Abstract
Background
Geriatric conditions may influence outcomes among patients receiving implantable cardioverter-defibrillators (ICDs). We sought to determine the prevalence of frailty and dementia among older adults receiving primary prevention ICDs, and to determine the impact of multimorbidity on mortality within one year of ICD implantation.
Methods and Results
The cohort included 83,792 Medicare patients from the National Cardiovascular Data Registry (NCDR®) ICD Registry™ who underwent first primary prevention ICD implantation between 2006–2009. These data were merged with Medicare analytic files to determine the prevalence of frailty, dementia and other conditions prior to ICD implantation, as well as one-year mortality. A validated claims-based algorithm was used to identify frail patients. Mutually exclusive patterns of chronic conditions were examined. The association of each pattern with one-year mortality was assessed using logistic regression models adjusted for selected patient characteristics. Approximately 1 in 10 Medicare patients with heart failure receiving a primary prevention ICD had frailty (10%) or dementia (1%). One-year mortality was 22% for patients with frailty, 27% for patients with dementia, and 12% in the overall cohort. Several multimorbidity patterns were associated with high one-year mortality rates: dementia with frailty (29%), frailty with COPD (25%), and frailty with diabetes (23%). These patterns were present in 8% of the cohort.
Conclusions
More than 10% of Medicare beneficiaries with heart failure receiving primary prevention ICDs have frailty or dementia. These patients had significantly higher one-year mortality than those with other common chronic conditions. Frailty and dementia should be considered in clinical decision-making and guideline development.
Keywords: implantable cardioverter-defibrillator, geriatrics
ICDs are widely used for primary prevention of sudden cardiac death in the large population of patients with systolic heart failure and left ventricular ejection fraction (LVEF) ≤35% who are at risk for lethal ventricular arrhythmias. Although ICDs for primary prevention effectively reduced mortality in clinical trials, subsequent observational studies found that patients with a 20% one-year mortality risk did not clearly benefit from ICDs.1, 2 Consequently, Medicare’s coverage policy includes the important exception of patients with non-cardiac diseases associated with a life expectancy less than one year.3
More than 140,000 ICDs are implanted in the United States annually for primary prevention indications, many in older adults.4 Over 40% of new ICD implants are in patients over age 70, and 10 to 20% are in patients over age 80, yet the mean age of patients in the major ICD trials was between 58 and 65 years.5 Older adults with heart failure often have multiple comorbidities and are frail, increasing their risk of death from non-cardiac causes. An understanding of outcomes in older persons, especially those with common comorbidities, is limited.5–7
Measures of geriatric impairment – multifactorial, non-disease specific conditions such as frailty and dementia8 – may enhance existing risk prediction strategies for mortality within one year of ICD implantation. Frailty is the accumulation of insults across multiple organ systems that leads to decreased physiologic reserves and resistance to stressors.9 ICD research has focused on single-disease risk factors to identify patients likely to live at least one year after ICD implantation. Geriatric conditions may affect patient outcomes and clinical decision-making regarding the use of ICDs. Furthermore, specific patterns of coexisting conditions (as opposed to a simple tally of the number of conditions that an individual has) may synergistically lead to worsened outcomes.10 However, little is known about the impact of geriatric conditions and patterns of comorbidity on one-year mortality after ICD implantation.
Our objective was to determine the prevalence of comorbid conditions among older adults undergoing primary prevention ICD placement, with a focus on two common geriatric conditions, frailty and dementia. We also aimed to determine the impact of patterns of these geriatric conditions and other common comorbidities on mortality within one year of ICD implantation.
Methods
Sources of Data
We used data from the NCDR ICD registry, which was created in 2006 in response to a mandate from the Centers for Medicare and Medicaid Services that required all hospitals to report data on primary prevention ICD implantations in Medicare patients.4, 11 The registry contains patient demographics, cardiac history, comorbidities, type of device, and adverse events during the initial hospitalization. Implanting centers must enter complete and accurate data to receive Medicare reimbursement and the data undergo quality-control checks.12 The present analysis used data from 2006 through the end of 2009.
Longitudinal outcomes were obtained by linking NCDR data with Medicare inpatient, outpatient and carrier analytic files using probabilistic matching.13 The inpatient files contain institutional claims for facility costs covered under Medicare Part A. The outpatient files contain claims from outpatient providers such as hospital outpatient departments, and the carrier files contain non-institutional provider claims for services covered under Medicare Part B (for example, physicians and physician assistants).
Study Population
All new (not replacement) ICD implants from 2006 through 2009 were included. Patients were excluded if they were younger than 65 years; enrolled in Medicare Advantage or not enrolled in Medicare at the time of implantation; not enrolled in Medicare for a full year prior to implantation; did not have an ICD for primary prevention; did not have a history of heart failure; or had a previous ICD.
