Abstract
Cerebral small vessel disease (SVD) gives rise to one in five strokes worldwide and constitutes a major source of cognitive decline in the elderly. SVD is known to occur in relation to hypertension, diabetes, smoking, radiation therapy and in a range of inherited and genetic disorders, autoimmune disorders, connective tissue disorders, and infections. Until recently, changes in capillary patency and blood viscosity have received little attention in the aetiopathogenesis of SVD and the high risk of subsequent stroke and cognitive decline. Capillary flow patterns were, however, recently shown to limit the extraction efficacy of oxygen in tissue and capillary dysfunction therefore proposed as a source of stroke-like symptoms and neurodegeneration, even in the absence of physical flow-limiting vascular pathology. In this review, we examine whether capillary flow disturbances may be a shared feature of conditions that represent risk factors for SVD. We then discuss aspects of capillary dysfunction that could be prevented or alleviated and therefore might be of general benefit to patients at risk of SVD, stroke or cognitive decline.
Keywords: Cerebral small vessel disease, stroke, dementia, capillary dysfunction, oxygenation
Introduction
Cerebral small vessel disease (SVD) denotes a range of pathological processes, which affect the small arteries, arterioles, capillaries and small veins of the brain.1 SVD is associated with small subcortical infarcts, lacunes, white matter hyperintensities, enlarged perivascular spaces, microbleeds, and cortical atrophy,2 gives rise to one in five strokes worldwide, and constitutes a major source of cognitive decline, particularly in the elderly.1
Until recently, changes in capillary morphology and blood–brain barrier (BBB) function have received little attention in a etiopathogenesis of SVD and associated stroke and cognitive decline.3,4 In addition, capillary flow patterns have now been shown to limit the extraction efficacy of oxygen in tissue,5 and capillary dysfunction proposed as a source of stroke-like symptoms6 and neurodegeneration,7 even in the absence of flow-limiting vascular pathology.
Here, we briefly review the properties of capillary dysfunction and the evidence for capillary involvement in SVD and in conditions that impose risk of SVD. We then examine whether capillary dysfunction may play a role in the aetiopathogenesis of SVD and the subsequent development of stroke or cognitive decline. Finally, we discuss whether capillary dysfunction may serve as a common therapeutic target in efforts to prevent or ameliorate stroke and cognitive decline across the diverse range of conditions associated with SVD.
Capillary dysfunction
In normal brain, only 30–40% of blood’s oxygen passes into the brain parenchyma from small arteries, arterioles and capillaries to fuel cerebral metabolism.8 Oxygen extraction is known to be inefficient from capillaries with high flow velocities.9 The heterogeneity of flow velocities across the capillary bed of normal, resting brain tissue is extremely high10–12 and therefore limits net oxygen extraction5,13,14 – a biophysical property referred to as functional shunting. In the normal brain, capillary flow patterns homogenize when cerebral blood flow (CBF) increases in relation to cortical activation10,12,15,16 and thereby facilitate more efficient oxygen extraction.5,14 This homogenization is partly a passive property of normal microvascular networks: as CBF increases, the blood tends to distribute in a more homogenous way across ‘ideal’ capillary networks.17 In a later section, we discuss how cerebral pericytes regulate capillary diameter during functional activation18 and possibly provide active regulation of capillary flow patterns.19
Capillary dysfunction refers to conditions in which changes in capillary function and/or patency, or in blood rheology, disturb either capillary flow patterns, their homogenization during hyperaemia or both. To determine the effects of capillary dysfunction on oxygen extraction, the distribution of erythrocyte velocities or transit times across the capillary bed must be known.5,14,17,20,21 For convenience, we quantify capillary dysfunction by the accompanying capillary transit time heterogeneity (CTH) and use accepted transit time distributions for which the standard deviation describes CTH by a single parameter.5,13,14,17,21 Figure 1 illustrates how CBF and its microvascular distribution (CTH), combined, affect oxygen uptake in brain tissue.
Figure 1.
