p = 0.000), IL-7 (p = 0.000), IL-8 (p = 0.000), IL-16 (p = 0.001), interferon-inducible protein–10 (0.044), SAA (p = 0.011), and IL-1 alpha (p = 0.084). However, compared to nondemented subjects, there were higher concentrations of the following analytes in dementia: bFGF (p = 0.002), ICAM-1 (p = 0.001), VEGF-C (p = 0.000), VEGF-D (p = 0.000). More interestingly, when all the samples for all brain regions from poststroke subjects were analyzed, relative to PSND subjects, PSD had lower concentrations of IL-6 (p = 0.000) and IL-8 (p = 0.000). In contrast, there were higher concentrations of IL-1 alpha (p = 0.040) in PSD compared to PSND subjects.
3.2. IL-6, IL-8, and CRP
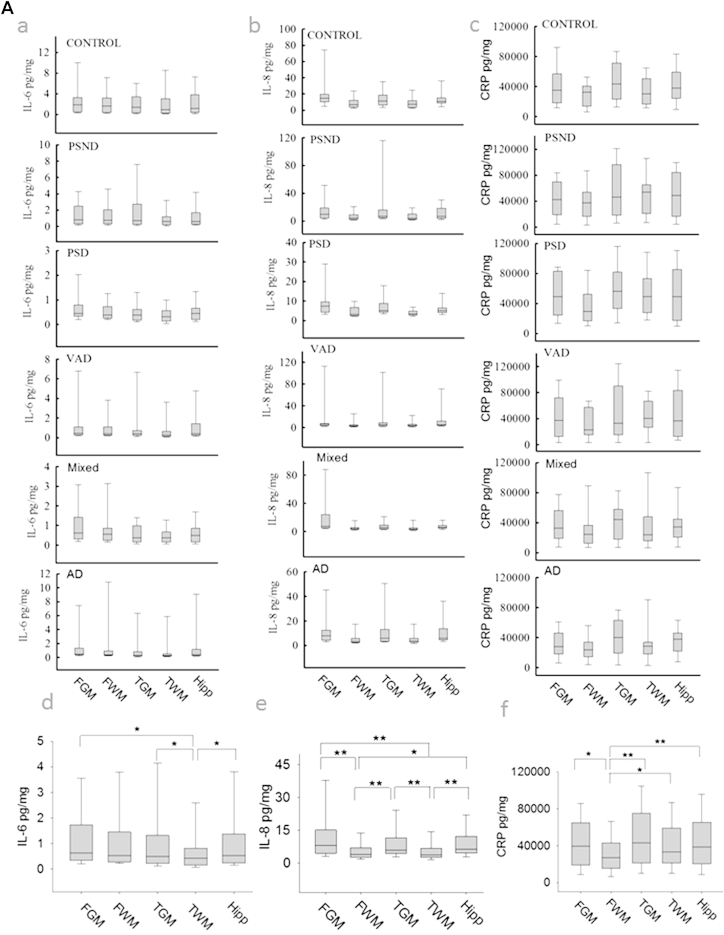
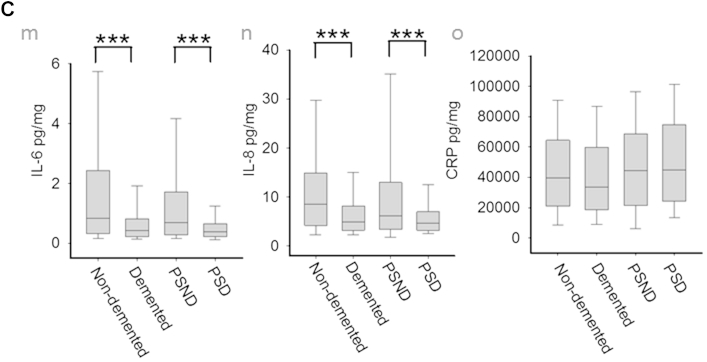
IL-6 and IL-8 were analyzed further to explore specificity for dementia types and regional differences based on the aforementioned findings. We reasoned that the concentrations of these cytokines and CRP might distinguish the demented and nondemented subjects, and in particular PSD and PSND groups (Fig. 3 and Table 4). We noted clear differences between demented and nondemented groups with or without stroke (Fig. 3C). Statistical analysis by one-way analysis of variance after Aligned Rank Transform (ART) showed that the normal controls had significantly higher levels of IL6 and IL-8 than the disease groups, including PSND, PSD, VaD, AD, and mixed, both when brain areas were analyzed separately, or grouped (p < 0.01). To exclude all possible stroke-induced influences (i.e., PSND and PSD subjects), only nonstroke samples were analyzed with independent sample t test after ART. It was found that nondemented samples (only controls without stroke) had higher levels of IL-6 (p = 0.000) and IL-8 (p = 0.000) than the entire sample of demented subjects (including AD, mixed dementia, and VaD). Moreover, the normal controls had significantly higher levels of IL-6 and IL-8 compared to AD or mixed dementia or VaD (p < 0.02 for all; data not shown).
