Abstract
In Brazil, colorectal cancer (CRC) is the fourth most common cause of cancer-related death among men, and the third most common among women. We aimed to examine CRC screening-related knowledge, attitudes, and practices among physicians and nurses working in Brazil’s network of health units, and to describe the capacity of these units for CRC screening. In 2011, 1600 health units were randomly selected from all 26 states and the Federal District. One coordinator and one health care provider were selected for the interview. Response rates were 78% for coordinators, 34% for physicians, and 65% for nurses. The Brazilian National Cancer Institute (INCA) recommendations for CRC screening were not often used in the health units, but screening outreach and use of CRC exams were more common in units that were using them. Physicians and nurses differed in most characteristics, and in their knowledge, attitudes, and practices of CRC screening. Forty-seven percent of physicians reported not conducting CRC screening compared to 65% of nurses. Fecal occult blood test was most often used by physicians and nurses, but fewer physicians than nurses perceived this exam as very effective in reducing CRC mortality. Physicians’ gender, years since graduation, and geographical region of practice in Brazil were associated to CRC screening practice. The findings may reflect the low influence of INCA CRC screening recommendations, physicians receiving their medical education when CRC burden in Brazil was of low concern, and the lack of CRC screening capacity in some regions of Brazil.
Keywords: Colorectal neoplasms; Health care surveys; Mass screening; Health Knowledge, Attitudes, Practice; Cross-Sectional Studies; Health Personnel
Introduction
In Brazil, colorectal cancer (CRC) is the fourth most common cause of cancer-related death among men, and the third most common among women (International Agency for Research on Cancer, 2013). In 2012, the age-standardized CRC mortality rate for both sexes was 8.0 per 100,000 people (International Agency for Research on Cancer, 2013). The Brazilian National Cancer Institute (INCA) estimated that over 30,000 new CRC cases were expected in 2012, with higher incidence in the South (38 per 100,000) and Southeast regions (45 per 100,000) compared to the North (9 per 100,000) (Instituto Nacional do Câncer, Ministério da Saúde, Brasil, 2011).
Increases in CRC incidence and mortality have been predicted for South American countries in the coming years (Center et al., 2009; Jemal et al., 2010). CRC incidence and mortality in adults aged 50–75 years can be reduced through screening exams such as a fecal occult blood test (FOBT), flexible sigmoidoscopy, or colonoscopy because these tests can detect early-stage cancer and precancerous adenomatous polyps (Calonge et al., 2008). Despite Brazil’s federally mandated Unified Health System with its network of basic health units, which offer comprehensive primary care services, the country does not have a national policy or government program that addresses CRC prevention and control (Paim et al., 2011; Pan-American Health Organization, 2013). Since 2002, INCA has recommended an annual FOBT for asymptomatic people aged 50 years or older and colonoscopy in case of a positive screening test (Instituto Nacional do Câncer, 2002); however, very little data about CRC screening rates are available (Coy, 2013; Perez et al., 2008; Schmidt et al., 2011).
Recommendations from physicians and nurses for patients to participate in CRC cancer screening are an important factor in patient CRC screening adherence (Brawarsky et al., 2004; Khalid-de Bakker et al., 2011; O’Malley et al., 2004; Vernon, 1997). In Brazil, knowledge, attitudes, and practices of physicians and nurses regarding CRC screening have not been extensively explored, especially by region. The primary aim of this study was to explore CRC screening knowledge, attitudes, and practices of physicians and nurses in the Brazilian network of health units at a time when the country has a growing CRC burden but no national policy or program. Another aim was to describe the capacity of health units for CRC screening throughout Brazil.
Methods
In 2011, a telephone survey was administered to health unit coordinators, physicians, nurses, and community health workers (CHW) as part of the Guide for Useful Interventions for Physical Activity in Brazil and Latin America (GUIA) project. The extensive questionnaire included themes related to physical activity, nutrition, and breast, cervical, and colorectal cancers. The questionnaire targeting health unit coordinators contained 54 questions, of which three were related to CRC. The questionnaire for health care providers had a total of 79 questions, of which five were related to CRC. Questions were drafted in English, translated to Portuguese by native speakers, and back-translated to English. The final Portuguese version of the questionnaire was piloted among a small group of participants in Brazil. From a list of 42,486 health care units provided by the Ministry of Health, 1600 were randomly sampled from all regions of Brazil. The sample was formed by targeting the coordinator and the physician in unit 1, the coordinator and the nurse in unit 2, and the coordinator and the community health worker in unit 3, so that the total target sample included 1600 coordinators, 534 physicians, 533 nurses, and 533 CHWs. If, for example, more than one physician worked in unit 1, the interviewer asked the coordinator to provide a list with all of them. Of that list, one physician would be sampled using a table with random numbers. One coordinator (n = 1600) and one physician (n = 534), nurse (n = 533), or CHW (n = 533) were selected from each unit for the interview. Interviews lasted 40 minutes on average. We limited our analyses to coordinators, physicians, and nurses’ responses since they are responsible for CRC screening activities in Brazil, and CHWs were not asked CRC-related questions. The overall response rate was 50% across all professional categories (78% for coordinators, 34% for physicians, and 65% for nurses). More details about the design and sampling methods can be found elsewhere (Florindo et al., 2013; Stormo et al., 2014). This study was reviewed and approved by the Research Ethics Committee of the Federal University of Pelotas, and the institutional review boards of Washington University in St. Louis and the U.S. Centers for Disease Control and Prevention.
Basic health units are formed by multidisciplinary teams of physicians, nurses and nurse assistants, and CHWs. Health unit coordinators are responsible for managing the work process in the unit, translating national health policies to the unit context, and maintaining the connection between these policies, health care providers, and the local population. Therefore, they were asked about unit characteristics, use of CRC screening recommendations, outreach for CRC screening, and use of CRC exams in the unit (Appendix A). Use of CRC screening recommendations and outreach were assessed only if coordinators concomitantly knew the INCA recommendations for cancer screening, and generally used cancer screening recommendations in the unit.