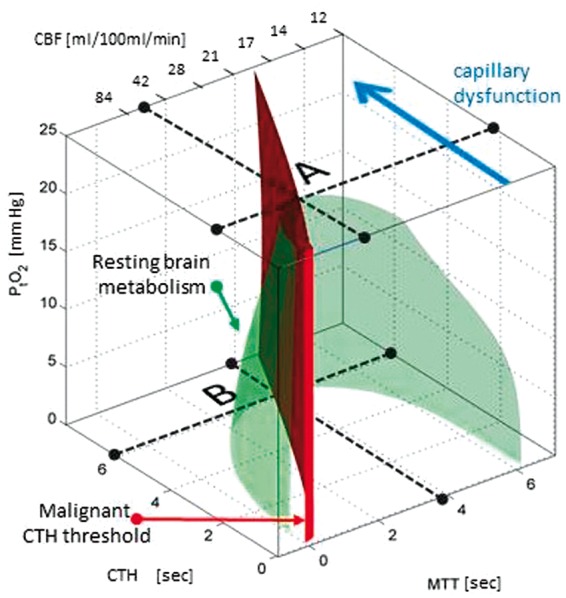
The green isocontour surface corresponds to all combinations of CBF, CTH, and PtO2 for which brain oxygenation – according to our model5 – matches the metabolic rate of oxygen in resting brain.187 Transitions to combinations of CBF, CTH and PtO2 that correspond to points located outside the resulting, bell-shaped surface are therefore predicted to result in immediate neurological symptoms, and tissue damage if they persist. The red plane marks the boundary, left of which vasodilation reduces tissue oxygen availability (dubbed malignant CTH). The maximum value that CTH can attain at a PtO2 of 25 mmHg, if oxygen availability is to support the metabolic needs of resting brain tissue, is indicated by the label A. As CTH increases further (progressive capillary dysfunction), CBF must be attenuated in order to reduce the level of ‘physiological shunting’. Importantly, continued tissue oxygen metabolism reduces tissue oxygen tension, and thereby improves blood-tissue concentration gradients and net extraction. As a result, the bell-shaped surface widens towards its base, reflecting that higher levels of CTH (more severe capillary dysfunction) can be accommodated by attenuating CBF and CBF responses. A critical limit is reached, however, as PtO2 approaches zero – label B. At this point, the metabolic needs of tissue are met by ‘delaying’ mean transit time (MTT) to a threshold of approximately 4 s, corresponding to CBF = 21 ml/100ml/min. As a result, slight increases in CTH (e.g. caused by an infection or dehydration) or a slight change in CBF (small flow reductions as well as flow increases) can trigger a critical reduction in tissue oxygen availability, and thereby stroke-like symptoms. The blue arrow indicates progressive capillary flow disturbances, which cause CTH to increase and tissue oxygen availability to approach the metabolic requirements of resting brain tissue (the green iso-contour). Note that the traditional notion of ischemia (which disregards capillary flow patterns) considers only a reduction in CBF (increase in MTT), that is, a transition along the x-axis in the three-dimensional plot. Source: Reproduced and modified from the literature.6 CBF: cerebral blood flow; CTH: capillary transit time heterogeneity; PtO2: tissue oxygen tension.
If changes in capillary patency or blood rheology become severe, our analyses predict that the flow-metabolism coupling, which is crucially required for tissue function and survival, modifies CBF in a counterintuitive direction.6 While homogenization of capillary flows (CTH reduction) maintains efficient oxygen extraction during hyperaemia in normal brain,5 only the suppression of CBF can reduce functional shunting if CTH can no longer be reduced. Attenuated CBF responses (reduced cerebrovascular reserve capacity) are therefore expected in both capillary dysfunction and flow-limiting conditions – but to represent the maintenance of flow-metabolism coupling in the former and flow limitations at the level of resistance vessels in the latter.
