Abstract
Movement disorders, which include disorders such as Parkinson’s disease, dystonia, Tourette’s syndrome, restless legs syndrome, and akathisia, have traditionally been considered to be disorders of impaired motor control resulting predominantly from dysfunction of the basal ganglia. This notion has been revised largely because of increasing recognition of associated behavioural, psychiatric, autonomic, and other non-motor symptoms. The sensory aspects of movement disorders include intrinsic sensory abnormalities and the effects of external sensory input on the underlying motor abnormality. The basal ganglia, cerebellum, thalamus, and their connections, coupled with altered sensory input, seem to play a key part in abnormal sensorimotor integration. However, more investigation into the phenomenology and physiological basis of sensory abnormalities, and about the role of the basal ganglia, cerebellum, and related structures in somatosensory processing, and its effect on motor control, is needed.
Introduction
The term movement disorders has often been used synonymously with motor disorders, but sensory aspects are increasingly recognised to be important components of nearly all movement disorders (panel). Movement disorders have traditionally been regarded as disorders of impaired motor control resulting predominantly from dysfunction of the basal ganglia, but this notion has been revised, largely because of increasing recognition of associated behavioural, psychiatric, autonomic, and other non-motor symptoms.1–4 Furthermore, the high frequency of sensory symptoms and sensory abnormalities suggests that the sensory system is involved in the pathophysiology and pathogenesis of various movement disorders.
Panel. Sensory aspects of movement disorders.
Parkinson’s disease
Pain, akathisia, olfactory loss, visual impairment, vestibular dysfunction, proprioceptive and kinaesthetic dysfunction, and sensory cueing
Dystonias
Pain, photosensitivity, alleviating manoeuvres, kinaesthetic dysfunction, abnormal temporal and spatial discrimination
Peripherally induced dystonia, tremor, other movement disorders, and complex regional pain syndrome
Pain, paraesthesias
Tics and Tourette’s syndrome
Premonitory urge phenomena, enhanced sensory perception, alleviating manoeuvres
Restless legs syndrome
Urge phenomena, reduction of urge with bright lights
Akathisia
Urge phenomena, reduction with passive motion (perception of movement)
Stereotypies
Urge phenomena
Tardive pain
Painful mouth and vagina syndrome, and phantom dyskinesias
Leg stereotypy disorder
Urge phenomena
Paroxysmal kinesigenic and non-kinesigenic dyskinesias
Numbness, paraesthesias, crawling sensations in legs
Epileptic automatism
Self-stimulatory behaviour
Self-stimulatory behaviours associated with normal development, or metabolic, genetic, and autistic disorders, and other neurological disorders (Lesch-Nyhan, neuroacanthocytosis, etc)
Self-stimulatory (masturbatory) behaviour
Painful limb (painful legs and moving toes and painful arms and moving fingers)
Pain and discomfort presumably due to peripheral nerve damage
Huntington’s disease
Abnormal nociception and visual perception
In addition to intrinsic sensory symptoms, the importance of peripheral sensory feedback in the execution and planning of voluntary movement is well recognised.5,6 This process is exemplified by the use of various manoeuvres, such as sensory cueing in patients with Parkinson’s disease to help them to overcome freezing, or alleviating manoeuvres (also referred to as sensory tricks or so-called geste antagoniste) used by patients with dystonia to transiently correct the abnormal posture or movement. These and other examples suggest that many movement disorders are modulated by internal and external sensory signals and that abnormal sensorimotor integration might alter normal motor control.7,8 Additionally, many studies have provided evidence for non-elemental sensory loss—abnormalities that are undetectable with standard sensory testing—in various movement disorders.7,9,10
In this Review we provide examples of movement disorders for which, on the basis of clinical or experimental findings, there is evidence of abnormal sensorimotor integration. We then review the role of the basal ganglia and cerebellar circuitry in sensory processing, and its effect on motor control.
Parkinson’s disease
Parkinson’s disease is a prototypical disorder of the basal ganglia circuitry that is primarily characterised by degeneration of the substantia nigra pars compacta, resulting in striatal dopamine deficiency, but other central and peripheral dopaminergic and non-dopaminergic systems are also involved, which account for the broad range of motor and non-motor symptoms. The cardinal features of Parkinson’s disease include rest tremor, rigidity, bradykinesia, and gait and balance dysfunction.11 In addition to these and other motor manifestations, there is increasing recognition of non-motor abnormalities that affect nearly all patients at various stages of Parkinson’s disease, even long before the onset of motor symptoms.12 Of the most prominent and most troublesome non-motor symptoms of Parkinson’s disease are various sensory disturbances, including pain,13–16 urge such as akathisia, impairments in sensory perception such as olfactory loss,17 and visual changes.2,18 One of the earliest manifestations of Parkinson’s disease, which often precedes motor symptoms by several years, is shoulder pain.14,19–21 In one study of 25 patients with Parkinson’s disease and 25 controls, patients with Parkinson’s disease were 21 times more likely to have shoulder pain than were those without the disease.20 Pain is increasingly recognised as a major cause of reduced health-related quality of life.22
Parkinson’s disease-related pain has many causes, including musculoskeletal, dystonic, radicular, neuropathical, and central pain.14,16 Many studies support central mechanisms of pain. For example, lower pain threshold, particularly for heat, in patients with Parkinson’s disease can be normalised with levodopa therapy23–26 and with subthalamic nucleus or pallidal stimulation,27 which provides evidence for abnormal central (particularly basal ganglia) sensory processing in Parkinson’s disease. Results of behavioural and lesioning studies in animals have shown that the substantia nigra, caudate, and globus pallidus play an important part in localisation, integration, and behavioural response to nociceptive stimuli.14,16,28,98 Furthermore, the presence of endorphins, endocannabinoids, and various neuropeptides within the basal ganglia, which are known to be involved in the modulation of pain, provides further support for the role of this structure in central pain processing in Parkinson’s disease.16,30
Haptic perception, defined as the ability to distinguish the shape, orientation, and texture of an object by active touch of a surface and manipulation of an object in space, is also impaired in patients with Parkinson’s disease. For example, an increased threshold for ascertaining convex curvature has been shown in Parkinson’s disease, providing additional evidence for defective proprioceptive processing in the disease.31 Although a reduction in haptic perception is a well recognised age-related condition, patients with Parkinson’s disease have a decreased sensitivity and acuity beyond the expected findings in older (mean age 63·3 years) healthy individuals.32
Several studies have shown abnormalities in sensory perception and proprioceptive integration mainly with the use of kinaesthetic sense, the conscious perception of limb position and motion in space.33–35 Poor recognition of limb displacement has been noted during testing of passive motion in patients with Parkinson’s disease.36,37 Other kinaesthetic abnormalities include impaired detection of applied force and weight required for single-joint displacement.38 Additionally, altered kinaesthetic sense of joint displacement has been implicated in the abnormal scaling of movement.39,40 This abnormality might improve with dopamine replacement but not with deep-brain stimulation.41 Finally, impaired discriminative sensory function in Parkinson’s disease, indicated by increased somaesthetic temporal discrimination threshold, has been correlated with striatal dopamine deficiency on positron emission tomography (PET).10 Whether dopaminergic therapy augments kinaesthetic perception is unclear,42,43 but it does seem to improve discriminative sensory function.10
Impairments in gait and balance, some of the most disabling symptoms in Parkinson’s disease,44 are multifactorial in origin and partly arise from impaired multimodal integration of sensory feedback from vestibular, visual, and proprioceptive sensory systems. Impaired vestibular responses,45 reduced internal representation of their bodies, and impaired proprioception37 clearly contribute to the impairments in gait and balance in patients with this disease.46
Patients with Parkinson’s disease rely on visual input, such as the perception of forward motion, for the generation and maintenance of coordinated movements,47,48 and freezing of gait can occur when visual input is disrupted.49,50 Impairments in balance51 and foot displacement in walking tasks are most notable when visual cues are blocked and patients are relying only on proprioceptive feedback.52 The well known occurrence of sensory cueing through auditory, visual, or tactile inputs to overcome akinesia or motor freezing also emphasises the role of multimodal integration of sensory input in Parkinson’s disease.53–56 Tricks or manoeuvres such as kicking a cane, bouncing a ball while walking, or stepping over a laser-generated line can be used to overcome gait freezing (also known as gait akinesia or motor blocks).57 Additionally, the curious occurrence of kinesia paradoxica, manifested by the sudden ability to overcome akinesia with a surge of emotional energy, often precipitated by a sensory stimulus, shows that the motor programmes in patients with Parkinson’s disease might be intact, but that the patients have difficulty using or accessing the programmes.11 Because initiation and execution of movement in Parkinson’s disease becomes more dependent on external cueing as the disease progresses, the increasingly defective use of proprioceptive input could have a role in the pathophysiology of Parkinson’s disease-related freezing, which also worsens with disease progression.8,9,58–60 Impaired kinaesthetic processing might explain why patients with Parkinson’s disease rely heavily on visual input as a compensatory process to generate and maintain automatic patterned movements necessary for gait.61–65 Impairment in automatic movements in Parkinson’s disease has been attributed to loss of neurons in the centromedian thalamus that project to the sensorimotor regions of the striatum66,67 and hyperactivation of the cerebellum.68 The cerebellum might be recruited in an attempt to compensate for the primary motor dysfunction in Parkinson’s disease, as suggested by increased cerebellar activation on functional MRI with visually cued finger taps in patients with Parkinson’s disease in the so-called off state compared with in healthy controls.68–70 Further investigation into the role of the cerebellum’s contribution to sensorimotor integration in Parkinson’s disease is needed.71
Dystonia
Dystonia encompasses a broad range of patterned movements—either focal, segmental, or generalised—produced by involuntary muscle contractions and causing twisting, squeezing, and other abnormal postures that are often initiated or worsened by voluntary action and associated with overflow activation into adjacent or even contralateral muscle groups (so-called mirror dystonia).72–74 The neuroanatomical basis of dystonia is unclear, but the basal ganglia have been implicated in its pathophysiology through observations of dystonia secondary to basal ganglia lesions75,76 and its presence in known basal ganglia disorders such as Parkinson’s disease77 and Huntington’s disease.78 Structural lesions in the thalamus, parietal lobe, brainstem, and cerebellum can also cause secondary dystonia. The absence of observable neurodegeneration in primary dystonias suggests an underlying neuronal dysfunction of connectivity, plasticity, and synaptic regulation involving the basal ganglia circuitry.79
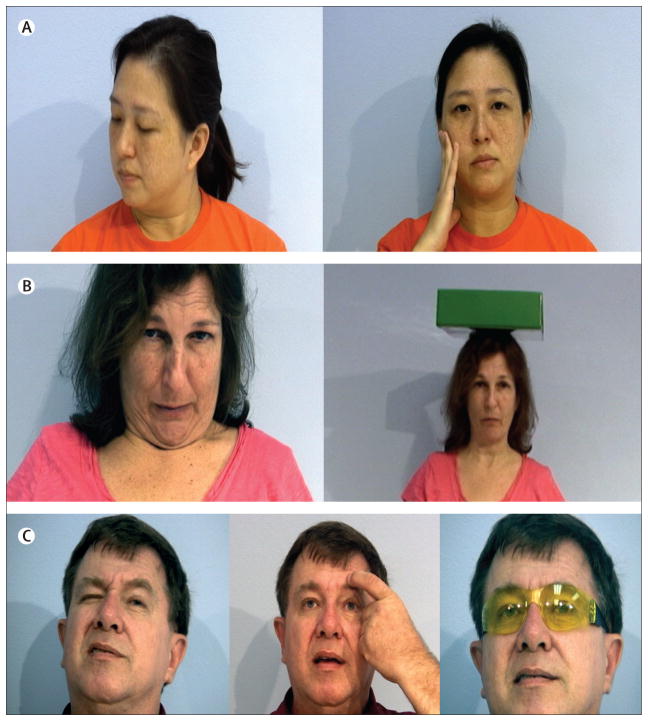
Pain, partly connected to the muscle spasm, is a well recognised symptom of dystonia. In two large series of patients with cervical dystonia, the frequency of pain was 68% and 75%.80,81 Many cases of dystonia, however, show no overt signs of sensory abnormalities on clinical examination, but sensory symptoms might exist—eg, patients with blepharospasm, a form of focal dystonia, frequently complain of photosensitivity and other ocular discomforts, and neck pain often precedes or is associated with cervical dystonia.4 In addition to pain, most patients with dystonia have noted that a certain voluntary movement or some alteration in sensory input temporarily improves the dystonic posture or movement,82 particularly early in the disease course.83 This well recognised phenomenon has traditionally been referred to as a geste antagoniste or a sensory trick. Because it does not always involve a sensory input and is real rather than fake, as implied by the word “trick”, we propose that a more appropriate term for this phenomenon is alleviating manoeuvre. This manoeuvre could be motor or sensory in nature. Most patients who use alleviating manoeuvres obtain partial or complete improvement of their dystonic posture or movement. A study showed that a complete resolution of dystonia with alleviating manoeuvres is associated with better visuotactile discrimination and shorter duration of dystonic symptoms compared with patients with less effective alleviating manoeuvres.84 Examples of alleviating manoeuvres include a light touch to certain areas of the face, chin, or neck that allows a patient with cervical dystonia to bring the head into a primary (normal) position; or pulling on the upper eyelid or an eyebrow, wearing tinted lenses, talking, or singing that enables a patient with blepharospasm to keep the eyes open (figure 1). The presence of these and other alleviating manoeuvres initially led to the misconception that cervical and other dystonias were of psychological origin; however, their presence is now a key diagnostic feature.85 Patients with generalised dystonia also use a variety of alleviating manoeuvres, such as placing their hands in their pockets, behind their neck or back, or on their hip; dancing or walking backwards; and placing objects on their head (figure 1B). Whereas many patients report that doing a particular task, such as playing a musical instrument86 or a particular sport, such a golf,87 triggers their dystonia (eg, task-specific dystonia), some experience paradoxical improvement of dystonia—eg, while playing a piano—which previously has been interpreted as a form of sensory trick.88
Figure 1. Alleviating manoeuvres.