Physicians and nurses answered questions about use of INCA CRC screening recommendations, familiarity with CRC screening exams, perceived exam effectiveness to reduce CRC mortality, and use of exams (Appendix B). Knowledge of CRC screening methods was determined through questions about familiarity with FOBT and sigmoidoscopy. Answers were dichotomized as “more familiar” (“very familiar,” “familiar”) and “less familiar” (“little familiarity,” “not familiar”). Attitudes about CRC screening were assessed with questions about effectiveness of FOBT, sigmoidoscopy, and colonoscopy, which were re-categorized as “very effective” and “not very effective” (including “little effectiveness,” “not effective,” “effectiveness unknown,” “I don’t know”); and perceived influence of INCA screening recommendations, for which a binary variable was created with responses “very influential” and “not very influential” (“little influential,” “not influential,” “I don’t know”). Screening practice was dichotomized as “screening” (“less than 50 years,” “50–55 years,” “56–61 years,” “62–67 years,” and “other”) and “no screening” (“I do not perform CRC screening”). Physicians and nurses who reported to start routine CRC screening for a specific age group were asked about use of FOBT, sigmoidoscopy, and colonoscopy in the unit.
Statistical analyses
Descriptive analyses were performed to summarize sample characteristics, health unit capacity, and CRC screening knowledge, attitudes, and practices among physicians and nurses. Continuous variables were presented as medians with 25th and 75th percentiles, and discrete variables as frequencies and percentages. The Pearson chi-square test was used for discrete variables. The Wilcoxon rank-sum test was used to test for differences among continuous variables. The main response variable analyzed was physicians’ screening practices. Bivariate analyses were performed to compare characteristics of physicians who screen to those who do not screen. Logistic regression was used to explore which factors contribute to physicians not conducting screening. Odds ratios (OR) with 95% confidence intervals (95% CI) were reported as an estimate of the magnitude of association. A full model was created and backward selection with α = 0.4 used as the significance level for maintaining the covariates in the model. Statistical testing in the models was performed using Wald chi-square tests. Covariates of interest were age, years since graduation, hours working in the unit per week, and patients seen per week, as continuous variables; gender, country region, influence of INCA recommendations, familiarity with FOBT exam, and perception of FOBT and colonoscopy effectiveness, as categorical variables. The linearity assumption for the continuous variables was assessed using restricted cubic spline functions. Because there was no evidence of nonlinearity, the continuous variables are presented as linear effects in the final model. Robust regression diagnostic methods were employed to assess the fit-ness and adequacy of regression models (Hosmer et al., 1991; Kleinbaum et al., 1987). Statistical analyses were performed with SAS v.9.3 (Cary, NC).
Results
Unit capacity for CRC screening
Out of 1600 coordinators, 1251 answered the survey. Knowledge of INCA cancer screening recommendations in general was reported by 655 coordinators (52%), and of these, 596 (90%) declared that their unit followed the recommendations. Twenty-five percent of units were leading CRC screening outreach activities. FOBT was used in 50% of the units, while sigmoidoscopy was used in 17%, and colonoscopy in 26% (data not shown). Thirty-six percent (n = 209) reported the use of INCA recommendations for CRC screening in their unit (Table 1). In the Central-West Region of Brazil, 41% of units were using INCA recommendations for CRC screening, while 36% were using them in the South and Northeast, 35% in the Southeast, and 28% in the North. In addition, use of INCA CRC recommendations was 37% among units that covered a population of 5000 people or less; 29% among those that covered between 5001 and 15,000, and 31% in units covering more than 15,000 people. For patients seen per month, 38% of units that received less than 500 monthly visits used INCA CRC screening recommendations, whereas 33% of those receiving between 501 and 1000 visits per month, and 31% of those with over 1000 monthly visits used them. However, the differences in use of INCA recommendations for CRC screening by region, population covered, or patients seen per month were not statistically significant. CRC screening outreach and use of CRC exams were higher (p < 0.001) in units using INCA recommendations for CRC screening compared to units that were not using them (64% vs. 3% for outreach; 69% vs. 39% for FOBT use; 26% vs. 12% for sigmoidoscopy use; and 38% vs. 20% for colonoscopy use).
Table 1.
Use of INCA recommendations for colorectal cancer screening in Brazil by unit characteristics and capacity.a Unit coordinators survey; Guide for Useful Interventions for Physical Activity 2011.
| Use of INCA recommendations for CRC screening
|
|||||
|---|---|---|---|---|---|
| Yes
|
No
|
|
|||
| N | % | N | % | P-valueb | |
| Total of units | 209 | 36 | 375 | 64 | |
| Unit characteristics | |||||
| Country region | 0.80 | ||||
| South | 39 | 36 | 70 | 64 | |
| Southeast | 65 | 35 | 119 | 65 | |
| Central-West | 21 | 41 | 30 | 59 | |
| Northeast | 73 | 36 | 128 | 64 | |
| North | 11 | 28 | 28 | 72 | |
| Total population covered | 0.36 | ||||
| ≤5000 | 142 | 37 | 241 | 63 | |
| 5001–15,000 | 23 | 29 | 55 | 71 | |
| >15,000 | 14 | 31 | 31 | 69 | |
| Patients per month | 0.35 | ||||
| ≤500 | 81 | 38 | 131 | 62 | |
| 501–1000 | 47 | 33 | 95 | 67 | |
| >1000 | 38 | 31 | 85 | 69 | |
| Unit capacity for CRC screening | |||||
| CRC screening outreach | <0.001 | ||||
| Yes | 134 | 64 | 10 | 3 | |
| No | 74 | 36 | 363 | 97 | |
| FOBT use | <0.001 | ||||
| Yes | 136 | 69 | 140 | 39 | |
| No | 62 | 31 | 223 | 61 | |
| Sigmoidoscopy use | <0.001 | ||||
| Yes | 49 | 26 | 43 | 12 | |
| No | 140 | 74 | 307 | 88 | |
| Colonoscopy use | <0.001 | ||||
| Yes | 75 | 38 | 70 | 20 | |
| No | 122 | 62 | 287 | 80 | |
CRC: colorectal cancer; INCA: Brazilian National Cancer Institute; FOBT: fecal occult blood test.