In cerebral ischemia, tissue function and survival are threatened by tissue hypoxia as a result of limited blood supply, whereas in capillary dysfunction, the source of hypoxia is inefficient oxygen extraction from the microcirculation. Notably, stroke-like symptoms and hypoxic tissue injury can therefore, in principle, be caused by capillary flow disturbances, in the absence of a flow-limiting condition.6,7 We have shown that CTH can reach a critical biophysical threshold CTHmax, above which net oxygen extraction can no longer meet the metabolic demands of resting brain tissue, although CBF is suppressed to minimize net functional shunting.6 Notably, the CBF value that optimizes net oxygen extraction for CTH = CTHmax seems almost identical to the classical ischemic threshold of approximately 20 ml/100ml/min,6 making critical capillary dysfunction and cerebral ischemia22 indistinguishable in terms of their low CBF and elevated oxygen extraction fraction (OEF).
If CBF suppression fails to compensate for capillary dysfunction, tissue hypoxia and injury can occur at CBF values above the ischemic threshold and even above normal brain perfusion. The ‘luxury perfusion syndrome’,23 which is sometimes observed upon reperfusion of tissue after prolonged or severe ischemia, may represent an instance of tissue damage caused by excessive functional shunting, keeping in mind that capillary constrictions can be observed after ischemia.19,24
Reductions of CBF to levels below the ischemic threshold can cause neurological symptoms and tissue injury irrespective of whether vascular patency is reduced at the arterial or the capillary level, but some vascular causes of neurological symptoms and tissue injury are specific to capillary dysfunction: capillary dysfunction can thus cause neurological deterioration and hypoxic tissue injury (a) in the absence of primary, flow-limiting pathology (e.g. no severe stenosis, thrombosis, embolism) (b) under conditions of augmented CBF (iatrogenic or spontaneous) and (c) due to increased blood viscosity if this increases CTH beyond CTHmax.
We discuss additional signatures of capillary dysfunction below.
Sources of capillary dysfunction in conditions that represent risk factors for SVD
We now review factors that may affect blood flow through individual capillaries in normal and diseased brain. In Tables 2 to 7, we list microvascular changes (middle column) in conditions considered risk factors for SVD according to Pantoni’s classification.1 Cutaneous leukocytoclastic angiitis is now classified as a single-organ vasculitis25 and therefore omitted from the list. We used Web of Science™ and PubMed to search for occurrences of the terms listed in the leftmost column of Tables 2 to 7 in combination with ‘capillary’, ‘endothelium’, ‘glycocalyx’, ‘basement membrane’, ‘pericyte’ or ’viscosity’. Examples of SVD-related infections are adapted from Younger.26 Literature searches for Table 5 were conducted between 30 December 2014 and 18 January 2015, while the remaining literature searches were conducted from 21 July 2014 to 26 September 2014.
Table 2.
Type 1: Arteriolosclerosis (age- and vascular risk-factor-related SVD). Type 2: CAA, sporadic or hereditary.
| Risk factor | Changes in capillary morphology or blood rheology | Signs of capillary dysfunction |
|---|---|---|
| Ageing | Human brain: Pericyte loss. Variable capillary diameters, increased capillary tortuosity, twisting, and looping. Thickened basement membranes with inclusions. Pericapillary fibrosis.194,195 Figure 2, panel B | |
| Hypertension | Animal brain: Pericyte degeneration, swelling of endothelium and surrounding astrocyte endfeet. Thickened basement membranes.196,197 In vitro: Angiotensin II, endothelin-1 constrict retinal pericytes.38,39 | Animal models: Elevated CBF and BOLD responses to hypercapnia in early-stage hypertension.97 Elevated resting flow at time of suppressed functional hyperemia.111 |
| Diabetes | Human: Thickened basement membranes.198,199 Hyperviscosity and reduced erythrocyte deformability in proportion to microvascular complications.92 Animal models: Pericyte loss and thickening of capillary basement membrane.196,200,201 Pericyte loss in STZ-induced diabetes is caused by oxidative stress in diabetic retinopathy.186 Glycocalyx degradation by oxidative stress, oxidized lipoproteins, hyperglycemia.83,87,88 In vitro: Hyperglycemia-induced oxidative stress in pericytic mitochondria cause pericyte apoptosis.202 | Animal models: Elevated CBF early after disease induction by STZ.94–96 |
| Cerebral amyloid angiopathy AD APOE ɛ4 genotype | Human brain: Pericyte degeneration, pericapillary fibrosis.190 In vitro: Cultured human pericytes undergo degeneration when exposed to certain subtypes of Aβ.62 Pericytes express Aβ receptors involved in amyloid internalization and pericyte death.63 | Human: Elevated resting and activity-related CBF in young APOE ɛ4 carriers,99,100 elevated BOLD.101,102,110 |
AD: Alzheimer’s disease; APOE: apolipoprotein; Aβ: amyloid β; BOLD: blood oxygen level dependent; CAA: cerebral amyloid angiopathy; CBF: cerebral blood flow; STZ: streptozotocin; SVD: small vessel disease.