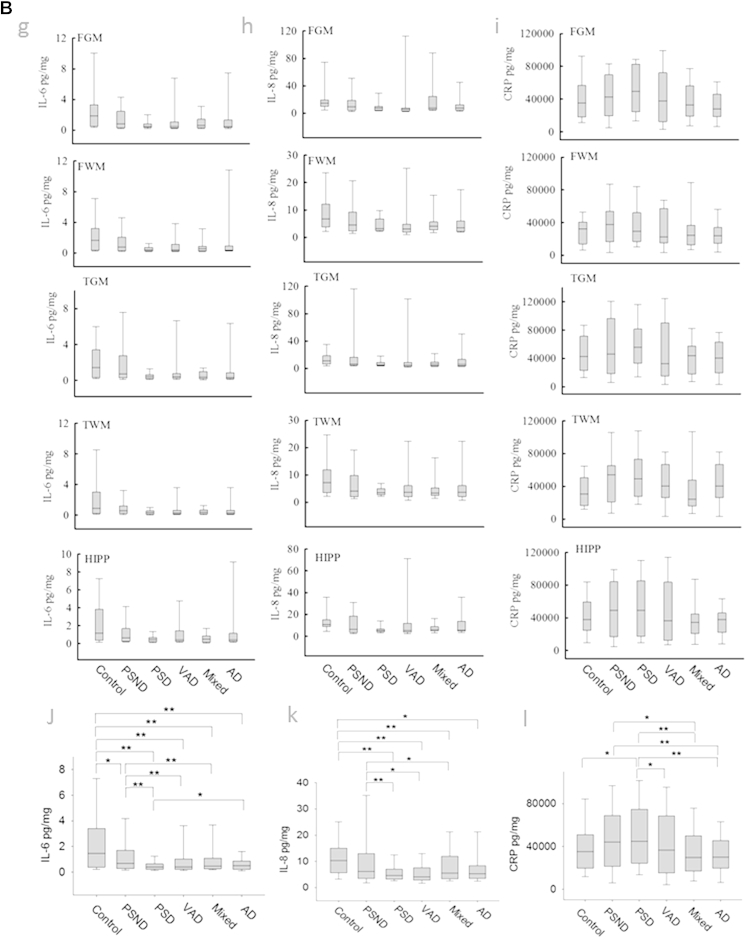
Fig. 3.
Concentrations of IL-6, IL-8, and CRP in different dementias and brain regions. (A) Boxplots from different disease group in each brain region for IL-6 (a), IL-8 (b), and CRP (c). Boxplots for all samples from different regions: IL-6 (d), IL-8 (e), CRP (f). (B) Boxplots from different areas in each disease group for IL-6 (g), IL-8 (h), and CRP (i). Boxplots for all samples from different diseases: IL-6 (j), IL-8 (k), CRP (l). (C) Boxplots showing IL-6 (m), IL-8 (n), and CRP (o) concentrations for samples from all nondemented and demented subjects and from PSND and PSD subjects. *p < 0.05, **p < 0.005, ***p < 0.001. Abbreviations: AD, Alzheimer's disease; CRP, C-reactive protein; FGM, frontal gray matter; FWM, frontal white matter; IL, interleukin; PSD, poststroke demented; PSND, poststroke nondemented; TGM, temporal gray matter; TWM, temporal white matter; VaD, vascular dementia.
Table 4.
Significance by one-way ANOVA test after ART ranking for all samples from each brain area and for all 5 brain areas of demented and nondemented subjects
| Brain regions and test groups | IL-6 | IL-8 | |
|---|---|---|---|
| FGM | Demented vs. nondemented | 0.013 | 0.014 |
| FWM | Demented vs. nondemented | 0.005 | 0.023 |
| TGM | Demented vs. nondemented | 0.004 | 0.003 |
| TWM | Demented vs. nondemented | 0.002 | 0.037 |
| Hipp | Demented vs. nondemented | 0.017 | 0.026 |
| All areas | Demented vs. nondemented | 0.000 | 0.000 |
Key: ANOVA, analysis of variance; FGM, frontal gray matter; FWM, frontal white matter; TGM, temporal gray matter; TWM, temporal white matter.
Between each disease group, statistical analysis showed that controls had higher concentrations of IL-6 than all other groups (p < 0.001, Supplementary Table 1). The PSND group also showed higher levels of IL-6 than PSD, VaD, and mixed dementia subjects, but not different from AD (Fig. 3G). Control and PSND subjects also had significantly higher concentrations of IL-8 than PSD, VaD, and mixed dementia groups, whereas no differences were apparent between control and PSND subjects (Supplementary Table 1, Fig. 3H). Although AD subjects showed significantly lower concentrations of IL-8 than controls, there was no difference with the PSND group. There were also no differences in the concentrations of both IL-6 and IL-8 between PSD, VaD, and mixed dementia; however, there was a difference between AD and PSD for IL-6 (p = 0.006) and a trend in IL-8 (p = 0.061). When analysis was limited to comparison of AD and normal controls, independent sample t tests (after ART) showed that AD subjects exhibited lower concentrations of both IL-6 (p = 0.001) and IL-8 (p = 0.019) compared to controls.
The concentrations of IL-6 were similar between the different brain areas analyzed, with exception of the TWM, which had significantly lower IL-6 in relation to FGM, TGM, and hippocampus (Hipp) (Fig. 3D). The white matter IL-8 content (both FWM and TWM) was significantly lower to that found in the gray matter (FGM, TGM, and Hipp). There were no differences found between FGM, TGM, and Hipp or between FWM and TWM (Fig. 3E). We also noted significant (p < 0.05) correlations between IL-6 and IL-8 concentrations and the MMSE and CAMCOG scores across all dementias and in all poststroke subjects (Supplementary Table 2).