Only coordinators who self-reported knowledge of INCA cancer screening recommendations and use of recommendations in the unit for cancer screening in general (N = 596 / 1251) were asked about use of colorectal cancer screening recommendations. Over 50% of the data are missing.
P value comparing units where INCA recommendations for CRC screening are used to those where they are not.
Provider characteristics and knowledge, attitudes, and practice of CRC screening
A total of 182 physicians and 347 nurses answered the survey. The majority of physicians were male, over 30 years old, and had graduated more than 5 years ago (Table 2). Nurses were mostly female, 30 years or younger, and had graduated less than 5 years ago. Physicians saw more patients per week than nurses (p < 0.001), while nurses more often worked 40 hours or more per week compared to physicians (p < 0.001). Regional distribution of physicians was similar to that of nurses, most of them practicing in the Southeast and Northeast regions.
Table 2.
Socio-demographic characteristics, and colorectal cancer screening knowledge, attitudes, and practices of physicians and nurses in Brazil; GUIA 2011.
| Characteristic | Physicians (N = 182)
|
Nurses (N = 347)
|
P-valueb
|
||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Gender | <0.001 | ||||
| Female | 79 | 43 | 294 | 85 | |
| Male | 103 | 57 | 53 | 15 | |
| Age (in years) | <0.001 | ||||
| ≤30 | 49 | 27 | 184 | 53 | |
| 31–45 | 73 | 40 | 130 | 38 | |
| 46–59 | 44 | 24 | 29 | 8 | |
| ≥60 | 16 | 9 | 3 | 1 | |
| Years since graduation | <0.001 | ||||
| ≤5 | 66 | 37 | 194 | 56 | |
| 6–15 | 54 | 30 | 117 | 34 | |
| ≥15 | 60 | 33 | 33 | 10 | |
| Hours worked per week | <0.001 | ||||
| <40 | 82 | 46 | 79 | 23 | |
| ≥40 | 98 | 54 | 267 | 77 | |
| Patients seen per week | <0.001 | ||||
| ≤50 | 33 | 19 | 138 | 43 | |
| 51–150 | 102 | 57 | 160 | 49 | |
| >150 | 43 | 24 | 26 | 8 | |
| Region | 0.07 | ||||
| North | 11 | 6 | 17 | 5 | |
| Northeast | 49 | 27 | 125 | 36 | |
| Central-West | 21 | 11 | 21 | 6 | |
| Southeast | 65 | 36 | 109 | 31 | |
| South | 36 | 20 | 75 | 22 | |
| Knowledge and attitudes regarding CRC screening | |||||
| Perception of INCA recommendations for CRC screening as very influential | 64 | 40 | 106a | 37a | 0.54 |
| More familiar with FOBT | 133 | 77 | 112 | 33 | <0.001 |
| More familiar with sigmoidoscopy | 88 | 51 | 36 | 11 | <0.001 |
| Perception of FOBT as very effective | 96 | 56 | 222a | 75a | <0.001 |
| Perception of sigmoidoscopy as very effective | 141 | 82 | 274a | 92a | <0.01 |
| Perception of colonoscopy as very effective | 163 | 94 | 309 | 97 | 0.22 |
| CRC screening practices | |||||
| Age of routine CRC screening initiation | <0.001 | ||||
| <50 | 34 | 19 | 61 | 18 | |
| 50–55 | 52 | 30 | 47 | 14 | |
| 56–61 | 3 | 2 | 2 | 1 | |
| 62–67 | 2 | 1 | 0 | – | |
| Other | 1 | 1 | 9 | 2 | |
| I do not conduct CRC screening | 83 | 47 | 220 | 65 | |
CRC: colorectal cancer; INCA: Brazilian National Cancer Institute; FOBT: fecal occult blood test.
Over 10% of observations are missing.
P-value for comparison between sample characteristics of physicians and nurses.
Forty percent of physicians and 37% of nurses perceived INCA recommendations for CRC screening as very influential (p = 0.54). Physicians were more familiar than nurses with FOBT (77% vs. 33%; p < 0.001) and with sigmoidoscopy (51% vs. 11%; p < 0.001). In addition, more nurses than physicians perceived FOBT (75% vs. 56%; p < 0.001) and sigmoidoscopy (92% vs. 82%; p < 0.01) as very effective exams to reduce CRC mortality. Colonoscopy was perceived as very effective by 94% of physicians and 97% of nurses (p = 0.22).
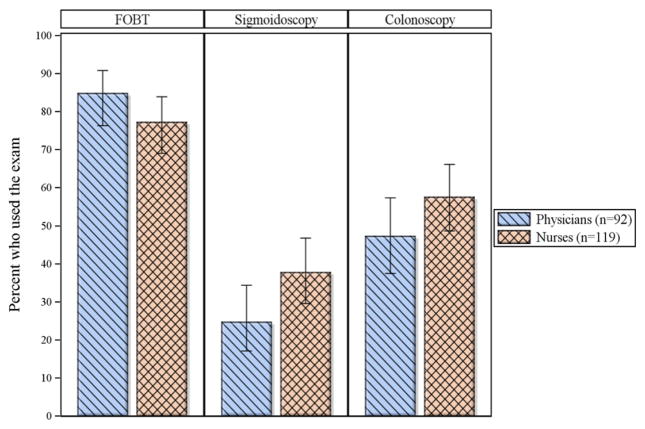
Thirty percent of physicians started routine CRC screening with patients aged 50–55 years compared to 14% of nurses (p < 0.001). Sixty-five percent of nurses said they did not conduct CRC screening compared to 47% of physicians (p < 0.001). Physicians and nurses who were conducting CRC screening identified FOBT as the exam most often used in their units (85% and 77%, respectively; p = 0.16) followed by colonoscopy (47% and 58%; p = 0.14) and sigmoidoscopy (25% and 38%; p = 0.04) (Fig. 1).