(A) The patient has near-complete resolution of her right torticollis by lightly touching the right side of her face. (B) The patient’s anterocollis improves when attempting to balance a heavy book on her head. (C) The blepharospasm in this patient is alleviated by lightly touching his eyebrow and by wearing yellow tinted goggles.
The mechanisms by which alleviating manoeuvres improve dystonia are not well understood, but results of several physiological and functional MRI studies have shown that a light touch in a specific area of the body (typically in relation to the location of dystonia) can attenuate muscle activity89,90 in association with reduced activation of the supplementary motor area and primary sensorimotor cortex.82,89–92 The physiological and functional imaging findings are difficult to interpret because less cortical activation would be expected with less muscle contraction. With use of electromyography in patients with cervical dystonia, Schramm, Reiners, and Naumann92 proposed a two-phase model in which abnormal head posture is first normalised by counter-pressure or volitional antagonistic muscle activity, and then the position is stabilised by changed sensory input. Although sensory mechanisms have been implicated in these manoeuvres, altered sensory input might not be needed at least for the first phase because electro-myography recruitment is altered even before the hand makes contact with the face.93 Further studies are needed to better understand the role of sensorimotor interaction in the mechanism of alleviating manoeuvres.
Photosensitivity in blepharospasm is an important, but poorly understood, sensory aspect of this focal dystonia. Patients with blepharospasm not only complain of pain associated with light sensitivity, but also often report worsening of eye spasms when exposed to bright lights, for which they often have to wear dark glasses, even when indoors. A distinct set of photoreceptors named the intrinsically photosensitive retinal ganglion cells have been identified and implicated in blepharospasm.94 These cells, present in the retina and iris, use the photopigment melanopsin, rather than rhodopsin, to detect light in a non-image-forming manner and have direct connections to the thalamic nuclei connected to somatic sensation and pain. These thalamic nuclei also receive convergent input from the trigeminal afferents, which might also provide an explanation for photophobia associated with migraine.94 Not all patients with blepharospasm have photosensitivity; however, those who have these symptoms have increased activity in the thalamus and dorsal midbrain with PET imaging.95 The association between photosensitivity in blepharospasm and, more generally, pain and dystonia needs to be further clarified.
Various sensory abnormalities have been identified in patients with primary focal dystonia. Recording of contact heat-evoked potentials in response to heat stimulation of the volar forearm and the dorsum of the hand at a temperature of 51°C in patients with focal hand dystonia showed lower N2–P2 amplitudes in the somatosensory cortex from the dystonic arm than in the unaffected side and healthy controls.96 Additionally, on quantitative sensory testing, increased thresholds of thermal detection and mechanical pain, and decreased mechanical pain sensitivity on the affected limb, suggest a loss of sensory function of the dystonic hand. This study indicates the potential contribution of the small-fibre A-δ-system, which underlies transmission of the thermal stimuli to the pathophysiology of dystonia. Other studies also used somatosensory-evoked potentials and transcranial magnetic stimulation to show impaired cortical somatosensory processing, abnormal sensorimotor integration, and maladaptive cortical plasticity in patients with dystonia.97–99 Patients with focal dystonia also have decreased kinaesthetic perception to passive joint movement,100 abnormal proprioceptive vibration-induced illusion of movements,101–104 and impaired integration of proprioceptive input with evidence of abnormal egocentric spatial representation.105,106 Further more, abnormal spatial discrimination, simultaneous two-point cutaneous stimuli, and impaired temporal discrimination (two stimuli at the same place separated in time), have been recorded in patients with dystonia.107–112 Abnormalities in temporal cutaneous discrimination have been noted not only in the affected limb but also in the unaffected limb of patients with unilateral focal dystonia, and in patients with blepharospasm and cervical dystonia and their unaffected relatives, which suggests a common underlying genetic endophenotype.110,113–115 The presence of these non-elemental sensory abnormalities, coupled with evidence of impaired sensory and motor processing and loss of so-called surround inhibition, provide strong support for the idea that dystonia is not only a motor but also a sensory disorder.7,116–118
Although in this Review we have focused on primary dystonia, dystonic movements can also occur in other basal ganglia disorders.74,119 The involvement and mechanism of sensory abnormalities in the secondary dystonias are probably heterogeneous, partly defined by the underlying pathogenic abnormality.
The most effective treatment of focal dystonia is local injection of botulinum neurotoxin, targeting the affected muscles.120,121 Botulinum neurotoxin not only relaxes the abnormally contracting muscle but also alters peripheral input by weakening the intrafusal muscle fibres, leading to reduced spindle afferent activity.122 Reduction of peripheral sensory input by cooling the affected limb to treat writer’s cramp has been noted to transiently improve this focal dystonia.123 Administration of local anaesthetic improved dystonic movements in medically refractory writer’s cramp.124 Sensory training through use of Braille reading in patients with focal hand dystonia transiently reduced the dystonic movement and improved writing.125,126 These findings not only provide additional support for the importance of the sensory system in the pathophysiology of dystonia, but also suggest new therapeutic strategies.
Peripherally induced dystonia and complex regional pain
Although the topic of peripherally induced movement disorders is controversial, several examples of involuntary movements—such as hemifacial spasm and amputation stump movements—are clearly related to altered peripheral input.127 Furthermore, focal injury preceding the development of dystonia has been well documented, although the cause–effect association is not understood.127–129 Experiments in primates and human beings have shown that digit amputation or other types of peripheral injury cause the cortical representations of adjacent digits to expand topographically and to occupy most or all of the cortical territories formerly representing the amputated digit.130–132 Thus, central reorganisation in response to altered peripheral input could be the mechanism that underlies peripherally induced movement disorders.127,133 Furthermore, sensorimotor training that includes several methods, such as imagery or mirror treatment, the use of prostheses, and other training procedures, have been used to improve dystonia and other disorders of sensorimotor integration.125,134,135
Another syndrome associated with dystonia is complex regional pain syndrome (CRPS), a severe, persistent pain syndrome that develops soon after an injury or immobilisation (eg, casting or splinting) and is associated with vasomotor, sudomotor, trophic changes, and mood and psychological disturbances.129,136–140 The pathophysiology of CRPS-related dystonia is largely unknown, although immunological mechanisms are increasingly implicated.141 One hypothesis for CRPS-related dystonia is that noxious stimuli interfere with joint and muscle proprioception of the affected body part, which disrupts neighbouring and distal muscle activation during voluntary and reflex movements.142 In a systematic study of the patterns of dystonia in 85 patients with CRPS, the observed flexion of distal joints was attributed to aberration in the normal feedback from the Golgi tendon organs in the regulation of force, ultimately leading to the abnormal fixed flexion.142 This mechanism is, however, highly speculative and more studies are needed to clarify the mechanism of abnormal movements and postures associated with CRPS. Because only a small group of patients develop dystonia in the setting of CRPS, some genetic predisposition might exist in patients who do develop this form of peripherally induced dystonia.140 The presence of affective disorders and of psychogenic features in these patients has led some researchers to propose that dystonia in the setting of peripheral injury is largely of psychogenic origin137,143 or a form of body integrity identity disorder.144 More data are needed to better understand the CRPS dystonia syndrome.