CRP was not significantly altered between demented and nondemented groups (p = 0.257), or between PSD and PSND subjects (p = 0.50). However, CRP was significantly higher in PSD compared to VaD, mixed, AD, and control (Fig. 3L). The FWM had significantly lower levels of CRP than all the other brain areas (Fig. 3F).
4. Discussion
Our report represents the first multiplex analyte assay in a large number of postmortem brain tissues from different dementias. The specificity of our results was demonstrated not only by the quantitative analyte changes but also by the varied regional distribution of several cytokines. Overall, we found decreases in many cytokines within both the gray and white matter across all dementia subtypes, suggesting inflammatory or immune factors are noteworthy substrates contributing to the pathogenesis of dementia per sé, irrespective of their underlying pathological changes.
We provide reliable estimates of individual cytokine concentrations in relation to brain protein in human brain tissues. They range from very high concentrations, for example, CRP, SAA, and bFGF to just barely detectable levels such as those of IFN-gamma, TNF-alpha, and TNF-beta. Because the periods of PMD were comparable between the dementia groups and controls, we surmise that postmortem alterations for any cytokine would be to a similar degree across samples. Therefore, the relative changes we observed between dementia samples and groups would be valid, especially that the cytokine assays were performed on the same occasion.
Most previous studies were performed in body fluids, primarily plasma (or sera) and CSF quantified as weight per unit volumes (Brosseron et al., 2014, Hu et al., 2012, Lee et al., 2009, Swardfager et al., 2010). Although it is not entirely adequate to compare cytokine concentrations between brain tissues and those reported previously in plasma and CSF samples, it may be of relevance here to consider the often studied cytokines such as IL-6 and IL-8. In a recent study (Leung et al., 2013), the median (range) plasma concentrations of IL-6 and IL-8 in elderly controls were reported to be 8.9 (1.3–36.6) and 8.3 (1.9–31.3) pg/mL. With reference to the CSF, the median concentrations of IL-6 and IL-8, in normal elderly subjects in another study (Westhoff et al., 2013), were reported to be 1.0 (0.2–2.2) and 28.4 (22.7–42.5) pg/mL, respectively. Given that widely published values for total protein in 1 mL of plasma and 1 mL CSF contain approximately 70 (60–80) and 0.45 (0.2–0.6) mg/mL protein, respectively (Felgenhauer, 1974), the aforementioned median (and range) concentrations of IL-6 in plasma reported by Leung et al. (2013) calculate to 0.13 (0.02–0.52) pg/mg protein, whereas that in CSF reported by Westhoff et al. (2013) calculate to 2.2 (0.44–4.9) pg/mg protein. Similarly, for IL-8, normal elderly plasma contains approximately 0.12 (0.03–0.45) pg/mg protein and CSF approximates to 63.1 (50.5–94.4) pg/mg protein. The values for CSF of 1.7 (0.7–14.8) and 34.9 (17.5–64.2) pg/mL for these cytokines reported by Kern et al. (2014) also fall within a comparable range. These calculations indicate that compared to plasma, CSF has greater amounts of both IL-6 and IL-8, and there is a 20-25 fold difference between these cytokines in the CSF but almost similar amounts in the plasma. Although we found a >7-fold difference in concentrations of these 2 cytokines within the brain, we report generally lower concentrations of IL-8 per mg protein, in particular, compared to the recently published CSF concentrations (Kern et al., 2014, Westhoff et al., 2013). The highest brain values of IL-6 and IL-8 were determined to be 8.9 and 40.0 pg/mg protein, respectively. Various factors could contribute to these differences. Because the total CSF protein in older (>70 years) than younger people (0.2–0.6 mg/mL) is generally greater, we suggest the mean concentrations we recorded in brain are realistic.
We specifically noted decreased concentrations of both IL-6 and IL-8 in brain tissues of demented subjects. As PSD and PSND subjects in our cohort had similar survival periods after stroke and exhibited comparable degrees of vascular burden (Allan et al., 2011), the lower production of IL-6 and IL-8 in PSD suggests a specific change related to a factor(s) which contribute to dementia per sé. When samples from stroke patients (PSND and PSD) were excluded from the cohort, all other forms of dementia also showed significantly lower levels of IL-6 and IL-8 than the normal controls, implying a possible similar diminished immune response in the context of dementia including AD. The origin of IL-6 in the brain is uncertain, with a number of studies hinting that neurons, glial cells, and the vascular endothelium could be the source of IL-6 (Jang et al., 2008, Suzuki et al., 2009). Indeed, immunostaining with IL-6 antibody was found within neuronal and glial cells in formalin fixed brain tissue sections (AC and RNK, data not shown). Although IL-6 is involved in the synthesis of acute phase proteins, it displays pleiotrophic effects (Frei et al., 1989). IL-6 was also shown to be essential for poststroke angiogenesis (Gertz et al., 2012) and a protectant of cerebral infarction (Herrmann et al., 2003, Loddick et al., 1998). IL-6 elevation in CSF of stroke patients and its further correlation with stroke severity have been reported in some studies (Beridze et al., 2011, Vila et al., 2000) although Sun et al. (2009) did not find any change in IL-6 in the CSF. Plasma elevated concentrations of IL-6, IL-1 beta, and TNF-alpha have also been reported in the elderly (Kern et al., 2014). Although this seems to contradict the expected functional defects, chronic subclinical inflammation may be caused by partial inability of the aged immune system to eliminate certain pathogens, such as products of degradation processes implicating an inefficient immune response in the elderly.