Geriatric Conditions: Dementia and Frailty
To be classified as having dementia, beneficiaries were required to have at least one Part A Medicare inpatient claim or two outpatient or carrier claims with International Classification of Diagnoses – 9th revision (ICD-9) codes 290.xx, 294.1x, or 331.2x.14 We ascertained the presence of frailty using a coding algorithm designed for the Johns Hopkins Adjusted Clinical Groups® (ACG®) System (Version 11), a tool that analyzes health insurance enrollment records and claims data to predict the intensity of a person’s future healthcare utilization. The ACG System is the most widely used population-based case mix system in the world.15 The ACG System frailty marker incorporates 99 ICD-9 codes, selected by a group of geriatricians, and categorized into 10 clusters: malnutrition, dementia, severe vision impairment, decubitus ulcer, incontinence of urine, incontinence of feces, weight loss, social support needs, difficulty walking, and falls (Appendix 1).16, 17 The marker is a binary variable that identifies individuals as frail if they have one of the diagnostic clusters. It has been validated using the Medicare Current Beneficiary Survey and has been shown to accurately identify patients with limitations in activities of daily living.16
Chronic Conditions
Comorbid chronic conditions were ascertained first from the NCDR. We chose conditions that were: 1) identifiable by either ICD-9 diagnosis codes or within the NCDR, and either 2) prevalent among older adults, or 3) associated with mortality after receipt of an ICD.18, 19 Diabetes, chronic obstructive pulmonary disease (COPD), renal disease (creatinine ≥2.0 or dialysis), cerebrovascular disease (CVA) and hyponatremia (serum sodium level <136) were identified in this manner. Comorbidities that were not identified in the NCDR were ascertained from Medicare data if they were present (as either the primary or a secondary diagnosis) on one Part A Medicare claim or two outpatient or carrier claims during the 365 days preceding the date of ICD implantation. Diagnoses were excluded if they were not present in the NCDR and were found on only one outpatient or carrier claim. This approach increases the specificity of diagnoses and eliminates “rule out” encounters.20 We used validated ICD-9 coding algorithms for diabetes (250.xx); COPD (416.8x, 416.9x, 490.xx–505.xx, 506.4x, 508.1x, or 508.8; at least one of these had to be in a Part A inpatient claim); renal disease (403.01, 403.11, 403.91, 404.02, 404.036, 404.12, 404.13, 404.92, 404.93, 582.xx, 583.0x–583.7x, 585.xx, 586.xx, 588.0x, V42.0x, V45.1x, V56.xx); CVA (362.34, 430.xx–438.xx); cirrhosis (571.2x, 571.5x, 571.6x, 571.4x–571.49, 572.2x–572.8x, 456.0x–456.21); metastatic solid tumor (196.xx–199.xx); and hyponatremia (276.1x).14, 21, 22
Patient Characteristics
We regressed death within one year following ICD implantation on the comorbidity clusters, with the group with no comorbidity as the reference group, adjusted for selected patient characteristics associated with mortality among primary prevention ICD recipients. These variables, obtained from the NCDR, included: age, sex, most recent LVEF, New York Heart Association (NYHA) classification, atrial fibrillation or flutter, and systolic blood pressure.
Primary Outcome
The outcome was death within one year following ICD implantation, determined from the Medicare Beneficiary Denominator data.
Statistical Analysis
First, we determined the prevalence and one-year mortality rate of each of the traditional comorbidities and of frailty and dementia, the geriatric conditions of interest. To determine the impact of multimorbidity patterns on one-year mortality, we used a stepwise multimorbidity pattern analysis technique that has been previously described.10, 23, 24 The first step was to specify our list of building block conditions. We used 7 chronic conditions and the 2 geriatric conditions described above.
Next, we sought to determine the most prevalent multimorbidity patterns. A multimorbidity pattern is a specific, mutually exclusive combination of building block conditions, such as dementia and chronic obstructive pulmonary disease (COPD). In a multimorbidity pattern analysis, individuals are sorted into mutually exclusive patterns using logic rules. Because 9 conditions were initially considered, a large number of patterns were derived; however most of the patterns included very few individuals. Prevalence was estimated for each of the 275 mutually exclusive patterns of conditions (all single disease occurrences as well as all combinations of ≥2 conditions).
The association of each multimorbidity pattern with one-year mortality after ICD implantation was then estimated using logistic regression models unadjusted and adjusted for selected patient characteristics. The initial pattern table was sorted from highest to lowest by: 1) prevalence, and 2) one-year mortality. The next phase involved identifying a reduced number of co-occurring conditions to include in the final pattern analysis. Three geriatricians (ARG, BL and DDM) adjudicated all 275 patterns and combined them into a reduced number of clinically meaningful, mutually exclusive patterns. This process was iterative and was guided by clinical experience in addition to the prevalence and mortality data.
Odds ratios (ORs) were modeled for one-year mortality in ICD recipients aged 65 and older with heart failure plus a multimorbidity pattern versus heart failure alone. The effects of covariates were examined through multivariate models. To address the large number of multiple comparisons, we evaluated significance at the p<0.001 level. Analyses were performed using the SAS statistical package version 9.2 (SAS Institute, Cary, NC). All analyses were approved by the Yale University Human Investigation Committee.
Results
Patient characteristics
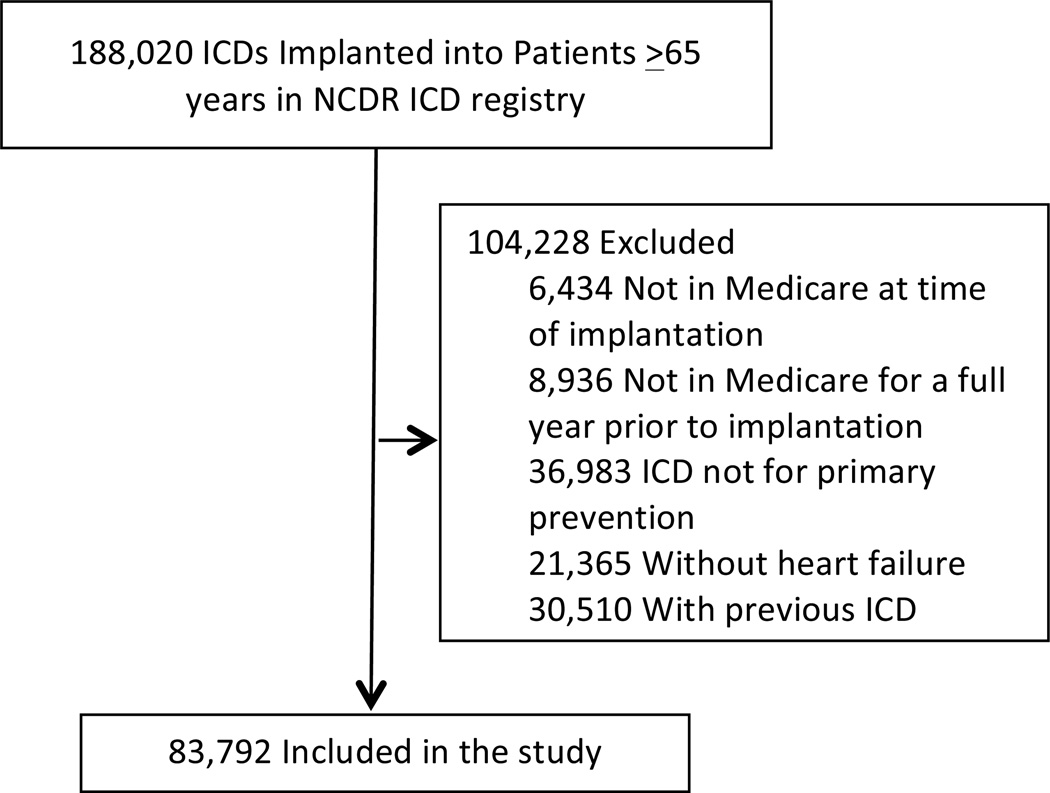
From January 2006 through December 2009, 188,020 ICDs were implanted in patients 65 years and older (Figure 1). Patients were excluded if they were not in Medicare at the time of implantation (n = 6,434; 3%), not in Medicare for a full year prior to implantation (n = 8,936; 5%), received an ICD for secondary prevention (n = 36,983; 21%), did not have a history of heart failure (n = 21,365; 16%), or had a previous ICD (n = 30,510; 27%). The final study cohort included 83,792 patients in 1,314 hospitals (73% of the initial sample).
