Abstract
Methylone, 3,4-methylenedioxypyrovalerone (MDPV), and mephedrone are psychoactive ingredients of ‘bath salts’ and their abuse represents a growing public health care concern. These drugs are cathinone derivatives and are classified chemically as β-ketoamphetamines. Because of their close structural similarity to the amphetamines, methylone, MDPV, and mephedrone share most of their pharmacological, neurochemical, and behavioral properties. One point of divergence in their actions is the ability to cause damage to the CNS. Unlike methamphetamine, the β-ketoamphetamines do not damage dopamine (DA) nerve endings. However, mephedrone has been shown to significantly accentuate methamphetamine neurotoxicity. Bath salt formulations contain numerous different psychoactive ingredients, and individuals who abuse bath salts also coabuse other illicit drugs. Therefore, we have evaluated the effects of methylone, MDPV, mephedrone, and methamphetamine on DA nerve endings. The β-ketoamphetamines alone or in all possible two-drug combinations do not result in damage to DA nerve endings but do cause hyperthermia. MDPV completely protects against the neurotoxic effects of methamphetamine while methylone accentuates it. Neither MDPV nor methylone attenuates the hyperthermic effects of methamphetamine. The potent neuroprotective effects of MDPV extend to amphetamine-, 3,4-methylenedioxymethamphetamine-, and MPTP-induced neurotoxicity. These results indicate that β-ketoamphetamine drugs that are non-substrate blockers of the DA transporter (i.e., MDPV) protect against methamphetamine neurotoxicity, whereas those that are substrates for uptake by the DA transporter and which cause DA release (i.e., methylone, mephedrone) accentuate neurotoxicity.
Keywords: dopamine nerve ending, dopamine transporter, neurotoxic amphetamines, neurotoxicity, β-ketoamphetamines
Methylone, 3,4-methylenedioxypyrovalerone (MDPV), and mephedrone are synthetic psychoactive drugs that have received worldwide notoriety as components of ‘bath salts’. These agents are abused singly or with other psychoactive drugs like alcohol, cannabis, and 3,4-methylenedioxymethamphetamine (Winstock et al. 2011; Miller and Stogner 2014). The high abuse potential of these illicit compounds and the clinically significant dangers associated with their intake has resulted in their classification as Schedule I compounds by the US Drug Enforcement Administration. These same drugs are also now banned by all member states of the European Monitoring Centre for Drugs and Drug Addiction. Despite regulatory efforts to restrict their distribution and sale, growing abuse of methylone, MDPV and mephedrone continues to be a significant public health, law enforcement, and societal concern (Miotto et al. 2013).
Bath salt constituents are cathinone derivatives and are also referred to as β-ketoamphetamines as they bear very close structural similarity to the amphetamines. In fact, their possession of a beta-keto moiety is the only feature that differentiates them from their respective amphetamine congeners [e.g., 3,4-methylenedioxymethamphetamine (MDMA) is the deketo form of methylone]. Not surprisingly, methylone, MDPV, and mephedrone share many of the pharmacological and behavioral characteristics commonly associated with the amphetamine psychostimulants to include increased locomotor activity (Huang et al. 2012; Lisek et al. 2012; Lopez-Arnau et al. 2012; Marusich et al. 2012, 2014; Motbey et al. 2012a; Wright et al. 2012; Aarde et al. 2013a,b; Fantegrossi et al. 2013; Gatch et al. 2013; Miller et al. 2013; Shortall et al. 2013b; Varner et al. 2013), altered learning and memory (Motbey et al. 2012b; den Hollander et al. 2014), disruptions in thermoregulation (Baumann et al. 2012; Aarde et al. 2013a; Fantegrossi et al. 2013; Shortall et al. 2013a; Lopez-Arnau et al. 2014), induction of behavioral sensitization (Gregg et al. 2013a, b), and the ability to serve as discriminative drug stimuli (Fantegrossi et al. 2013; Gatch et al. 2013; Varner et al. 2013). The abuse potential of these drugs has been affirmed in pre-clinical studies that document their ability to support the formation of a conditioned place preference (Lisek et al. 2012; Karlsson et al. 2014), sustain self-administration (Hadlock et al. 2011; Watterson et al. 2012; Aarde et al. 2013a,b), and enhance intracranial self-stimulation (Robinson et al. 2012; Watterson et al. 2012; Bonano et al. 2014). These drugs also elicit the release of dopamine (DA), serotonin (5-HT), and norepinephrine and block the uptake of these monoamines by their respective transporters (Hadlock et al. 2011; Baumann et al. 2012; Eshleman et al. 2013; Marusich et al. 2014).
Based on the close chemical and pharmacological similarities shared by the amphetamines and β-ketoamphetamines, we hypothesized that the β-ketoamphetamines would cause neurotoxicity to monoamine nerve terminals in the striatum, hippocampus, and cortex like methamphetamine, amphetamine, and MDMA. Initial studies revealed the surprising finding that at least mephedrone did not cause damage to DA nerve endings, even when administered in a high-dose binge regimen (Angoa-Perez et al. 2012). Some studies have documented mild damage to 5-HT nerve endings by mephedrone (Hadlock et al. 2011) and the toxicity of this drug can be unmasked to a small degree when given in high doses over a 2-day span at elevated ambient temperature (Lopez-Arnau et al. 2014; Martinez-Clemente et al. 2014). In balance, a large number of emerging studies indicate that methylone, MDPV, and mephedrone do not appear to cause chronic depletions of DA, 5-HT, or norepinephrine that would be indicative of neurotoxicity (Kehr et al. 2011; Angoa-Perez et al. 2012, 2014; Baumann et al. 2012; Motbey et al. 2012b; Shortall et al. 2013b). Failure to document a neurotoxic profile for mephedrone prompted the alternative hypothesis that its ability to block the uptake of DA by the DA transporter (DAT) would provide protection against methamphetamine neurotoxicity as is seen with more ‘classical’ DAT blockers (Schmidt and Gibb 1985; Pu et al. 1994). This prediction also proved incorrect when we found that mephedrone caused a significant enhancement of the neurotoxicity caused by methamphetamine, amphetamine, and MDMA (Angoa-Perez et al. 2013).
Illicit bath salt formulations are not pure and forensic analyses reveal that they contain mixtures of various psychoactive ingredients (Spiller et al. 2011). In light of this fact and considering that individuals who abuse bath salts coabuse numerous other substances (Winstock et al. 2011; Miller and Stogner 2014), we expanded the study of β-ketoamphetamine effects on the DA nerve ending by treating mice with combinations of methylone, MDPV, mephedrone, and methamphetamine. The results revealed that any combination of bath salt agents did not lead to neurotoxicity. On the other hand, MDPV potently and completely protected against methamphetamine neurotoxicity, whereas methylone, like mephedrone (Angoa-Perez et al. 2013), significantly enhanced the toxic effects of methamphetamine. These results indicate that the non-neurotoxic β-ketoamphetamines can be differentiated as protectants or enhancers of neuro-toxicity by virtue of their interaction with the DAT as non-substrate blockers or substrates, respectively.