Our results are consistent with some previous findings, reporting decreases in IL-6 in the brain tissues from the frontal gray and white matter of VaD and mixed dementia in comparison to controls (Mulugeta et al., 2008). Kim et al. (2011) reported significantly lower plasma IL-8 levels in subjects with mild cognitive impairment and AD than controls. Similar findings of significant decreases in TNF-alpha and IL-6 in CSF and serum, respectively, were reported in AD subjects with a mean duration of dementia for 2.5 years (1.5–3.4 years) (Richartz et al., 2005). Yamada et al. (1995) also reported decreased CSF IL-6 in AD, with the magnitude of CSF IL-6 reduction significantly greater in early onset dementia. However, serum concentrations of some proteins may increase initially but decline in later M2 stages of disease like AD, as elegantly demonstrated by Sudduth et al. (2013). In our cohort, most AD patients had severe degree of dementia, with MMSE score lower than 8, which may indicate that they are more fragile and exhibit more intense immunosenesence.
In AD, higher levels of cytokines (IL-6 and IFN-gamma) were reported in lymphocytes, and these were related to more advanced age (Jabbari Azad et al., 2014). Similarly, in another study (Licastro et al., 2000), higher concentrations of IL-6 in peripheral blood of AD subjects were reported, but this was not evident in the CSF (Engelborghs et al., 1999). Baune et al. (2008) reported that increased serum concentrations of IL-8 were associated with poor cognitive performance on cognitive tests in healthy elderly individuals. These inconsistent findings in the context of our findings in postmortem brain tissue may be attributed to differences in study populations, for example, inclusion criteria of subjects (different degrees of pathogenesis), sample size, and different protocols for sample type and preparation and variability in assay procedures. For most of the studies, cytokines were sampled from peripheral blood (serum, plasma, or whole blood cells) or CSF and often have short half-lives. They may reach higher concentrations at or near sites of release and much lower concentrations after dilution into blood and CSF.
Remarkably, when whole blood supernatants were stimulated by lipopolysaccharide (bacterial virulence factors that induce inflammation), there was a diminished production of proinflammatory cytokines (TNF-α and IL-1β) in older people compared to younger individuals (Bruunsgaard et al., 1999). This lack of response in cytokine production on stimulation reflects the general dysfunction of particular immune cells on inflammatory stimuli, indicating the presence of immunosenescence, with an attenuated secretory activity for IL-6, IL-12, IFN-gamma, TNF-alpha of monocytes and/or macrophages within whole blood cell cultures of AD subjects. This suggests a systemic, possibly age-related alteration of immune mechanisms involved in AD pathogenesis (Richartz et al., 2005). De Luigi et al. (2002) found that dementia patients exhibited an upregulation of circulating cytokines and a downregulation of cytokines released by blood cells after exposure to lipopolysaccharide, suggesting a similar mechanism is present in both AD and multi-infarct dementia. In addition, there is further evidence of premature immunosenescence with decreased T- and B-cell numbers and insufficient microglial phagocytosis in AD subjects (Richartz-Salzburger et al., 2007). Thus, better cognitive performance was associated with less effector memory CD4+ T cells, more naïve CD8+ T cells, and more B cells in healthy elderly people. In contrast, significantly lower levels of CD4+ naïve T cells and an increase in the ratio of the activated and/or naïve CD4+ T cells were found in AD subjects (Tan et al., 2002). It is not unlikely there are shared pathways leading to cognitive dysfunction among various types of dementia. Immunosenescence could be one possible cause, promoter, or result of dementia pathology. Alternatively, they occur simultaneously and are interrelated and interact with each other (De Luigi et al., 2002).
The dampening of the inflammatory and/or immune responses in relation to pathological changes may not only occur in AD, but also in other types of dementias, such as VaD and PSD as evident from our study. This phenomenon has been linked to other diseases. For example, the percentages of CD8+ naïve and CD8+ recent thymic emigrant cells, and T-cell receptor rearrangement excision circle levels in peripheral blood cells were significantly lower in cancer patients than in age-matched controls (Falci et al., 2013). Cancer patients also have significant shorter telomere lengths and significant lower level of CRP and IL-6 and nutritional markers in their peripheral blood samples, when compared to the controls (Falci et al., 2013). It is plausible that subjects destined to develop dementia have a diminished inflammatory response, encompassing immunosenescence. These subjects who are generally frail develop greater susceptibility to infection but reduced responses and impaired elimination of pathogens (Boraschi and Italiani, 2014, Grubeck-Loebenstein et al., 2009). Although more evidence needs to be gathered to demonstrate that immunosenescence causes dementia, studies using strategies to rejuvenate the immune system partially have demonstrated memory recovery in immune-deficient mice (Ron-Harel et al., 2008).