Urge sensations in movement disorders
Urge phenomenon
Although an urge preceding a movement might not necessarily represent a sensory event because it is often associated with underlying obsessive-compulsive disorder,145 some evidence exists that the urge phenomenon might occur secondary to abnormalities in sensorimotor integration.146,147 Thus, the movement that follows and temporarily relieves an urge might not be truly involuntary but rather unvoluntary, occurring in response to an inner feeling.148
Tics and Tourette’s syndrome
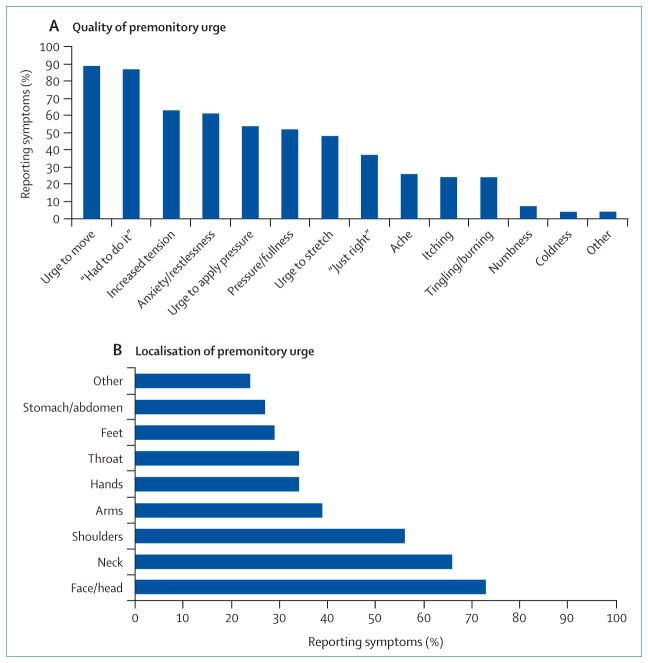
Tics—defined as abrupt, brief, involuntary movements or vocalisations—are the clinical hallmark of Tourette’s syndrome.149 Tics manifest as repetitive contractions of an isolated muscle group (focal tics) occurring out of a normal background, or as complex tics involving a wide variety of muscle jerks in different muscle groups in a stereotypical and patterned sequence.150 In addition to motor tics, people with Tourette’s syndrome also produce sounds, such as sniffing, grunting, or throat clearing (simple phonic tics), or utterances with semantic meaning (complex phonic tics), including shouting of obscenities or profanities (coprolalia). A distinguishing feature of tics that separates them from other hyperkinetic movements is the presence of a premonitory urge, described as an unpleasant sensation often preceding the motor tic in a crescendo manner until the tic is executed. Although some children have difficulty describing and articulating these abnormal sensations, most adults with Tourette’s syndrome can provide an adequate description to allow categorisation of the premonitory phenomena as either regional, localised to the area of the tic, or generalised, manifested by a non-specific inner tension, urge, or discomfort, not necessarily anatomically connected to the tic.151 The Premonitory Urge for Tics Scale (PUTS), originally validated in children, also has good psychometric properties in adults with Tourette’s syndrome.152 The sensations might be vague and poorly localised feelings such as an urge, anxiety, or a need to do something, whereas other sensations are regional, localised to a specific area, sometimes referred to as sensory or compulsive tics (figure 2).153–156 Although the premonitory sensation is involuntary, the movement is typically perceived by the patient as a voluntary action to relieve the preceding, underlying discomfort.152,157 Additional support for the voluntary nature of tics includes the ability to transiently suppress tics at the expense of worsening internal discomfort.150 By contrast with the localised premonitory urge, such as a sensation of dry throat preceding throat clearing or a tension in the neck preceding neck extension (so-called whiplash) tics, there are rare instances when the patient perceives sensations in other objects or people that are relieved when the patient touches or scratches them—defined as extracorporeal phantom tics.158 Patients with Tourette’s syndrome have described increased sensitivity to faint external stimuli, such as clothing tags and textures, and light and sound, which often evolves into a disabling, compulsive need to adjust or remove the clothing.159 Similar to dystonia, some patients have used alleviating manoeuvres to control tics,160 and habit-reversal techniques have been incorporated into behavioural modification of tics.161 In a study of 19 patients with Tourette’s syndrome and 19 age-matched healthy volunteers, 80% of patients had heightened subjective sensitivity to external stimuli to all five senses except taste.162 However, sensory thresholds and psychophysical response curves for the subjective intensity of stimuli were normal. The investigators concluded that the results indicate that patients’ perceived sensitivity derives from altered central processing rather than enhanced peripheral detection.
Figure 2. Characteristics of urge phenomena in Tourette’s syndrome.
These charts represent the localisation and descriptive quality of the sensory urge in a survey of 50 patients. Data from Kwak and colleagues.157
Another unique observation regarding premonitory sensations is that botulinum neurotoxin injections targeting the area of premonitory sensation improve not only the intensity and frequency of tics but also the premonitory sensation, thus removing the need to perform the tic,157,163,164 or even essentially eliminating coprolalia.165 Although evidence for the effects of botulinum neurotoxin on peripheral nociceptive transmission and reduction of central sensitisation is growing,166 future research needs to address the potential mechanism of botulinum neurotoxin to relieve premonitory sensory urge.
The reduction in tic frequency and severity with centrally acting dopamine receptor blockers, dopamine depleters such as tetrabenazine,167 and pallidal or thalamic deep brain stimulation168 provides strong evidence that tics are centrally generated. A post-mortem study of brains of patients with Tourette’s syndrome showed reduction in the number of cholinergic interneurons in the associative and sensorimotor regions of the striatum.169 In Tourette’s syndrome, a breakdown in the gating mechanisms for sensory inputs and reduced efficiency of synaptic inhibition have been implicated in the urge phenomenon and release of tics.170–172 Abnormal gating of sensory stimuli and increased sensory feedback in Tourette’s syndrome have been shown through self-paced finger-movement tasks,173 and by decreased inhibition of the blink reflex.174,175 Furthermore, an increased dependence on visual pathways to complete tasks supports the role of the sensory system in the pathophysiology of Tourette’s syndrome.176
Restless legs syndrome
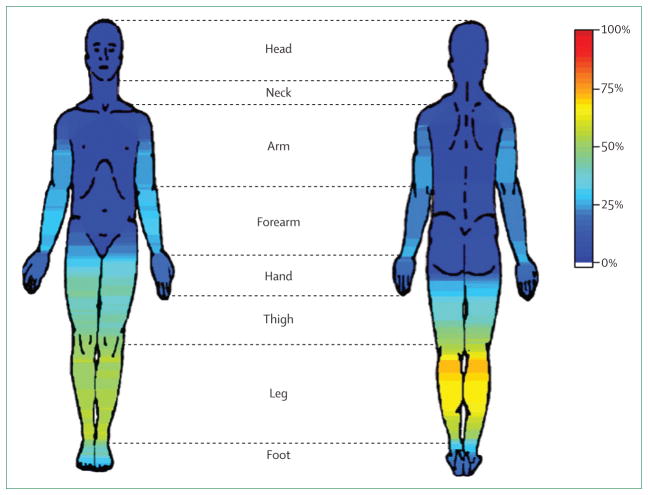
Restless legs syndrome is characterised by an ill-defined unpleasant crawling sensation in the legs, predominantly in the evening when the patient is attempting to fall asleep.177 In one study the most frequent spontaneous descriptors were “urge to move” (24%), “irritating” (17%), and “painful” (17%).178 The most frequent prompted descriptors were “restless” (88%), “uncomfortable” (78%), and “need to stretch” (76%).178 Deep muscular pain mainly localised to both legs has been described in up to 86% of patients, although these sensations can occasionally be localised to other regions of the body (figure 3).179 The sensory symptoms can also often be relieved by rubbing the area or by immersion in hot water. A key diagnostic feature of these unpleasant sensations is that they are partially or totally relieved by movements such as walking or stretching for as long as the activity continues,180 which suggests that restless legs syndrome, like tics, can be thought of as a disorder of sensation relieved by movement.
Figure 3. Topography of sensations in restless legs syndrome.
The frequency and distribution of sensory symptoms associated with restless legs syndrome. Figure reproduced from Karroum and colleagues,179 by permission of the Society for Neuroscience.
The pathophysiology of the sensory urge in restless legs syndrome is largely unknown. Although symptoms similar to those of restless legs syndrome can be present in patients with sensory neuropathies,181,182 the presence of similar symptoms in other basal ganglia disorders such as Parkinson’s disease, evidence of reduced endogenous dopamine with increased dopamine D2 receptor availability on PET imaging,183 and partial alleviation of symptoms with globus pallidus internus deep brain stimulation146 implies that the basal ganglia have a role in modulation of this sensorimotor condition.9 The potential role of melatonin is suggested by the finding of reduced urge phenomenon in patients with restless legs syndrome when exposed to bright lights.184 Alteration of pain perception with chronic dopaminergic therapy with evidence of low ferritin and iron concentrations in the substantia nigra further suggests basal ganglia involvement through disruption of the basal ganglia, descending spinal dopaminergic pathways, or both.185–189 Although the association between iron abnormalities and restless legs syndrome has not yet been fully elucidated, a deficit of iron transport into the CNS has been suggested as the fundamental metabolic abnormality in the syndrome.190 Only a few studies have formally addressed sensorimotor integration in restless legs syndrome.188,191 Reduced short-latency afferent inhibition, a marker for sensorimotor integration, has been shown with transcranial magnetic stimulation in patients with the syndrome, and this abnormality normalised with dopaminergic therapy.188 Future neurophysiological studies should be directed to improve our understanding of the urge phenomenon and its pathophysiological mechanisms.
Akathisia
Akathisia refers to an abnormal state of excessive restlessness or an urge or need to move about. These symptoms are relieved during movement. By contrast with many other movement disorders that might be primary or idiopathic, akathisia is almost always seen as a result of particular drugs, such as selective serotonin reuptake inhibitors,192 dopamine-receptor blockers, or dopamine depleters such as tetrabenazine,167,193 although it might also be encountered in patients with Parkinson’s disease, even those not taking medication.194 Although the pathophysiology of akathisia is largely unknown, the exacerbation of symptoms in low-dopamine states, as seen in Parkinson’s disease, and the association with the use of dopamine-receptor blockers or dopamine depleters suggests that this disorder is a symptom of abnormal dopamine transmission. Improvement with zolpidem, which binds to the GABA-benzodiazepine receptor complex, suggests that the GABAergic system could also be involved in akathisia.195 A unique aspect of this sensorimotor disorder is the amelioration of the sensory symptoms with passive motion (eg, as a passenger in a moving car), which suggests that passive sensory input (eg, a perception of movement via visual and vestibular input) rather than voluntary movement alleviates the sensory discomfort associated with akathisia.196 Future studies should be directed at assessment of whether sensory impairments and abnormalities exist in sensorimotor integration.
Stereotypies
Stereotypies are defined as coordinated, patterned, repetitive movements or sounds that are typically involuntary although are mainly recognised to be in response to or induced by an inner sensory urge, stimulus, or unwanted feeling.197 Stereotypies have many causes, including autistic and psychiatric disorders, but tardive dyskinesia is probably the most common cause of adult-onset stereotypy.198 This group of iatrogenic hyperkinetic movement disorders, caused by dopamine-receptor blocking agents, is typically manifested by oro-buccal-lingual stereotypy, but various movement disorders can be encountered in patients with tardive dyskinesia, including akathisia, dystonia, tics, tremor, and chorea. In addition to movement disorders (including involuntary vocalisations), patients with tardive dyskinesia can have various sensory symptoms, such as an urge to move (as in akathisia), paraesthesias, and pain, particularly involving the oral and genital areas.148 Another example of a sensory aspect of tardive dyskinesia is phantom dyskinesia, which was first reported in a 58-year-old woman who had persistent post-amputation stump chorea and the perception of involuntary movement in the phantom left arm.199
Another example of abnormal sensorimotor integration is a syndrome that we have named ‘leg stereotypy disorder’. Although not previously well described in published literature, this syndrome is often observed in people who, while seated in meetings, exhibit stereotypical 1–2 Hz rhythmical flexion–extension movements of the hips with toes resting on the floor. The movement might last for a few seconds, minutes, or hours, and goes away when standing or walking. People can stop easily, at will. Many individuals with this syndrome describe the intense need to move their legs in response to an inner tension or state of anxiety, which is transiently relieved by the movement. Although frequently familial, this syndrome is different from restless legs syndrome in that it is not diurnal in pattern and is not associated with unpleasant sensations. Further clinical, physiological, and pharmacological characterisation of this disorder is needed.