Materials and methods
Drugs and reagents
Methylone hydrochloride, MDPV hydrochloride, (±) mephedrone hydrochloride, and (±) MDMA hydrochloride were obtained from the NIDA Research Resources Drug Supply Program. (+) methamphetamine hydrochloride, D-amphetamine sulfate, MPTP hydrochloride, DA, polyclonal antibodies against glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and all buffers and HPLC reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Bicinchoninic acid protein assay kits were obtained from Pierce (Rockford, IL, USA). Polyclonal antibodies against rat tyrosine hydroxylase (TH) were produced as described previously (Kuhn and Billingsley 1987). Monoclonal antibodies against rat DAT were generously provided by Dr Roxanne A. Vaughan (University of North Dakota, Grand Forks, ND, USA). Polyclonal antibodies against glial fibrillary acidic protein (GFAP) were obtained from Thermo Scientific (Rockford, IL, USA). IRDye secondary antibodies for Odyssey Imaging Systems were purchased from LiCor Biosciences (Lincoln, NE, USA).
Animals
Female C57BL/6 mice (Harlan, Indianapolis, IN, USA) weighing 20–25 g at the time of experimentation were housed five per cage in large shoe-box cages in a light (12-h light/dark) and temperature-controlled room. Female mice were used because they are known to be very sensitive to neuronal damage by the neurotoxic amphetamines and to maintain consistency with our previous studies of methamphetamine and β-ketoamphetamine interactions (Angoa-Perez et al. 2012, 2013, 2014). Mice had free access to food and water. The Institutional Care and Use Committee of Wayne State University approved the animal care and experimental procedures. All procedures were also in compliance with the NIH Guide for the Care and Use of Laboratory Animals and were conducted in compliance with ARRIVE guidelines.
Pharmacological and physiological procedures
Mice were treated with methylone (30 mg/kg), MDPV (30 mg/kg), or mephedrone (40 mg/kg) using a binge-like regimen comprised four injections with a 2-h interval between each injection. This binge treatment regimen, when used to inject substituted amphetamines results in extensive DA nerve ending damage. Doses of all drugs used fall well within the range used in similar published studies (Angoa-Perez et al. 2012, 2013; Marusich et al. 2012; Fantegrossi et al. 2013; den Hollander et al. 2013; Karlsson et al. 2014). Independent groups of mice were treated with each drug separately or with all possible combinations of two of the drugs. For combination treatment of mice with methylone or MDPV with methamphetamine, mice were treated with varying doses of either β-ketoamphetamine (4′ – 10, 20, or 30 mg/kg) concurrent each injection of varying doses of methamphetamine (4′ – 2.5, 5, or 10 mg/kg). The combination treatment of mephedrone + methamphetamine was carried out in a previous study (Angoa-Perez et al. 2013) and was not repeated presently. Controls received injections of physiological saline on the same schedule used for the β-ketoamphetamines and methamphetamine (alone or in combination). The effect of MDPV (4′ – 30 mg/kg) on amphetamine (4′ –5 mg/kg)- and MDMA (4′ – 20 mg/kg)-induced damage to DA nerve endings was also tested. The latter drugs were administered using the same binge regimen described above for the β-ketoamphetamines and methamphetamine. To determine if MDPV neuroprotection would extend to non-amphetamine neurotoxins, mice were treated with MDPV (2′ –10 mg/kg) prior to each of two injections of MPTP (20 mg/kg). All injections were given via the i.p. route. Mice were killed 2 days after the last drug treatment when amphetamine-associated neurotoxicity has reached maximum. Body temperature was monitored by telemetry using IPTT-300 implantable temperature transponders from Bio Medic Data Systems, Inc. (Seaford, DE, USA). Temperatures were recorded non-invasively every 20 min starting 60 min before the first drug injection and continuing for 9 h thereafter using the DAS-5001 console system from Bio Medic Data Systems, Inc.
Determination of striatal DA content
Striatal tissue was dissected from the brain after treatment and stored at −80°C. Frozen tissues were weighed and sonicated in 10 volumes of 0.16 N perchloric acid at 4°C. Insoluble protein was removed by centrifugation and DA was determined by HPLC with electrochemical detection as described previously (Angoa-Perez et al. 2012, 2013).
Determination of DAT, TH, and GFAP protein levels by immunoblotting
The effects of drug treatments on striatal DAT and TH levels, highly specific markers for striatal DA nerve endings, were determined by immunoblotting as an index of toxicity. GFAP levels were used as an index of nerve ending damage as described previously (Angoa-Perez et al. 2012). Mice were killed by decapitation after treatment and striatum was dissected bilaterally. Tissue was stored at −80°C. Frozen tissue was disrupted by sonication in 1% sodium dodecyl sulfate at 95°C and insoluble material was sedimented by centrifugation. Protein was determined by the bicinchoninic acid method and equal amounts of protein (70 μg/lane) were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then electroblotted to nitrocellulose. Blots were blocked in Odyssey blocking buffer (phosphate-buffered saline) for 1 h at 23°C. Primary antibodies against DAT (1 : 1000), TH (1 : 1000), GFAP (1 : 2000) or GAPDH (1 : 10 000) were added to blots and allowed to incubate for 16 h at 4°C. Blots were washed three times in Tris-buffered saline to remove unreacted antibodies and then incubated with IRDye secondary antibodies (1 : 4000) for 1 h at 23°C. Immunoreactive bands were visualized by enhanced fluorescence and the relative densities of TH-, DAT-, GFAP-, and GAPDH-reactive bands were determined by imaging with an Odyssey CLx Infrared Image System (LiCor Biosciences) and quantified using ImageJ software (NIH, Bethesda, MD, USA). TH, DAT and GFAP relative densities were normalized to GAPDH.
Data analysis
The effects of drug treatments on core body temperature over time were analyzed using two-way ANOVAs and post hoc comparisons were carried out using Bonferroni’s test. Two-way ANOVAs were performed to analyze the dose effects of methylone, MDPV, and methamphetamine, and their combinations, on striatal levels of DA, DAT, TH, and GFAP. The effects of individual drug treatments on DA, DAT, TH, and GFAP content were also tested for significance by one-way ANOVA and post hoc comparisons were carried out with the Holm–Sidak test for multiple comparisons. The effects of MDPV on amphetamine, MDMA, and MPTP toxicity were analyzed with a one-way ANOVA and post hoc comparisons were carried out with the Holm–Sidak test for multiple comparisons. Differences were considered significant if p < 0.05. Because many of the treatments using multiple doses of methylone or MDPV in combination with methamphetamine resulted in such large numbers of planned comparisons, the outcomes of the statistical tests are described minimally in Results (i.e., only p values presented) and detailed descriptions of all statistical outcomes are presented in Tables S1–S6. Likewise, the details of the statistical analyses of methylone, MDPV, and mephedrone (single and combined treatments) effects on body temperature are presented in Supporting information. All statistical analyses were carried out using GraphPad Prism version 6.01 for Windows (GraphPad Software, San Diego, CA, USA, www.graphpad.com).
Results
Effects of treatment with individual or combinations of β-ketoamphetamines on DA nerve endings
Mice were treated with methylone (30 mg/kg), MDPV, (30 mg/kg) or mephedrone (40 mg/kg) singly or in all possible combinations of two of these drugs and the effects on striatal DA, DAT, and TH levels are presented in Fig. 1. It can be seen that none of the drugs changed the levels from control of any marker for DA nerve endings to include DA (Fig. 1a), DAT (Fig. 1b) or TH (Fig. 1c). Likewise, combined treatment with MDPV + mephedrone, methylone + mephedrone, or methylone + MDPV did not alter striatal levels of DA, DAT, or TH. The effects of these drugs singly or in combination on striatal GFAP are presented in Fig. 1(d) and show that this marker for astrogliosis was not changed from control values by any treatment. In agreement with published accounts, each β-ketoamphetamine alone and in all two-drug combinations resulted in significant hyperthermia and these results are described in more detail in Figures S1–S3.
Fig. 1.