Table 3.
Type 3: Inherited or genetic SVD other than CAA.
| Risk factor | Changes in capillary morphology or blood rheology | Capillary dysfunction signs |
|---|---|---|
| CADASIL/CARASIL | Human brain: Deposits of N3ECD48 and GOM in capillary walls, pericytes.204,49,205 Human skin, muscle: Pericyte loss, thickened capillary basement membrane with pericyte fragments. Pericyte-endothelial peg-socket contact disruption. Endothelial swelling and luminal processes.49 Animal models: Reduced capillary density.50 Pericyte degeneration.51 | Mouse model: Attenuated functional hyperemia prior to arteriolar damage.50 |
| Swedish-type hereditary multi-infarct dementia203 | No data available | No data available |
| MELAS | Human: Endothelial protrusions due to mitochondrial aggregates in the cerebrum192 and cerebellum.206 Pericytes contain aggregates of enlarged mitochondria in brain192 and muscle191,207 – Figure 2, panels E and F. | Globally elevated CBF, low OEF, and reduced CMRO2 before130–133 and after131,132,134 stroke. Preserved CBF and vasoreactivity in lesions.135 |
| Fabry’s disease | Human brain: Endothelial cell swelling, vacuolization, and deposits in arteries, arterioles, capillaries and veins.208 Granulomatous and ‘zebra’ deposits (approximately 1 µm) in endothelial cells and pericytes.209 Animal model: In lesions, storage material in relation to pericytes.210 | Hyperperfusion128 and enhanced vasodilation212 reversed126,127 by therapy that removes capillary deposits.189 Lesions develop in previously hyper-perfused tissue.129 |
| Hereditary cerebroretinal vasculopathy | Human retina: Capillary obliterations with fluorescent leakage, shunt vessels with leakage.211 | |
| HERNS | Human brain: Multilayered, thickened capillary basement membrane.213,214 Human retina: Capillary obliterations with tortuous telangiectatic microaneurisms.213,214 Widespread capillary closure and fluorescent leakage.213 | |
| COL4A1 gene mutations (codes for type IV collagen α1 basement membrane protein) | Human retina: Arteriolar (no capillary) involvement215. Animal models of collagen IV deficiency: Normal vascular system, but aberrant capillary organization216 during development. | |
| PADMAL | Small vessel (but no capillary) changes reported.217,218 |
CADASIL/CARASIL: erebral autosomal dominant/recessive arteriopathy with subcortical ischemic strokes and leuko-encephalopathy; GOM: granular osmophilic material; HERNS: Hereditary endotheliopathy with retinopathy, nephropathy and stroke; MELAS: Mitochondrial encephalo-pathy with lactic acidosis and stroke-like episodes; N3ECD: NOTCH3 extracellular domains; PADMAL: Pontine autosomal dominant angiopathy and leukoencephalopathy; SVD: small vessel disease.
Table 4.