The basal ganglia, cerebellum, and sensory processing
The somatosensory system is a complex network of neurons, synapses, and receptors, through which we perceive and navigate our environment. Cajal200 provided a detailed description of the somatosensory network in animals, including frogs, cats, and mice, and in human beings, in Histology of the nervous system of man and vertebrates. According to Cajal, this elaborate somatosensory system is made up of six mechanoreceptors—Meissner corpuscle, Pacinian corpuscle, Golgi tendon organ, Merkel discs, Ruffini organs, and muscle spindles.201,202 These sensory receptors and neurons with cell bodies in the dorsal root ganglion mediate sensory input, including pain, to the spinal cord and brain through the spinothalamic, spinoreticular, spinohypothalamic, and spinocerebellar tracts.201,202 The afferent sensory system interacts via direct and indirect projections with the brainstem, cerebellum, subcortical, and cortical structures. Sensory information eventually affects the motor system and the choice and pattern of movement. Thus, sensory information has two roles: first to inform consciousness about the state of the world (exteroceptive) and the state of the body (interoceptive), and second to guide the driving of the motor system. Abnormalities in this sensorimotor integration underlie many hypokinetic and hyperkinetic movement disorders.8
Although the basal ganglia do not directly receive sensory information, processing of indirect information by the basal ganglia has a distinct effect on movement. Various models of the basal ganglia suggest two major roles for it in the generation and maintenance of movements: co-activation of agonist–antagonist muscles to maintain equilibrium and balance; and sequential activation of agonist and then antagonist muscles for implementation of fast movements.203 Additionally, and perhaps most importantly, the basal ganglia enable specific movements and selectively inhibit competing motor programmes that could interfere with the intended voluntary movement.116 Several neuro physiological studies provide support for the emerging idea that the basal ganglia serve as a gate-keeper for sensory inputs at various levels along the CNS, and that abnormal sensorimotor integration is a key feature in the pathogenesis of many movement disorders.8,9,156 The role of the basal ganglia extends beyond motor control to the recognition of anatomically distinct loops that have reciprocal connections with the frontal, limbic, and sensory systems affecting cognitive, emotional, and sensorimotor processing. Abnormal sensorimotor processing could lead not only to sensory symptoms but also to sensory and motor abnormalities. Increasing evidence suggests that proprioceptive sensory input plays a crucial part in the generation and coordination of movements. The motor circuit comprised of the substantia nigra, subthalamic nucleus, globus pallidus, and putamen is the most studied basal ganglia loop, which is mediated mainly via the direct, indirect, and hyperdirect pathways, the first two of which are affected by specific dopamine receptors. Reinforcement of automatic movements through sensory-evoked phasic release of dopamine is hypothesised to be crucial to the maintenance of automatic movements.204 In fact, the dopamine D2 receptors have been implicated in the regulation of affective and motivational aspects of sensory processing.183,205 The progressive loss of dopamine signalling in Parkinson’s disease results in reduced capacity to generate normal automatic movements, whereas goal-directed motor actions remain intact, as seen with external cueing.66 Alternatively, aberrantly increased dopaminergic transmission might result in increased repetitive and stereotyped movements such as those associated with urge phenomena.66 Abnormalities in striatal dopaminergic anatomy have also been shown in animal models of Tourette’s syndrome206—rodent and primate models of Tourette’s syndrome have shown that tics are associated with phasic changes of neuronal activity throughout the cortico-basal ganglia circuitry, and that tics, and their sensory components, are linked to abnormalities in the sensorimotor network, mostly in the prefrontal dorsolateral cortex and sensorimotor parts of the basal ganglia.207,208
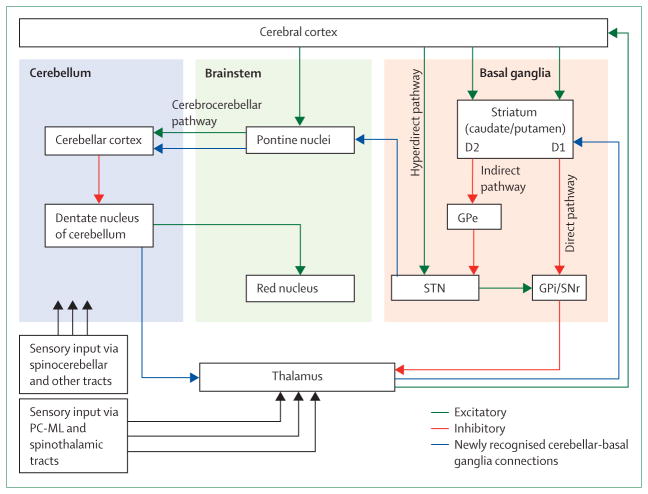
The cerebellum receives considerable sensory information directly and it seems to play an important part in the guidance of movement, particularly online corrections for coordination.71 The cortico-cerebellar circuit, mainly involved in the coordination of movement, connects the frontal lobe, pontine nuclei, cerebellar cortex, deep cerebellar nuclei, red nucleus, ventrolateral thalamic nucleus, and motor cortex. Novel imaging techniques such as functional MRI and diffusion tractography imaging have drawn attention not only to the basal ganglia but also to the cerebellum and its projections in the pathophysiology of Parkinson’s disease, essential tremor, and dystonia.208–211 Results of transcranial magnetic stimulation studies show abnormal cerebellar functioning, including reduced effect on cortical plasticity in primary dystonia,212,213 and viral tracing techniques have shown reciprocal connections between the basal ganglia and the sensorimotor cortex and associative and limbic regions of the cerebellum (figure 4).214,215 Thus, the cortical-basal ganglia-cerebellar connections have important implications for motor, cognitive, affective, and sensory aspects of movement disorders.
Figure 4. Subcortical and cortical pathways for motor control.
This diagram of the basal ganglia and cerebellum shows pathways involved in sensorimotor integration. The blue pathway is that described by Bostan and Strick.215 D1 and D2=dopamine receptors. GPe=globus pallidus pars externa. GPi=globus pallidus pars interna. SNr=substantia nigra pars reticularis. STN=subthalamic nucleus. PC-ML=posterior column-medial lemiscus.
Search strategy and selection criteria.
The disorders that we have discussed in this Review were selected as the most illustrative examples of sensory abnormalities encountered in or contributing to movement disorders. We identified references through searches of PubMed with the search terms “sensory”, “sensorimotor integration”, “basal ganglia”, “cerebellum”, “movement disorders”, “Parkinson’s disease”, “dystonia”, “complex regional pain”, “Tourette’s syndrome”, “tics”, “akathisia”, “stereotypies”, and “restless legs syndrome”. We included articles published between January, 2010, and May, 2013. Articles were also identified by searches of the reference lists of the articles identified by this search, and of our own files. We used additional searches to elaborate on specific topics at the authors’ discretion. We reviewed only papers published in English. The final reference list was generated on the basis of originality and relevance to the scope of this Review.
Conclusions
Increasing numbers of movement disorders have been recognised to exhibit sensory symptoms or abnormalities that might be integral to the pathophysiology of the abnormal movements or postures. The basal ganglia, cerebellum, thalamus, and their connections, coupled with altered sensory input, seem to play a key part in abnormal sensorimotor integration in various movement disorders. Additional clinical, neurophysiological, and imaging studies are needed to better understand the mechanisms of sensory abnormalities in patients with movement disorders.
Acknowledgments
MH is an employee of the National Institutes of Health. JJ acknowledges the support of the National Parkinson Foundation and the Huntington Disease Association of America. NP has received support for a movement disorder fellowship from the Dystonia Medical Research Foundation.
Footnotes
Contributors
NP did the literature search, interpreted the data, and wrote the initial draft of the paper. JJ and MH provided input for the concept and design of the manuscript, revised drafts, and supervised the project.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Bayulkem K, Lopez G. Clinical approach to nonmotor sensory fluctuations in Parkinson’s disease. J Neurol Sci. 2011;310:82–85. doi: 10.1016/j.jns.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhuri KR, Odin P, Antonini A, Martinez-Martin P. Parkinson’s disease: the non-motor issues. Parkinsonism Relat Disord. 2011;17:717–23. doi: 10.1016/j.parkreldis.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Kuyper DJ, Parra V, Aerts S, Okun MS, Kluger BM. Nonmotor manifestations of dystonia: a systematic review. Mov Disord. 2011;26:1206–17. doi: 10.1002/mds.23709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stamelou M, Edwards MJ, Hallett M, Bhatia KP. The non-motor syndrome of primary dystonia: clinical and pathophysiological implications. Brain. 2012;135:1668–81. doi: 10.1093/brain/awr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nijs J, Daenen L, Cras P, Struyf F, Roussel N, Oostendorp RA. Nociception affects motor output: a review on sensory-motor interaction with focus on clinical implications. Clin J Pain. 2012;28:175–81. doi: 10.1097/AJP.0b013e318225daf3. [DOI] [PubMed] [Google Scholar]
- 6.Seki K, Fetz EE. Gating of sensory input at spinal and cortical levels during preparation and execution of voluntary movement. J Neurosci. 2012;32:890–902. doi: 10.1523/JNEUROSCI.4958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallett M. Is dystonia a sensory disorder? Ann Neurol. 1995;38:139–40. doi: 10.1002/ana.410380203. [DOI] [PubMed] [Google Scholar]
- 8.Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov Disord. 2003;18:231–40. doi: 10.1002/mds.10327. [DOI] [PubMed] [Google Scholar]
- 9.Kaji R, Murase N. Sensory function of basal ganglia. Mov Disord. 2001;16:593–94. doi: 10.1002/mds.1137. [DOI] [PubMed] [Google Scholar]
- 10.Lyoo CH, Ryu YH, Lee MJ, Lee MS. Striatal dopamine loss and discriminative sensory dysfunction in Parkinson’s disease. Acta Neurol Scand. 2012;126:344–49. doi: 10.1111/j.1600-0404.2012.01657.x. [DOI] [PubMed] [Google Scholar]
- 11.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–76. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 12.Khoo TK, Yarnall AJ, Duncan GW, et al. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology. 2013;80:276–81. doi: 10.1212/WNL.0b013e31827deb74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Defazio G, Berardelli A, Fabbrini G, et al. Pain as a nonmotor symptom of Parkinson disease: evidence from a case-control study. Arch Neurol. 2008;65:1191–94. doi: 10.1001/archneurol.2008.2. [DOI] [PubMed] [Google Scholar]
- 14.Ha AD, Jankovic J. Pain in Parkinson’s disease. Mov Disord. 2012;27:485–91. doi: 10.1002/mds.23959. [DOI] [PubMed] [Google Scholar]