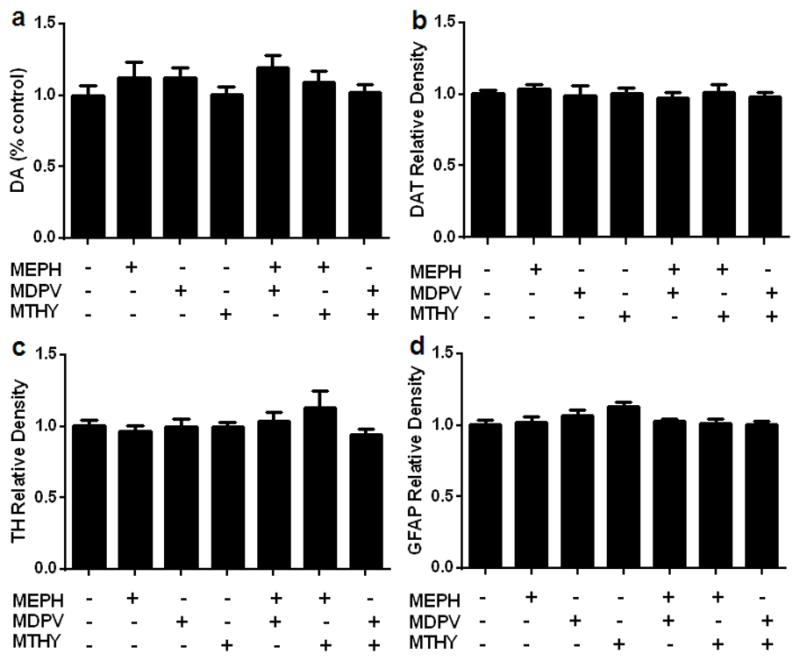
Effects of mephedrone (4′ – 40 mg/kg), methylone (4′ –30 mg/kg), and MDPV (4′ – 30 mg/kg) alone or in combination on DA nerve endings of the striatum. Mice were treated with mephedrone (MEPH; 4′ – 40 mg/kg), MDPV (4′ – 30 mg/kg), or methylone (MTHY; 4′ – 30 mg/kg) singly or in the indicated two-drug combinations and the levels of DA (a), dopamine transporter (DAT) (b), tyrosine hydroxylase (TH), (c) and glial fibrillary acidic protein (GFAP) (d) were determined 2 days after treatment. Controls were injected with physiological saline on the same binge schedule used for the β-ketoamphetamines. DA levels were determined by HPLC and are reported as % control. Relative pixel densities for immunoblots of DAT, TH, and GFAP were quantified using ImageJ, normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and expressed as relative band density by comparison to the respective control. Data are expressed as mean ± SEM for n = 4–5 mice per group.
Effects of MDPV on methamphetamine-, amphetamine-, and MPTP-induced toxicity to DA nerve endings
Mice were treated with MDPV and methamphetamine in varying doses of each drug alone or in combination and Fig. 2 shows the results of these treatments on DA (Fig. 2a), DAT (Fig. 2b), TH (Fig. 2c), and GFAP (Fig. 2d). The main effects of both drugs and their interactions on DA, DAT, TH, and GFAP were all highly statistically significant (p < 0.01–0.0001). MDPV alone at any dose did not change the levels of any of the dependent variables, whereas with only one exception, all doses of methamphetamine alone significantly reduced DA, DAT, and TH and significantly increased GFAP (p < 0.05–0.0001). The 2.5 mg/kg dose of methamphetamine alone did not lower TH levels significantly. All doses of MDPV provided complete protection against the effects of each dose of methamphetmine on all DA nerve ending markers and GFAP as evidenced by two different statistical comparisons. First, none of the MDPV + methamphetamine treatments differed from control at any dose combination for both drug. Second, with only a few exceptions, all MDPV + methamphetamine treatments were significantly different from methamphetamine alone for each respective dose of this drug (p < 0.05–0.0001). The exceptions involved the lowest dose of methamphetamine. The specific outcomes for the statistical tests of all main effects and interactions as well as all post hoc comparisons for the data in Fig. 2 are presented in detail in Tables S1 and S2.
Fig. 2.
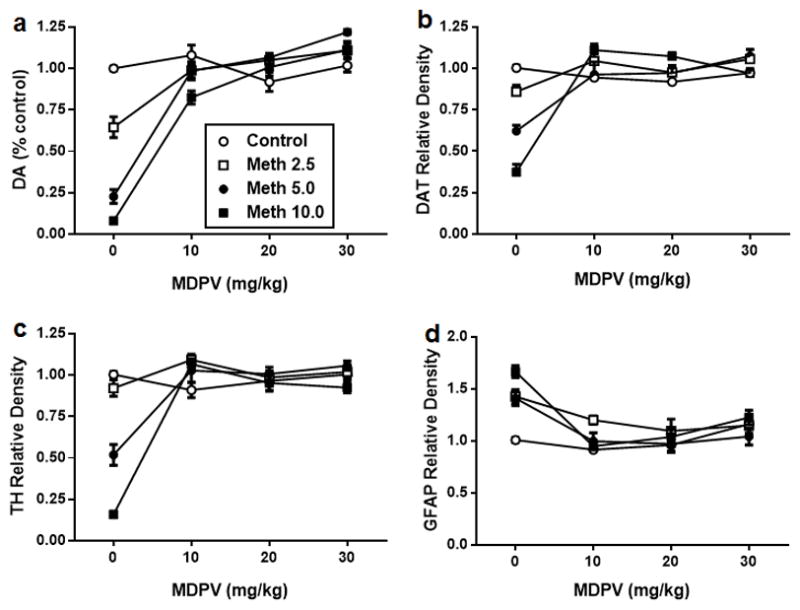
Effects of MDPV (4′ – 10, 20, or 30 mg/kg) on methamphetamine (2.5, 5, or 10 mg/kg)-induced neurotoxicity to DA nerve endings. Mice were treated with MDPV (4′ – 10, 20 or 30 mg/kg), methamphetamine (Meth; 2.5, 5 or 10 mg/kg), or their combination in the indicated doses and the levels of DA (a), dopamine transporter (DAT) (b), tyrosine hydroxylase (TH) (c), and glial fibrillary acidic protein (GFAP) (d) were determined 2 days after treatment. Controls were injected with physiological saline on the same binge schedule used for MDPV and methamphetamine. DA levels were determined by HPLC and are reported as % control. Relative pixel densities for immunoblots of DAT, TH, and GFAP were quantified using ImageJ, normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and expressed as relative band density by comparison with the respective control. Data are means for n = 4–8 mice per group. SEM bars (< 5% of means) and symbols indicating p values are omitted from the figure for the sake of clarity. Specific details of all statistical comparisons for the data in this figure are included in Tables S1 and S2.
The effects on body temperature of the highest doses of MDPV (30 mg/kg) given in combination with methamphetamine (10 mg/kg) were measured and the results are presented in Fig. 3. The main effects of time (F(30,480) = 7.861, p < 0.0001) and treatment (F(3,16) = 157.1, p < 0.0001) as well as their interaction (F(90,480) = 6.789, p < 0.0001) were statistically significant. When given alone, methamphetamine (p < 0.0001) and MDPV (p < 0.0001) caused significant increases in body temperature by comparison to controls. Combined treatment with methamphetamine + MDPV significantly increased body temperature by comparison to control (p < 0.0001) and Fig. 3 also shows that MDPV did not reduce the hyperthermic effect of methamphetamine (p > 0.05). The effects of MDPV on body temperature differed significantly from both methamphetamine alone (p < 0.0001) and from methamphetamine + MDPV (p < 0.0001). Taken together, these results indicate that MDPV protects against methamphetamine neurotoxicity without interfering with its hyperthermic effects.