Fig. 1.
Use of colorectal cancer screening exams by provider type in Brazil; GUIA 2011.a. aOnly providers who self-reported performing CRC screening were asked the question—53% of physicians (n = 92); 35% of nurses (n = 119). Error bars represent 95% confldence intervals. FOBT: fecal occult blood test.
Screening practice among physicians
Compared to physicians who were not conducting CRC screening, physicians who were conducting it were younger (p < 0.001), had graduated more recently from medical school (p < 0.001), were more familiar with FOBT (p = 0.03), and less often perceived FOBT as very effective in reducing CRC mortality (p = 0.03; Table 3). After adjusting for gender, years since graduation, region, patients seen per week, influence of INCA CRC recommendations, and perception of FOBT effectiveness (Table 4), female physicians (OR 2.18, 95% CI 1.07–4.42), and those practicing in the North compared to the South (OR 5.99, 95% CI 1.10–32.67), were more likely to not conduct screening. The more years since medical school graduation, the less likely physicians said they would screen for colorectal cancer, with odds of not screening increasing by 1.37 (95% CI 1.17–1.60) for each 5-year increase in years since graduation.
Table 3.
Physicians’ demographic characteristics, colorectal cancer screening knowledge, and attitudes by screening practice in Brazil; GUIA 2011.a
| Physicians who do not screen (n = 83)
|
Physicians who screen (n = 92)
|
P-valueb
|
|||
|---|---|---|---|---|---|
| Characteristics
| |||||
| Median | Q1, Q3 | Median | Q1, Q3 | ||
| Age | 42 | 33, 54 | 33 | 28, 44.5 | <0.001 |
| Years since graduation | 13 | 6, 28 | 6 | 1, 14 | <0.001 |
| Hours worked per week | 40 | 12, 40 | 40 | 25, 40 | 0.08 |
| Patients per week | 100 | 60, 150 | 120 | 90, 160 | 0.12 |
| N | % | N | % | ||
| Gender | 0.13 | ||||
| Male | 42 | 51 | 57 | 62 | |
| Female | 41 | 49 | 35 | 38 | |
| Region | 0.07 | ||||
| South | 11 | 13 | 23 | 25 | |
| Southeast | 27 | 33 | 36 | 39 | |
| Central-West | 13 | 16 | 6 | 7 | |
| Northeast | 25 | 30 | 23 | 25 | |
| North | 7 | 8 | 4 | 4 | |
| Knowledge and attitudes regarding CRC screening | N | % | N | % | |
| Perception of INCA recommendations for CRC screening | 0.29 | ||||
| Very influential | 27 | 33 | 37 | 40 | |
| Not very influential | 56 | 67 | 55 | 60 | |
| Familiarity with FOBT | 0.03 | ||||
| More familiar | 56 | 70 | 77 | 84 | |
| Less familiar | 24 | 30 | 15 | 16 | |
| Familiarity with sigmoidoscopy | 0.13 | ||||
| More familiar | 36 | 45 | 52 | 57 | |
| Less familiar | 44 | 55 | 40 | 43 | |
| Perception of FOBT effectiveness | 0.03 | ||||
| Very effective | 52 | 63 | 43 | 47 | |
| Not very effective | 31 | 37 | 49 | 53 | |
| Perception of sigmoidoscopy effectiveness | 0.96 | ||||
| Very effective | 67 | 81 | 74 | 80 | |
| Not very effective | 16 | 19 | 18 | 20 | |
| Perception of colonoscopy effectiveness | 0.20 | ||||
| Very effective | 75 | 91 | 87 | 97 | |
| Not very effective | 7 | 9 | 3 | 3 | |
CRC: colorectal cancer; INCA: Brazilian National Cancer Institute; FOBT: fecal occult blood test.
Median and quartiles reported for continuous variables. Counts and percentages reported for categorical variables.
P-value for physicians who do not screening vs. those who screen.
Table 4.
Association between selected factors and lack of screening practice among physicians in Brazil; GUIA 2011.
| Crude OR |
CI | Adj ORa |
CI | P-valueb | |
|---|---|---|---|---|---|
| Gender | 0.03 | ||||
| Male | Ref. | Ref. | |||
| Female | 1.59 | 0.87–2.90 | 2.18 | 1.07–4.42 | |
| Years since graduation (5-year increase) | 1.28 | 1.12–1.47 | 1.37 | 1.17–1.60 | <0.001 |
| Patients/week (10-patient increase) | 0.97 | 0.94–1.00 | 0.97 | 0.94–1.01 | 0.16 |
| Region | 0.10 | ||||
| South | Ref. | Ref. | |||
| Southeast | 1.56 | 0.65–3.76 | 1.18 | 0.44–3.18 | |
| Central-West | 4.53 | 1.36–15.11 | 3.72 | 0.99–14.05 | |
| Northeast | 2.27 | 0.91–5.67 | 1.67 | 0.60–4.63 | |
| North | 3.66 | 0.88–15.18 | 5.99 | 1.10–32.67 | |
| Perception of INCA recommendations for CRC screening | 0.09 | ||||
| Very influential | Ref. | Ref. | |||
| Not very influential | 1.40 | 0.75–2.59 | 1.89 | 0.90–3.96 | |
| Perception of FOBT effectiveness | 0.27 | ||||
| Very effective | Ref. | Ref. | |||
| Not very effective | 0.52 | 0.29–0.96 | 0.68 | 0.33–1.36 |
Adj: adjusted; Ref.: reference; OR: odds ratio; CI: 95 % confldence interval; CRC: colorectal cancer; INCA: Brazilian National Cancer Institute; FOBT: fecal occult blood test
Adjusted for gender, years since graduation, region, patients seen per week, influence of INCA recommendations, and perception of FOBT effectiveness where appropriate.
P-value from Wald chi-square tests in the model backward selection.
