Abstract
Purpose
The few studies to date that have examined the relationship between hemoglobin and fracture risk have focused on low hemoglobin values. The present study examined hip fracture risk across the hemoglobin distribution in older non-Hispanic white adults from the third National Health and Nutrition Examination Survey (NHANES III, 1988–1994).
Methods
Hemoglobin was measured using a Coulter S-plus Jr.® (Coulter Electronics, Hialeah, FL) in 2122 non-Hispanic whites age 65 years and older. Hip fracture cases were identified using linked Medicare and mortality records obtained through 2007. Cox proportional hazards models were used to assess the best-fitting model and to estimate the hazards ratio (HR) for hip fracture by hemoglobin decile before and after adjusting for selected confounders.
Results
There were 239 hip fracture cases in the analytic sample. The best fitting model was quadratic. When compared to values in the middle of the distribution, those with hemoglobin in the lowest and highest deciles had increased hip fracture risk (HRlowest decile =2.96, 95% CI 1.44–6.08; HRhighest decile = 2.06, 95% CI 1.09–3.92) after adjusting for age and sex. Both HRs remained significant after adjusting for additional confounders (HRlowest decile =2.24, 95% CI 1.09–3.92; HRhighest decile = 2.37, 95% CI 1.35–4.16).
Conclusions
Both low and high hemoglobin values were associated with increased hip fracture risk. The mechanism underlying the relationship is not clear, but there was some suggestion that it may differ for low versus high hemoglobin.
Keywords: Epidemiology, hemoglobin, hip fracture risk
Introduction
Low hemoglobin has been linked to impaired bone turnover and poor bone strength in animal models [1–3], and a small number of studies in humans have reported an association between low hemoglobin and low BMD [4–6]. Although anemia is common in the elderly [7], to date only two prospective studies and one retrospective open-cohort study have examined the relationship between low hemoglobin and risk of fracture or injurious falls that included fracture [8–10]. All three studies found low hemoglobin increased risk of fracture or injurious falls, but the two prospective studies differed as to whether the relationship remained significant in women after adjusting for selected confounders. In addition, these studies did not examine the possible role of common causes of anemia in the elderly [7], such as biochemically-defined iron or folate deficiency, inflammation, or renal insufficiency, as possible mechanisms underlying the low hemoglobin-fracture risk relationship.
The previous studies of hemoglobin and risk of fracture or injurious falls focused on risk among those with low hemoglobin, but high hemoglobin levels have also been associated with poor outcomes, including mobility disability and mortality [11–13]. The present study used linked Medicare and mortality data for non-Hispanic white respondents age 65 years and older from the third National Health and Nutrition Examination Survey (NHANES III) to assess hip fracture risk across the hemoglobin distribution. The relationship between hemoglobin and hip fracture risk was examined before and after adjusting for selected variables that included common causes of anemia in the elderly, as well as possible causes of elevated hemoglobin.
Methods
Sample
The baseline data for this study came from NHANES III, which was conducted in 1988–1994 by the National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention, to assess the health and nutritional status of a representative sample of the non-institutionalized, civilian population of the U.S. Data were collected via household interviews and physical examinations conducted in specially equipped mobile examination centers [14]. All procedures in NHANES III were approved by the NCHS Institutional Review Board, and written informed consent was obtained from all subjects [14].
NHANES III was designed to provide statistically reliable estimates for three race/ethnic groups: non-Hispanic whites (NHW), non-Hispanic blacks (NHB), and Mexican Americans (MA). Race and ethnicity were self-reported.
NHANES III was linked with data from mortality files created by NCHS and with Medicare enrollment and claims records in order to provide a follow-up component to the survey. Vital status of study participants from the date of their participation in NHANES III through December 31, 2006 was determined from the NHANES III Linked Mortality File [15]. This file contains mortality follow-up data based on a probabilistic match between the eligible NHANES III sample and the National Death Index (NDI). Vital status for the year 2007 was based on status from the Medicare Denominator file [16].
Medicare enrollment and utilization data were available for NHANES respondents who agreed to provide personal identification [16]. There were 8,585 persons in NHANES III who were age 65 years and older at the time of the Medicare linkage, of which 8,303 (97%) were eligible to be linked with Medicare. Of these 8,303 eligible respondents, 8,024 (97%) were successfully validated and matched with Medicare administrative records. Medicare claims data were provided from respondents who participated in fee-for-service care from 1991 through 2007. Claims data were not available for those in Medicare managed care plans. In the present study, claims data from the following files were used: Medicare Provider Analysis and Review (MEDPAR) Inpatient Hospital Stay File/Skilled Nursing Facility (SNF) File, Carrier Standard Analytic File, and Outpatient Standard Analytic File. The Medicare Denominator files were used to restrict the analytic sample to those who were successfully matched to the Medicare claims data, to obtain information about enrollment in managed care plans and to provide vital status for the year 2007.
The analytic sample in this study was restricted to respondents aged 65 years and older at the time of their NHANES interview at baseline. Preliminary analyses indicated that a significant interaction in the hemoglobin-hip fracture relationship existed by race/ethnicity, but there were insufficient hip fracture cases among non-Hispanic blacks and Mexican Americans to permit separate analyses in those groups. As a result, the analytic sample was also restricted to non-Hispanic whites.
Of the 3407 non-Hispanic white individuals age 65 years and older who were interviewed in NHANES III, 2122 (62%) remained in the analytic sample after exclusions had been made. Respondents were excluded if they: a) were interviewed but not examined in the mobile examination centers (n=867); b) had a prior hip fracture at baseline (n=121); c) were hip fracture cases with concurrent codes indicating care for a previous fracture or other bone diseases (n=24); d) were not eligible for linkage to the CMS Medicare denominator file2 (n=40); e) were eligible but could not be linked to the CMS Medicare denominator file (n=64); f) were enrolled in managed care at the time of the baseline examination (n=78); g) were decedents whose death certificate lacked cause of death information (n=8); or i) were missing hemoglobin (n=83).