Fig. 3.
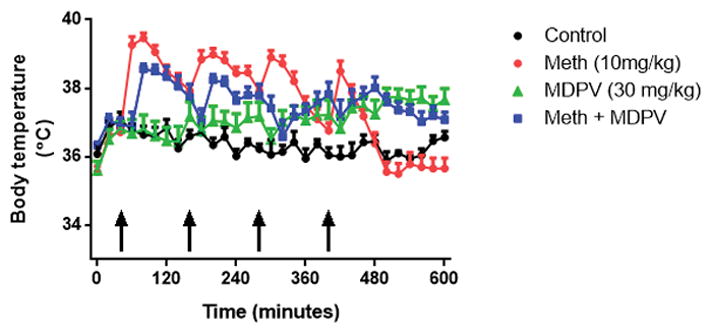
Effects of MDPV (4′ – 30 mg/kg), methamphetamine (4′ –10 mg/kg), and their combination on core body temperature. Mice were treated with MDPV (4′ – 30 mg/kg), methamphetamine (4′ –10 mg/kg), or their combination and body temperature was measured via telemetry at 20-min intervals starting 40 min before the first drug injection and continuing for 560 min during drug treatments. Controls were injected with physiological saline on the same binge schedule used for MDPV and methamphetamine. Data are means for n = 5–6 mice per group. SEM bars (< 5% of means) and symbols indicating p values are omitted from the figure for the sake of clarity.
The generality of the neuroprotective effects of MDPV was examined by testing it for protection against amphetamine-, MDMA-, and MPTP-induced damage to DA nerve endings and the results are presented in Fig. 4. Because MDPV does not change DA nerve ending markers (see Figs 1 and 2), data from groups treated with this drug alone are omitted from Fig. 4. The main effect of drug treatment on striatal levels of DA (Fig. 4a), DAT (Fig. 4b) and TH (Fig. 4c) was significant for amphetamine (p < 0.009–0.0001), MDMA (p < 0.0002–0.0001), and MPTP (p < 0.0025–0.0001). Individually, all test drugs significantly decreased DA, DAT, and TH, and increases in GFAP by comparison to controls (p < 0.05–0.0001) were observed after all drugs except MPTP. MDPV provided protection against each treatment by comparison to drug alone (p < 0.05–0.0001), with a few exceptions. In most cases, the protective effect of MDPV was complete (i.e., drug + MDPV did not differ from control values). Exceptions were observed for amphetamine + MDPV, where DA levels were actually elevated above those of controls, for MDMA + MDPV effects on DAT and for MDMA + MDPV effects on TH, where protection was significant but partial. Although MDPV provided some protection against the MDMA-induced reduction in TH, this effect did not reach statistical significance. MPTP alone did not elevate GFAP levels presently so it was not possible to observe a protective effect of MDPV on this dependent variable. The specific outcomes for the statistical tests of all main effects and interactions as well as all post hoc comparisons for the data in Fig. 4 are presented in detail in Tables S3 and S4.
Fig. 4.
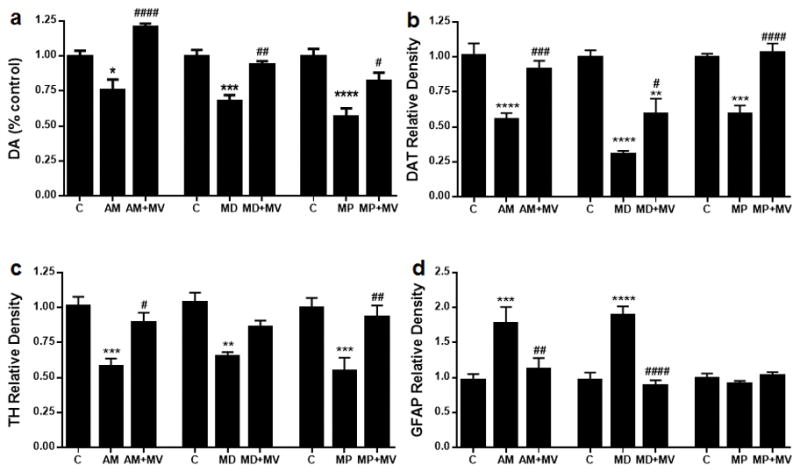
Effects of MDPV (4′ – 30 mg/kg) on amphetamine (4′ – 5 mg/ kg)-, 3,4-methylenedioxymethamphetamine (MDMA) (4′ – 20 mg/kg)-, and MPTP (2′ – 20 mg/kg)-induced neurotoxicity to DA nerve endings. Mice were treated with MDPV (MV; 4′ – 30 mg/kg) in combination with amphetamine (AM; 4′ – 5 mg/kg), MDMA (MD; 4′ –20 mg/kg) or MPTP (MP; 2′ – 20 mg/kg) and the levels DA (a), dopamine transporter (DAT) (b), tyrosine hydroxylase (TH) (c), and glial fibrillary acidic protein (GFAP) (d) were determined 2 days after treatment. Controls were injected with physiological saline on the same binge schedule used for all drugs. DA levels were determined by HPLC and are reported as % control. Relative pixel densities for immunoblots of DAT, TH and GFAP were quantified using ImageJ, normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and expressed as relative band density by comparison with the respective control. Data are mean ± SEM for n = 5–6 mice per group. *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 by comparison with untreated controls. #p < 0.05, ##p < 0.01, ###p < 0.001 and ####p < 0.0001 by comparison with AMPH, MDMA or MPTP alone. Specific details of all statistical comparisons for the data in this figure are included in Tables S3 and S4.
Effects of methylone on methamphetamine-induced toxicity to DA nerve endings
Mice were treated with varying doses of methylone (4′ – 10, 20 or 30 mg/kg) alone or in combination with 2.5 mg/kg methamphetamine and Fig. 5 shows the results of the effects of these treatments on DA (Fig. 5a), DAT (Fig. 5b), TH (Fig. 5c), and GFAP (Fig. 5d). The main effect of treatment on DA, DAT, TH, and GFAP was highly significant (p < 0.01–0.0001). Methylone alone at any dose did not change the levels of any dependent variable. Methamphetamine (2.5 mg/kg) significantly changed the levels of DA (p < 0.0001) and GFAP (p < 0.01) and while DAT and TH were reduced by this low dose of methamphetamine, the changes were not significant. All doses of methylone significantly accentuated the effects of methamphetamine on DA, DAT, TH, and GFAP by comparison with controls (p < 0.05–0.0001) with the exception of the 10 mg/kg dose, which did not significantly increase the methamphetamine effect on TH. Figure 5 also shows that methylone significantly enhanced methamphetamine effects on DA and DAT at all doses by comparison with methamphetamine alone. The enhancing effect of methylone was observed only at the 30 mg/kg dose in the case of TH but not for GFAP. Doses of methamphetamine higher than 2.5 mg/kg were not tested to avoid a floor effect on depletion of DA nerve ending markers. The specific outcomes for the statistical tests of all main effects and interactions as well as all post hoc comparisons for the data in Fig. 5 are presented in detail in Tables S5 and S6.
Fig. 5.