- 15.Rana AQ, Siddiqui I, Mosabbir A, et al. Association of pain, Parkinson’s disease, and restless legs syndrome. J Neurol Sci. 2013;327:32–34. doi: 10.1016/j.jns.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 16.Wasner G, Deuschl G. Pains in Parkinson disease--many syndromes under one umbrella. Nat Rev Neurol. 2012;8:284–94. doi: 10.1038/nrneurol.2012.54. [DOI] [PubMed] [Google Scholar]
- 17.Siderowf A, Jennings D, Eberly S, et al. Impaired olfaction and other prodromal features in the Parkinson At-Risk Syndrome Study. Mov Disord. 2012;27:406–12. doi: 10.1002/mds.24892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uc EY, Rizzo M, Anderson SW, Qian S, Rodnitzky RL, Dawson JD. Visual dysfunction in Parkinson disease without dementia. Neurology. 2005;65:1907–13. doi: 10.1212/01.wnl.0000191565.11065.11. [DOI] [PubMed] [Google Scholar]
- 19.Stamey W, Davidson A, Jankovic J. Shoulder pain: a presenting symptom of Parkinson disease. J Clin Rheumatol. 2008;14:253–54. doi: 10.1097/RHU.0b013e3181826d43. [DOI] [PubMed] [Google Scholar]
- 20.Madden MB, Hall DA. Shoulder pain in Parkinson’s disease: a case-control study. Mov Disord. 2010;25:1105–06. doi: 10.1002/mds.23048. [DOI] [PubMed] [Google Scholar]
- 21.Yucel A, Kusbeci OY. Magnetic resonance imaging findings of shoulders in Parkinson’s disease. Mov Disord. 2010;25:2524–30. doi: 10.1002/mds.23310. [DOI] [PubMed] [Google Scholar]
- 22.Muller T, Muhlack S, Woitalla D. Pain perception, pain drug therapy and health status in patients with Parkinson’s disease. Neuroepidemiology. 2011;37:183–87. doi: 10.1159/000331911. [DOI] [PubMed] [Google Scholar]
- 23.Gerdelat-Mas A, Simonetta-Moreau M, Thalamas C, et al. Levodopa raises objective pain threshold in Parkinson’s disease: a RIII reflex study. J Neurol Neurosurg Psychiatry. 2007;78:1140–42. doi: 10.1136/jnnp.2007.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djaldetti R, Shifrin A, Rogowski Z, Sprecher E, Melamed E, Yarnitsky D. Quantitative measurement of pain sensation in patients with Parkinson disease. Neurology. 2004;62:2171–75. doi: 10.1212/01.wnl.0000130455.38550.9d. [DOI] [PubMed] [Google Scholar]
- 25.Schestatsky P, Kumru H, Valls-Sole J, et al. Neurophysiologic study of central pain in patients with Parkinson disease. Neurology. 2007;69:2162–69. doi: 10.1212/01.wnl.0000295669.12443.d3. [DOI] [PubMed] [Google Scholar]
- 26.Lefaucheur R, Berthelot L, Sénant J, Borden A, Maltête D. Acute genital pain during non-motor fluctuations improved by apomorphine. Mov Disord. 2013;28:687–88. doi: 10.1002/mds.25379. [DOI] [PubMed] [Google Scholar]
- 27.Ciampi de Andrade D, Lefaucheur JP, Galhardoni R, et al. Subthalamic deep brain stimulation modulates small fiber-dependent sensory thresholds in Parkinson’s disease. Pain. 2012;153:1107–13. doi: 10.1016/j.pain.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Fil A, Cano-de-la-Cuerda R, Munoz-Hellin E, Vela L, Ramiro-Gonzalez M, Fernandez-de-Las-Penas C. Pain in Parkinson disease: a review of the literature. Parkinsonism Related Disord. 2013;19:285–94. doi: 10.1016/j.parkreldis.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Perrotta A, Sandrini G, Serrao M, et al. Facilitated temporal summation of pain at spinal level in Parkinson’s disease. Mov Disord. 2011;26:442–48. doi: 10.1002/mds.23458. [DOI] [PubMed] [Google Scholar]
- 30.Morera L, Labar G, Ortar G, Lambert DM. Development and characterization of endocannabinoid hydrolases FAAH and MAGL inhibitors bearing a benzotriazol-1-yl carboxamide scaffold. Bioorg Med Chem. 2012;20:6260–75. doi: 10.1016/j.bmc.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Konczak J, Li KY, Tuite PJ, Poizner H. Haptic perception of object curvature in Parkinson’s disease. PLoS One. 2008;3:e2625. doi: 10.1371/journal.pone.0002625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konczak J, Sciutti A, Avanzino L, et al. Parkinson’s disease accelerates age-related decline in haptic perception by altering somatosensory integration. Brain. 2012;135:3371–79. doi: 10.1093/brain/aws265. [DOI] [PubMed] [Google Scholar]
- 33.Chang EF, Turner RS, Ostrem JL, Davis VR, Starr PA. Neuronal responses to passive movement in the globus pallidus internus in primary dystonia. J Neurophysiol. 2007;98:3696–707. doi: 10.1152/jn.00594.2007. [DOI] [PubMed] [Google Scholar]
- 34.Seiss E, Praamstra P, Hesse CW, Rickards H. Proprioceptive sensory function in Parkinson’s disease and Huntington’s disease: evidence from proprioception-related EEG potentials. Exp Brain Res. 2003;148:308–19. doi: 10.1007/s00221-002-1291-6. [DOI] [PubMed] [Google Scholar]
- 35.Snider SR, Fahn S, Isgreen WP, Cote LJ. Primary sensory symptoms in parkinsonism. Neurology. 1976;26:423–29. doi: 10.1212/wnl.26.5.423. [DOI] [PubMed] [Google Scholar]
- 36.Konczak J, Krawczewski K, Tuite P, Maschke M. The perception of passive motion in Parkinson’s disease. J Neurol. 2007;254:655–63. doi: 10.1007/s00415-006-0426-2. [DOI] [PubMed] [Google Scholar]
- 37.Wright WG, Gurfinkel VS, King LA, Nutt JG, Cordo PJ, Horak FB. Axial kinesthesia is impaired in Parkinson’s disease: effects of levodopa. Exp Neurol. 2010;225:202–09. doi: 10.1016/j.expneurol.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maschke M, Tuite PJ, Krawczewski K, Pickett K, Konczak J. Perception of heaviness in Parkinson’s disease. Mov Disord. 2006;21:1013–18. doi: 10.1002/mds.20876. [DOI] [PubMed] [Google Scholar]
- 39.Demirci M, Grill S, McShane L, Hallett M. A mismatch between kinesthetic and visual perception in Parkinson’s disease. Ann Neurol. 1997;41:781–88. doi: 10.1002/ana.410410614. [DOI] [PubMed] [Google Scholar]
- 40.Fiorio M, Stanzani C, Rothwell JC, et al. Defective temporal discrimination of passive movements in Parkinson’s disease. Neurosci Lett. 2007;417:312–15. doi: 10.1016/j.neulet.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 41.Conte A, Modugno N, Lena F, et al. Subthalamic nucleus stimulation and somatosensory temporal discrimination in Parkinson’s disease. Brain. 2010;133:2656–63. doi: 10.1093/brain/awq191. [DOI] [PubMed] [Google Scholar]
- 42.Li KY, Pickett K, Nestrasil I, Tuite P, Konczak J. The effect of dopamine replacement therapy on haptic sensitivity in Parkinson’s disease. J Neurol. 2010;257:1992–98. doi: 10.1007/s00415-010-5646-9. [DOI] [PubMed] [Google Scholar]
- 43.Mongeon D, Blanchet P, Messier J. Impact of Parkinson’s disease and dopaminergic medication on proprioceptive processing. Neuroscience. 2009;158:426–40. doi: 10.1016/j.neuroscience.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Coelho M, Marti MJ, Tolosa E, et al. Late-stage Parkinson’s disease: the Barcelona and Lisbon cohort. J Neurol. 2010;257:1524–32. doi: 10.1007/s00415-010-5566-8. [DOI] [PubMed] [Google Scholar]
- 45.Reichert WH, Doolittle J, McDowell FH. Vestibular dysfunction in Parkinson disease. Neurology. 1982;32:1133–38. doi: 10.1212/wnl.32.10.1133. [DOI] [PubMed] [Google Scholar]
- 46.Barnett-Cowan M, Dyde RT, Fox SH, Moro E, Hutchison WD, Harris LR. Multisensory determinants of orientation perception in Parkinson’s disease. Neuroscience. 2010;167:1138–50. doi: 10.1016/j.neuroscience.2010.02.065. [DOI] [PubMed] [Google Scholar]
- 47.Sacrey LA, Whishaw IQ. Subsystems of sensory attention for skilled reaching: vision for transport and pre-shaping and somatosensation for grasping, withdrawal and release. Behav Brain Res. 2012;231:356–65. doi: 10.1016/j.bbr.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 48.Tan T, Almeida QJ, Rahimi F. Proprioceptive deficits in Parkinson’s disease patients with freezing of gait. Neuroscience. 2011;192:746–52. doi: 10.1016/j.neuroscience.2011.06.071. [DOI] [PubMed] [Google Scholar]
- 49.van der Hoorn A, Beudel M, de Jong BM. Interruption of visually perceived forward motion in depth evokes a cortical activation shift from spatial to intentional motor regions. Brain Res. 2010;1358:160–71. doi: 10.1016/j.brainres.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 50.Suarez H, Geisinger D, Ferreira ED, et al. Balance in Parkinson’s disease patients changing the visual input. Braz J Otorhinolaryngol. 2011;77:651–55. doi: 10.1590/S1808-86942011000500019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colnat-Coulbois S, Gauchard GC, Maillard L, et al. Management of postural sensory conflict and dynamic balance control in late-stage Parkinson’s disease. Neuroscience. 2011;193:363–69. doi: 10.1016/j.neuroscience.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 52.Martens KA, Almeida QJ. Dissociating between sensory and perceptual deficits in PD: more than simply a motor deficit. Mov Disord. 2012;27:387–92. doi: 10.1002/mds.24042. [DOI] [PubMed] [Google Scholar]
- 53.Marchese R, Diverio M, Zucchi F, Lentino C, Abbruzzese G. The role of sensory cues in the rehabilitation of parkinsonian patients: a comparison of two physical therapy protocols. Mov Disord. 2000;15:879–83. doi: 10.1002/1531-8257(200009)15:5<879::aid-mds1018>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 54.Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson’s disease. Normalisation strategies and underlying mechanisms. Brain. 1996;119:551–68. doi: 10.1093/brain/119.2.551. [DOI] [PubMed] [Google Scholar]
- 55.Siegert RJ, Harper DN, Cameron FB, Abernethy D. Self-initiated versus externally cued reaction times in Parkinson’s disease. J Clin Exp Neuropsychol. 2002;24:146–53. doi: 10.1076/jcen.24.2.146.991. [DOI] [PubMed] [Google Scholar]
- 56.Spildooren J, Vercruysse S, Meyns P, et al. Turning and unilateral cueing in Parkinson’s disease patients with and without freezing of gait. Neuroscience. 2012;207:298–306. doi: 10.1016/j.neuroscience.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 57.Van Gerpen JA, Rucker CT, Matthews M, Saucier MA. Lifting the “FOG” with laser generated visual-cueing. Neurologist. 2012;18:298–301. doi: 10.1097/NRL.0b013e318266f919. [DOI] [PubMed] [Google Scholar]
- 58.Berardelli A, Curra A, Fabbrini G, Gilio F, Manfredi M. Pathophysiology of tics and Tourette syndrome. J Neurol. 2003;250:781–87. doi: 10.1007/s00415-003-1102-4. [DOI] [PubMed] [Google Scholar]
- 59.Konczak J, Corcos DM, Horak F, et al. Proprioception and motor control in Parkinson’s disease. J Mot Behav. 2009;41:543–52. doi: 10.3200/35-09-002. [DOI] [PubMed] [Google Scholar]
- 60.Pessiglione M, Guehl D, Rolland AS, et al. Thalamic neuronal activity in dopamine-depleted primates: evidence for a loss of functional segregation within basal ganglia circuits. J Neurosci. 2005;25:1523–31. doi: 10.1523/JNEUROSCI.4056-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Almeida QJ, Frank JS, Roy EA, et al. An evaluation of sensorimotor integration during locomotion toward a target in Parkinson’s disease. Neuroscience. 2005;134:283–93. doi: 10.1016/j.neuroscience.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 62.Inzelberg R, Korczyn AD. Concerning “visual control of arm movement in Parkinson’s disease”. Mov Disord. 1996;11:115. doi: 10.1002/mds.870110129. [DOI] [PubMed] [Google Scholar]