Figure 1.
Flow diagram of the study population.
Characteristics of the 83,792 patients are listed in Table 1. The mean patient age was 75.42 (6.08) years. Most patients were male (72%) and white (85%). One-third of the patients had an LVEF less than 20% (this cut-off was chosen because patients with a 20% one-year mortality risk do not clearly benefit from ICDs),1, 2 and 92% had NYHA Class II or III heart failure.
Table 1.
Baseline Characteristics of Patients in Study
| Description | Died Within 1 Year after ICD Implantation | P | |||
|---|---|---|---|---|---|
| No (N=73,605) | Yes (N=10,187) | ||||
| N | % | N | % | ||
| Age: Mean (±SD) | 75.23 | 6.03 | 76.80 | 6.28 | <0.001 |
| Gender: Female | 21095 | 28.66 | 2690 | 26.41 | <0.001 |
| Race | <0.001 | ||||
| Hispanic | 2949 | 4.01 | 488 | 4.79 | |
| White non-Hispanic | 63000 | 85.59 | 8420 | 82.65 | |
| Black non-Hispanic | 5850 | 7.95 | 1008 | 9.89 | |
| Other | 1806 | 2.45 | 271 | 2.66 | |
| History and Risk Factors | |||||
| Hemodialysis | 2411 | 3.28 | 1149 | 11.28 | <0.001 |
| Syncope | 9663 | 13.13 | 1607 | 15.78 | <0.001 |
| CHF Duration* | <0.001 | ||||
| No | 80 | 0.11 | 15 | 0.15 | |
| Less than 3 months | 11796 | 16.03 | 1782 | 17.49 | |
| 3 to 9 months | 11239 | 15.27 | 1311 | 12.87 | |
| Greater than 9 months | 50490 | 68.60 | 7079 | 69.49 | |
| Most Recent LVEF >20% | <0.001 | ||||
| Unknown | 618 | 0.84 | 108 | 1.06 | |
| No | 23345 | 31.72 | 4123 | 40.47 | |
| Yes | 49642 | 67.44 | 5956 | 58.47 | |
| NYHA Class | <0.001 | ||||
| Missing | 51 | 0.07 | 5 | 0.05 | |
| Class I | 2232 | 3.03 | 193 | 1.89 | |
| Class II | 22873 | 31.08 | 1991 | 19.54 | |
| Class III | 45239 | 61.46 | 6980 | 68.52 | |
| Class IV | 3210 | 4.36 | 1018 | 9.99 | |
| Atrial Fibrillation/Atrial Flutter | 29693 | 40.34 | 5063 | 49.70 | <0.001 |
| Ventricular Tachycardia | 18011 | 24.47 | 3236 | 31.77 | <0.001 |
| Previous MI | 40850 | 55.50 | 6141 | 60.28 | <0.001 |
| Prior Pacemaker Insertion | 0.004 | ||||
| Unknown | 20 | 0.03 | 4 | 0.04 | |
| No | 60893 | 82.73 | 8548 | 83.91 | |
| Yes - Atrial Chamber | 333 | 0.45 | 52 | 0.51 | |
| Yes - Ventricular Chamber | 2081 | 2.83 | 296 | 2.91 | |
| Yes - Dual Chamber | 9614 | 13.06 | 1187 | 11.65 | |
| Yes - Biventricular | 664 | 0.90 | 100 | 0.98 | |
| CRT-D (current implant) | 39114 | 53.14 | 5608 | 55.05 | <0.001 |
Time since the initial CHF diagnosis.
Geriatric conditions and chronic conditions
The absence of all diseases other than heart failure was the most common pattern (19%). Overall, 10% of patients with ICDs were frail and 1% had dementia. The prevalence of comorbid chronic conditions was 47% for diabetes, 35% for COPD, 27% for cerebrovascular accident (CVA), 26% for chronic kidney disease (CKD) and 19% for hyponatremia. Metastatic solid tumor and cirrhosis each accounted for less than 1% of the population.