Descriptive characteristics and risk factors were compared between non-Hispanic white respondents who were retained versus excluded from the analytic sample to assess the potential for nonresponse bias in the study results. The excluded respondents were more likely to be women and have lower hemoglobin, femur neck BMD and BMI than respondents who were included. They were also more likely to have renal insufficiency, be less active, and self-report having been told by a physician that they had non-skin cancer.
Fracture case identification
Hip fracture risk was examined in the present study because results from the Women’s Health Initiative from Chen et al [8] indicated that, of the various skeletal sites examined, the strongest relationship between hemoglobin and fracture risk occurred for hip fracture [8]. Hip fracture cases were identified using methods developed by Ray et al [17] and Taylor et al [18]. International Classification of Disease (ICD), Healthcare Common Procedure Coding System (HCPCS) or Current Procedural Terminology (CPT) codes for the years 1991–2007 [19, 20] were used to define cases. Cases identified from the MedPAR Hospital Inpatient/Skilled Nursing Facility files were based on ICD-9 diagnosis codes for any of 10 discharge diagnoses. Cases identified from the Carrier file were based on ICD-9 diagnosis codes for any of 5 diagnoses and a concurrent relevant HCPCS/CPT procedure code. Cases identified from the Outpatient File were based on relevant ICD-9 diagnosis codes for any of 11 diagnoses or on relevant ICD-9 surgical procedure codes; both required a concurrent relevant HCPCS/CPT code for any of 45 procedure variables. Respondents with codes indicating care of previous fracture or other bone diseases, neoplasm or hip arthroplasty for arthritis were excluded from the analyses. Detailed information describing the definition of hip fracture cases from Medicare records, including specific codes, have been published elsewhere [21].
Cause of death information from the NHANES III Linked Mortality File was also used to identify hip fracture. Persons with an ICD-9 code 820 or ICD-10 code S72.0-S72.2 listed as an underlying or multiple cause of death were defined as hip fracture cases (n=22 in the analytic sample).
A total of 239 hip fracture cases were identified in the analytic sample used in the present study. The majority of fracture cases (n=193, or 81% of hip fracture cases) in the analytic sample had diagnoses consistent with hip fracture on records from more than one file. Of the 230 hip fracture cases that were identified via inpatient hospital records, 205, or 89% also had relevant surgical codes for hip fracture.
Hemoglobin and hematocrit
Hemoglobin and hematocrit were measured on venous blood in the mobile examination centers using a Coulter S-plus Jr.® (Coulter Electronics, Hialeah, FL). Hematocrit was included in the present study to examine the prevalence of erythrocytosis, or excess red blood cells. Definitive erythrocytosis was defined as hematocrit > 60% for males and >56% for females, while suspected erythrocytosis was defined as hematocrit >51% for males and >48% for females [22].
Potential confounding variables
Potential confounding variables examined in the present study included factors that have previously been linked with hip fracture risk or with hemoglobin, including important causes of anemia among the elderly in the US [7] and secondary causes of elevated hemoglobin associated with erythrocytosis [22].
Biochemical indicators of iron, folate and vitamin B12 status
Serum biochemical indicators of iron, folate, and vitamin B12 status were measured at the National Center of Environmental Health, CDC, Atlanta GA. Details of the methods have been published elsewhere [23]. Briefly, serum ferritin was measured using a two site immunoradiometric assay (IRMA) (BioRad Laboratories, Hercules, CA). Transferrin saturation was calculated as serum iron divided by total iron-binding capacity, as measured by a CDC modification of the automated Technicon AAII-25® ferrozine colorimetric method (Alpkem TFA® analyzer; Alpkem, Clackamas, OR). Free erythrocyte protoporphyrin was measured in whole blood by fluorescence extraction using a modification of the Sassa method. Serum and red cell folate and serum vitamin B12 were measured with a radioimmunoassay kit ((QuantaPhase-I and QuantaPhase-II Folate Radioassay Kit, Bio-Rad Laboratories, Hercules, CA). Serum vitamin B12 was only measured during the second phase of NHANES III (1991–1994).
Iron deficiency was defined as having two or more of the following conditions: serum ferritin< 12 ng/mL, transferrin saturation< 15%, or free erythrocyte protoporphyrin > 70 [24]. Low folate status was defined as serum folate < 3 ng/mL or red cell folate < 140 ng/mL, while low vitamin B12 status was defined as serum vitamin B12 < 200 pg/mL [25].
Inflammation
Respondents were considered to have inflammation if they met any one of the following three conditions: serum C-reactive protein (CRP) > 1 mg/dL; plasma fibrinogen > 400 g/L, or low serum iron (defined as a value < 60 μg/dL) without evidence of iron deficiency as described in the previous section. The latter approach was used by Guralnik et al [7] to define anemia of chronic inflammation in their study of important causes of anemia in older persons. Serum CRP was measured at the University of Washington, Seattle WA by latex-enhanced nephelometry, while plasma fibrinogen was measured at White Sands Research Center, Alamogordo NM by comparing the clotting time of the plasma specimen with the clotting time of a standardized fibrinogen preparation [23].
Thyroid function
Serum thyroid stimulating hormone (TSH) was measured via a chemiluminescence immunometric assay kit (Nichols Instsitute Diagnostics, San Juan Capistrano, CA) at the University of Southern California Endocrine Services laboratory, Los Angeles CA.
Bone density and body mass index
Femur neck bone mineral density (BMD) was measured by dual energy x-ray absorptiometry (Hologic QDR 1000, Bedford MA) [26]. Body weight was measured to the nearest 0.01 kg using an electronic load cell scale, and standing height was measured with a fixed stadiometer. Body mass index (BMI) was calculated as body weight (kilograms) divided by height (meters squared).
Renal insufficiency
Renal insufficiency was defined using the approach of Guralnik et al [7]. Specifically, creatinine clearance was estimated using the Cockroft-Gault equation [27]:
Values for women were reduced by 15% [27]. Serum creatinine was measured on a Hitachi model 737 multichannel analyzer (Boehringer Mannheim Diagnostics, Indianapolis IN) by the Jaffe reaction. Renal insufficiency was defined as an estimated creatinine clearance < 30 mL/min.