- 63.Klockgether T, Dichgans J. Visual control of arm movement in Parkinson’s disease. Mov Disord. 1994;9:48–56. doi: 10.1002/mds.870090108. [DOI] [PubMed] [Google Scholar]
- 64.Levy-Tzedek S, Krebs HI, Arle JE, Shils JL, Poizner H. Rhythmic movement in Parkinson’s disease: effects of visual feedback and medication state. Exp Brain Res. 2011;211:277–86. doi: 10.1007/s00221-011-2685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luessi F, Mueller LK, Breimhorst M, Vogt T. Influence of visual cues on gait in Parkinson’s disease during treadmill walking at multiple velocities. J Neurol Sci. 2012;314:78–82. doi: 10.1016/j.jns.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 66.Redgrave P, Rodriguez M, Smith Y, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci. 2010;11:760–72. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Galvan A, Smith Y. The primate thalamostriatal systems: Anatomical organization, functional roles and possible involvement in Parkinson’s disease. Basal Ganglia. 2011;1:179–89. doi: 10.1016/j.baga.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu T, Hallett M. The cerebellum in Parkinson’s disease. Brain. 2013;136:696–709. doi: 10.1093/brain/aws360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cerasa A, Hagberg GE, Peppe A, et al. Functional changes in the activity of cerebellum and frontostriatal regions during externally and internally timed movement in Parkinson’s disease. Brain Res Bull. 2006;71:259–69. doi: 10.1016/j.brainresbull.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 70.Catalan MJ, Ishii K, Honda M, Samii A, Hallett M. A PET study of sequential finger movements of varying length in patients with Parkinson’s disease. Brain. 1999;122:483–95. doi: 10.1093/brain/122.3.483. [DOI] [PubMed] [Google Scholar]
- 71.Koziol LF, Budding DE, Chidekel D. From movement to thought: executive function, embodied cognition, and the cerebellum. Cerebellum. 2012;11:505–25. doi: 10.1007/s12311-011-0321-y. [DOI] [PubMed] [Google Scholar]
- 72.Colosimo C, Berardelli A. Clinical phenomenology of dystonia. Int Rev Neurobiol. 2011;98:509–24. doi: 10.1016/B978-0-12-381328-2.00018-3. [DOI] [PubMed] [Google Scholar]
- 73.Phukan J, Albanese A, Gasser T, Warner T. Primary dystonia and dystonia-plus syndromes: clinical characteristics, diagnosis, and pathogenesis. Lancet Neurol. 2011;10:1074–85. doi: 10.1016/S1474-4422(11)70232-0. [DOI] [PubMed] [Google Scholar]
- 74.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863–73. doi: 10.1002/mds.25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trompetto C, Avanzino L, Marinelli L, et al. Corticospinal excitability in patients with secondary dystonia due to focal lesions of the basal ganglia and thalamus. Clin Neurophysiol. 2012;123:808–14. doi: 10.1016/j.clinph.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 76.Wijemanne S, Jankovic J. Hemidystonia-hemiatrophy syndrome. Mov Disord. 2009;24:583–89. doi: 10.1002/mds.22415. [DOI] [PubMed] [Google Scholar]
- 77.Wickremaratchi MM, Knipe MD, Sastry BS, et al. The motor phenotype of Parkinson’s disease in relation to age at onset. Mov Disord. 2011;26:457–63. doi: 10.1002/mds.23469. [DOI] [PubMed] [Google Scholar]
- 78.Louis ED, Anderson KE, Moskowitz C, Thorne DZ, Marder K. Dystonia-predominant adult-onset Huntington disease: association between motor phenotype and age of onset in adults. Arch Neurol. 2000;57:1326–30. doi: 10.1001/archneur.57.9.1326. [DOI] [PubMed] [Google Scholar]
- 79.Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–34. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- 80.Chan J, Brin MF, Fahn S. Idiopathic cervical dystonia: clinical characteristics. Mov Disord. 1991;6:119–26. doi: 10.1002/mds.870060206. [DOI] [PubMed] [Google Scholar]
- 81.Jankovic J, Leder S, Warner D, Schwartz K. Cervical dystonia: clinical findings and associated movement disorders. Neurology. 1991;41:1088–91. doi: 10.1212/wnl.41.7.1088. [DOI] [PubMed] [Google Scholar]
- 82.Poisson A, Krack P, Thobois S, et al. History of the ‘geste antagoniste’ sign in cervical dystonia. J Neurol. 2012;259:1580–84. doi: 10.1007/s00415-011-6380-7. [DOI] [PubMed] [Google Scholar]
- 83.Kagi G, Katschnig P, Fiorio M, et al. Sensory tricks in primary cervical dystonia depend on visuotactile temporal discrimination. Mov Disord. 2013;28:356–61. doi: 10.1002/mds.25305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kägi G, Katschnig P, Fiorio M. Sensory tricks in primary cervical dystonia depend on visuotactile temporal discrimination. Mov Disord. 2013;28:356–61. doi: 10.1002/mds.25305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Defazio G, Hallett M, Jinnah HA, Berardelli A. Development and validation of a clinical guideline for diagnosing blepharospasm. Neurology. 2013;81:236–40. doi: 10.1212/WNL.0b013e31829bfdf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jankovic J, Ashoori A. Movement disorders in musicians. Mov Disord. 2008;23:1957–65. doi: 10.1002/mds.22255. [DOI] [PubMed] [Google Scholar]
- 87.Dhungana S, Jankovic J. Yips and other movement disorders in golfers. Mov Disord. 2013;28:576–81. doi: 10.1002/mds.25442. [DOI] [PubMed] [Google Scholar]
- 88.Kojovic M, Parees I, Sadnicka A, et al. The brighter side of music in dystonia. Arch Neurol. 2012;69:917–19. doi: 10.1001/archneurol.2012.33. [DOI] [PubMed] [Google Scholar]
- 89.Wissel J, Muller J, Ebersbach G, Poewe W. Trick maneuvers in cervical dystonia: investigation of movement- and touch-related changes in polymyographic activity. Mov Disord. 1999;14:994–99. doi: 10.1002/1531-8257(199911)14:6<994::aid-mds1013>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 90.Muller J, Wissel J, Masuhr F, Ebersbach G, Wenning GK, Poewe W. Clinical characteristics of the geste antagoniste in cervical dystonia. J Neurol. 2001;248:478–82. doi: 10.1007/s004150170156. [DOI] [PubMed] [Google Scholar]
- 91.Naumann M, Magyar-Lehmann S, Reiners K, Erbguth F, Leenders KL. Sensory tricks in cervical dystonia: perceptual dysbalance of parietal cortex modulates frontal motor programming. Ann Neurol. 2000;47:322–28. [PubMed] [Google Scholar]
- 92.Schramm A, Reiners K, Naumann M. Complex mechanisms of sensory tricks in cervical dystonia. Mov Disord. 2004;19:452–58. doi: 10.1002/mds.10689. [DOI] [PubMed] [Google Scholar]
- 93.Tang JK, Mahant N, Cunic D, et al. Changes in cortical and pallidal oscillatory activity during the execution of a sensory trick in patients with cervical dystonia. Exp Neurol. 2007;204:845–48. doi: 10.1016/j.expneurol.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 94.Digre KB, Brennan KC. Shedding light on photophobia. J Neuroophthalmol. 2012;32:68–81. doi: 10.1097/WNO.0b013e3182474548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Emoto H, Suzuki Y, Wakakura M, et al. Photophobia in essential blepharospasm—a positron emission tomographic study. Mov Disord. 2010;25:433–39. doi: 10.1002/mds.22916. [DOI] [PubMed] [Google Scholar]
- 96.Suttrup I, Oberdiek D, Suttrup J, Osada N, Evers S, Marziniak M. Loss of sensory function in patients with idiopathic hand dystonia. Mov Disord. 2011;26:107–13. doi: 10.1002/mds.23425. [DOI] [PubMed] [Google Scholar]
- 97.Belvisi D, Suppa A, Marsili L, et al. Abnormal experimentally- and behaviorally-induced LTP-like plasticity in focal hand dystonia. Exp Neurol. 2013;240:64–74. doi: 10.1016/j.expneurol.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 98.Tamura Y, Matsuhashi M, Lin P, et al. Impaired intracortical inhibition in the primary somatosensory cortex in focal hand dystonia. Mov Disord. 2008;23:558–65. doi: 10.1002/mds.21870. [DOI] [PubMed] [Google Scholar]
- 99.Tamura Y, Ueki Y, Lin P, et al. Disordered plasticity in the primary somatosensory cortex in focal hand dystonia. Brain. 2009;132:749–55. doi: 10.1093/brain/awn348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Putzki N, Stude P, Konczak J, Graf K, Diener HC, Maschke M. Kinesthesia is impaired in focal dystonia. Mov Disord. 2006;21:754–60. doi: 10.1002/mds.20799. [DOI] [PubMed] [Google Scholar]
- 101.Fiorio M, Weise D, Onal-Hartmann C, Zeller D, Tinazzi M, Classen J. Impairment of the rubber hand illusion in focal hand dystonia. Brain. 2011;134:1428–37. doi: 10.1093/brain/awr026. [DOI] [PubMed] [Google Scholar]
- 102.Rome S, Grunewald RA. Abnormal perception of vibration-induced illusion of movement in dystonia. Neurology. 1999;53:1794–800. doi: 10.1212/wnl.53.8.1794. [DOI] [PubMed] [Google Scholar]
- 103.Frima N, Rome SM, Grunewald RA. The effect of fatigue on abnormal vibration induced illusion of movement in idiopathic focal dystonia. J Neurol Neurosurg Psychiatry. 2003;74:1154–56. doi: 10.1136/jnnp.74.8.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Frima N, Nasir J, Grunewald RA. Abnormal vibration-induced illusion of movement in idiopathic focal dystonia: an endophenotypic marker? Mov Disord. 2008;23:373–77. doi: 10.1002/mds.21838. [DOI] [PubMed] [Google Scholar]
- 105.Tinazzi M, Fiorio M, Fiaschi A, Rothwell JC, Bhatia KP. Sensory functions in dystonia: insights from behavioral studies. Mov Disord. 2009;24:1427–36. doi: 10.1002/mds.22490. [DOI] [PubMed] [Google Scholar]
- 106.Muller SV, Glaser P, Troger M, Dengler R, Johannes S, Munte TF. Disturbed egocentric space representation in cervical dystonia. Mov Disord. 2005;20:58–63. doi: 10.1002/mds.20293. [DOI] [PubMed] [Google Scholar]
- 107.Tinazzi M, Fiorio M, Stanzani C, et al. Temporal discrimination of two passive movements in writer’s cramp. Mov Disord. 2006;21:1131–55. doi: 10.1002/mds.20892. [DOI] [PubMed] [Google Scholar]
- 108.Sanger TD, Tarsy D, Pascual-Leone A. Abnormalities of spatial and temporal sensory discrimination in writer’s cramp. Mov Disord. 2001;16:94–99. doi: 10.1002/1531-8257(200101)16:1<94::aid-mds1020>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 109.Fiorio M, Tinazzi M, Scontrini A, et al. Tactile temporal discrimination in patients with blepharospasm. J Neurol Neurosurg Psychiatry. 2008;79:796–98. doi: 10.1136/jnnp.2007.131524. [DOI] [PubMed] [Google Scholar]
- 110.Bradley D, Whelan R, Walsh R, et al. Comparing endophenotypes in adult-onset primary torsion dystonia. Mov Disord. 2010;25:84–90. doi: 10.1002/mds.22889. [DOI] [PubMed] [Google Scholar]
- 111.Morgante F, Tinazzi M, Squintani G, et al. Abnormal tactile temporal discrimination in psychogenic dystonia. Neurology. 2011;77:1191–97. doi: 10.1212/WNL.0b013e31822f0449. [DOI] [PubMed] [Google Scholar]