Mortality
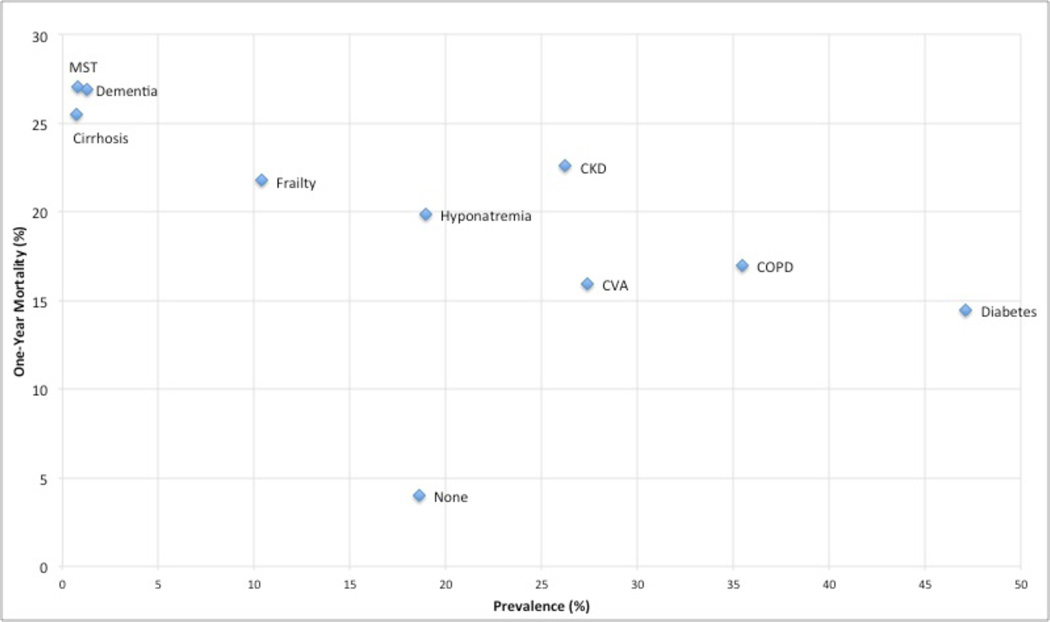
The overall one-year mortality following ICD implantation was 12%. Geriatric conditions were highly associated with mortality within one year of ICD implantation. A total of 27% of patients with dementia died within the first year, and 22% of patients with frailty. Of the traditional comorbidities, only metastatic solid tumor and cirrhosis had one-year mortality rates comparable to dementia. CKD had a higher one-year mortality (23%) than frailty. Hyponatremia, diabetes, COPD and CVA all had lower one-year mortality rates than dementia and frailty (Figure 2). Among patients with no conditions other than heart failure, 4% died within the first year.
Figure 2.
MST=metastatic solid tumor; CKD=chronic kidney disease; CVA=cerebrovascular accident; COPD=chronic obstructive pulmonary disease.
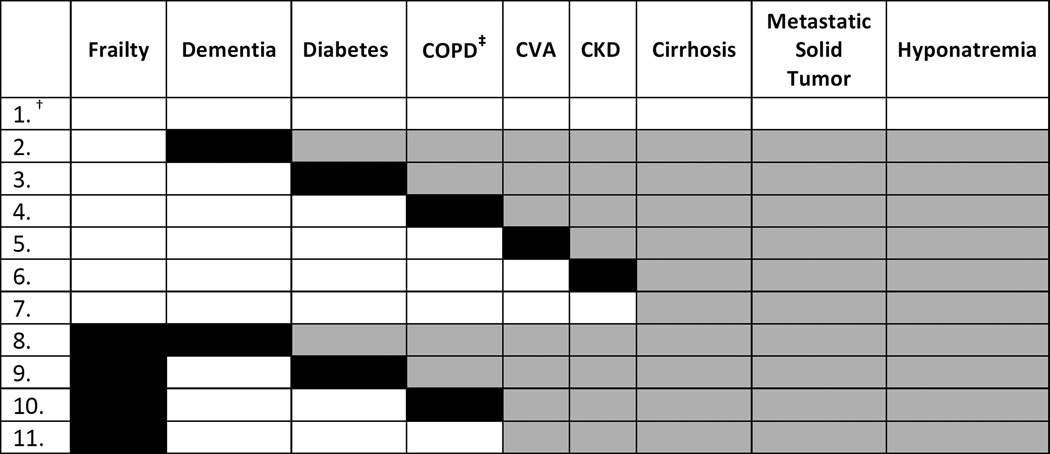
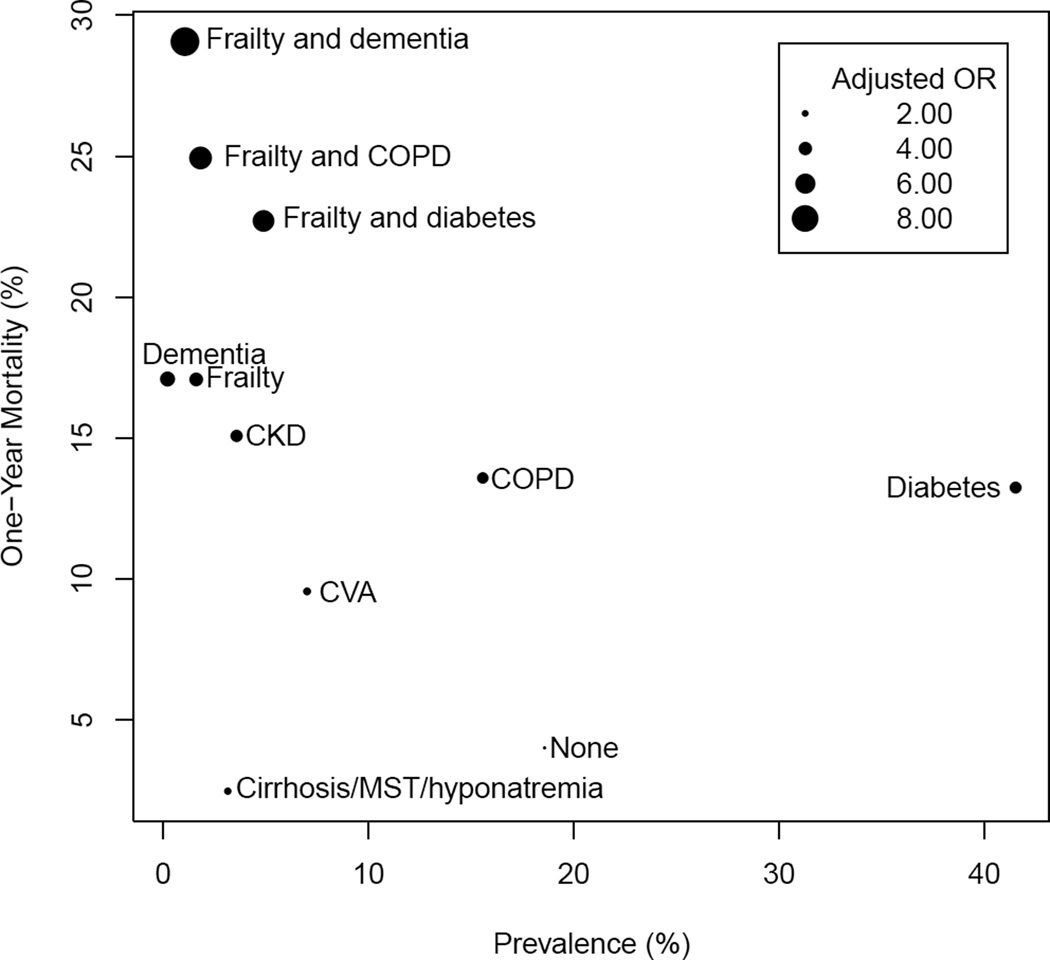
In multimorbidity patterns analysis, 11 mutually exclusive patterns were examined (Figure 3). Several multimorbidity patterns had one-year mortality rates after ICD implantation greater than 20%: dementia with frailty (29%), frailty with COPD (25%), and frailty with diabetes (23%). These three patterns together accounted for 8% of patients. Patients with both frailty and dementia had the highest odds of death compared to patients with no comorbidity (OR 8.68; 95% CI 7.33–10.27) (Figure 4). Dementia and frailty consistently elevated the risk of death from a patient’s traditional comorbidities. For example, patients with frailty and diabetes (the most prevalent coexisting chronic condition in the cohort) were 6 times as likely to die within one year of ICD implantation than people with heart failure alone (OR = 6.49; 95% CI 5.81–7.24). In contrast, patients with diabetes who were not frail were nearly 4 times as likely to die within one year of ICD implantation (OR 3.60; 95% CI 3.30–3.92). A similar pattern was found for the combination of COPD and frailty.