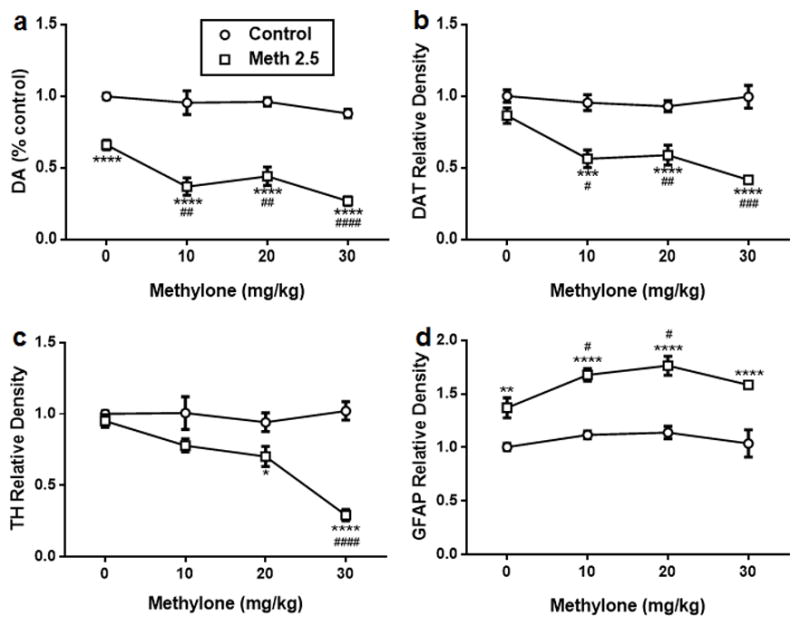
Effects of methylone (4′ – 10, 20 or 30 mg/kg) on methamphetamine (4′ – 2.5 mg/kg)-induced neurotoxicity to DA nerve endings. Mice were treated with methylone (4′ – 10, 20, or 30 mg/ kg) alone or in combination with methamphetamine (4′ – 2.5 mg/kg) and the levels DA (a), dopamine transporter (DAT) (b), tyrosine hydroxylase (TH) (c), and glial fibrillary acidic protein (GFAP) (d) were determined 2 days after treatment. Controls were injected with physiological saline on the same binge schedule used for all drugs. DA levels were determined by HPLC and are reported as % control. Relative pixel densities for immunoblots of DAT, TH and GFAP were quantified using ImageJ, normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and expressed as relative band density by comparison with the respective control. Data are mean ± SEM for n = 4–6 mice per group. **p < 0.01, ***p < 0.001 and ****p < 0.0001 by comparison with untreated controls. #p < 0.05, ##p < 0.01, ###p < 0.001 and ####p < 0.0001 by comparison with methamphetamine alone. Specific details of all statistical comparisons for the data in this figure are included in Tables S5 and S6.
The effect on body temperature of the highest doses of methylone (30 mg/kg) and methamphetamine (2.5 mg/kg) given in combination were measured and the results presented in Fig. 6 indicate that the main effects of time (F (30,480) = 7.16, p < 0.0001) and treatment (F(3,16) = 480.7, p < 0.0001) as well as their interaction (F(90,480) = 6.77, p < 0.0001) were statistically significant. Compared to controls, methamphetamine (p < 0.0001), methylone (p < 0.0001), and their combination (p < 0.0001) significantly increased body temperature. The effects of methamphetamine on body temperature also differed significantly from those of methylone (p < 0.0001) and the combination of methamphetamine + methylone (p < 0.0001). Finally, methylone effects on body temperature were significantly different from those caused by methamphetamine + methylone (p < 0.01).
Fig. 6.
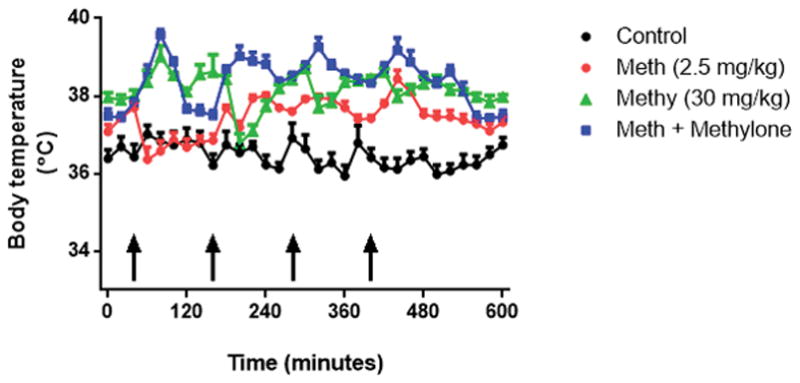
Effects of methylone (4′ 30 mg/kg) and methamphetamine (4′ – 2.5 mg/kg) and their combination on core body temperature. Mice were treated with methylone (4′ – 30 mg/kg), methamphetamine (4′ – 2.5 mg/kg), or their combination and body temperature was measured via telemetry at 20-min intervals starting 40 min before the first drug injection and continuing for 560 min during drug treatments. Controls were injected with physiological saline on the same binge schedule used for MDPV and methamphetamine. Data are means for n = 5–8 mice per group. SEM bars (< 5% of means) and symbols indicating p values are omitted from the figure for the sake of clarity.
Discussion
The goal of the present work was to expand our previous studies of how mephedrone influences DA nerve ending damage caused by methamphetamine. Most pre-clinical investigations of the neuropharmacological actions of the β-ketoamphetamines have studied the effects of single drugs on the CNS. However, illicit bath salt formulations generally contain mixtures of psychoactive ingredients (Spiller et al. 2011) and individuals who abuse these drugs often coabuse other substances along with bath salts (Winstock et al. 2011; Miller and Stogner 2014). Therefore, a compelling rationale exists for testing bath salts in combination as well as with other psychostimulants like methamphetamine, amphetamine, and MDMA. Mephedrone, despite its close structural and pharmacological similarities to the neurotoxic amphetamines, does not cause neurotoxicity when administered to mice in a high-dose binge regimen (Angoa-Perez et al. 2012). Instead, this drug exerts the surprising property of enhancing the depletions in striatal DA, DAT, and TH caused by methamphetamine, amphetamine, and MDMA (Angoa-Perez et al. 2013). These results raise at least two very interesting questions about the mechanisms of action of the β-ketoamphetamines and their amphetamine congeners that cause damage to the CNS. First, if mephedrone shares with methamphetamine the ability to release DA, inhibit its uptake, and cause hyperthermia, factors that are thought to be essential for methamphetamine neurotoxicity, why is it not neurotoxic? Second, if mephedrone can block DA uptake via its interaction with the DAT, why does it not prevent methamphetamine-induced neurotoxicity like other DAT blockers (Schmidt and Gibb 1985; Pu et al. 1994; Angoa-Perez et al. 2013)? The answer to these questions may lie in the mechanisms by which mephedrone and other bath salt constituents interact with the DAT.
When given alone or in two-drug combinations, mephedrone, MDPV, and methylone cause significant increases in body temperature. The most remarkable effect is associated with mephedrone which causes a profound hypothermia immediately after injection that reverts to hyperthermia after 30–40 min, in agreement with our previous studies with this drug (Angoa-Perez et al. 2012, 2013). When methylone or MDPV are given with mephedrone, this hypothermic effect of mephedrone is retained and slightly exaggerated. Combined treatment with MDPV and methylone results in a 1–2°C steady increase in core body temperature that becomes evident within 15 min of treatment and persists for at least 8–9 h. Each of these drugs increases the synaptic levels of DA by stimulating release and blocking uptake (i.e., mephedrone, methylone) or by acting as a pure DAT antagonist (i.e., MDPV). Despite exerting significant hyperthermic effects overall, none of the β-ketoamphetamines alone or in combination result in changes in striatal DA, DAT, TH, or GFAP that would be indicative of neurotoxicity. Taken together, these results leave open the question of why the β-ketoamphetamines do not cause DA nerve ending damage despite exerting most of the effects that are considered important for methamphetamine-induced neurotoxicity.