Type 4: Inflammatory and immunologically mediated SVD. Vasculitis caused by infection.
| Risk factor | Changes in capillary morphology or blood rheology | Capillary dysfunction signs |
|---|---|---|
| Varicella Zoster | Reactivated Varicella Zoster Virus travels across axons to infect vessel walls.226 | |
| Hepatitis C | HCV can infect capillary endothelium.227 HCV-related vasculitis is often the result of cryoglobulinaemia – See Table 3. | |
| HIV-1 | HIV infection is associated with vasculitis228 and ischemic stroke,229 and vasculitis is identified as the stroke mechanism in many HIV-related ischemic strokes.230 The role HIV-1 virus, as opposed those of immunosuppression, coinfections, co-morbidities, and antiviral therapy, in conferring high risk of SVD231 or stroke232 remains controversial. HAND is associated with infection of perivascular macrophages and microglia which then release viral proteins (gp120, Tat and Vpr) which are neurotoxic in vitro.233 HIV virus infects cerebral capillary endothelial cells234 HIV proteins cause human brain microvascular endothelial cell apoptosis.235 HIV proteins induce pericyte migration and reduce pericyte coverage.236 | Elevated BOLD signals in asymptomatic HIV patients.105 Elevated BOLD signal in seropositive patients with low CNS penetration of antiretroviral therapy compared to patients with high penetrance, and controls.107 Suppression of functional hyperemia partly reversed by viscosity-lowering phosphodiesterase-inhibitor.239 |
| Treponema pallidum | Meningovascular neurosyphilis is dominated by lymphoplasmacytic infiltrates and intima-proliferation (endarteritis obliterans) in the walls of leptomeningeal arteries, veins and vasa vasorum and associated with the development of multiple cerebral infarcts. In Parenchymatous neurosyphilis, meninges and nervous tissue of the brain and/or spinal cord is invaded by spirochetes parenchymal. Associated with cortical atrophy, amyloid deposits, neurofibrillary tangles and dementia. Spirochetes frequently aggregate around capillaries/microvessels.237 | Significant increase in CMRO2 in the absence of increases in CBF after antibacterial treatment147 indicating improved oxygen extraction. |
| Borrelia burgdorferi | Similar to tertiary neurosyphilis (above), Lyme neuroborreliosis exists in a meningovascular form, associated with endarteritis obliterans and multiple cerebral infarcts,238 and in a parenchymatous form, with slowly progressive dementia. Intraparenchymal, perivascular lymphoplasmacytic infiltrates is a frequent finding.237 | Reduced cerebrovascular reserve capacity.240 Cerebral hypoperfusion is partially reversed by antibiotic therapy.148 |
BOLD: blood oxygen level dependent; CBF: cerebral blood flow; CMRO2: cerebral metabolic rate of oxygen; HAND: HIV associated neurocognitive disorder; HCV: hepatitis C virus; HIV: human immunodeficiency virus; SVD: small vessel disease.
Table 5.
Type 4: Inflammatory and immunologically mediated SVD.
| Risk factor | Changes in capillary morphology or blood rheology | Capillary dysfunction signs |
|---|---|---|
| ANCA-associated vasculitides: Granulomatosis with poly-angiitis (formerly Wegener’s) Churg-Strauss syndrome Microscopic polyangiitis | ANCAs cause neutrophil adhesion to capillary endothelium and trigger their release of toxic proteases and oxidants in close proximity to the endothelium, resulting in endothelial cell necrosis and increased capillary permeability.79,219 The diseases are dominated by capillaritis in the kidney, lung or heart, but fibrinoid necrosis of intracerebral small arteries, arterioles, capillaries and venules also occurs.26 | BOLD responses to fatiguing stimulus was attenuated in patients with granulomatosis with poly-angiitis compared to controls with similar degree of fatigue.225 |
| Henoch-Schönlein purpura | Arteriolar, capillary and venular interstitial infiltration by polymorphonuclear lymphocytes, eosinophils and mononuclear cells, with fibrinoid necrosis and perivascular granuloma formation.26 | |
| Cryoglobulinaemic vasculitis | Cryoglobulins, composed of IgG, IgM, complement, lipoprotein and antigenic moieties, precipitate and lead to hyperviscosity in serum excess.26 Intravascular activation of complement- and clotting cascades in arterioles and capillaries.26 | |
| Primary angiitis of the CNS | Affects medium-sized arteries, arterioles, capillaries and venules of the brain parenchyma and leptomeninges.220 Exists in a granulomatous form with frequent Aβ deposit, in a lymphocytic form with occasional vessel destruction, and a necrotizing form with transmural fibrinoid necrosis. Strokes and transient ischemic attacks occur in 30–50% of patients.220 | |
| Sneddon’s syndrome| (Livedo reticuaris) | Human skin: Skin discoloration caused by capillary stasis and thickening of the arteriolar wall.221 Proliferating capillary endothelial cells with luminal and abluminal protrusions.222 Human retina: Capillary occlusions/neovascularization,223 arteriolar occlusions.224 |
ANCA: antineutrophil cytoplasmic antibodies; Aβ: amyloid β; BOLD: blood oxygen level dependent; CNS: central nervous system; SVD: small vessel disease.