Self-reported diagnosis of selected diseases
Variables related to selected heart or respiratory conditions and cancer were included as possible covariates since they may be secondary causes of high hemoglobin or erythrocytosis [22]. Specifically, self-reported doctor’s diagnosis of heart conditions, respiratory conditions, or cancer other than skin cancer were based on positive responses to the question: “Has a doctor ever told you that you had”: a) heart attack, congestive heart failure, or stroke; b) asthma, bronchitis, emphysema or hay fever; and c) cancers other than skin cancer.
Falls and timed chair stand
Falls were defined as the number of times in the previous 12 months that the respondent reported falling and landing on the floor or hitting an object. Timed chair stand was defined as time in seconds to rise five times from an armless chair [28].
Lifestyle behaviors
Respondents rated their current physical activity level as more active, less active, or about the same when compared to others of their same age and sex. Respondents also rated their general health status as poor, fair, good, very good or excellent. Cigarette use was categorized as ever smoked versus never smoked based on responses to questionnaire items asking “Have you ever smoked at least 100 cigarettes in your lifetime?” Alcohol users were defined as respondents who self-reported that they usually consumed three or more drinks per day when they drank alcohol.
Statistical Analysis
All analyses were performed using SAS 9.3 (SAS Institute, Cary NC) and SUDAAN software [29] for analysis of data from complex sample surveys. Descriptive characteristics and risk factors at baseline were screened for possible inclusion as confounders in subsequent analysis by using linear regression and chi-square analyses to examine their relationship with hip fracture status and hemoglobin (expressed as a continuous variable and also grouped into two categories).
Cox proportional hazards models were used to model time to event and to calculate estimates of the hazard ratio (HR) of hip fracture by hemoglobin while controlling for all risk factors simultaneously and accounting for unequal length of follow-up. Length of follow-up was calculated for hip fracture cases as the time from date of examination to date of diagnosis or procedure for those cases identified by Medicare records or date of death for those cases identified by death certificates. Follow-up time for non-cases was calculated as time from baseline exam to date of death for decedents, date of entry into managed care for those who enrolled in a Medicare managed care program after their baseline examination, or end of follow-up on December 31, 2007 for those who did not fall into the first two categories. A test of the proportional hazard assumption indicated no significant trend in HR with time (p=0.15), which suggests the assumption was not violated.
Preliminary analyses to assess significant interactions in the hemoglobin-hip fracture relationship by demographic characteristics identified a significant interaction by race/ethnicity, but not by age or sex in the non-Hispanic white sample, so subsequent analyses were performed for the total non-Hispanic white sample after adjusting for age and sex.
Preliminary analyses were also performed to evaluate the shape of the relationship between hemoglobin and fracture risk by fitting a series of Cox models that successively included linear, quadratic and cubic terms for hemoglobin in addition to adjusting for age and sex [30]. The best model was chosen based on the statistical significance of the linear, quadratic or cubic term for hemoglobin. Results of these analyses indicated that the relationship between hemoglobin and hip fracture risk was quadratic.
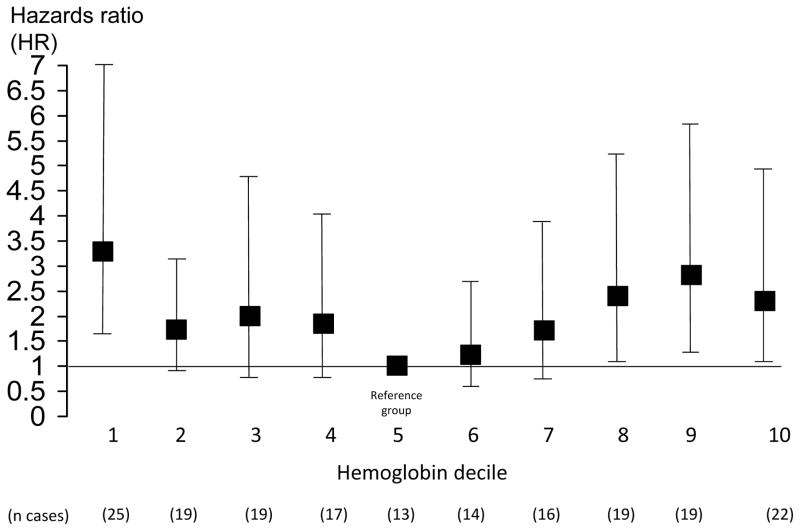
To further evaluate the shape of the relationship, the hemoglobin distribution was divided into sex-specific deciles, in order to use the smallest quantiles possible while still maintaining at least 10 hip fracture cases per quantile. The goal was to obtain reasonably stable estimates that could be used to visualize the relationship without making assumptions about its exact shape or location of possible inflection points. HRs were plotted using the fifth hemoglobin decile as the initial reference group (Figure 1). In subsequent analyses, the fifth and sixth deciles were combined to form the reference group in order to increase the number of hip fracture cases in that category. Cox proportional hazards models were used to calculate HRs for hip fracture by hemoglobin deciles after adjusting for age and sex (base model) as well as after adjusting simultaneously for all additional risk factors that were significantly related to fracture status or hemoglobin (full model).
Figure 1.
Hazard ratio (HR) for hip fracture by sex-specific hemoglobin decile
The potential role of the risk factors used in the full model to explain the hemoglobin-hip fracture relationship was evaluated by examining the change in the HR for the hemoglobin deciles when tested in the same model after adding each risk factor singly to the base Cox model, and comparing the HR for the base + individual risk factor with the HR for the base model alone.
Secondary analyses were performed to assess the impact of including serum ferritin in the multivariate Cox model as a continuous variable, since elevated ferritin can indicate inflammation, which can reduce hemoglobin values [7]. High ferritin has also been associated with low BMD [31]. HRs for the model that included ferritin as a continuous variable were compared with HRs from the main analysis, where low ferritin was used as part of the definition of iron deficiency. Secondary analyses were also conducted in women only, in order to facilitate a comparison with results from previous studies of hemoglobin and fracture risk [8, 9]. Finally, secondary analyses were performed to examine the effect of including serum vitamin B12 deficiency in the Cox model in addition to iron and folate deficiency, since vitamin B12 deficiency can result in anemia. This analysis was limited to 1051 respondents in the analytic sample who were examined in the second phase of NHANES III (1991–1994), and thus had serum vitamin B12 data. HRs for the multivariate model that included vitamin B12 deficiency in addition to iron and folate deficiency were compared with HRs from the multivariate model without vitamin B12 deficiency in this analytic subsample.