There are some limitations to our study. It is possible that the long postmortem interval could have contributed to related global or differential changes in cytokines. The cause of death and antemortem status including any comorbidities could be additional factors influencing the observed results. The main cause of death in our sample was bronchopneumonia (Allan et al., 2011). However, there was also no correlation between cytokine levels and PMD, as indicated by multiple regression analysis, and there were no differences in PMD between the groups. We also could not delineate any of the groups from controls based on any known antemortem comorbidities. We would expect all brain regions to have been affected equally, but clearly, there were differential responses in cytokines. Another factor is that prior medications could have modulated the cerebral expression of cytokines. However, as previously reported, all subjects had similar use of medication in respect to their mental and physical health (Allan et al., 2011).
Our study provides strong evidence of impaired inflammatory mechanisms in the pathogenesis of dementia. It is possible that the decreased inflammatory response reflects cellular changes in reactive cells or simply global brain atrophy related to dementia. Further assessment of cell specific markers of microglia and astrocyte markers could demonstrate whether there are any changes in reactive cells and whether the decreased cytokine levels were because of dampened cellular responses. The investigation of inflammatory markers in bloods from the same cohort may be of further use. Better understanding of the association between the immune system and cognitive function will help us to develop new strategies to counter against cognitive dysfunction.
Disclosure statement
The coauthors have no disclosures with regard to this report. The study was not industry sponsored. There are no conflicts of interest.
Acknowledgements
The authors are grateful to the patients, families, and clinical house staff for their cooperation in the investigation of this study. The authors thank Michelle Widdrington, Carein Todd, Jean Scott, Deborah Lett, and Anne Nicholson for assistance in managing and screening the cohort. The authors thank Janet Y Slade and Roslyn Hall for excellent technical assistance. The authors are most grateful to the staff of the Newcastle Brain Tissue Resource (NBTR) for assisting us to undertake this complicated study. The authors would like to acknowledge the profound support of Meso-Scale Discovery (MSD) (Dr John Butler and Dr James Parry). This work is primarily supported by a grant from the Dunhill Medical Trust UK (R277/0213). The authors also acknowledge continued support of the Medical Research Council (MRC, G0500247), Newcastle Centre for Brain Ageing and Vitality (BBSRC, EPSRC, ESRC and MRC, LLHW), and Alzheimer's Research (ARUK). The CogFAST study was originally supported by the MRC in 1999. Tissue for this study was collected by the Newcastle Brain Tissue Resource, which is funded in part by a grant from the UK MRC (G0400074), by the Newcastle NIHR Biomedical Research Centre in Ageing and Age Related Diseases award to the Newcastle Upon Tyne Hospitals NHS Foundation Trust, and by a grant from the Alzheimer's Society and ARUK as part of the Brains for Dementia Research Project.
Aiqing Chen led the study, performed most of the described experiments, developed relevant methodology, undertook the analysis, and wrote the first draft of the article. Arthur E. Oakley advised on and interpreted the morphological analysis, constructed the figures, and corrected drafts of the article. Maria Monteiro assisted with the retrieval and dissection of the brain tissues used in the study. Katri Tuomela assisted with the setup and performance of the multiplex assays. Louise Allan provided clinical input to the CogFAST study, participated in diagnostic consensus conferences, and gave intellectual support. Elizabeta Mukaetova-Ladinska provided intellectual advice on the study and corrected drafts of the article. John T. O'Brien clinical input to the CogFAST study, participated in diagnostic consensus conferences, and gave intellectual support. Raj N. Kalaria conceived the original study, performed some of the neuropathological analysis, corrected several drafts, and obtained the funding.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.neurobiolaging.2015.10.021.
Contributor Information
Aiqing Chen, Email: Aiqing.chen@ncl.ac.uk.
Raj N. Kalaria, Email: raj.kalaria@ncl.ac.uk.
Appendix A. Supplementary data
References
- Allan L.M., Rowan E.N., Firbank M.J., Thomas A.J., Parry S.W., Polvikoski T.M., O'Brien J.T., Kalaria R.N. Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain. 2011;134(Pt 12):3716–3727. doi: 10.1093/brain/awr273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez X.A., Franco A., Fernandez-Novoa L., Cacabelos R. Blood levels of histamine, IL-1 beta, and TNF-alpha in patients with mild to moderate Alzheimer disease. Mol. Chem. Neuropathol. 1996;29:237–252. doi: 10.1007/BF02815005. [DOI] [PubMed] [Google Scholar]