Figure 3.
* Because our sorting and ranking step had confirmed that many of the patterns with high one-year mortality included frailty or dementia, and because these were the geriatric conditions of interest, we first classified patients into a multimorbidity pattern based on the presence of one of these two geriatric conditions. Patients who did not have dementia or frailty were placed into another multimorbidity pattern based on their individual combination of building block conditions.
† Each row is a distinct pattern. Shading identifies which comorbidities comprise the pattern. Each pattern does include the comorbidity shaded in black, and may include any of the other comorbidities in that row shaded in gray. For example, all individuals represented in row 2 have dementia, but any individual in row 2 may also have the conditions shaded in gray. Individuals in row 7 have either cirrhosis, metastatic solid tumor, hyponatremia, or a combination of these three comorbidities and no others.
‡ COPD = chronic obstructive pulmonary disease. CKD = chronic kidney disease. CVA = cerebrovascular disease.
Figure 4.
MST=metastatic solid tumor; CKD=chronic kidney disease; CVA=cerebrovascular accident; COPD=chronic obstructive pulmonary disease. Size of bubble corresponds to odds ratio, calculated using adults with heart failure alone as referent (odds if have heart failure and multimorbidity pattern/odds if have heart failure alone). Models adjusted for age, gender, left ventricular ejection fraction, atrial fibrillation/flutter, systolic blood pressure, and New York Heart Association functional classification.
In total, 17% of implants were upgrades from pacemakers to ICDs. As upgrade procedures have been associated with greater risk of complications,25 we performed a sensitivity analysis to examine the extent to which this may have contributed to our results. Incorporating prior pacemaker as a covariate in the multivariate analyses did not alter our findings.
Two multimorbidity patterns were associated with higher rates of in-hospital complications or death after ICD implantation, COPD (OR = 1.21; 95% CI 1.08–1.35) and frailty with COPD (OR = 1.35; 95% CI 1.08–1.69).
Discussion
The main finding of this study is that more than 10% of patients receiving primary prevention ICDs are frail or have dementia. Patients with these geriatric conditions had substantially higher mortality within the first year after ICD implantation than those without these conditions. Several multimorbidity patterns were associated with high one-year mortality rates: dementia with frailty, frailty with COPD, and frailty with diabetes. Frailty and dementia were more strongly associated with mortality than were traditional comorbidities.
To our knowledge, this is the first study to determine the prevalence of frailty among older adults receiving primary prevention ICDs. We identified frail patients using an algorithm designed for use with Medicare claims. Analyses of large claims databases can advance the evidence base regarding ICD therapy, geriatric conditions and treatment effect heterogeneity. Several studies have shown that frailty is independently associated with mortality among older adults with heart failure, and that frailty assessment may identify patients at risk of poor outcomes after invasive procedures.26–29 For older adults with multiple coexisting illnesses, ICD therapy may make life and death more onerous through post-procedural complications, increased hospitalizations, inappropriate shocks, and futile end-of-life shocks among patients dying of non-arrhythmic causes.30 Viewed in this context, our findings point toward a greater role for frailty screening among older adults who are being considered for primary prevention ICDs based on cardiac criteria. Frailty screening may improve appropriate ICD utilization as well as patient outcomes.
Our study also confirms the importance of dementia for short-term mortality outcomes after ICD implantation. Previous research has shown that 6% of patients hospitalized for heart failure have dementia, and that the use of ICDs to prevent sudden cardiac death would provide limited benefit among such patients.31 Another study demonstrated that the 5-year survival of patients with cognitive impairment or dementia who underwent pacemaker or ICD insertion was significantly lower than that of matched controls without cognitive impairment or dementia.32 In our study, patients with dementia who underwent ICD implantation had a one-year mortality rate comparable to that of patients with metastatic solid tumors. Although the one-year mortality rates for these two conditions were clinically similar, they were statistically different due to our large sample size. We found that dementia was present in only 1% of Medicare beneficiaries undergoing primary prevention ICD placement. This suggests that physicians referring patients for ICD implantation may already be identifying patients with significant cognitive impairment who are likely to derive limited benefit from ICDs.