Results
Table 1 compares selected risk factors at baseline by hip fracture and hemoglobin status. Hemoglobin was divided into two categories (above/below the median) so that results for hemoglobin and hip fracture status could be summarized in a single table. Variables that differed significantly either by hip fracture status or hemoglobin group included age, sex, femur neck BMD, BMI, timed chair stands, iron or folate deficiency, inflammation, renal insufficiency, and smoking. These variables were subsequently included in the full multivariate Cox models used to assess hip fracture risk by hemoglobin status.
Table 1.
Relationship between baseline characteristics and hip fracture status or hemoglobin among non-Hispanic white adults age 65+ years, NHANES III follow-up
| Hip fracture
|
Hemoglobin (g/dL)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | < sex-specific median | >= sex-specific median | |||||
|
|
|
|||||||
| n | Mean or % | n | Mean or % | n | Mean or % | n | Mean or % | |
| Age (years) | 239 | 75.4 | 1833 | 73.4* | 905 | 74.5 | 1217 | 73* |
| Hemoglobin (g/dL) | 239 | 13.8 | 1883 | 14* | 905 | 12.9 | 1217 | 14.7* |
| Serum thyroid stim hormone (uU/mL) | 223 | 2.7 | 1797 | 2.7 | 878 | 2.8 | 1142 | 2.6 |
| Femur neck BMD (g/cm2) | 216 | 0.600 | 1734 | 0.695* | 822 | 0.673 | 1128 | 0.693* |
| Body mass index (height in cm2/weight in kg) | 239 | 25.3 | 1881 | 26.9* | 903 | 26.1 | 1217 | 27.1* |
| Times fallen in past year (n) | 237 | 0.42 | 1875 | 0.41 | 900 | 0.42 | 1212 | 0.41 |
| Time to complete five chair stands (seconds) | 208 | 14.3 | 1660 | 13.3* | 774 | 13.7 | 1094 | 13.2 |
| Sex | ||||||||
| Male | 87 | 29.8 | 957 | 45.9* | 461 | 43.6 | 583 | 44.8 |
| Female | 152 | 70.2 | 926 | 54.1 | 444 | 56.4 | 634 | 55.2 |
| Iron or folate deficiency | ||||||||
| Yes | 65 | 27.9 | 378 | 20.3* | 219 | 24.7 | 224 | 18.6* |
| No | 168 | 72.1 | 1475 | 79.7 | 673 | 75.3 | 970 | 81.4 |
| Inflammation | ||||||||
| Yes | 78 | 32.0† | 557 | 29.8 | 319 | 34.8 | 316 | 26.9* |
| No | 145 | 68 | 1217 | 70.2 | 543 | 65.2 | 819 | 73.1 |
| Renal insufficiency | ||||||||
| Yes | 20 | 6.1† | 131 | 4.9 | 103 | 7.8 | 48 | 3.2†* |
| No | 210 | 93.9 | 1694 | 95.1 | 779 | 92.2 | 1125 | 96.8 |
| Ever smoked | ||||||||
| Yes | 107 | 47.6 | 992 | 55.5* | 436 | 48.6 | 663 | 58.7* |
| No | 132 | 52.4 | 891 | 44.5 | 469 | 51.4 | 554 | 41.3 |
| Drink 3+ units per drinking occasion | ||||||||
| Yes | 9 | ‡ | 96 | 5.4 | 42 | 5.3† | 63 | 5.3† |
| No | 223 | 95.8 | 1751 | 94.6 | 838 | 94.7 | 1136 | 94.7 |
| Activity level compared to others of same age and sex | ||||||||
| More | 113 | 46 | 869 | 47 | 420 | 46.8 | 562 | 46.7 |
| Less | 28 | 12† | 224 | 12 | 111 | 11.9 | 141 | 12.4 |
| Same | 90 | 42† | 726 | 41 | 336 | 41.3 | 480 | 40.9 |
| Self-rated health status | ||||||||
| Poor/fair | 85 | 37.4 | 713 | 40.1 | 327 | 38.4 | 471 | 40.7 |
| Excellent/very good/good | 154 | 62.6 | 1165 | 59.9 | 576 | 61.6 | 743 | 59.3 |
| Self-reported diagnosed heart, lung, or non-skin cancer | ||||||||
| Yes | 104 | 44.1 | 847 | 43.4 | 405 | 43.3 | 546 | 43.6 |
| No | 134 | 55.9 | 1018 | 56.6 | 493 | 56.7 | 659 | 56.4 |
p < 0.05 based on linear regression (means) or chi-square (percents)
May be unreliable, relative standard error =30–39% or based on less than 12 degrees of freedom.
Unreliable; relative standard error ≥ 40% or insufficient degrees of freedom
The prevalence of suspected erythrocytosis, defined as a hematocrit value >51% for males and > 48% for females, was 1.2% (data not shown). No individuals in the analytic sample met the criteria for definitive erythrocytosis (hematocrit > 60% for males and > 56% for females).
HRs by hemoglobin categories are shown in Table 2 after adjusting for age and sex (base model) and after adjusting for the significant confounders identified as well (full model). When compared to the reference group (5th and 6th decile), those with hemoglobin in both the lowest and highest deciles had significantly increased hip fracture risk after adjusting for age and sex. The HR for hemoglobin decile 9 was also significantly higher than the reference group. Adjusting for the additional risk factors reduced the HR point estimate for the lowest hemoglobin decile, but it remained statistically significant. Adjusting for the additional risk factors increased the HR point estimate somewhat in the highest hemoglobin decile.
Table 2.