- 112.Scontrini A, Conte A, Defazio G, et al. Somatosensory temporal discrimination in patients with primary focal dystonia. J Neurol Neurosurg Psychiatry. 2009;80:1315–19. doi: 10.1136/jnnp.2009.178236. [DOI] [PubMed] [Google Scholar]
- 113.Walsh R, O’Dwyer JP, Sheikh IH, O’Riordan S, Lynch T, Hutchinson M. Sporadic adult onset dystonia: sensory abnormalities as an endophenotype in unaffected relatives. J Neurol Neurosurg Psychiatry. 2007;78:980–83. doi: 10.1136/jnnp.2006.105585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tinazzi M, Fasano A, Di Matteo A, et al. Temporal discrimination in patients with dystonia and tremor and patients with essential tremor. Neurology. 2013;80:76–84. doi: 10.1212/WNL.0b013e31827b1a54. [DOI] [PubMed] [Google Scholar]
- 115.Kimmich O, Bradley D, Whelan R, et al. Sporadic adult onset primary torsion dystonia is a genetic disorder by the temporal discrimination test. Brain. 2011;134:2656–63. doi: 10.1093/brain/awr194. [DOI] [PubMed] [Google Scholar]
- 116.Mink JW. The basal ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol. 2003;60:1365–68. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- 117.Beck S, Hallett M. Surround inhibition in the motor system. Exp Brain Res. 2011;210:165–72. doi: 10.1007/s00221-011-2610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hallett M. Dystonia: a sensory and motor disorder of short latency inhibition. Ann Neurol. 2009;66:125–27. doi: 10.1002/ana.21762. [DOI] [PubMed] [Google Scholar]
- 119.Schneider SA, Dusek P, Hardy J, Westenberger A, Jankovic J, Bhatia KP. Genetics and pathophysiology of neurodegeneration with brain iron accumulation (NBIA) Curr Neuropharmacol. 2013;11:59–79. doi: 10.2174/157015913804999469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hallett M, Albanese A, Dressler D, et al. Evidence-based review and assessment of botulinum neurotoxin for the treatment of movement disorders. Toxicon. 2013;67:94–114. doi: 10.1016/j.toxicon.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 121.Jankovic J. Medical treatment of dystonia. Mov Disord. 2013;28:1001–12. doi: 10.1002/mds.25552. [DOI] [PubMed] [Google Scholar]
- 122.Trompetto C, Curra A, Buccolieri A, Suppa A, Abbruzzese G, Berardelli A. Botulinum toxin changes intrafusal feedback in dystonia: a study with the tonic vibration reflex. Mov Disord. 2006;21:777–82. doi: 10.1002/mds.20801. [DOI] [PubMed] [Google Scholar]
- 123.Pohl C, Happe J, Klockgether T. Cooling improves the writing performance of patients with writer’s cramp. Mov Disord. 2002;17:1341–44. doi: 10.1002/mds.10251. [DOI] [PubMed] [Google Scholar]
- 124.Kaji R, Kohara N, Katayama M, et al. Muscle afferent block by intramuscular injection of lidocaine for the treatment of writer’s cramp. Muscle Nerve. 1995;18:234–35. doi: 10.1002/mus.880180214. [DOI] [PubMed] [Google Scholar]
- 125.Zeuner KE, Bara-Jimenez W, Noguchi PS, Goldstein SR, Dambrosia JM, Hallett M. Sensory training for patients with focal hand dystonia. Ann Neurol. 2002;51:593–98. doi: 10.1002/ana.10174. [DOI] [PubMed] [Google Scholar]
- 126.Zeuner KE, Hallett M. Sensory training as treatment for focal hand dystonia: a 1-year follow-up. Mov Disord. 2003;18:1044–47. doi: 10.1002/mds.10490. [DOI] [PubMed] [Google Scholar]
- 127.Jankovic J. Peripherally induced movement disorders. Neurol Clin. 2009;27:821–32. doi: 10.1016/j.ncl.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 128.van Rooijen DE, Geraedts EJ, Marinus J, Jankovic J, van Hilten JJ. Peripheral trauma and movement disorders: a systematic review of reported cases. J Neurol Neurosurg Psychiatry. 2011;82:892–98. doi: 10.1136/jnnp.2010.232504. [DOI] [PubMed] [Google Scholar]
- 129.Kumar H, Jog M. Peripheral trauma induced dystonia or post-traumatic syndrome? Can J Neurol Sci. 2011;38:22–29. doi: 10.1017/s0317167100011057. [DOI] [PubMed] [Google Scholar]
- 130.Allard T, Clark SA, Jenkins WM, Merzenich MM. Reorganization of somatosensory area 3b representations in adult owl monkeys after digital syndactyly. J Neurophysiol. 1991;66:1048–58. doi: 10.1152/jn.1991.66.3.1048. [DOI] [PubMed] [Google Scholar]
- 131.Cohen LG, Bandinelli S, Findley TW, Hallett M. Motor reorganization after upper limb amputation in man. A study with focal magnetic stimulation. Brain. 1991;114:615–27. doi: 10.1093/brain/114.1.615. [DOI] [PubMed] [Google Scholar]
- 132.Braune S, Schady W. Changes in sensation after nerve injury or amputation: the role of central factors. J Neurol Neurosurg Psychiatry. 1993;56:393–99. doi: 10.1136/jnnp.56.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Baizabal-Carvallo JF, Jankovic J. Deep brain stimulation of the subthalamic nucleus for peripherally induced Parkinsonism. Neuromodulation. 2013 doi: 10.1111/ner.12071. published online May 10. [DOI] [PubMed] [Google Scholar]
- 134.Flor H, Diers M. Sensorimotor training and cortical reorganization. NeuroRehabilitation. 2009;25:19–27. doi: 10.3233/NRE-2009-0496. [DOI] [PubMed] [Google Scholar]
- 135.Westlake KP, Byl NN. Neural plasticity and implications for hand rehabilitation after neurological insult. J Hand Ther. 2013;26:87–93. doi: 10.1016/j.jht.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 136.Nagler RM. CRPS: Central aspects related to locus of pain, pathophysiology, and mood. Neurology. 2010;75:109–10. doi: 10.1212/WNL.0b013e3181e7cae3. [DOI] [PubMed] [Google Scholar]
- 137.Hawley JS, Weiner WJ. Psychogenic dystonia and peripheral trauma. Neurology. 2011;77:496–502. doi: 10.1212/WNL.0b013e3182287aaf. [DOI] [PubMed] [Google Scholar]
- 138.Marinus J, Moseley GL, Birklein F, et al. Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol. 2011;10:637–48. doi: 10.1016/S1474-4422(11)70106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van Hilten JJ. Movement disorders in complex regional pain syndrome. Pain Med. 2010;11:1274–77. doi: 10.1111/j.1526-4637.2010.00916.x. [DOI] [PubMed] [Google Scholar]
- 140.van Rooijen DE, Roelen DL, Verduijn W, et al. Genetic HLA associations in complex regional pain syndrome with and without dystonia. J Pain. 2012;13:784–89. doi: 10.1016/j.jpain.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 141.Parkitny L, McAuley JH, Di Pietro F, et al. Inflammation in complex regional pain syndrome: a systematic review and meta-analysis. Neurology. 2013;80:106–17. doi: 10.1212/WNL.0b013e31827b1aa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Munts AG, Mugge W, Meurs TS, et al. Fixed dystonia in complex regional pain syndrome: a descriptive and computational modeling approach. BMC Neurol. 2011;11:53. doi: 10.1186/1471-2377-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lang AE, Voon V. Psychogenic movement disorders: past developments, current status, and future directions. Mov Disord. 2011;26:1175–86. doi: 10.1002/mds.23571. [DOI] [PubMed] [Google Scholar]
- 144.Edwards MJ, Alonso-Canovas A, Schrag A, Bloem BR, Thompson PD, Bhatia K. Limb amputations in fixed dystonia: a form of body integrity identity disorder? Mov Disord. 2011;26:1410–14. doi: 10.1002/mds.23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ferrao YA, Shavitt RG, Prado H, et al. Sensory phenomena associated with repetitive behaviors in obsessive-compulsive disorder: an exploratory study of 1001 patients. Psychiatry Res. 2012;197:253–58. doi: 10.1016/j.psychres.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 146.Ondo WG, Jankovic J, Simpson R, Jimenez-Shahed J. Globus pallidus deep brain stimulation for refractory idiopathic restless legs syndrome. Sleep Med. 2012;13:1202–04. doi: 10.1016/j.sleep.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 147.Mazzone SB, McGovern AE, Yang SK, et al. Sensorimotor circuitry involved in the higher brain control of coughing. Cough. 2013;9:7. doi: 10.1186/1745-9974-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fahn S, Jankovic J, Hallett M. Principles and practice of movement disorders. China: Elsevier; 2011. [Google Scholar]
- 149.Jankovic J, Kurlan R. Tourette syndrome: evolving concepts. Mov Disord. 2011;26:1149–56. doi: 10.1002/mds.23618. [DOI] [PubMed] [Google Scholar]
- 150.Jankovic J. Tourette’s syndrome. N Engl J Med. 2001;345:1184–92. doi: 10.1056/NEJMra010032. [DOI] [PubMed] [Google Scholar]
- 151.Jankovic J, Gelineau-Kattner R, Davidson A. Tourette’s syndrome in adults. Mov Disord. 2010;25:2171–75. doi: 10.1002/mds.23199. [DOI] [PubMed] [Google Scholar]
- 152.Crossley E, Seri S, Stern JS, Robertson MM, Cavanna AE. Premonitory urges for tics in adult patients with Tourette syndrome. Brain Dev. 2013 doi: 10.1016/j.braindev.2012.12.010. published online Jan 28. [DOI] [PubMed] [Google Scholar]
- 153.Kurlan R, Lichter D, Hewitt D. Sensory tics in Tourette’s syndrome. Neurology. 1989;39:731–34. doi: 10.1212/wnl.39.5.731. [DOI] [PubMed] [Google Scholar]
- 154.Leckman JF, Walker DE, Cohen DJ. Premonitory urges in Tourette’s syndrome. Am J Psychiatry. 1993;150:98–102. doi: 10.1176/ajp.150.1.98. [DOI] [PubMed] [Google Scholar]
- 155.Steinberg T, Harush A, Barnea M, et al. Tic-related cognition, sensory phenomena, and anxiety in children and adolescents with Tourette syndrome. Compr Psychiatry. 2013;54:462–66. doi: 10.1016/j.comppsych.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 156.Rajagopal S, Seri And S, Cavanna AE. Premonitory urges and sensorimotor processing in Tourette syndrome. Behav Neurol. 2013;27:65–73. doi: 10.3233/BEN-120308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kwak C, Dat Vuong K, Jankovic J. Premonitory sensory phenomenon in Tourette’s syndrome. Mov Disord. 2003;18:1530–33. doi: 10.1002/mds.10618. [DOI] [PubMed] [Google Scholar]
- 158.Karp BI, Hallett M. Extracorporeal ‘phantom’ tics in Tourette’s syndrome. Neurology. 1996;46:38–40. doi: 10.1212/wnl.46.1.38. [DOI] [PubMed] [Google Scholar]
- 159.Cohen AJ, Leckman JF. Sensory phenomena associated with Gilles de la Tourette’s syndrome. J Clin Psychiatry. 1992;53:319–23. [PubMed] [Google Scholar]
- 160.Gilbert RW. Tic modulation using sensory tricks. Tremor Other Hyperkinet Mov (NY) 2013 doi: 10.7916/D81G0KZR. published online Mar 26. tre-03-115-3129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Himle MB, Woods DW, Piacentini JC, Walkup JT. Brief review of habit reversal training for Tourette syndrome. J Child Neurol. 2006;21:719–25. doi: 10.1177/08830738060210080101. [DOI] [PubMed] [Google Scholar]
- 162.Belluscio BA, Jin L, Watters V, Lee TH, Hallett M. Sensory sensitivity to external stimuli in Tourette syndrome patients. Mov Disord. 2011;26:2538–43. doi: 10.1002/mds.23977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jankovic J. Botulinum toxin in the treatment of dystonic tics. Mov Disord. 1994;9:347–49. doi: 10.1002/mds.870090315. [DOI] [PubMed] [Google Scholar]