Traditional multivariate regression does not account for the specific patterns of co-occurring conditions prevalent among older adults with heart failure. Previous studies used comorbidity indices such as the Charlson to examine multimorbidity in older adults with ICDs.18 However, the Charlson is difficult to use in clinical practice. Furthermore, such indices were not developed in older adults with heart failure, and conditions that may have the most bearing on outcomes, such as frailty and dementia, are not incorporated or not appropriately weighted. Clinicians do not treat Charlson scores, they treat individuals. We used a stepwise multimorbidity pattern analysis technique to gain an in-depth understanding of the comorbidity burden of older adults with heart failure, ICDs, dementia and frailty. We sorted the 275 mutually exclusive patterns of conditions by prevalence and one-year mortality, and then combined patterns into mutually exclusive groups. This revealed that the combination of frailty and another comorbidity signified a state of heightened vulnerability after ICD implantation. For example, 23% of patients with frailty and diabetes died within one year of ICD implantation, compared with 13% of patients who had diabetes but were not frail.
Several multimorbidity patterns had one-year mortality rates after ICD implantation greater than 20%: dementia with frailty, frailty with COPD, and frailty with diabetes. This finding is important because it is not clear that ICD therapy benefits patients with a ≥20% baseline annual mortality risk.2, 33 Retrospective studies that used data from the primary prevention trials found that patients with a predicted annual mortality ≥20% (using risk prediction tools such as the Seattle Heart Failure Model) did not appear to benefit from ICD therapy. The development of risk prediction models to identify patients who are unlikely to benefit from ICDs is an active area of investigation. We do not yet know the best method of identifying patients with high one-year mortality risk who are unlikely to benefit from ICD therapy. Furthermore, older adults with multiple comorbidities were under-represented in the primary prevention trials, underscoring the challenge of generalizing the results to the large numbers of older adults receiving ICDs. Our study adds to the literature by taking a clinically relevant approach to examining multimorbidity in older adults with ICDs, and making risk prediction relevant to older adults with multiple comorbidities and geriatric conditions.
Current guidelines for the use of primary prevention ICDs, including guidelines for the care of vulnerable older adults with heart failure, have not incorporated assessment of frailty.8, 34, 35 Our analysis suggests additional elements to include in the National ICD Registry data collection process, such as a physical performance test, measurement of gait speed, or composite frailty scale. These data are already being collected from patients undergoing aortic valve replacement and cardiac surgery.36 Our findings highlight the need for subsequent studies involving detailed clinical assessments of frailty among older adults being considered for primary prevention ICD therapy. Identification of geriatric conditions should improve risk prediction and foster patient-centered clinical decision-making. Future primary prevention ICD eligibility guidelines should incorporate frailty and dementia. In addition, frailty assessment should be taught as part of training and recertification for implanting physicians and general cardiologists who are likely to refer patients for ICDs.
Our findings should be interpreted in the context of some limitations. The main drawback is the lack of a consensus on how frailty should be evaluated. The claims-based algorithm we used to identify clinically frail patients has been used in two other studies, but it has not been tested in a heart failure population.16, 17 Although there is no gold standard for the assessment of frailty, numerous definitions have been proposed. The Fried frailty phenotype is the most widely-used. It is standardized, physiologically-based, and independently predicts adverse outcomes such as falls, hospitalizations and death in older adults. Using this definition, 6.9% of older adults in the Cardiovascular Health Study were identified as frail.9 Adaptations of this clinical phenotype abound. Rockwood’s Frailty Index operationalizes frailty as the number of accumulated deficits; among a sample of elderly Canadians, 22.7% were identified as frail using the Frailty Index.37 Still others propose using solitary objective measures, such as gait speed, as a proxy for frailty.38 In the present study, use of the ACG System frailty measure was motivated by the available data and was intended to be hypothesis-generating rather than definitive. Patients may have been classified as frail as a consequence of heart failure alone. It was beyond the scope and aims of our study to determine whether certain clusters, such as falls or incontinence, were more prevalent among patients identified as frail. It is unlikely that urinary incontinence is synonymous with frailty, or that social support needs can be accurately measured in administrative claims. We recognize that these are inherent problems with the frailty marker we used. However, despite these qualifications, our use of the ACG System frailty measure had face validity because we identified a subset of previously unrecognized patients with heightened vulnerability, as evidenced by their 22% mortality in the first year after ICD implantation. These findings are novel and clinically-significant. We did not have data on the cause of death and therefore cannot say whether patients identified as frail using our measure died as a result of causes unrelated to the ICD; this is an important avenue for future research.
The proportion of ICD recipients who had frailty was much higher than the proportion who had dementia. Frail older adults should not be excluded a priori from ICD implantation, and we cannot say what the correct rate of ICD implantation among frail older adults should be. We were unable to determine the benefit (or lack thereof) of ICDs in frail patients because the available data only allowed us compare outcomes among patients who all received the device. But the high one-year mortality rate among patients with frailty undergoing ICD implantation should be incorporated into the highly preference-sensitive decision of whether to implant an ICD for primary prevention. Our finding that primary prevention ICD recipients with frailty have high one-year mortality is crucial information for patients and clinicians to have in the shared decision-making process. Future research should confirm the significance of our findings by prospectively evaluating clinical measures of frailty among patients with ICDs, and comparing quality of life and clinical outcomes for ICD-eligible patients with dementia and frailty who either do or do not receive an ICD.
Another limitation is that we have probably under-estimated the true prevalence of dementia among ICD recipients because dementia is undercoded in Medicare claims data.39 Dementia, as identified in claims, is often advanced. We do not intend to imply that there is a correct rate of ICD implantation in persons with dementia; it probably should not be zero. Rather, we hope our findings will support meaningful, patient-centered discussions about ICD implantation between physicians, older adults with cognitive impairment, and their families.
Our analysis of geriatric conditions was limited to frailty and dementia. We chose these conditions because they could be identified using ICD-9 codes. However, other geriatric conditions, such as polypharmacy, may play an important role in modifying patient outcomes after receipt of an ICD. In addition, the available data did not enable us to examine the influence of geriatric conditions on other outcomes that may be valued by older adults with ICDs, such as functional status and quality of life. Finally, although we restricted the cohort to primary prevention patients, an appreciable number had a history of ventricular tachycardia (25%; primarily non-sustained) and NYHA Class IV heart failure (5%). The inclusion of these patients, who were likely to be sicker, may have affected our findings. Importantly, however, these data reflect real-world use of ICDs.