Hazard ratios (HR) for hip fracture by sex-specific hemoglobin decile Non-Hispanic white adults age 65+ years, NHANES III follow-up
| Sex-specific Hb percentile | Base model (Age, sex-adjusted) | Full multivariate model* | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n cases | HR | LL | UL | HR | LL | UL | |
| Sex-specific hemoglobin decile | |||||||
| 1 | 25 | 2.96 | 1.44 | 6.08 | 2.24 | 1.09 | 4.63 |
| 2 | 19 | 1.56 | 0.95 | 2.55 | 1.54 | 0.97 | 2.46 |
| 3 | 19 | 1.79 | 0.90 | 3.57 | 1.40 | 0.62 | 3.14 |
| 4 | 17 | 1.66 | 0.71 | 3.88 | 1.74 | 0.74 | 4.06 |
| 5–6 (reference) | 27 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 7 | 16 | 1.54 | 0.71 | 3.36 | 1.60 | 0.71 | 3.61 |
| 8 | 19 | 2.16 | 0.95 | 4.91 | 2.01 | 0.82 | 4.98 |
| 9 | 19 | 2.53 | 1.28 | 5.00 | 2.44 | 1.21 | 4.90 |
| 10 | 22 | 2.06 | 1.09 | 3.92 | 2.37 | 1.35 | 4.16 |
| p=0.05** | p=0.008** | ||||||
adjusted for age, sex, ever smoked, femur neck BMD, iron/folate deficiency, inflammation, renal insufficiency, BMI, timed chair stand.
p value for the overall relationship between hemoglobin and hip fracture risk
LL=lower limit of 95% confidence interval; UL=upper limit of 95% confidence interval
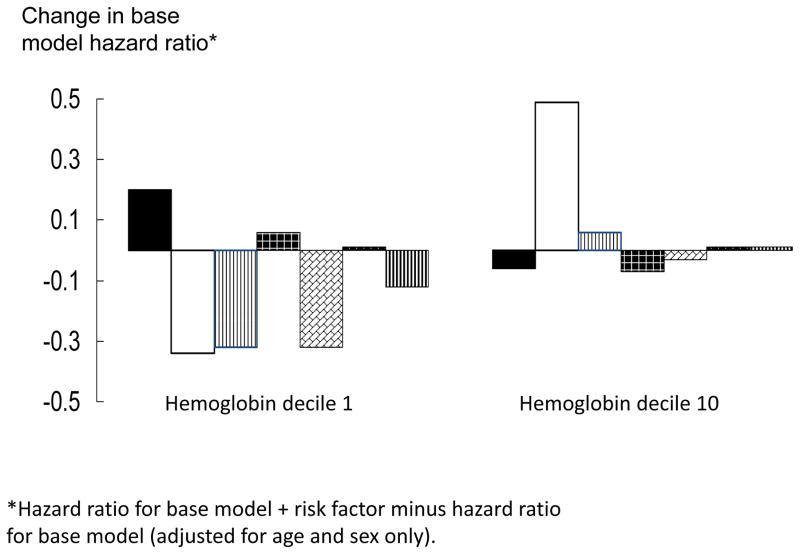
Table 3 summarizes results from analyses performed to examine the potential contribution of the risk factors used in the full model on the hemoglobin-hip fracture relationship in more detail. The impact of adding the risk factors singly was tested for all hemoglobin deciles in the same model, but the results are shown only for the first and tenth hemoglobin deciles because those deciles had the largest number of hip fracture cases. In decile 1, adding femur neck BMD, BMI and iron/folate deficiency separately to the base model each lowered the HR by approximately 0.3–0.4 units, suggesting that they had a similar effect on hemoglobin-hip fracture risk relationship in this decile. Adding smoking to the base model increased the HR in decile 1 by approximately 0.2 units, while the remaining risk factors changed the HR by 0.14 units or less. In hemoglobin decile 10, adding FNBMD to the base model increased the HR by 0.6 units, while the other variables changed the base model HR by 0.14 units or less. It is important to note that, while adding the risk factors to the base model changed the HR point estimates by these amounts, the 95% CI for the HRs from the base + one additional risk factor models overlapped with the base model HRs in each decile group.
Table 3.
Change in hazard ratio (HR) for risk factors added singly to base model for first and tenth hemoglobin decile, Non-Hispanic white adults age 65+ years, NHANES III follow-up
| Hemoglobin decile | HR | LL | UL | Difference from base model HR* |
|---|---|---|---|---|
| Decile 1 | ||||
| Base model (adjusted for age and sex) | 2.96 | 1.44 | 6.08 | |
| Base model + smoking | 3.14 | 1.51 | 6.56 | 0.18 |
| Base model + femur neck BMD | 2.57 | 1.29 | 5.12 | −0.39 |
| Base model + BMI | 2.66 | 1.29 | 5.48 | −0.30 |
| Base model + timed chair stand | 2.96 | 1.40 | 6.22 | 0 |
| Base model + iron/folate deficiency | 2.61 | 1.27 | 5.37 | −0.35 |
| Base model + inflammation | 2.97 | 1.49 | 5.92 | 0.01 |
| Base model + renal impairment | 2.82 | 1.34 | 5.90 | −0.14 |
| Decile 10 | ||||
| Base model (adjusted for age and sex) | 2.06 | 1.09 | 3.92 | |
| Base model + smoking | 1.97 | 1.04 | 3.74 | −0.09 |
| Base model + femur neck BMD | 2.65 | 1.49 | 4.71 | 0.59 |
| Base model + BMI | 2.20 | 1.19 | 4.06 | 0.14 |
| Base model + timed chair stand | 1.94 | 1.00 | 3.73 | −0.12 |
| Base model + iron/folate deficiency | 2.05 | 1.09 | 3.84 | −0.01 |
| Base model + inflammation | 2.06 | 1.09 | 3.92 | 0 |
| Base model + renal impairment | 2.06 | 1.09 | 3.91 | 0 |
Hazard ratio for base model + risk factor minus hazard ratio for base model only.
LL=lower limit of 95% confidence interval; UL=upper limit of 95% confidence interval
HRs obtained from the secondary analysis that included serum ferritin in the multivariate Cox model as a continuous variable were similar to those obtained in the main analyses which had focused on low ferritin only (data not shown). The relationship between hemoglobin and hip fracture risk also remained significant before and after adjusting for the selected risk factors when the analyses were limited to women only (data not shown). Finally, HRs for the multivariate model which included serum B12 deficiency were similar to those from the multivariate model without vitamin B12 deficiency (data not shown). Because conclusions were unchanged in all three sets of secondary analyses, only results from the main analyses are shown in the tables.