We found presently that methylone shares with mephedrone the ability to enhance methamphetamine-induced damage to DA nerve terminals, whereas MDPV provides complete protection against neurotoxicity. This neuroprotective effect of MDPV extends to amphetamine and MDMA, and to MPP+, the neurotoxic metabolite of MPTP that is structurally unrelated to the neurotoxic amphetamines but which depends on uptake by the DAT to exert its damaging effects on DA neurons (Javitch et al. 1985). These differing effects of mephedrone, methylone, and MDPV on methamphetamine neurotoxicity cannot be explained by their effects on body temperature because neither interferes with the ability of methamphetamine to cause hyperthermia. Recent studies of the mechanisms by which β-ketoamphetamines interact with the DAT offer significant insight into why these drugs have such divergent effects on neurotoxicity. The bath salts have been classified as substrates and non-substrates based on whether or not they are transported by the DAT. Mephedrone and methylone, like methamphetamine, are substrates for DAT-mediated uptake and they cause release of DA via carrier-mediated exchange (Baumann et al. 2012; Cameron et al. 2013a,b; Eshleman et al. 2013). MDPV is not a substrate for transport and interacts with the DAT strictly as a blocker, like cocaine (Baumann et al. 2013; Cameron et al. 2013a,b; Eshleman et al. 2013; Kolanos et al. 2013; Simmler et al. 2013a). This dichotomy of interaction with the DAT by mephedrone and methylone on one hand and by MDPV on the other can explain their opposing effects on methamphetamine-induced neurotoxicity. Mephedrone and methylone enhance the effects of methamphetamine, most likely by increasing the release of DA above that caused by either drug alone. This possibility has not yet been tested but is supported by prior results showing that treatments resulting in an increase in the releasable pool of DA significantly accentuate methamphetamine-induced damage in DA nerve endings (Thomas et al. 2008, 2009). MDPV has an effect that is similar to more classical DAT blockers and protects against methamphetamine-induced neurotoxicity. Compared to amfonelic acid (Schmidt and Gibb 1985; Pu et al. 1994) and nomifensine (Angoa-Perez et al. 2013), which provide partial but significant protection, MDPV completely prevents the damaging effects of methamphetamine on DA nerve endings. By blocking DAT-mediated transport (inward or outward), MDPV blocks methamphetamine-induced efflux of DA (Simmler et al. 2013b). This property alone represents an important mechanism by which it protects against DA nerve ending damage caused by drugs that depend on inward DAT transport to exert their toxicity to include the neurotoxic amphetamines and MPTP (i.e., MPP+). MDPV may be one of the most powerful blockers of the DAT yet described (Baumann et al. 2013; Eshleman et al. 2013; Simmler et al. 2013a). It is also more effective than bupropion or methyl-phenidate in blocking methamphetamine-induced DA release (Simmler et al. 2013b) and is the most potent drug identified to date for protecting against methamphetamine-induced damage to DA nerve endings.
Methamphetamine is probably the prototypical neurotoxic amphetamine. Its ability to flood the synapse with DA, especially after binge administration (O’Dell et al. 1993), is thought to be the first step in a cascade that leads rapidly to mitochondrial dysfunction, enhanced excitatory neurotransmission, increases in glial reactivity and oxidative stress, nerve ending damage, and apoptosis (Halpin et al. 2014). The numerous facets of methamphetamine-induced neurotoxicity have been studied in great detail over the past three decades, whereas the dangers associated with bath salts have emerged only recently. However, the β-ketoamphetamines should offer new possibilities for achieving a better understanding of the mechanisms by which methamphetamine, amphetamine, and MDMA cause damage to monoamine nerve endings. Mephedrone and methylone cause little or no neurotoxicity yet they cause significant efflux of DA and inhibit its re-uptake through their interactions with the DAT. The interactions of mephedrone and methylone with the DAT are very similar to those of methamphetamine and this leaves unanswered the question posed above of why they enhance the neurotoxicity of the amphetamines as opposed to offering protection. It could be predicted that mephedrone and methylone would dilute the effects of methamphetamine on DA release by substituting less toxic DAT substrates for a more toxic one. This does not appear to be the case.
Several other possibilities offer sources for speculation regarding enhanced neurotoxicity when mephedrone or methylone are combined with methamphetamine and include the following points. First, the β-ketoamphetamines could alter the pharmacokinetics or metabolism of methamphetamine such that blood and brain drug levels are increased in amount and/or for longer periods of time over those seen after methamphetamine alone. The β-keto group increases the polarity of mephedrone and methylone and reduces their relative penetration into the brain (Hill and Thomas 2011). It is therefore possible that methamphetamine-induced alteration in the integrity of the blood–brain barrier (Northrop and Yamamoto 2012) could make it more permeable to the β-ketoamphetamines. The net result of these possible effects would be similar to treatment with higher doses of both drugs. Second, at the level of the DA nerve ending, it is possible that the bath salts enhance methamphetamine toxicity because of increased release of DA over that seen after either drug alone. Methamphetamine collapses the pH gradient across the synaptic vesicle membrane, allowing DA leakage into the cytoplasm and subsequent efflux via reverse transport (Sulzer et al. 2005). Methylone and mephedrone release cytoplasmic DA via reverse transport through the DAT, but they differ significantly from methamphetamine in that they have little if any affinity for the vesicle monoamine transporter and thus their inhibition of uptake and stimulation of release from synaptic vesicles is far lower than that of methamphetamine (Eshleman et al. 2013). Because methylone and mephedrone do not likely release DA from synaptic vesicles, combined treatment with either mephedrone or methylone + methamphetamine could recruit greater numbers of DAT molecules to result in heightened DA efflux into the synapse because amphetamine-induced release is greater when originating from both synaptic vesicle and cytoplasmic stores versus cytoplasmic stores only (Pifl et al. 1995). Third, mephedrone and methylone could enhance methamphetamine toxicity by inhibiting monoamine oxidase A. We have shown previously that the monoamine oxidase A inhibitor clorgyline significantly increases methamphetamine-induced depletion of DA (Thomas et al. 2008) but it is not known if the bath salts inhibit monoamine oxidase. Ongoing studies in our laboratory are directed at achieving a better understanding of these possible mechanisms by which the β-ketoamphetamines accentuate methamphetamine-induced neurotoxicity.
From a strictly pre-clinical, mechanistic perspective, MDPV has the potential to be an effective neuroprotectant in drug-induced neurotoxicity and in neurodegenerative conditions such as Parkinson’s disease, or for treatment of stimulant dependence. MDPV is not neurotoxic and is far more potent in preventing methamphetamine-induced DA release than bupropion or methylphenidate as mentioned above (Simmler et al. 2013b). The latter two drugs have been tested as treatments for stimulant dependence (Tiihonen et al. 2007; Elkashef et al. 2008; Shoptaw et al. 2008) with mixed outcomes so far. However, as a powerful DAT antagonist, the abuse potential of MDPV is high in humans (Coppola and Mondola 2012) as well as in animal models of addiction (Watterson et al. 2012; Aarde et al. 2013b; Bonano et al. 2014; Karlsson et al. 2014) and this property alone would limit its use as a therapeutic agent. Despite its abuse potential in the illicit market, MDPV or a structural variant with lower abuse potential could have clinical effectiveness in treating substance abuse disorders as do methadone and buprenorphine, drugs that also possess high abuse potential in humans.