Despite these limitations, our study is an important first step in establishing the prognostic importance of geriatric conditions, particularly frailty, in older adults undergoing ICD implantation. There is growing awareness of the need for a more comprehensive approach to clinical decision-making regarding the use of recent innovations in heart failure management, such as ICDs, transcatheter aortic valve replacements and left ventricular assist devices. Decision-making regarding the use of recent innovations in heart failure management must go beyond individual diseases and consider geriatric conditions as well as the context of the patient.8 Future research should confirm the influence of frailty on patient outcomes after ICD implantation using different frailty measures, explore patient-centered outcomes in addition to mortality, and test frailty assessment tools that can be implemented in a heart failure clinic.
Supplementary Material
What Is Known
ICDs for primary prevention of sudden cardiac death are not recommended for patients with a life expectancy less than one year.
ICD research has focused on single-disease risk factors to identify patients likely to live at least one year after ICD implantation.
What the Study Adds
More than 10% of patients receiving primary prevention ICDs are frail or have dementia. Frailty and dementia are more strongly associated with mortality within the first year after ICD implantation than are traditional comorbidities.
The high one-year mortality rate among patients with frailty and dementia undergoing ICD implantation should be incorporated into the highly preference-sensitive decision of whether to implant an ICD for primary prevention.
Acknowledgments
This study made use of the Johns Hopkins Adjusted Clinical Groups (ACG®) System that is copyrighted by The Johns Hopkins University. Any reference to the ACG® System herein is purely factual in nature, and does not represent an endorsement or confirmation by The Johns Hopkins University of the views expressed herein.
Sources of Funding
This research was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR®). The views expressed in this manuscript represent those of the author(s), and do not necessarily represent the official views of the NCDR® or its associated professional societies identified at www.ncdr.com.
Dr. Green is supported by a Johns Hopkins Clinical Research Scholars Mentored Career Development Award (KL2). This project was completed while she had funding from the Agency for Healthcare Research and Quality (HHSA290201200007I).
Dr. Pamela Peterson is supported by grant K08 HS019814-01 from the Agency for Healthcare Research and Quality.
Dr. Daugherty is supported by grant K08HL103776-01 from the National Heart Lung and Blood Institute of the NIH.
Dr. Matlock is supported by a career development award from the National Institutes on Aging (K23 AG040696).
Footnotes
Disclosures
Dr. Masoudi has a contract with the American College of Cardiology for his role as Senior Medical Officer of the National Cardiovascular Data Registries.
Drs. Leff and Spatz and Mr. Wang have no relevant disclosures.
Partners and Sponsors
ICD Registry™ is an initiative of the American College of Cardiology Foundation and the Heart Rhythm Society.
References
- 1.Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol. 2012;59:2075–2079. doi: 10.1016/j.jacc.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 2.Levy WC, Lee KL, Hellkamp AS, Poole JE, Mozaffarian D, Linker DT, Maggioni AP, Anand I, Poole-Wilson PA, Fishbein DP, Johnson G, Anderson J, Mark DB, Bardy GH. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation. 2009;120:835–842. doi: 10.1161/CIRCULATIONAHA.108.816884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Medicare & Medicaid Services. Decision Memo for Implantable Defibrillators (CAG-00268R3) [Accessed November 16, 2015]; https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=148.
- 4.Kremers MS, Hammill SC, Berul CI, Koutras C, Curtis JS, Wang Y, Beachy J, Blum Meisnere L, Conyers del M, Reynolds MR, Heidenreich PA, Al-Khatib SM, Pina IL, Blake K, Norine Walsh M, Wilkoff BL, Shalaby A, Masoudi FA, Rumsfeld J. The National ICD Registry Report: Version 2.1 including leads and pediatrics for years 2010 and 2011. Heart Rhythm. 2013;10:e59–e65. doi: 10.1016/j.hrthm.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 5.Cutro R, Rich MW, Hauptman PJ. Device therapy in patients with heart failure and advanced age: too much too late? Int J Cardiol. 2012;155:52–55. doi: 10.1016/j.ijcard.2011.01.078. [DOI] [PubMed] [Google Scholar]
- 6.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics C and Stroke Statistics S. Heart disease and stroke statistics--2012 update: A report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santangeli P, Di Biase L, Dello Russo A, Casella M, Bartoletti S, Santarelli P, Pelargonio G, Natale A. Meta-analysis: age and effectiveness of prophylactic implantable cardioverter-defibrillators. Ann Intern Med. 2010;153:592–599. doi: 10.7326/0003-4819-153-9-201011020-00009. [DOI] [PubMed] [Google Scholar]
- 8.Dodson JA, Chaudhry SI. Geriatric conditions in heart failure. Curr Cardiovasc Risk Rep. 2012;6:404–410. doi: 10.1007/s12170-012-0259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA Cardiovascular Health Study Collaborative Research G. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 10.Boyd CM, Leff B, Weiss CO, Wolff JL, Hamblin A, Martin L. Faces of Medicaid: Clarifying Multimorbidity Patterns to Improve Targeting and Delivery of Clinical Services for Medicaid Populations. [Accessed November 16, 2015]; http://www.chcs.org/media/Clarifying_Multimorbidity_for_Medicaid_report-FINAL.pdf. [Google Scholar]
- 11.McClellan MB, Tunis SR. Medicare coverage of ICDs. N Engl J Med. 2005;352:222–224. doi: 10.1056/NEJMp048354. [DOI] [PubMed] [Google Scholar]