- Baune B.T., Ponath G., Golledge J., Varga G., Arolt V., Rothermundt M., Berger K. Association between IL-8 cytokine and cognitive performance in an elderly general population—the MEMO-Study. Neurobiol. Aging. 2008;29:937–944. doi: 10.1016/j.neurobiolaging.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Beridze M., Sanikidze T., Shakarishvili R., Intskirveli N., Bornstein N.M. Selected acute phase CSF factors in ischemic stroke: findings and prognostic value. BMC Neurol. 2011;11:41. doi: 10.1186/1471-2377-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraschi D., Italiani P. Immunosenescence and vaccine failure in the elderly: strategies for improving response. Immunol. Lett. 2014;162(1 Pt B):346–353. doi: 10.1016/j.imlet.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Brosseron F., Krauthausen M., Kummer M., Heneka M.T. Body fluid cytokine levels in mild cognitive impairment and Alzheimer's disease: a comparative overview. Mol. Neurobiol. 2014;50:534–544. doi: 10.1007/s12035-014-8657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H., Pedersen A.N., Schroll M., Skinhoj P., Pedersen B.K. Impaired production of proinflammatory cytokines in response to lipopolysaccharide (LPS) stimulation in elderly humans. Clin. Exp. Immunol. 1999;118:235–241. doi: 10.1046/j.1365-2249.1999.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luigi A., Pizzimenti S., Quadri P., Lucca U., Tettamanti M., Fragiacomo C., De Simoni M.G. Peripheral inflammatory response in Alzheimer's disease and multiinfarct dementia. Neurobiol. Dis. 2002;11:308–314. doi: 10.1006/nbdi.2002.0556. [DOI] [PubMed] [Google Scholar]
- Deramecourt V., Slade J.Y., Oakley A.E., Perry R.H., Ince P.G., Maurage C.A., Kalaria R.N. Staging and natural history of cerebrovascular pathology in dementia. Neurology. 2012;78:1043–1050. doi: 10.1212/WNL.0b013e31824e8e7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelborghs S., De Brabander M., De Cree J., D'Hooge R., Geerts H., Verhaegen H., De Deyn P.P. Unchanged levels of interleukins, neopterin, interferon-gamma and tumor necrosis factor-alpha in cerebrospinal fluid of patients with dementia of the Alzheimer type. Neurochem. Int. 1999;34:523–530. doi: 10.1016/s0197-0186(99)00031-5. [DOI] [PubMed] [Google Scholar]
- Falci C., Gianesin K., Sergi G., Giunco S., De Ronch I., Valpione S., Solda C., Fiduccia P., Lonardi S., Zanchetta M., Keppel S., Brunello A., Zafferri V., Manzato E., De Rossi A., Zagonel V. Immune senescence and cancer in elderly patients: results from an exploratory study. Exp. Gerontol. 2013;48:1436–1442. doi: 10.1016/j.exger.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Felgenhauer K. Protein size and cerebrospinal fluid composition. Klinische Wochenschrift. 1974;52:1158–1164. doi: 10.1007/BF01466734. [DOI] [PubMed] [Google Scholar]
- Frei K., Malipiero U.V., Leist T.P., Zinkernagel R.M., Schwab M.E., Fontana A. On the cellular source and function of interleukin 6 produced in the central nervous system in viral diseases. Eur. J. Immunol. 1989;19:689–694. doi: 10.1002/eji.1830190418. [DOI] [PubMed] [Google Scholar]
- Fulop T., Kotb R., Fortin C.F., Pawelec G., de Angelis F., Larbi A. Potential role of immunosenescence in cancer development. Ann. N. Y. Acad. Sci. 2010;1197:158–165. doi: 10.1111/j.1749-6632.2009.05370.x. [DOI] [PubMed] [Google Scholar]
- Gertz K., Kronenberg G., Kalin R.E., Baldinger T., Werner C., Balkaya M., Eom G.D., Hellmann-Regen J., Krober J., Miller K.R., Lindauer U., Laufs U., Dirnagl U., Heppner F.L., Endres M. Essential role of interleukin-6 in post-stroke angiogenesis. Brain. 2012;135(Pt 6):1964–1980. doi: 10.1093/brain/aws075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno D., Marmot M.G., Singh-Manoux A. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology. 2008;33(10):1322–1334. doi: 10.1016/j.psyneuen.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B., Della Bella S., Iorio A.M., Michel J.P., Pawelec G., Solana R. Immunosenescence and vaccine failure in the elderly. Aging Clin. Exp. Res. 2009;21:201–209. doi: 10.1007/BF03324904. [DOI] [PubMed] [Google Scholar]
- Herrmann O., Tarabin V., Suzuki S., Attigah N., Coserea I., Schneider A., Vogel J., Prinz S., Schwab S., Monyer H., Brombacher F., Schwaninger M. Regulation of body temperature and neuroprotection by endogenous interleukin-6 in cerebral ischemia. J. Cereb. Blood Flow Metab. 2003;23:406–415. doi: 10.1097/01.WCB.0000055177.50448.FA. [DOI] [PubMed] [Google Scholar]
- Higgins J.J., Tashtoush S. An aligned rank transform test for interaction. Nonlinear World. 1994;1:201–211. [Google Scholar]
- Hoozemans J.J., Veerhuis R., Rozemuller J.M., Eikelenboom P. Soothing the inflamed brain: effect of non-steroidal anti-inflammatory drugs on Alzheimer's disease pathology. CNS Neurol. Disord. Drug Targets. 2011;10:57–67. doi: 10.2174/187152711794488665. [DOI] [PubMed] [Google Scholar]