Discussion
Unit capacity for CRC screening is still low in Brazil. This finding is further supported by a recent assessment of CRC programs globally which found a general lack of infrastructure for CRC screening in South American countries even though national CRC screening guidelines may be in place (Schreuders et al., 2015). CRC screening outreach activities were not common in Brazilian health units and use of CRC screening exams was infrequent, especially for sigmoidoscopy and colonoscopy. However, units using INCA CRC screening recommendations had higher use of FOBT, sigmoidoscopy, and colonoscopy, and more screening outreach.
Our study revealed that approximately half of surveyed physicians in Brazil were screening their eligible patients for CRC. Only 30% were routinely starting screening with patients aged 50–55 years, reinforcing our findings that INCA recommendations for CRC screening were not often used in the units and that their influence among physicians was low. Regarding the method of screening, physicians were fairly familiar with FOBT and sigmoidoscopy, and almost all of them perceived colonoscopy as being very effective in reducing CRC mortality. Physicians were less certain, however, about the effectiveness of FOBT. Although colonoscopy has been shown to reduce CRC mortality (Zauber et al., 2012), its effectiveness has not been verified in randomized controlled trials (RCT), and the quality of the evidence is fair (Calonge et al., 2008; Lieberman et al., 2012; National Cancer Institute; U.S. Preventive Services Task Force, 2008). The magnitude of FOBT effectiveness in reducing CRC mortality may be smaller than that of colonoscopy, but it has been assessed through good-quality RCTs (National Cancer Institute; Pignone et al., 2002). FOBT and other similarly less invasive tests could play a fundamental role in reaching more people because of better acceptability (Benson et al., 2008; Hardcastle et al., 1996; Multicentre Australian Colorectal-neoplasia Screening Group, 2006; Segnan et al., 2005). In Thailand, where 62% of primary care physicians routinely recommend CRC screening to asymptomatic average-risk patients (Thanapirom et al., 2012), results of a pilot implementation program using fecal immunochemical tests (FIT) demonstrated the possibility of screening a large number of people without straining the health system, and considerably reducing the need for more complex exams, such as sigmoidoscopy and colonoscopy (Khuhaprema et al., 2014). This may be particularly beneficial to Brazil, where health units might adequately offer a secondary prevention service of lower complexity, increasing the breadth of their services and expanding their capacity for cancer control.
Nurses were not very familiar with FOBT and sigmoidoscopy, but most considered all CRC exams very effective in reducing CRC mortality. However, the majority of nurses did not conduct CRC screening, an expected result since the initiation of a screening routine is the responsibility of physicians in Brazil. It is important to note that we were not able to distinguish if nurses who reported starting routing CRC screening were doing it directly or simply recommending that the patient talked to his or her physician. The pilot study in Thailand illustrates the opportunity of engaging nurses, and also CHWs, in CRC screening by training patients for successful collection of FIT stool samples and other outreach activities (Khuhaprema et al., 2014). Similar engagement could be promoted in Brazil via the Unified Health System and its health units.
Overall, our findings were not surprising since Brazil does not have CRC screening programs or a national policy to guide CRC prevention and control. Cancer prevention and control in Brazil regained strength in the 1990s when the newly created Unified Health System was being structured (Parada et al., 2008). INCA was then in charge of developing national cancer policies, which included the creation of the first national program for cervical cancer control, in 1998 (Instituto Nacional do Câncer, Ministério da Saúde, Brasil, 2010). More recently, the Brazilian Ministry of Health has taken the lead in program development with the introduction of a national policy for the prevention and early detection of breast and cervical cancers in 2008 (Presidência da República, Casa Civil, Brasil, 2008) and in 2012 with the creation of a national plan to direct resources to cancer prevention and control (Presidência da República, Casa Civil, Brasil, 2012). Of note, the survey was conducted in the public sector’s network of primary health units. They form an important part of the Brazilian health system: public health units were reported as the usual source of care for 57% of the population in 2008, and the public sector employs over half of the health care force in Brazil (Paim et al., 2011). Yet the private health care sector is responsible for 62% of hospital care, 92% of tertiary diagnostic health services, and at least 20% of ambulatory care in Brazil (Santos et al., 2008). Differences in knowledge, attitudes, and practice of CRC screening may exist among physicians and nurses practicing in public versus private sector. Souza et al. did not find differences between physicians’ source of income (public, private, or both) and their knowledge and practice of CRC screening; however, the study had a small sample size and was based in one hospital (Souza et al., 2012). National provider-based studies analyzing possible differences between sectors are needed in order to understand the full picture of CRC screening in the Brazilian health system.
Given that, in Brazil, CRC may not be as high a priority as other cancers (Coy, 2013; Secretaria Municipal da Saúde de São Paulo-Coordenação de Epidemiologia e Informação (SMS/SP). Boletim CEInfo Análise n°06. São Paulo: SMS/SP, 2012), the low number of physicians conducting CRC screening was expected. Physicians less likely to conduct CRC screening were women, physicians who graduated longer ago from medical school, and those who were practicing in the North compared to the South. The last comparison is especially important since access to health care has been historically dependent on socioeconomic and geographic characteristics in Brazil. Although efforts have been made in the past decades to accelerate the development of North, Northeast, and Center-West regions, these are traditionally poorer and with less health infrastructure compared to the South and Southeast (Paim et al., 2011). In addition, the Brazilian health system faces challenges related to lack of CRC awareness in the population and the high costs of exams (Dias et al., 2007). Female physicians usually offer more preventive counseling and screening recommendations than male physicians (Henderson and Weisman, 2001). Based on our survey results, we were not able to explain the lower screening practice among female physicians, and that should be further investigated. Supporting our finding that lack of CRC screening increases with number of years since graduation, a study done in Southern Brazil revealed that physicians who graduated more than 15 years ago were less aware of CRC screening recommendations than the ones who graduated more recently (Souza et al., 2012). This is possibly due to the relatively recent introduction in Brazil of INCA recommendations for CRC screening in 2002. Before, physicians followed international recommendations, such as the ones provided by the American Cancer Society, and their influence in CRC screening practice has been similarly low (Secretaria Municipal da Saúde de São Paulo-Coordenação de Epidemiologia e Informação (SMS/SP). Boletim CEInfo Análise n°06. São Paulo: SMS/SP, 2012; da Silva et al., 2011; Tucunduva et al., 2004).