- 164.Kwak CH, Hanna PA, Jankovic J. Botulinum toxin in the treatment of tics. Arch Neurol. 2000;57:1190–93. doi: 10.1001/archneur.57.8.1190. [DOI] [PubMed] [Google Scholar]
- 165.Scott BL, Jankovic J, Donovan DT. Botulinum toxin injection into vocal cord in the treatment of malignant coprolalia associated with Tourette’s syndrome. Mov Disord. 1996;11:431–33. doi: 10.1002/mds.870110413. [DOI] [PubMed] [Google Scholar]
- 166.Marinelli S, Vacca V, Ricordy R, et al. The analgesic effect on neuropathic pain of retrogradely transported botulinum neurotoxin A involves Schwann cells and astrocytes. PLoS One. 2012;7:e47977. doi: 10.1371/journal.pone.0047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jimenez-Shahed J, Jankovic J. Tetrabenazine for treatment of chorea associated with Huntington’s disease. Expert Opin Orphan Drugs. 2013 in press. [Google Scholar]
- 168.Viswanathan A, Jimenez-Shahed J, Baizabal Carvallo JF, Jankovic J. Deep brain stimulation for Tourette syndrome: target selection. Stereotact Funct Neurosurg. 2012;90:213–24. doi: 10.1159/000337776. [DOI] [PubMed] [Google Scholar]
- 169.Kataoka Y, Kalanithi PS, Grantz H, et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518:277–91. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Swerdlow NR, Sutherland AN. Using animal models to develop therapeutics for Tourette syndrome. Pharmacol Ther. 2005;108:281–93. doi: 10.1016/j.pharmthera.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 171.Orth M, Munchau A. Transcranial magnetic stimulation studies of sensorimotor networks in Tourette syndrome. Behav Neurol. 2013;27:57–64. doi: 10.3233/BEN-120289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sutherland Owens AN, Miguel EC, Swerdlow NR. Sensory gating scales and premonitory urges in Tourette syndrome. Scientific World Journal. 2011;11:736–41. doi: 10.1100/tsw.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Biermann-Ruben K, Miller A, Franzkowiak S, et al. Increased sensory feedback in Tourette syndrome. Neuroimage. 2012;63:119–25. doi: 10.1016/j.neuroimage.2012.06.059. [DOI] [PubMed] [Google Scholar]
- 174.Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M. Sensorimotor gating in boys with Tourette’s syndrome and ADHD: preliminary results. Biol Psychiatry. 1996;39:33–41. doi: 10.1016/0006-3223(95)00101-8. [DOI] [PubMed] [Google Scholar]
- 175.Swerdlow NR, Karban B, Ploum Y, Sharp R, Geyer MA, Eastvold A. Tactile prepuff inhibition of startle in children with Tourette’s syndrome: in search of an “fMRI-friendly” startle paradigm. Biol Psychiatry. 2001;50:578–85. doi: 10.1016/s0006-3223(01)01164-7. [DOI] [PubMed] [Google Scholar]
- 176.Georgiou N, Bradshaw JL, Phillips JG, Bradshaw JA, Chiu E. Advance information and movement sequencing in Gilles de la Tourette’s syndrome. J Neurol Neurosurg Psychiatry. 1995;58:184–91. doi: 10.1136/jnnp.58.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Karroum EG, Golmard JL, Leu-Semenescu S, Arnulf I. Sensations in restless legs syndrome. Sleep Med. 2012;13:402–08. doi: 10.1016/j.sleep.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 178.Kerr S, McKinon W, Bentley A. Descriptors of restless legs syndrome sensations. Sleep Med. 2012;13:409–13. doi: 10.1016/j.sleep.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 179.Karroum EG, Leu-Semenescu S, Arnulf I. Topography of the sensations in primary restless legs syndrome. J Neurol Sci. 2012;320:26–31. doi: 10.1016/j.jns.2012.05.051. [DOI] [PubMed] [Google Scholar]
- 180.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 181.Gemignani F, Vitetta F, Brindani F, Contini M, Negrotti A. Painful polyneuropathy associated with restless legs syndrome. Clinical features and sensory profile. Sleep Med. 2013;14:79–84. doi: 10.1016/j.sleep.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 182.Shah RS, Rajabally YA. Restless legs syndrome in sensory axonal neuropathy: a case-control study. Rev Neurol. 2013;169:228–33. doi: 10.1016/j.neurol.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 183.Cervenka S, Palhagen SE, Comley RA, et al. Support for dopaminergic hypoactivity in restless legs syndrome: a PET study on D2-receptor binding. Brain. 2006;129:2017–28. doi: 10.1093/brain/awl163. [DOI] [PubMed] [Google Scholar]
- 184.Whittom S, Dumont M, Petit D, et al. Effects of melatonin and bright light administration on motor and sensory symptoms of RLS. Sleep Med. 2010;11:351–55. doi: 10.1016/j.sleep.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 185.Barraud Q, Obeid I, Aubert I, et al. Neuroanatomical study of the A11 diencephalospinal pathway in the non-human primate. PLoS One. 2010;5:e13306. doi: 10.1371/journal.pone.0013306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Connor JR, Boyer PJ, Menzies SL, et al. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61:304–09. doi: 10.1212/01.wnl.0000078887.16593.12. [DOI] [PubMed] [Google Scholar]
- 187.Earley CJ, Allen RP, Beard JL, Connor JR. Insight into the pathophysiology of restless legs syndrome. J Neurosci Res. 2000;62:623–28. doi: 10.1002/1097-4547(20001201)62:5<623::AID-JNR1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 188.Rizzo V, Arico I, Liotta G, et al. Impairment of sensory-motor integration in patients affected by RLS. J Neurol. 2010;257:1979–85. doi: 10.1007/s00415-010-5644-y. [DOI] [PubMed] [Google Scholar]
- 189.Trenkwalder C, Paulus W. Restless legs syndrome: pathophysiology, clinical presentation and management. Nat Rev Neurol. 2010;6:337–46. doi: 10.1038/nrneurol.2010.55. [DOI] [PubMed] [Google Scholar]
- 190.Dusek P, Jankovic J, Le W. Iron dysregulation in movement disorders. Neurobiol Dis. 2012;46:1–18. doi: 10.1016/j.nbd.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 191.McEchron MD, Alexander DN, Smith ME, Hoffman DL, Podskalny GD, Connor JR. Altered eyeblink reflex conditioning in restless legs syndrome patients. Sleep Med. 2010;11:314–19. doi: 10.1016/j.sleep.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 192.Poyurovsky M, Meerovich I, Weizman A. Beneficial effect of low-dose mianserin on fluvoxamine-induced akathisia in an obsessive-compulsive patient. Int Clin Psychopharmacol. 1995;10:111–14. doi: 10.1097/00004850-199506000-00008. [DOI] [PubMed] [Google Scholar]
- 193.Jankovic J, Clarence-Smith K. Tetrabenazine for the treatment of chorea and other hyperkinetic movement disorders. Expert Rev Neurother. 2011;11:1509–23. doi: 10.1586/ern.11.149. [DOI] [PubMed] [Google Scholar]
- 194.Mohr P, Volavka J. Ladislav Haskovec and akathisia: 100th anniversary. Br J Psychiatry. 2002;181:537. doi: 10.1192/bjp.181.6.537-a. [DOI] [PubMed] [Google Scholar]
- 195.Waln O, Jankovic J. Zolpidem improves tardive dyskinesia and akathisia. Mov Disord. 2013;28:1748–49. doi: 10.1002/mds.25480. [DOI] [PubMed] [Google Scholar]
- 196.Smith M. Case report: akathisia abolished by passive movement. Acta Psychiatr Scand. 1998;97:168–69. doi: 10.1111/j.1600-0447.1998.tb09982.x. [DOI] [PubMed] [Google Scholar]
- 197.Edwards MJ, Lang AE, Bhatia KP. Stereotypies: a critical appraisal and suggestion of a clinically useful definition. Mov Disord. 2012;27:179–85. doi: 10.1002/mds.23994. [DOI] [PubMed] [Google Scholar]
- 198.Waln O, Jankovic J. An update on tardive dyskinesia: from phenomenology to treatment. Tremor and Other Hyperkinet Mov (NY) 2013 doi: 10.7916/D88P5Z71. published online July 12. pii: tre-03-161-4138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jankovic J, Glass JP. Metoclopramide-induced phantom dyskinesia. Neurology. 1985;35:432–35. doi: 10.1212/wnl.35.3.432. [DOI] [PubMed] [Google Scholar]
- 200.Cajal R, Swanson N, Swanson L. Histology of the nervous system of man and vertebrates. New York: Oxford University Press; 1953. [Google Scholar]
- 201.Willis WD., Jr The somatosensory system, with emphasis on structures important for pain. Brain Res Rev. 2007;55:297–313. doi: 10.1016/j.brainresrev.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 202.McGlone F, Reilly D. The cutaneous sensory system. Neurosci Biobehav Rev. 2010;34:148–59. doi: 10.1016/j.neubiorev.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 203.Hemami H, Moussavi Z. A model of the basal ganglia in voluntary movement and postural reactions. Comput Methods Biomech Biomed Engin. 2013 doi: 10.1080/10255842.2012.751983. published online Jan 3. [DOI] [PubMed] [Google Scholar]
- 204.Redgrave P, Vautrelle N, Reynolds JN. Functional properties of the basal ganglia’s re-entrant loop architecture: selection and reinforcement. Neuroscience. 2011;198:138–51. doi: 10.1016/j.neuroscience.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 205.Paulus W, Dowling P, Rijsman R, Stiasny-Kolster K, Trenkwalder C, de Weerd A. Pathophysiological concepts of restless legs syndrome. Mov Disord. 2007;22:1451–56. doi: 10.1002/mds.21533. [DOI] [PubMed] [Google Scholar]
- 206.Baldan Ramsey LC, Xu M, Wood N, Pittenger C. Lesions of the dorsomedial striatum disrupt prepulse inhibition. Neuroscience. 2011;180:222–28. doi: 10.1016/j.neuroscience.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bronfeld M, Bar-Gad I. Tic disorders: what happens in the basal ganglia? Neuroscientist. 2013;19:101–08. doi: 10.1177/1073858412444466. [DOI] [PubMed] [Google Scholar]
- 208.Worbe Y, Sgambato-Faure V, Epinat J, et al. Towards a primate model of Gilles de la Tourette syndrome: anatomo-behavioural correlation of disorders induced by striatal dysfunction. Cortex. 2013;49:1126–40. doi: 10.1016/j.cortex.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 209.Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. NeuroImage. 2007;35:222–33. doi: 10.1016/j.neuroimage.2006.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Argyelan M, Carbon M, Niethammer M, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci. 2009;29:9740–47. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Barĕs M, Husárová I, Lungu OV. Essential tremor, the cerebellum, and motor timing: towards integrating them into one complex entity. Tremor Other Hyperkinet Mov (NY) 2012 published online Sept 12. tre-62-93-6523-1. [PMC free article] [PubMed] [Google Scholar]
- 212.Kojovic M, Pareés I, Kassavetis P, Palomar FJ, et al. Secondary and primary dystonia: pathophysiological differences. Brain. 2013;136:2038–49. doi: 10.1093/brain/awt150. [DOI] [PubMed] [Google Scholar]
- 213.Hubsch C, Roze E, Popa T, et al. Defective cerebellar control of cortical plasticity in writer’s cramp. Brain. 2013;136:2050–62. doi: 10.1093/brain/awt147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–93. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- 215.Bostan AC, Strick PL. The cerebellum and basal ganglia are interconnected. Neuropsychol Rev. 2010;20:261–70. doi: 10.1007/s11065-010-9143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]