- Hu W.T., Holtzman D.M., Fagan A.M., Shaw L.M., Perrin R., Arnold S.E., Grossman M., Xiong C., Craig-Schapiro R., Clark C.M., Pickering E., Kuhn M., Chen Y., Van Deerlin V.M., McCluskey L., Elman L., Karlawish J., Chen-Plotkin A., Hurtig H.I., Siderowf A., Swenson F., Lee V.M., Morris J.C., Trojanowski J.Q., Soares H., Alzheimer's Disease Neuroimaging Initiative Plasma multianalyte profiling in mild cognitive impairment and Alzheimer disease. Neurology. 2012;79:897–905. doi: 10.1212/WNL.0b013e318266fa70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman M., Shalit F., Roth-Deri I., Gutman B., Brodie C., Kott E., Sredni B. Correlation of cytokine secretion by mononuclear cells of Alzheimer patients and their disease stage. J. Neuroimmunol. 1994;52:147–152. doi: 10.1016/0165-5728(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Huberman M., Sredni B., Stern L., Kott E., Shalit F. IL-2 and IL-6 secretion in dementia: correlation with type and severity of disease. J. Neurol. Sci. 1995;130:161–164. doi: 10.1016/0022-510x(95)00016-u. [DOI] [PubMed] [Google Scholar]
- Ihara M., Polvikoski T.M., Hall R., Slade J.Y., Perry R.H., Oakley A.E., Englund E., O'Brien J.T., Ince P.G., Kalaria R.N. Quantification of myelin loss in frontal lobe white matter in vascular dementia, Alzheimer's disease, and dementia with Lewy bodies. Acta Neuropathol. 2010;119:579–589. doi: 10.1007/s00401-009-0635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbari Azad F., Talaei A., Rafatpanah H., Yousefzadeh H., Jafari R., Talaei A., Farid Hosseini R. Association between cytokine production and disease severity in Alzheimer's disease. Iran J. Allergy Asthma Immunol. 2014;13:433–439. [PubMed] [Google Scholar]
- Jang S., Kelley K.W., Johnson R.W. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7534–7539. doi: 10.1073/pnas.0802865105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaria R.N., Kenny R.A., Ballard C.G., Perry R., Ince P., Polvikoski T. Towards defining the neuropathological substrates of vascular dementia. J. Neurol. Sci. 2004;226:75–80. doi: 10.1016/j.jns.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Kern S., Skoog I., Borjesson-Hanson A., Blennow K., Zetterberg H., Ostling S., Kern J., Gudmundsson P., Marlow T., Rosengren L., Waern M. Higher CSF interleukin-6 and CSF interleukin-8 in current depression in older women. Results from a population-based sample. Brain Behav. Immun. 2014;41:55–58. doi: 10.1016/j.bbi.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Kim S.M., Song J., Kim S., Han C., Park M.H., Koh Y., Jo S.A., Kim Y.Y. Identification of peripheral inflammatory markers between normal control and Alzheimer's disease. BMC Neurol. 2011;11:51. doi: 10.1186/1471-2377-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.S., Chung J.H., Choi T.K., Suh S.Y., Oh B.H., Hong C.H. Peripheral cytokines and chemokines in Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2009;28:281–287. doi: 10.1159/000245156. [DOI] [PubMed] [Google Scholar]
- Leung R., Proitsi P., Simmons A., Lunnon K., Guntert A., Kronenberg D., Pritchard M., Tsolaki M., Mecocci P., Kloszewska I., Vellas B., Soininen H., Wahlund L.O., Lovestone S. Inflammatory proteins in plasma are associated with severity of Alzheimer's disease. PLoS One. 2013;8:e64971. doi: 10.1371/journal.pone.0064971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licastro F., Pedrini S., Caputo L., Annoni G., Davis L.J., Ferri C., Casadei V., Grimaldi L.M. Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with Alzheimer's disease: peripheral inflammation or signals from the brain? J. Neuroimmunol. 2000;103:97–102. doi: 10.1016/s0165-5728(99)00226-x. [DOI] [PubMed] [Google Scholar]
- Loddick S.A., Turnbull A.V., Rothwell N.J. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J. Cereb. Blood Flow Metab. 1998;18:176–179. doi: 10.1097/00004647-199802000-00008. [DOI] [PubMed] [Google Scholar]
- Montine T.J., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Dickson D.W., Duyckaerts C., Frosch M.P., Masliah E., Mirra S.S., Nelson P.T., Schneider J.A., Thal D.R., Trojanowski J.Q., Vinters H.V., Hyman B.T., National Institute on Aging. Alzheimer's Association National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulugeta E., Molina-Holgado F., Elliott M.S., Hortobagyi T., Perry R., Kalaria R.N., Ballard C.G., Francis P.T. Inflammatory mediators in the frontal lobe of patients with mixed and vascular dementia. Dement. Geriatr. Cogn. Disord. 2008;25:278–286. doi: 10.1159/000118633. [DOI] [PubMed] [Google Scholar]
- Narasimhalu K., Lee J., Leong Y.L., Ma L., De Silva D.A., Wong M.C., Chang H.M., Chen C. Inflammatory markers and their association with post stroke cognitive decline. Int. J. Stroke. 2015;10:513–518. doi: 10.1111/ijs.12001. [DOI] [PubMed] [Google Scholar]
- Rafnsson S.B., Deary I.J., Smith F.B., Whiteman M.C., Rumley A., Lowe G.D., Fowkes F.G. Cognitive decline and markers of inflammation and hemostasis: the Edinburgh Artery Study. J. Am. Geriatr. Soc. 2007;55:700–707. doi: 10.1111/j.1532-5415.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- Richartz E., Batra A., Simon P., Wormstall H., Bartels M., Buchkremer G., Schott K. Diminished production of proinflammatory cytokines in patients with Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2005;19:184–188. doi: 10.1159/000083497. [DOI] [PubMed] [Google Scholar]