Supplementary Material
Figure S1. Effects of methylone or MDPV singly or in combination on body temperature.
Figure S2. Effects of mephedrone or methylone singly or in combination on body temperature.
Figure S3. Effects of mephedrone or MDPV singly or in combination on body temperature.
Table S1. Two-way ANOVAs for treatment effects of MDPV, methamphetamine, or their combination on DA, DAT, TH, and GFAP.
Table S2. Post hoc statistical comparisons (Holm–Sidak test for multiple comparisons) for treatment of mice with MDPV, methamphetamine, or MDPV + methamphetamine.
Table S3. One-way ANOVAs for treatment effects of amphetamine, MDMA, and MPTP given singly or in combination with MDPV on DA, DAT, TH, and GFAP.
Table S4. Post hoc statistical comparisons (Holm–Sidak test for multiple comparisons) for treatment of mice with amphetamine, MDMA, or MPTP singly or in combination with MDPV.
Table S5. One-way ANOVAs for treatment effects of methylone, methamphetamine, and their combination on DA, DAT, TH, and GFAP.
Table S6. Post hoc statistical comparisons (Holm–Sidak test for multiple comparisons) for treatment of mice with methylone, methamphetamine, or methylone + methamphetamine.
Acknowledgments
This work was supported by grants from the Department of Veterans Affairs. We thank Dr Roxanne Vaughn for her generous gift of DAT antibodies. We would also like to thank the NIDA Drug Supply Program for providing methylone, MDPV, mephedrone, and MDMA for use in these studies.
Abbreviations used
- 5-HT
serotonin
- DA
dopamine
- DAT
dopamine transporter
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GFAP
glial fibrillary acidic protein
- MDMA
3,4-methylenedioxymeth-amphetamine
- MDPV
3,4-methylenedioxypyrovalerone
- TH
tyrosine hydroxylase
Footnotes
Conflict of interest disclosure
The authors declare no conflict of interest.
Author contributions
JHA, MAP, and DMK designed the experiments; JHA and MAP performed the experiments and collected the data; JHA, MAP, and DMK analyzed the data; DMK wrote the first draft of the manuscript and all authors contributed significantly to the writing of the final version of the article.
References
- Aarde SM, Angrish D, Barlow DJ, Wright MJ, Jr, Vandewater SA, Creehan KM, Houseknecht KL, Dickerson TJ, Taffe MA. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addict Biol. 2013a;18:786–799. doi: 10.1111/adb.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013b;71C:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Perez M, Kane MJ, Francescutti DM, Sykes KE, Shah MM, Mohammed AM, Thomas DM, Kuhn DM. Mephedrone, an abused psychoactive component of ‘bath salts’ and methamphetamine congener, does not cause neurotoxicity to dopamine nerve endings of the striatum. J Neurochem. 2012;120:1097–1107. doi: 10.1111/j.1471-4159.2011.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Perez M, Kane MJ, Briggs DI, Francescutti DM, Sykes CE, Shah MM, Thomas DM, Kuhn DM. Mephedrone does not damage dopamine nerve endings of the striatum, but enhances the neurotoxicity of methamphetamine, amphetamine, and MDMA. J Neurochem. 2013;125:102–110. doi: 10.1111/jnc.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Perez M, Kane MJ, Herrera-Mundo N, Francescutti DM, Kuhn DM. Effects of combined treatment with mephedrone and methamphetamine or 3,4-methylenedioxymethamphetamine on serotonin nerve endings of the hippocampus. Life Sci. 2014;97:31–36. doi: 10.1016/j.lfs.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS. Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxy-pyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology. 2014;231:199–207. doi: 10.1007/s00213-013-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron K, Kolanos R, Vekariya R, De Felice L, Glennon RA. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts”, produce opposite effects at the human dopamine transporter. Psychopharmacology. 2013a;227:493–499. doi: 10.1007/s00213-013-2967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron KN, Kolanos R, Solis E, Jr, Glennon RA, De Felice LJ. Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. Br J Pharmacol. 2013b;168:1750–1757. doi: 10.1111/bph.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola M, Mondola R. 3,4-methylenedioxypyrovalerone (MDPV): chemistry, pharmacology and toxicology of a new designer drug of abuse marketed online. Toxicol Lett. 2012;208:12–15. doi: 10.1016/j.toxlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33:1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol. 2013;85:1803–1815. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2013;38:563–573. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013;24:437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Tallarida CS, Reitz A, McCurdy C, Rawls SM. Mephedrone (4-methylmethcathinone), a principal constituent of psychoactive bath salts, produces behavioral sensitization in rats. Drug Alcohol Depend. 2013a;133:46–50. doi: 10.1016/j.drugalcdep.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Tallarida CS, Reitz AB, Rawls SM. Mephedrone interactions with cocaine: prior exposure to the ‘bath salt’ constituent enhances cocaine-induced locomotor activation in rats. Behav Pharmacol. 2013b;24:684–688. doi: 10.1097/FBP.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, et al. 4-Methylmethcathinone(mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin LE, Collins SA, Yamamoto BK. Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine. Life Sci. 2014;97:37–44. doi: 10.1016/j.lfs.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin Toxicol. 2011;49:705–719. doi: 10.3109/15563650.2011.615318. [DOI] [PubMed] [Google Scholar]
- den Hollander B, Rozov S, Linden AM, Uusi-Oukari M, Ojanpera I, Korpi ER. Long-term cognitive and neurochemical effects of “bath salt” designer drugs methylone and mephedrone. Pharmacol Biochem Behav. 2013;103:501–509. doi: 10.1016/j.pbb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- den Hollander B, Sundstrom M, Pelander A, Ojanpera I, Mervaala E, Korpi ER, Kankuri E. Keto amphetamine toxicity-focus on the redox reactivity of the cathinone designer drug mephedrone. Toxicol Sci. 2014;141:120–131. doi: 10.1093/toxsci/kfu108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug Alcohol Depend. 2012;126:168–175. doi: 10.1016/j.drugalcdep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitch JA, D’Amato RJ, Strittmatter SM, Snyder SH. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci USA. 1985;82:2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson L, Andersson M, Kronstrand R, Kugelberg FC. Mephedrone, methylone and 3,4-methylenedioxypyrovalerone (MDPV) induce conditioned place preference in mice. Basic Clin Pharmacol Toxicol. 2014;115:411–416. doi: 10.1111/bcpt.12253. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared to MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and serotonin levels in nucleus accumbens of awake rats. Br J Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanos R, Solis E, Jr, Sakloth F, De Felice LJ, Glennon RA. “Deconstruction” of the abused synthetic cathinone methylenedioxypyrovalerone (MDPV) and an examination of effects at the human dopamine transporter. ACS Chem Neurosci. 2013;4:1524–1529. doi: 10.1021/cn4001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DM, Billingsley ML. Tyrosine hydroxylase: purification from PC -12 cells, characterization, and production of antibodies. Neurochem Int. 1987;11:463–475. doi: 10.1016/0197-0186(87)90036-2. [DOI] [PubMed] [Google Scholar]