Discussion
Hemoglobin was significantly related to hip fracture risk in a nonlinear fashion among the older US white adults examined in the present study before and after adjusting for several potential confounding factors. The relationship appeared to be U-shaped, as both low and high hemoglobin values were associated with higher hip fracture risk than hemoglobin values in center of the distribution. The HRs for high or low hemoglobin were similar in magnitude to those reported for several of the risk factors currently used in the FRAX© model, such as femur neck BMD, previous fracture, or smoking [32–35]. U-shaped and J-shaped relationships have been reported previously between hemoglobin and mortality [11–13] or mobility disability [11], but the present study appears to be the first to report a U-shaped relationship between hemoglobin and fracture risk. Previous studies of the hemoglobin-fracture risk relationship focused on risk in anemic versus non-anemic women [8, 10] or used a linear model in which the highest hemoglobin tertile was the reference group [9].
The mechanism through which hemoglobin affects hip fracture risk is not clear. Results from the present study suggest that different factors may play a role at the two tails of the hemoglobin distribution. For example, adjusting for various risk factors reduced the magnitude of the point estimate for the hemoglobin in the lowest decile when compared to hemoglobin in the middle of the distribution, but increased the HR point estimate in the highest hemoglobin decile. The analyses in which risk factors were added singly to the base model also suggested different roles for the risk factors at low versus high hemoglobin. In the lowest decile, adding femur neck BMD, BMI, and iron/folate deficiency each attenuated the base model HR point estimate by a similar amount, while the other risk factors had smaller separate impacts. In contrast, adjusting separately for BMI or iron/folate deficiency had little impact on the base model HR estimate in the highest hemoglobin decile, while adjusting for femur neck BMD increased the base model HR point estimate. It is also interesting to note that, of the important causes of anemia in older person that could be considered in this study, iron/folate deficiency appeared to have more impact than inflammation or renal insufficiency on the hemoglobin-hip fracture relationship in the lowest HB group. However, the HR estimates for these “base plus single risk factor” models had 95% CI that overlapped the base model HR, which precludes firm conclusions.
Some have postulated that low hemoglobin is linked to poor outcomes directly via its effect on oxygen delivery to tissues [36], while others have suggested that, rather than being causative, low hemoglobin reflects poor general health [37]. Some data suggest anemia has an independent effect on mortality and that correcting anemia may reduce morbidity from cardiovascular or kidney disease and certain cancers [38]. Anemia may also act together with common comorbidities to increase risk of outcomes like frailty. Anemia has been linked to a number of potential pathways to fracture, including falls, reduced cognition, decreased physical activity and decreased BMD [36].
Mechanisms linking high hemoglobin to poor outcomes have also not been clearly identified. High hemoglobin is related to blood viscosity, which may compromise cardiovascular function by causing endothelial damage in blood vessels. The resulting inflammation could possibly impact bone in a negative manner. Blood viscosity could not be assessed in the present study, but, based on hematocrit criteria, no respondents had definitive erythrocytosis and only 1% had suspected erythrocytosis. Thus, excessive blood viscosity seems unlikely to explain the higher fracture risk seen among those with high hemoglobin. Inflammation also seems unlikely to have played an important role, since adjusting for inflammation did not noticeably decrease HRs in the higher hemoglobin categories. High hemoglobin can also be a secondary outcome of various heart, renal and lung conditions [22], which in turn could potentially play a role in the link between high hemoglobin and fracture. Renal insufficiency and self-reported heart, lung and non-skin cancer diagnoses were among the variables considered for inclusion in the full model in the present study, but only renal insufficiency met the criteria of being significantly related to either hip fracture status or hemoglobin. Its inclusion in in the full model had little effect on the HR for the highest hemoglobin group.
In addition to finding a nonlinear relationship between hemoglobin and hip fracture risk, results from the present study differed from two of the previous studies on this topic regarding differences in the relationship by some demographic variables. For example, Jorgensen et al [9] reported that the relationship between hemoglobin and fracture risk was no longer significant in women after adjusting for confounding variables, but adjusting for confounders did not remove the relationship among women in the present study. Use of different confounders and fracture outcomes in the two studies could potentially explain these differences. The present study also found a significant interaction in the hemoglobin-fracture relationship by race/ethnicity, whereas Chen et al [9] found no race/ethnic differences in the relationship. The reason for these different findings is not clear, but race/ethnic differences in the relationship between hemoglobin and other outcomes, such as mortality or mobility disability [39–41], have been reported. Finally, Chen et al [8] noted that the hemoglobin-fracture relationship was stronger in women over age 70 than in younger women, but no difference by age was found in the present study when results were compared by age 65–79 years versus 80+ years. Use of different age categories in the two studies could potentially explain the difference in findings by age.
Strengths of the present study include use of a cohort derived from a nationally representative sample and the availability of variables that have been identified as important causes of both low and high hemoglobin levels. However, not all relevant variables could be considered in the study. For example, genetic disorders that lead to high hemoglobin were not assessed in NHANES III, and data for secondary causes of elevated hemoglobin, such as certain cardiovascular and respiratory conditions or selected cancers, were limited to self-reported data for broad categories of cancer and heart or lung conditions. The very low prevalence of erythrocytosis observed in the study sample suggests that the genetic disorders were unlikely to play an important role in explaining the observed relationship between high hemoglobin and hip fracture risk, but the self-reported data on heart, lung and non-skin cancers may have been too broad to capture the relevant information for those conditions. Living at an altitude over 3000 feet can also elevate hemoglobin [42], but there were only two data collection locations in NHANES III over this altitude, so altitude appears unlikely to have played a significant role in the study results. Serum vitamin B12 data were not available for the entire analytic sample, but results from the secondary analyses using the serum vitamin B12 subsample from second phase of NHANES III suggested that its inclusion would not have significantly altered study results. Finally, the number of hip fracture cases in each hemoglobin decile was small, which likely limited power to detect significant relationships.