Table 6.
Type 4: Inflammatory and immunologically mediated SVD. Vasculitis caused by connective tissue disorders.
| Risk factor | Changes in capillary morphology or blood rheology | Capillary dysfunction signs |
|---|---|---|
| SLE | Increased capillary length, tortuosity, looping and hemorrhage on NCM.241 | Elevated task-active and task-negative BOLD responses compared to controls in childhood-onset SLE partients with little or no cognitive defects.104 |
| Systemic sclerosis (scleroderma) | Mega-capillaries and reduced capillary density by NCM is a strong predictor of the development of systemic sclerosis in patients with Raynaud’s phenomenon242. In brain, perivascular lymphocytic infiltrates243 and calcifications of arterial and arteriolar walls244 have been observed. The development of cerebral hypoperfusion243 parallels disease development as defined by NCM in some245 but not all246 patients. | Negative CBF response to pain in areas with pre-existing hypoperfusion/MRI lesions.141 |
| Dermatomyositis | Necrosis of capillary endothelium247 | Negative CBF response to pain in areas with pre-existing hypoperfusion/MRI lesions141. |
| Sjögren’s syndrome | ||
| Rheumatoid vasculitis | Rare complication of longstanding, severe rheumatoid arthritis. |
BOLD: blood oxygen level dependent; MRI: magnetic resonance imaging; NCM: nailfold capillary microscopy; SLE: systemic lupus erythematosus; SVD: small vessel disease.
Table 7.
Type 5: Venous collagenosis, and type 6: Other SVDs.
| Risk factor | Changes in capillary morphology or blood rheology | Capillary dysfunction signs |
|---|---|---|
| Post-radiation angiopathy | Post-radiation angiopathy is associated with capillary rarefaction and tissue hypoxia and thought to be the result of endothelial vulnerability to radiation.136,137 | Mineura et al.138 found hyperperfusion and low OEF early after radiation therapy in humans, and Hahn et al.139 demonstrated a dose-dependent CBF increase three months after irradiation, both consistent with elevated CTH caused by capillary endothelial damage. |
CTH: capillary transit time heterogeneity; OEF: oxygen extraction fraction; SVD: small vessel disease.
Pericyte dysfunction
Pericytes are embedded in layers of the basement membrane that surround the capillary endothelium.27 Pericytes are thought to cover most capillaries in the central nervous system where they regulate BBB function28 and aspects of the brain’s immune response.29 Pericytes are involved in the regulation of capillary development (angiogenesis), stabilization, maturation and remodeling30 and communicate with endothelial cells through peg-socket contacts as they – among other functions – jointly form and maintain the basement membrane.27 Neurogenic locus notch homolog protein 3 (NOTCH3) signalling is crucial for the postnatal differentiation of vascular cells into their ‘correct’ arterial, capillary and venous phenotypes27,31 and their ability to adapt to changes in pressure and vascular strain.32,33 While pericytes are characterized by their relation to microvessels, they share cellular and functional characteristics with vascular smooth muscle cells (VSMCs) encircling arterioles and venules. The distinction between VSMC and pericytes recently became a matter of debate with regards to the attribution of CBF-regulation19,34,35 – see discussion in the literature.36
Cerebral pericytes are contractile, and they have been shown to contract and relax in response to neuromediators, vasoactive drugs and, importantly, to sensory stimulation in brain slices as well as in vivo.18,19,35 Thus, cerebral pericytes have been shown to regulate capillary diameter during functional activation,18 dilating about 1 s before arteriolar dilation and thereby possibly controlling both CBF and CTH.19 Retinal pericytes have been characterized extensively in vitro: retinal pericytes constrict in response to high oxygen tension and relax in response to lactate and low pH,37 possibly providing a mechanism by which capillary flows can redistribute to meet local cellular metabolic demands during activation.37 Much like VSMCs, retinal pericytes react to a range of vasoactive substances, constricting when exposed to mechanical stretch, angiotensin II (AT2)38 and endothelin-1 (ET1)39 by a Ca++-dependent mechanism40 and relaxing when exposed to adenosine,41 ATP42 and nitric oxide (NO)43,44 and in response to cholinergic45 and adrenergic40 stimulation. See the study by Attwell et al.46 for a review of neurovascular coupling mechanisms and the control of VSMC and pericyte tone.