Study limitations
Limitations of our study include the low response rate among physicians, which may have biased our estimates. Adherence to INCA-recommended age of CRC screening initiation could not be analyzed because of the small number of observations among physicians available for subgroup analyses. In addition, self-report may have allowed physicians, nurses, and coordinators to give answers that would be expected and acceptable by their supervisors, instead of their actual practice or knowledge. Study strengths included the random assessment of units throughout all regions, even in more remote municipalities, which may offer a representative picture of the Brazilian public health system. Moreover, the prediction model for screening practice allowed for better insights on why physicians may not screen for CRC.
Conclusion
Latin–American countries are moving toward prioritizing cancer prevention and control but often lack policy and programs that address CRC specifically. Brazil has the potential to be a leading example in the region, drawing upon examples of pilot CRC screening programs available abroad (Khuhaprema et al., 2014; Seeff and Rohan, 2013), and in-country (Perez et al., 2008).
Our study aimed to understand current CRC screening knowledge, attitudes, and practices of providers in the Brazilian network of health units. INCA currently recommends annual FOBT exams for asymptomatic people age 50 or older and a colonoscopy in case of a positive result, but its influence in the screening practice of physicians is limited. The majority of physicians and nurses are aware of the effectiveness of CRC screening in reducing mortality, and physicians are more often familiar with CRC exams. However, almost half of physicians did not conduct CRC screening, which may reflect the low influence of screening recommendations, physicians receiving their medical education when CRC was not of high concern in Brazil, lack of capacity for CRC screening in certain parts of the country, and lack of national or local CRC screening programs. Given the increasing burden of colorectal cancer, this baseline information might be useful for Brazil.
Acknowledgments
This study was supported by Cooperative Agreement U48/DP001903 from the Centers for Disease Control and Prevention with the Prevention Research Center at Washington University in St. Louis. The analysis was supported by Cooperative Agreement number U36/CCU300430 from the Centers for Disease Control and Prevention and the Association of Schools and Programs of Public Health. The findings and conclusions of this publication are those of the authors and do not necessarily represent the official views of CDC or ASPPH.
Abbreviations
- 95% CI
95% confidence interval
- CHW
Community health worker
- CRC
Colorectal cancer
- FOBT
Fecal occult blood test
- GUIA
Guide for Useful Interventions for Physical Activity in Brazil and Latin America
- iFOBT
Immunochemical fecal occult blood test
- INCA
Brazilian National Cancer Institute
- OR
Odds ratio
- RCT
Randomized controlled trial
Appendix A. Survey questions related to colorectal cancer screening for coordinators; GUIA, 2011, Brazil
| Possible answers
|
|
|---|---|
| Knowledge of INCA cancer screening recommendations | |
| 1. Do you know the INCA recommendations for cancer screening? | (0) No (Skip to question 5) (1) Yes (9) I don’t know/ N/A |
| 2. Does your unit follow the INCA recommendations for cancer screening? | (0) No (Skip to question 5) (1) Yes (9) I don’t know/ N/A |
3. For what type of cancers does your unit use the INCA recommendations for early detection?
|
(0) No (1) Yes (9) I don’t know |
| Cancer screening outreach | |
4. For what type of cancers does your unit conduct outreach activities?
|
(0) No (1) Yes (9) I don’t know |
| Use of CRC screening exams | |
5. What colorectal cancer screening methods are used in your unit with asymptomatic patients?
|
(0) No (1) Yes (9) I don’t know |
CRC: colorectal cancer; INCA: Brazilian National Cancer Institute
Appendix B. Survey questions related to colorectal cancer screening for physicians and nurses; GUIA, 2011, Brazil
| Possible answers
|
|
|---|---|
| Perception of CRC screening effectiveness | |
1. How effective do you believe the following methods are to reduce colorectal cancer mortality?
|
(1) Very effective (2) Little effective (3) Not effective (4) Effectiveness unknown (9) I don’t know |
| Perception of influence of INCA recommendations | |
| 2. INCA released recommendations about colorectal cancer screening. In your unit, would you say the recommendations for colorectal cancer screening are: | (1) Very influential (2) Little influential (3) Not influential (4) I don’t know |
| Familiarity with CRC screening exams | |
3. For each of the following exams, please indicate how familiar you are with:
|
(1) Very familiar (2) Familiar (3) Little familiar (4) Not familiar |
| CRC screening practice and adherence with recommendations | |
| 4. At what age do you start routine colorectal cancer screening? | (1) Less than 50 years (2) 50–55 years (3) 56–61 years (4) 62–67 years (5) Other (6) I don’t do colorectal cancer screening (Ends the interview) |