Other limitations include hip fracture identification based on Medicare and death records without confirmation by x-ray. However the vast majority of cases had codes indicating hip fracture diagnosis on multiple sources of Medicare records, and hospitalized cases had concurrent codes indicating relevant surgical procedures for hip fracture. A small number of hip fracture cases may be mistakenly classified as non-cases due to lack of Medicare records prior to 1991--we previously estimated that 13 undetected hip fracture cases may have occurred during 1988–90 [21]. In addition, study results apply only to the segment of the population ages 65+ years that was not institutionalized at baseline and participated in Medicare fee-for-service programs. Exclusions for missing data or loss to follow-up were also made. Excluded respondents were more likely to have lower hemoglobin and lower BMD than respondents in the analytic sample, which could impact the generalizability of results for hip fracture risk among those with low hemoglobin. Nonrespondents were also more likely to be female, and have lower BMI and other selected characteristics associated with poor health than respondents who were included. Thus, although based on a cohort derived from nationally representative sample at baseline, results from the present study cannot be generalized to the entire adult population over age 65 years.
In summary, both high and low hemoglobin values were related to increased hip fracture risk in older non-Hispanic whites. Reasons for this relationship are unclear, although there was some suggestion that the factors underlying this relationship may differ for low versus high hemoglobin. Others have noted that, despite being relatively common, anemia in older persons may be overlooked in clinical settings and have called for more research and better guidelines for addressing this condition in this age group [36–38, 43]. Results from the present study support a focus on both low and high hemoglobin levels among older persons.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Respondents were ineligible for linkage to CMS administrative records if they were missing key identification data and/or if they did not provide their Social Security or Medicare number at baseline or did not have a Social Security number verified by the Social Security Administration's Enumeration Verification System.
Conflict of Interest: Anne Looker declares that she has no conflicts of interest
References
- 1.Lobo A, Gaievski E, Colli C. Hemoglobin Regeneration Efficiency in Anemic Rats: Effects on Bone Mineral Composition and Biomechanical Properties. Biol Trace Elem Res. 2011;143:403–411. doi: 10.1007/s12011-010-8871-2. [DOI] [PubMed] [Google Scholar]
- 2.Katsumata S-i, Tsuboi R, Uehara M, Suzuki K. Dietary Iron Deficiency Decreases Serum Osteocalcin Concentration and Bone Mineral Density in Rats. Bioscience, Biotechnology, and Biochemistry. 2006;70:2547–2550. doi: 10.1271/bbb.60221. [DOI] [PubMed] [Google Scholar]
- 3.Katsumata S-i, Katsumata-Tsuboi R, Uehara M, Suzuki K. Severe Iron Deficiency Decreases Both Bone Formation and Bone Resorption in Rats. The Journal of Nutrition. 2009;139:238–243. doi: 10.3945/jn.108.093757. [DOI] [PubMed] [Google Scholar]
- 4.Cesari M, Pahor M, Lauretani F, et al. Bone density and hemoglobin levels in older persons: results from the InCHIANTI study. Osteoporosis International. 2005;16:691–699. doi: 10.1007/s00198-004-1739-6. [DOI] [PubMed] [Google Scholar]
- 5.Laudisio A, Marzetti E, Pagano F, Bernabei R, Zuccala G. Haemoglobin levels are associated with bone mineral density in the elderly: a population-based study. Clin Rheumatol. 2009;28:145–151. doi: 10.1007/s10067-008-0998-6. [DOI] [PubMed] [Google Scholar]
- 6.Korkmaz U, Korkmaz N, Yazici S, Erkan M, Baki AE, Yazici M, Ozhan H, Ataoglu S. Anemia as a risk factor for low bone mineral density in postmenopausal Turkish women. Eur J Intern Med. 2012;23:154–158. doi: 10.1016/j.ejim.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Thomson CA, Aickin M, Nicholas JS, Van Wyck D, Lewis CE, Cauley JA, Bassford T, Investigato WHI. The Relationship Between Incidence of Fractures and Anemia in Older Multiethnic Women. Journal of the American Geriatrics Society. 2010;58:2337–2344. doi: 10.1111/j.1532-5415.2010.03183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jorgensen L, Skjelbakken T, Lochen ML, Ahmed L, Bjornerem A, Joakimsen R, Jacobsen BK. Anemia and the risk of non-vertebral fractures: the Tromso Study. Osteoporosis International. 2010;21:1761–1768. doi: 10.1007/s00198-009-1131-7. [DOI] [PubMed] [Google Scholar]
- 10.Duh MS, Mody SH, Lefebvre P, Woodman RC, Buteau S, Piech CT. Anaemia and the risk of injurious falls in a community-dwelling elderly population. Drugs & aging. 2008;25:325–334. doi: 10.2165/00002512-200825040-00005. [DOI] [PubMed] [Google Scholar]
- 11.Chaves PHM, Ashar B, Guralnik JM, Fried LP. Looking at the Relationship Between Hemoglobin Concentration and Prevalent Mobility Difficulty in Older Women. Should the Criteria Currently Used to Define Anemia in Older People be Reevaluated? Journal of the American Geriatrics Society. 2002;50:1257–1264. doi: 10.1046/j.1532-5415.2002.50313.x. [DOI] [PubMed] [Google Scholar]
- 12.Zakai Na KRHC, et al. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: The cardiovascular health study. Archives of internal medicine. 2005;165:2214–2220. doi: 10.1001/archinte.165.19.2214. [DOI] [PubMed] [Google Scholar]
- 13.Culleton BF, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Hemmelgarn BR. Impact of anemia on hospitalization and mortality in older adults. Blood. 2006;107:3841–3846. doi: 10.1182/blood-2005-10-4308. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention National Center for Health Statistics; Department of Health and Human Services, editor. Series 1: Programs and collection procedures. Centers for Disease Control and Prevention, National Center for Health Statistics; Hyattsville MD: 1994. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–94; pp. 1–407. [Google Scholar]