Pericyte loss and basement membrane thickening are observed in conditions that represent major risk factors for SVD, such as ageing, hypertension and diabetes – see Table 2. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL – Table 3) is associated with mutations in the NOTCH3 gene.47 The receptor protein encoded by the NOTCH3 gene is expressed in both VSMCs and pericytes,48 and recent evidence suggests that CADASIL is associated not only with degeneration and damage to VSMCs in small arteries and arterioles but also to microvascular pericytes. Dziewulska and Lewandowska49 observed pericyte loss and pericyte fragments within thickened basement membranes in skin and muscle biopsies in CADASIL. They reported that pericyte-endothelial peg-socket contacts were disrupted, seemingly giving rise to basement membrane thickening, endothelial swelling and protrusions into the capillary lumen.49 Recent animal models of CADASIL show evidence of reduced capillary density50 and pericyte degeneration51 and suggest that reduced pericyte coverage is related to impaired BBB function.52
When exposed to viral and bacterial proteins, pericytes initiate inflammatory responses that facilitate the recruitment of immune cells from the blood stream – see the study by Hill et al.53 for a recent review. As neutrophils pass through the surrounding basement membrane, they pass between embedded pericytes, which in turn remodel the laminin-rich basement membrane to permit extravasation.54 Importantly, pericytes seemingly change their phenotype during inflammation to become migratory,54 a phenomenon also observed in CNS injury.55 The ability of pericytes to undertake BBB function28 and capillary flow control19 may therefore be compromised during infection and inflammation.
Pericyte exposure to parenchymal waste: Amyloid and hemoglobin
Molecules the size of haemoglobin and amyloid β (Aβ) are cleared from the subarachnoid space and brain parenchyma along the basement membranes of arteries and capillaries.56,57 Indeed, impaired glymphatic clearance has recently emerged as a potential therapeutic target.58 Located in the layers of capillary basement membrane, pericytes are therefore exposed to amyloid during its perivascular removal and clearance across the BBB.59–61 Pericytes undergo degeneration when exposed to certain Aβ types in vitro,62,63 and although the role of pericytes in the pathogenesis of cerebral amyloid angiopathy (CAA) and Alzheimer’s disease (AD) remains poorly understood, evidence from Aβ transgenic mice and in vitro models suggest they may be involved in neurodegeneration.64,65 For recent reviews, see the study by Hamilton et al.66 and Winkler et al.67
Microbleeds are associated with SVD, and the vasoactive properties of hemoglobin breakdown-products are described in detail in the literature.68,69 Briefly, spontaneous autoxidation of oxyhemoglobin (HgbO) to methemoglobin and the iron released from hemoglobin cause the release of highly reactive superoxide radicals.68,69 Superoxides are thought to cause vasoconstriction by depleting vascular NO levels70,71 and to induce lipid peroxidation and peroxynitrite formation, which in turn cause vasoconstriction and structural damage to cerebral microvessels, including the endothelial cell layer.72 The breakdown of heme into bilirubin under such oxidative conditions results in the formation of bilirubin oxidation products (BOXes) that change the contractility, signalling and metabolism in large vessels – see also the study by Pyne-Geithman et al.73
Endothelial function
Endothelial cells are mechanically coupled by tight junctions, which ensure BBB integrity and prevent leakage of toxic molecules into the brain interstitium – see discussion in the literature.3,4 Endothelial cells are also electrically and metabolically coupled to each other as well as to nearby VSMCs via gap junctions composed of connexins.74 This rapid, bidirectional signalling pathway seemingly provides efficient coordination of vessel function across the microvascular bed.75–77 Disruption of the signalling between endothelial cells has been shown to cause profound breakdowns in vascular control across the capillary bed, resulting in extreme degrees of capillary shunting through the shortest arteriolo-venular pathways.78
In small-vessel vasculitides (Table 4) associated with antineutrophilic cytoplasmic antibodies (ANCAs), neutrophils adhere to capillary endothelial cells and cause the release of reactive oxygen species (ROS) and lysosomal enzymes. This abnormal inflammatory reaction causes endothelial cells to undergo necrosis and detach from the basement membrane, after which they can be found in peripheral blood.79 While the activation of neutrophils and endothelial cells in the capillary lumen may disturb capillary flow patterns in itself, the disruption of endothelial cell-to-cell signalling described above is expected to cause severe capillary dysfunction.