5. Which of the following screening exams are used in your unit to screen patients?
|
(0) No (1) Yes (8) N/A |
CRC: colorectal cancer; INCA: Brazilian National Cancer Institute
Footnotes
The findings and conclusions in this report do not necessarily represent the official position of Centers for Disease Control and Prevention.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- Benson VS, Patnick J, Davies AK, Nadel MR, Smith RA, Atkin WS. International Colorectal Cancer Screening Network. Colorectal cancer screening: a comparison of 35 initiatives in 17 countries. [Mar 15];Int J Cancer. 2008 122(6):1357–1367. doi: 10.1002/ijc.23273. http://dx.doi.org/10.1002/ijc.23273. [DOI] [PubMed] [Google Scholar]
- Brawarsky P, Brooks D, Mucci L, Wood P. Effect of physician recommendation and patient adherence on rates of colorectal cancer testing. Cancer Detect Prev. 2004;28(4):260–268. doi: 10.1016/j.cdp.2004.04.006. http://dx.doi.org/10.1016/j.cdp.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Calonge N, Petitti DB, DeWitt TG, Dietrich AJ, Gregory KD, Harris R, Isham G, LeFevre ML, Leipzig RM, Loveland-Cherry C, Marion LN, Melnyk B, Moyer VA, Ockene JK, Sawaya GF, Yawn BP U.S. Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. http://dx.doi.org/10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59(6):366–378. doi: 10.3322/caac.20038. http://dx.doi.org/10.3322/caac.20038. [DOI] [PubMed] [Google Scholar]
- Coy CSR. Colorectal cancer prevention in Brazil—where are we? J Coloproctol. 2013;33:111–112. http://dx.doi.org/10.1016/j.jcol.2013.09.001. [Google Scholar]
- da Silva LMC, da Fonseca AJ, Ferreira LP, Dalla-Benetta AC, Navarro C. Atitude e conhecimento de médicos da estratégia saúde da família sobre prevenção e rastreamento do câncer. Rev Bras Cancerol. 2011;57(4):525–534. [Google Scholar]
- Dias APTP, Gollner AM, Teixeira MTB. Câncer colorretal: rastreamento, prevenção, e controle. HU Rev. 2007;33(4):125–131. [Google Scholar]
- Florindo AA, Mielke GI, Gomes GA, Ramos LR, Bracco MM, Parra DC, Simoes EJ, Lobelo F, Hallal PC. Physical activity counseling in primary health care in Brazil: a national study on prevalence and associated factors. BMC Public Health. 2013;13(1):1–10. doi: 10.1186/1471-2458-13-794. http://dx.doi.org/10.1186/1471-2458-13-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. [Nov 30];Lancet. 1996 348(9040):1472–1477. doi: 10.1016/S0140-6736(96)03386-7. http://dx.doi.org/10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- Henderson JT, Weisman CS. Physician gender effects on preventive screening and counseling: an analysis of male and female patients’ health care experiences. Med Care. 2001;39(12):1281–1292. doi: 10.1097/00005650-200112000-00004. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Tabber S, Lemeshow S. The importance of assessing the fit of logistic regression models: a case study. Am J Public Health. 1991;81:1630–1635. doi: 10.2105/ajph.81.12.1630. http://dx.doi.org/10.2105/AJPH.81.12.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instituto Nacional do Câncer. Normas e recomendações do INCA: prevenção e controle de câncer. Rev Bras Cancerol. 2002;48(3):317–332. [Google Scholar]
- Instituto Nacional do Câncer, Ministério da Saúde, Brasil. Plano de ação para redução da incidência e mortalidade por câncer do colo do útero: sumário executivo. INCA; Rio de Janeiro: 2010. [Accessed 2015 July 23]. Available at: http://bvsms.saude.gov.br/bvs/publicacoes/plano_acao_reducao_cancer_colo.pdf. [Google Scholar]
- Instituto Nacional do Câncer, Ministério da Saúde, Brasil. Estimativa 2012: incidência de câncer no Brasil. INCA; Rio de Janeiro: 2011. [Accessed 2015 July 23]. Available at: http://portal.saude.sp.gov.br/resources/ses/perfil/gestor/homepage/estimativas-de-incidencia-de-cancer-2012/estimativas_incidencia_cancer_2012.pdf. [Google Scholar]
- International Agency for Research on Cancer. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. IARC; Lyon: 2013. [Accessed 2015 July 23]. Available at: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. [Google Scholar]
- Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. http://dx.doi.org/10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- Khalid-de Bakker C, Jonkers D, Smits K, Mesters I, Masclee A, Stockbrügger R. Participation in colorectal cancer screening trials after first-time invitation: a systematic review. Endoscopy. 2011;43(12):1059–1086. doi: 10.1055/s-0031-1291430. http://dx.doi.org/10.1055/s-0031-1291430. [DOI] [PubMed] [Google Scholar]
- Khuhaprema T, Sangrajrang S, Lalitwongsa S, Chokvanitphong V, Raunroadroong T, Ratanachu-Ek T, Muwonge R, Lucas E, Wild C, Sankaranarayanan R. Organised colorectal cancer screening in Lampang Province, Thailand: preliminary results from a pilot implementation programme. BMJ Open. 2014;4(1):e003671. doi: 10.1136/bmjopen-2013-003671. http://dx.doi.org/10.1136/bmjopen-2013-003671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinbaum DG, Kupper LL, Muller KE. Applied regression analysis and other multivariable methods. PWS-KENT Publishing Company; Boston: 1987. [Google Scholar]
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. [Sep];Gastroenterology. 2012 143(3):844–857. doi: 10.1053/j.gastro.2012.06.001. http://dx.doi.org/10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Multicentre Australian Colorectal-neoplasia Screening Group. A comparison of colo-rectal neoplasia screening tests: a multicentre community-based study of the impact of consumer choice. Med J Aust. 2006 Jun 5;184(11):546–550. doi: 10.5694/j.1326-5377.2006.tb00377.x. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. d. Colorectal cancer screening (PDQ®) [Accessed 2015 July 23]; Avaliable at:, http://www.cancer.gov/cancertopics/pdq/screening/colorectal/HealthProfessional/page1.