- Lisek R, Xu W, Yuvasheva E, Chiu YT, Reitz AB, Liu-Chen LY, Rawls SM. Mephedrone (‘bath salt’) elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend. 2012;126:257–262. doi: 10.1016/j.drugalcdep.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Arnau R, Martinez-Clemente J, Pubill D, Escubedo E, Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. Br J Pharmacol. 2012;167:407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Arnau R, Martinez-Clemente J, Abad S, Pubill D, Camarasa J, Escubedo E. Repeated doses of methylone, a new drug of abuse, induce changes in serotonin and dopamine systems in the mouse. Psychopharmacology. 2014;231:3119–3129. doi: 10.1007/s00213-014-3493-6. [DOI] [PubMed] [Google Scholar]
- Martinez-Clemente J, Lopez-Arnau R, Abad S, Pubill D, Escubedo E, Camarasa J. Dose and time-dependent selective neurotoxicity induced by mephedrone in mice. PLoS ONE. 2014;9:e99002. doi: 10.1371/journal.pone.0099002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL. Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neurotoxicology. 2012;33:1305–1313. doi: 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2014;87:206–213. doi: 10.1016/j.neuropharm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BL, Stogner JM. Not-so-clean fun: a profile of bath salt users among a college sample in the United States. J Psychoactive Drugs. 2014;46:147–153. doi: 10.1080/02791072.2013.876520. [DOI] [PubMed] [Google Scholar]
- Miller ML, Creehan KM, Angrish D, Barlow DJ, Houseknecht KL, Dickerson TJ, Taffe MA. Changes in ambient temperature differentially alter the thermoregulatory, cardiac and locomotor stimulant effects of 4-methylmethcathinone (mephedrone) Drug Alcohol Depend. 2013;127:248–253. doi: 10.1016/j.drugalcdep.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto K, Striebel J, Cho AK, Wang C. Clinical and pharmacological aspects of bath salt use: a review of the literature and case reports. Drug Alcohol Depend. 2013;132:1–12. doi: 10.1016/j.drugalcdep.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Motbey CP, Hunt GE, Bowen MT, Artiss S, McGregor IS. Mephedrone (4-methylmethcathinone, ‘meow’): acute behavioural effects and distribution of Fos expression in adolescent rats. Addict Biol. 2012a;17:409–422. doi: 10.1111/j.1369-1600.2011.00384.x. [DOI] [PubMed] [Google Scholar]
- Motbey CP, Karanges E, Li KM, et al. Mephedrone in adolescent rats: residual memory impairment and acute but not lasting 5-HT depletion. PLoS ONE. 2012b;7:e45473. doi: 10.1371/journal.pone.0045473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop NA, Yamamoto BK. Persistent neuroinflammatory effects of serial exposure to stress and methamphetamine on the blood-brain barrier. J Neuroimmune Pharmacol. 2012;7:951–968. doi: 10.1007/s11481-012-9391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell SJ, Weihmuller FB, Marshall JF. Methamphetamine-induced dopamine overflow and injury to striatal dopamine terminals: attenuation by dopamine D1 or D2 antagonists. J Neurochem. 1993;60:1792–1799. doi: 10.1111/j.1471-4159.1993.tb13405.x. [DOI] [PubMed] [Google Scholar]
- Pifl C, Drobny H, Reither H, Hornykiewicz O, Singer EA. Mechanism of the dopamine-releasing actions of amphetamine and cocaine: plasmalemmal dopamine transporter versus vesicular monoamine transporter. Mol Pharmacol. 1995;47:368–373. [PubMed] [Google Scholar]
- Pu C, Fisher JE, Cappon GD, Vorhees CV. The effects of amfonelic acid, a dopamine uptake inhibitor, on methamphetamine-induced dopaminergic terminal degeneration and astrocytic response in rat striatum. Brain Res. 1994;649:217–224. doi: 10.1016/0006-8993(94)91067-7. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Agoglia AE, Fish EW, Krouse MC, Malanga CJ. Mephedrone (4-methylmethcathinone) and intracranial self-stimulation in C57BL/6J mice: comparison to cocaine. Behav Brain Res. 2012;234:76–81. doi: 10.1016/j.bbr.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CJ, Gibb JW. Role of the dopamine uptake carrier in the neurochemical response to methamphetamine: effects of amfonelic acid. Eur J Pharmacol. 1985;109:73–80. doi: 10.1016/0014-2999(85)90541-2. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, De La Garza R, Newton T, Ling W. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96:222–232. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortall SE, Green AR, Swift KM, Fone KC, King MV. Differential effects of cathinone compounds and MDMA on body temperature in the rat, and pharmacological characterization of mephedrone-induced hypothermia. Br J Pharmacol. 2013a;168:966–977. doi: 10.1111/j.1476-5381.2012.02236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortall SE, Macerola AE, Swaby RT, et al. Behavioural and neurochemical comparison of chronic intermittent cathinone, mephedrone and MDMA administration to the rat. Eur Neuropsychopharmacol. 2013b;23:1085–1095. doi: 10.1016/j.euroneuro.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013a;169:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Wandeler R, Liechti ME. Bupropion, methylphenidate, and 3,4-methylenedioxypyrovalerone antagonize methamphetamine-induced efflux of dopamine according to their potencies as dopamine uptake inhibitors: implications for the treatment of methamphetamine dependence. BMC Res Notes. 2013b;6:220–224. doi: 10.1186/1756-0500-6-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J Neurochem. 2008;105:605–616. doi: 10.1111/j.1471-4159.2007.05155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. Increases in cytoplasmic dopamine compromise the normal resistance of the nucleus accumbens to methamphetamine neurotoxicity. J Neurochem. 2009;109:1745–1755. doi: 10.1111/j.1471-4159.2009.06094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Kuoppasalmi K, Fohr J, Tuomola P, Kuikanmaki O, Vorma H, Sokero P, Haukka J, Meririnne E. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164:160–162. doi: 10.1176/ajp.2007.164.1.160. [DOI] [PubMed] [Google Scholar]
- Varner KJ, Daigle K, Weed PF, Lewis PB, Mahne SE, Sankaranarayanan A, Winsauer PJ. Comparison of the behavioral and cardiovascular effects of mephedrone with other drugs of abuse in rats. Psychopharmacology. 2013;225:675–685. doi: 10.1007/s00213-012-2855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addict Biol. 2012;19:165–174. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J. Mephedrone: use, subjective effects and health risks. Addiction. 2011;106:1991–1996. doi: 10.1111/j.1360-0443.2011.03502.x. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Jr, Angrish D, Aarde SM, et al. Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in Wistar and Sprague-Dawley rats. PLoS ONE. 2012;7:e44652. doi: 10.1371/journal.pone.0044652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effects of methylone or MDPV singly or in combination on body temperature.
Figure S2. Effects of mephedrone or methylone singly or in combination on body temperature.
Figure S3. Effects of mephedrone or MDPV singly or in combination on body temperature.
Table S1. Two-way ANOVAs for treatment effects of MDPV, methamphetamine, or their combination on DA, DAT, TH, and GFAP.
Table S2. Post hoc statistical comparisons (Holm–Sidak test for multiple comparisons) for treatment of mice with MDPV, methamphetamine, or MDPV + methamphetamine.
Table S3. One-way ANOVAs for treatment effects of amphetamine, MDMA, and MPTP given singly or in combination with MDPV on DA, DAT, TH, and GFAP.
Table S4. Post hoc statistical comparisons (Holm–Sidak test for multiple comparisons) for treatment of mice with amphetamine, MDMA, or MPTP singly or in combination with MDPV.
Table S5. One-way ANOVAs for treatment effects of methylone, methamphetamine, and their combination on DA, DAT, TH, and GFAP.
Table S6. Post hoc statistical comparisons (Holm–Sidak test for multiple comparisons) for treatment of mice with methylone, methamphetamine, or methylone + methamphetamine.