- 15.Centers for Disease Control and Prevention National Center for Health Statistics. NCHS data linked to mortality files. Centers for Disease Control and Prevention, National Center for Health Statistics; 2012. http://www.cdc.gov/nchs/data_access/data_linkage/mortality.htm. [Google Scholar]
- 16.Centers for Disease Control and Prevention National Center for Health Statistics. NCHS Data linked to CMS Medicare enrollment and claims files. Centers for Disease Control and Prevention, National Center for Health Statistics; 2012. http://www.cdc.gov/nchs/data_access/data_linkage/CMS_Medicare.htm. [Google Scholar]
- 17.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. Journal of Clinical Epidemiology. 1992;45:703–714. doi: 10.1016/0895-4356(92)90047-q. [DOI] [PubMed] [Google Scholar]
- 18.Taylor AJ, Gary LC, Arora T, et al. Clinical and demographic factors associated with fractures among older Americans. Osteoporosis International. 2011;22:1263–1274. doi: 10.1007/s00198-010-1300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Association AM. CPT (Current Procedural Terminology) Assistant Archives 1990–2009. American Medical Association; Chicago IL: 2010. [Google Scholar]
- 20.National Center for Health Statistics. Conversion Table of New ICD-9-CM Codes. National Center for Health Statistics; 2010. http://www.cdc.gov/nchs/data/icd/ICD-9-CM_FY14_CNVTBL_Final.pdf. [Google Scholar]
- 21.Looker AC. Serum 25-hydroxyvitamin D and risk of major osteoporotic fractures in older U.S. adults. J Bone Miner Res. 2013;28:997–1006. doi: 10.1002/jbmr.1828. [DOI] [PubMed] [Google Scholar]
- 22.McMullin MF. The classification and diagnosis of erythrocytosis. International journal of laboratory hematology. 2008;30:447–459. doi: 10.1111/j.1751-553X.2008.01102.x. [DOI] [PubMed] [Google Scholar]
- 23.Gunter EWLB, Kokickowski SM. Centers for Disease Control NCfHS, editor. Laboratory methods used for the third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Centers for Disease Control, National Center for Health Statistics; 1996. http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/labman.pdf. [Google Scholar]
- 24.Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA. 1997;277:973–976. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- 25.Pfeiffer CM, Johnson CL, Jain RB, Yetley EA, Picciano MF, Rader JI, Fisher KD, Mulinare J, Osterloh JD. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988–2004. Am J Clin Nutr. 2007;86:718–727. doi: 10.1093/ajcn/86.3.718. [DOI] [PubMed] [Google Scholar]
- 26.Wahner HW, Looker A, Dunn WL, Walters LC, Hauser MF, Novak C. Quality control of bone densitometry in a national health survey (NHANES III) using three mobile examination centers. J Bone Miner Res. 1994;9:951–960. doi: 10.1002/jbmr.5650090621. [DOI] [PubMed] [Google Scholar]
- 27.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 28.Ostchega Y, Harris TB, Hirsch R, Parsons VL, Kington R, Katzoff M. Reliability and prevalence of physical performance examination assessing mobility and balance in older persons in the US: data from the Third National Health and Nutrition Examination Survey. J Am Geriatr Soc. 2000;48:1136–1141. doi: 10.1111/j.1532-5415.2000.tb04792.x. [DOI] [PubMed] [Google Scholar]
- 29.Research Triangle Institute. SUDAAN Language Manual, Release 10.0. Research Triangle Institute; Research Triangle Park, NC: 2008. [Google Scholar]
- 30.Korn EG, BI . Analysis of Health Surveys. John Wiley & Sons; New York: 1999. [Google Scholar]
- 31.Li GF, Pan YZ, Sirois P, Li K, Xu YJ. Iron homeostasis in osteoporosis and its clinical implications. Osteoporosis International. 2012;23:2403–2408. doi: 10.1007/s00198-012-1982-1. [DOI] [PubMed] [Google Scholar]
- 32.Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. Journal of Bone and Mineral Research. 2005;20:1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 33.Kanis JA, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Kanis JA, Johnell O, Oden A, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16:155–162. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 35.Kanis JA. Assessment of osteoporosis at the primary health-care level. World Health Organization; Geneva: 2007. WHO Scientific Group on Assessment of Osteoporosis at the Primary Health-care Level. [Google Scholar]
- 36.Chaves PHM. Functional Outcomes of Anemia in Older Adults. Seminars in Hematology. 2008;45:255–260. doi: 10.1053/j.seminhematol.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Merchant AA, Roy CN. Not so benign haematology: anaemia of the elderly. British Journal of Haematology. 2012;156:173–185. doi: 10.1111/j.1365-2141.2011.08920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nissenson AR, Goodnough LT, Dubois RW. Anemia: Not just an innocent bystander? Archives of internal medicine. 2003;163:1400–1404. doi: 10.1001/archinte.163.12.1400. [DOI] [PubMed] [Google Scholar]
- 39.Patel KV, Harris TB, Faulhaber M, Angleman SB, Connelly S, Bauer DC, Kuller LH, Newman AB, Guralnik JM. Racial variation in the relationship of anemia with mortality and mobility disability among older adults. Blood. 2007;109:4663–4670. doi: 10.1182/blood-2006-10-055384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong X, de Leon CM, Artz A, Tang Y, Shah R, Evans D. A Population-Based Study of Hemoglobin, Race, and Mortality in Elderly Persons. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2008;63:873–878. doi: 10.1093/gerona/63.8.873. [DOI] [PubMed] [Google Scholar]
- 41.Patel KV, Longo DL, Ershler WB, Yu B, Semba RD, Ferrucci L, Guralnik JM. Haemoglobin concentration and the risk of death in older adults: differences by race/ethnicity in the NHANES III follow-up. British Journal of Haematology. 2009;145:514–523. doi: 10.1111/j.1365-2141.2009.07659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prevention CfDCa. Recommendations to prevent and control iron deficiency in the United States. Centers for Disease Control and Prevention. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control. 1998;47:1–29. [PubMed] [Google Scholar]
- 43.Guralnik JM, Ershler WB, Schrier SL, Picozzi VJ. Anemia in the Elderly: A Public Health Crisis in Hematology. ASH Education Program Book. 2005;2005:528–532. doi: 10.1182/asheducation-2005.1.528. [DOI] [PubMed] [Google Scholar]