- O’Malley AS, Beaton E, Yabroff KR, Abramson R, Mandelblatt J. Patient and provider barriers to colorectal cancer screening in the primary care safety-net. Prev Med. 2004;39:56–63. doi: 10.1016/j.ypmed.2004.02.022. http://dx.doi.org/10.1016/j.ypmed.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Paim J, Travassos C, Almeida C, Bahia L, Macinko J. The Brazilian health system: history, advances, and challenges. Lancet. 2011;377(9779):1778–1797. doi: 10.1016/S0140-6736(11)60054-8. http://dx.doi.org/10.1016/S0140-6736(11)60054-8. [DOI] [PubMed] [Google Scholar]
- Pan-American Health Organization. Cancer in the Americas: Country Profiles 2013. PAHO; Washington, DC: 2013. [Accessed 2015 July 23]. Available at: http://www.paho.org/hq/index.php?option=com_docman&task=doc_download&gid=23456&Itemid= [Google Scholar]
- Parada R, de Assis M, da Silva RCF, Abreu MF, da Silva MAF, Dias MBK, Tomazelli JG. Brazilian cancer control policy and the role of primary care in cancer prevention and control. Rev APS. 2008;11(2):199–206. [Google Scholar]
- Perez RO, Proscurshim I, Julião G, Picolo M, Gama-Rodrigues J, Habr-Gama A. Instalação e resultados preliminares de programa de rastreamento populacional de câncer colorretal em município brasileiro. Arq Bras Cir Dig. 2008;21(1):12–15. http://dx.doi.org/10.1590/S0102-67202008000100003. [Google Scholar]
- Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. [Jul 16];Ann Intern Med. 2002 137(2):132–141. doi: 10.7326/0003-4819-137-2-200207160-00015. http://dx.doi.org/10.7326/0003-4819-137-2-200207160-00015. [DOI] [PubMed] [Google Scholar]
- Presidência da República, Casa Civil, Brasil. Diário Oficial da República Federativa do Brasil. PRB; Brasilia: 2008. [Accessed 2015 July 23]. Lei n° 11.664, de 29 de abril de 2008. Available at: http://www.planalto.gov.br/ccivil_03/_ato2007-2010/2008/lei/l11664.htm. [Google Scholar]
- Presidência da República, Casa Civil, Brasil. Diário Oficial da República Federativa do Brasil. PRB; Brasilia: 2012. [Accessed 2015 July 23]. Lei n° 12.715, de 17 de setembro de 2012. Available at: http://www.planalto.gov.br/ccivil_03/_ato2011-2014/2012/lei/l12715.htm. [Google Scholar]
- Santos IS, Uga MAD, Porto SM. The public-private mix in the Brazilian Health System: financing, delivery and utilization of health services. Cien Saude Colet. 2008;13(5):1431–1440. doi: 10.1590/s1413-81232008000500009. http://dx.doi.org/10.1590/S1413-81232008000500009. [DOI] [PubMed] [Google Scholar]
- Schmidt MI, Duncan BB, Menezes AM, Monteiro CA, Barreto SM, Chor D, Menezes PR. Chronic non-communicable diseases in Brazil: burden and current challenges. Lancet. 2011;377(9781):1949–1961. doi: 10.1016/S0140-6736(11)60135-9. http://dx.doi.org/10.1016/S0140-6736(11)60135-9. [DOI] [PubMed] [Google Scholar]
- Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, Kuipers EJ. Colorectal cancer screening: a global overview of existing programmes. [Oct];Gut. 2015 64(10):1637–1649. doi: 10.1136/gutjnl-2014-309086. http://dx.doi.org/10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- Secretaria Municipal da Saúde de São Paulo-Coordenação de Epidemiologia e Informação (SMS/SP) Boletim CEInfo Análise n°06. São Paulo: SMS/SP; 2012. [Accessed 2015 July 23]. Available at:, http://www.prefeitura.sp.gov.br/cidade/secretarias/upload/saude/arquivos/publicacoes/Boletim_CEInfo_Analise_06.pdf. [Google Scholar]
- Seeff LC, Rohan EA. Lessons learned from the CDC’s Colorectal Cancer Screening Demonstration Program. Cancer. 2013;119(Suppl 15):2817–2819. doi: 10.1002/cncr.28165. http://dx.doi.org/10.1002/cncr.28165. [DOI] [PubMed] [Google Scholar]
- Segnan N, Senore C, Andreoni B, Arrigoni A, Bisanti L, Cardelli A, Castiglione G, Crosta C, DiPlacido R, Ferrari A, Ferraris R, Ferrero F, Fracchia M, Gasperoni S, Malfitana G, Recchia S, Risio M, Rizzetto M, Saracco G, Spandre M, Turco D, Turco P, Zappa M SCORE2 Working Group-Italy. Randomized trial of different screening strategies for colorectal cancer: patient response and detection rates. [Mar 2];J Natl Cancer Inst. 2005 97(5):347–357. doi: 10.1093/jnci/dji050. http://dx.doi.org/10.1093/jnci/dji050. [DOI] [PubMed] [Google Scholar]
- Souza ECR, Lise M, Santos TP, de Carvalho LP. Knowledge and practice of physicians regarding colorectal cancer screening. J Coloproctol. 2012;32(4):385–394. http://dx.doi.org/10.1590/S2237-93632012000400005. [Google Scholar]
- Stormo AR, de Moura L, Saraiya M. Cervical cancer-related knowledge, attitudes, and practices of health professionals working in Brazil’s network of primary care units. Oncologist 2013–0318. 2014 doi: 10.1634/theoncologist.2013-0318. http://dx.doi.org/10.1634/theoncologist.2013-0318. [DOI] [PMC free article] [PubMed]
- Thanapirom K, Treeprasertsuk S, Rerknimitr R. Awareness of colorectal cancer screening in primary care physicians. J Med Assoc Thail. 2012;95(7):859–865. [PubMed] [Google Scholar]
- Tucunduva LTCM, de Sá VHLC, Koshimura ET, Prudente FVB, dos Santos AF, Samano EST, Costa LJM, del Giglio A. Estudo da atitude e do conhecimento dos médicos não oncologistas em relação às medidas de prevenção e rastreamento do câncer. Rev Assoc Med Bras. 2004;50(3):257–262. doi: 10.1590/s0104-42302004000300030. http://dx.doi.org/10.1590/S0104-42302004000300030. [DOI] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force. [October Accessed 2015 July 23];Final Evidence Summary: Colorectal Cancer: Screening. 2008 Available at:, http://www.uspreventiveservicestaskforce.org/Page/SupportingDoc/colorectal-cancer-screening/final-evidence-summary4.
- Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst. 1997;89(19):1406–1422. doi: 10.1093/jnci/89.19.1406. http://dx.doi.org/10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. [Feb 23];N Engl J Med. 2012 366(8):687–696. doi: 10.1056/NEJMoa1100370. http://dx.doi.org/10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]