- Richartz E., Stransky E., Batra A., Simon P., Lewczuk P., Buchkremer G., Bartels M., Schott K. Decline of immune responsiveness: a pathogenetic factor in Alzheimer's disease? J. Psychiatr. Res. 2005;39(5):535–543. doi: 10.1016/j.jpsychires.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Richartz-Salzburger E., Batra A., Stransky E., Laske C., Kohler N., Bartels M., Buchkremer G., Schott K. Altered lymphocyte distribution in Alzheimer's disease. J. Psychiatr. Res. 2007;41:174–178. doi: 10.1016/j.jpsychires.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Ron-Harel N., Segev Y., Lewitus G.M., Cardon M., Ziv Y., Netanely D., Jacob-Hirsch J., Amariglio N., Rechavi G., Domany E., Schwartz M. Age-dependent spatial memory loss can be partially restored by immune activation. Rejuvenation Res. 2008;11:903–913. doi: 10.1089/rej.2008.0755. [DOI] [PubMed] [Google Scholar]
- Schmidt R., Schmidt H., Curb J.D., Masaki K., White L.R., Launer L.J. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann. Neurol. 2002;52:168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- Schram M.T., Euser S.M., de Craen A.J., Witteman J.C., Frolich M., Hofman A., Jolles J., Breteler M.M., Westendorp R.G. Systemic markers of inflammation and cognitive decline in old age. J. Am. Geriatr. Soc. 2007;55:708–716. doi: 10.1111/j.1532-5415.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- Singh V.K., Guthikonda P. Circulating cytokines in Alzheimer's disease. J. Psychiatr. Res. 1997;31:657–660. doi: 10.1016/s0022-3956(97)00023-x. [DOI] [PubMed] [Google Scholar]
- Smith C.J., Denes A., Tyrrell P.J., Di Napoli M. Phase II anti-inflammatory and immune-modulating drugs for acute ischaemic stroke. Expert Opin. Investig. Drugs. 2015;24:623–643. doi: 10.1517/13543784.2015.1020110. [DOI] [PubMed] [Google Scholar]
- Sudduth T.L., Schmitt F.A., Nelson P.T., Wilcock D.M. Neuroinflammatory phenotype in early Alzheimer's disease. Neurobiol. Aging. 2013;34:1051–1059. doi: 10.1016/j.neurobiolaging.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Lu C.J., Lin C.H., Wen L.L. Interleukin-1beta is increased in the cerebrospinal fluid of patients with small infarcts. Eur. J. Neurol. 2009;16:858–863. doi: 10.1111/j.1468-1331.2009.02609.x. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Tanaka K., Suzuki N. Ambivalent aspects of interleukin-6 in cerebral ischemia: inflammatory versus neurotrophic aspects. J. Cereb. Blood Flow Metab. 2009;29:464–479. doi: 10.1038/jcbfm.2008.141. [DOI] [PubMed] [Google Scholar]
- Swardfager W., Lanctot K., Rothenburg L., Wong A., Cappell J., Herrmann N. A meta-analysis of cytokines in Alzheimer's disease. Biol. Psychiatry. 2010;68:930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Tan J., Town T., Abdullah L., Wu Y., Placzek A., Small B., Kroeger J., Crawford F., Richards D., Mullan M. CD45 isoform alteration in CD4+ T cells as a potential diagnostic marker of Alzheimer's disease. J. Neuroimmunol. 2002;132:164–172. doi: 10.1016/s0165-5728(02)00309-0. [DOI] [PubMed] [Google Scholar]
- Vila N., Castillo J., Davalos A., Chamorro A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke. 2000;31:2325–2329. doi: 10.1161/01.str.31.10.2325. [DOI] [PubMed] [Google Scholar]
- Weaver J.D., Huang M.H., Albert M., Harris T., Rowe J.W., Seeman T.E. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- Westhoff D., Witlox J., Koenderman L., Kalisvaart K.J., de Jonghe J.F., van Stijn M.F., Houdijk A.P., Hoogland I.C., Maclullich A.M., van Westerloo D.J., van de Beek D., Eikelenboom P., van Gool W.A. Preoperative cerebrospinal fluid cytokine levels and the risk of postoperative delirium in elderly hip fracture patients. J. Neuroinflammation. 2013;10:122. doi: 10.1186/1742-2094-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley W., Chong W.L., Sengupta A., Sandercock P. Blood markers for the prognosis of ischemic stroke: a systematic review. Stroke. 2009;40:e380–e389. doi: 10.1161/STROKEAHA.108.528752. [DOI] [PubMed] [Google Scholar]
- Whiteley W., Wardlaw J., Dennis M., Lowe G., Rumley A., Sattar N., Welsh P., Green A., Andrews M., Sandercock P. The use of blood biomarkers to predict poor outcome after acute transient ischemic attack or ischemic stroke. Stroke. 2012;43:86–91. doi: 10.1161/STROKEAHA.111.634089. [DOI] [PubMed] [Google Scholar]
- Wright C.B., Sacco R.L., Rundek T., Delman J., Rabbani L., Elkind M. Interleukin-6 is associated with cognitive function: the Northern Manhattan Study. J. Stroke Cerebrovasc. Dis. 2006;15:34–38. doi: 10.1016/j.jstrokecerebrovasdis.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Kono K., Umegaki H., Yamada K., Iguchi A., Fukatsu T., Nakashima N., Nishiwaki H., Shimada Y., Sugita Y., Yamamoto T., Hasegawa T., Nabeshima T. Decreased interleukin-6 level in the cerebrospinal fluid of patients with Alzheimer-type dementia. Neurosci. Lett. 1995;186:219–221. doi: 10.1016/0304-3940(95)11318-q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.