- 12.Messenger JC, Ho KK, Young CH, Slattery LE, Draoui JC, Curtis JP, Dehmer GJ, Grover FL, Mirro MJ, Reynolds MR, Rokos IC, Spertus JA, Wang TY, Winston SA, Rumsfeld JS, Masoudi FA, Science N Quality Oversight Committee Data Quality W. The National Cardiovascular Data Registry (NCDR) Data Quality Brief: the NCDR Data Quality Program in 2012. J Am Coll Cardiol. 2012;60:1484–1488. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Peterson PN, Varosy PD, Heidenreich PA, Wang Y, Dewland TA, Curtis JP, Go AS, Greenlee RT, Magid DJ, Normand SL, Masoudi FA. Association of single- vs dual-chamber ICDs with mortality, readmissions, and complications among patients receiving an ICD for primary prevention. JAMA. 2013;309:2025–2034. doi: 10.1001/jama.2013.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 15.Weiner JP, Starfield BH, Steinwachs DM, Mumford LM. Development and application of a population-oriented measure of ambulatory care case-mix. Med Care. 1991;29:452–472. doi: 10.1097/00005650-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman R, Abrams C, Weiner JP. Final report to Centers for Medicare and Medicaid Services. Baltimore, MD: US Department of Health and Human Services; 2003. Development and evaluation of the Johns Hopkins University risk adjustment models for Medicare+Choice plan payment. [Google Scholar]
- 17.Sternberg SA, Bentur N, Abrams C, Spalter T, Karpati T, Lemberger J, Heymann AD. Identifying frail older people using predictive modeling. Am J Manag Care. 2012;18:e392–e397. [PubMed] [Google Scholar]
- 18.Lee DS, Tu JV, Austin PC, Dorian P, Yee R, Chong A, Alter DA, Laupacis A. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol. 2007;49:2408–2415. doi: 10.1016/j.jacc.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 19.Chan PS, Nallamothu BK, Spertus JA, Masoudi FA, Bartone C, Kereiakes DJ, Chow T. Impact of age and medical comorbidity on the effectiveness of implantable cardioverter-defibrillators for primary prevention. Circ Cardiovasc Qual Outcomes. 2009;2:16–24. doi: 10.1161/CIRCOUTCOMES.108.807123. [DOI] [PubMed] [Google Scholar]
- 20.Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R, Wu AW. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 21.Al-Khatib SM, Greiner MA, Peterson ED, Hernandez AF, Schulman KA, Curtis LH. Patient and implanting physician factors associated with mortality and complications after implantable cardioverter-defibrillator implantation, 2002–2005. Circ Arrhythm Electrophysiol. 2008;1:240–249. doi: 10.1161/CIRCEP.108.777888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 23.Weiss CO, Boyd CM, Yu Q, Wolff JL, Leff B. Patterns of prevalent major chronic disease among older adults in the United States. JAMA. 2007;298:1160–1162. doi: 10.1001/jama.298.10.1160-b. [DOI] [PubMed] [Google Scholar]
- 24.Boyd CM, Leff B, Wolff JL, Yu Q, Zhou J, Rand C, Weiss CO. Informing clinical practice guideline development and implementation: prevalence of coexisting conditions among adults with coronary heart disease. J Am Geriatr Soc. 2011;59:797–805. doi: 10.1111/j.1532-5415.2011.03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poole JE, Gleva MJ, Mela T, Chung MK, Uslan DZ, Borge R, Gottipaty V, Shinn T, Dan D, Feldman LA, Seide H, Winston SA, Gallagher JJ, Langberg JJ, Mitchell K, Holcomb R, Investigators RR. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122:1553–1561. doi: 10.1161/CIRCULATIONAHA.110.976076. [DOI] [PubMed] [Google Scholar]
- 26.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, Fried LP. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 27.Cacciatore F, Abete P, Mazzella F, Viati L, Della Morte D, D'Ambrosio D, Gargiulo G, Testa G, Santis D, Galizia G, Ferrara N, Rengo F. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest. 2005;35:723–730. doi: 10.1111/j.1365-2362.2005.01572.x. [DOI] [PubMed] [Google Scholar]
- 28.Boxer R, Kleppinger A, Ahmad A, Annis K, Hager D, Kenny A. The 6-minute walk is associated with frailty and predicts mortality in older adults with heart failure. Congest Heart Fail. 2010;16:208–213. doi: 10.1111/j.1751-7133.2010.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lupon J, Gonzalez B, Santaeugenia S, Altimir S, Urrutia A, Mas D, Diez C, Pascual T, Cano L, Valle V. Prognostic implication of frailty and depressive symptoms in an outpatient population with heart failure. Rev Esp Cardiol. 2008;61:835–842. [PubMed] [Google Scholar]
- 30.Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: Management of implantable cardioverter-defibrillators in hospice: A nationwide survey. Ann Intern Med. 2010;152:296–299. doi: 10.1059/0003-4819-152-5-201003020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Setoguchi S, Nohria A, Rassen JA, Stevenson LW, Schneeweiss S. Maximum potential benefit of implantable defibrillators in preventing sudden death after hospital admission because of heart failure. CMAJ. 2009;180:611–616. doi: 10.1503/cmaj.080769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jama A, Rabinstein A, Hodge D, Herges R, Asirvatham S, Cha YM, Powell B, Rea RF, Friedman P. Cardiac device complications in the cognitively impaired. Pacing Clin Electrophysiol. 2013;36:1061–1067. doi: 10.1111/pace.12205. [DOI] [PubMed] [Google Scholar]
- 33.Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, Zareba W, McNitt S, Andrews ML, Investigators M-I. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–296. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 34.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW American College of Cardiology/American Heart Association Task Force on Practice G, American Association for Thoracic S and Society of Thoracic S. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1–62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 35.Heidenreich PA, Fonarow GC. Quality indicators for the care of heart failure in vulnerable elders. J Am Geriatr Soc. 2007;55(Suppl 2):S340–S346. doi: 10.1111/j.1532-5415.2007.01341.x. [DOI] [PubMed] [Google Scholar]
- 36.Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA, Popma JJ, Ferrucci L, Forman DE. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–762. doi: 10.1016/j.jacc.2013.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681–687. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 38.Chen MA. Frailty and cardiovascular disease: potential role of gait speed in surgical risk stratification in older adults. J Geriatr Cardiol. 2015;12:44–56. doi: 10.11909/j.issn.1671-5411.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fillit H, Geldmacher DS, Welter RT, Maslow K, Fraser M. Optimizing coding and reimbursement to improve management of Alzheimer's disease and related dementias. J Am Geriatr Soc. 2002;50:1871–1878. doi: 10.1046/j.1532-5415.2002.50519.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.