Glycocalyx dysfunction
The luminal surface of the capillary endothelium is covered by a 0.5-µm thick glycocalyx.80,81 This carbohydrate-rich matrix affects the passage of blood cells through the capillary bed.82 reducing capillary haematocrit to 20–50% of that found in the systemic circulation. Glycocalyx damage, in turn, disrupts capillary flow regulation, causing capillary hematocrit to approach that of the systemic circulation.83,84 The glycocalyx constitutes a fluid barrier in the vascular system and has been implicated in oedema formation.85,86 The glycocalyx is degraded by direct oxidative stress and exposure to oxidized lipoproteins,83,87,88 by hyperglycaemia89 and by ischemia.88,90
Blood viscosity
The dimensions of white blood cells (WBC) and erythrocytes exceed the average capillary diameter. Experimental studies have shown that capillary flow patterns are sensitive to the size, deformability, viscosity, number and endothelial adhesion of blood cells. In infections and low-grade vascular inflammation, blood is hence increasingly redirected to thoroughfare channels which act as functional shunts.91 In a classical study, hyperviscosity was demonstrated in diabetic patients (Table 2) compared to age-matched controls and the viscosity found to correlate with the extent of microvascular diabetic complications. Accordingly, erythrocyte deformability was lower in those diabetic patients who had the most extensive microangiopathy compared to either diabetics with no complications or to controls.92
In cryoglobulinaemic vasculitis (Table 5), cryoglobulins precipitate and lead to hyperviscosity.26 Hematocrit is lower in capillaries than in the systemic circulation, and since cryoglobulins precipitate more easily under conditions of serum excess, capillary flows may be particularly sensitive to this phenomenon.
Signs of capillary dysfunction
The previous section suggests that capillary morphology and function may be disturbed in conditions considered risk factors for SVD. Neuropathological data and direct in vivo observations of the microcirculation in these conditions are sparse, however, and it remains unclear whether capillary dysfunction might antedate changes in the morphology and function of upstream arteries and arterioles.
The neurovascular coupling mechanisms,93 which control local CBF according to the metabolic needs of tissue, are expected to account for the oxygen extraction efficacy downstream, including changes related to capillary dysfunction. Below we discuss how CBF has to be adjusted in order to compensate for the reduced oxygen extraction efficacy that accompanies various degrees of capillary dysfunction. Some CBF changes are highly suggestive of capillary flow disturbances as opposed to a flow-limiting pathology, and neurovascular coupling studies may therefore serve as an indirect means of addressing the role of capillary dysfunction in the evolution of SVD from its risk factors. These changes are listed in the right-most column in Tables 2–7.
We used Web of Science™ and PubMed to search for occurrences of the terms listed in the leftmost column of Tables 2–7 in combination with ‘CBF’, ‘blood-oxygen-level-dependent (BOLD)’, ‘oxygen’, ‘metabolism’, ‘functional hyperemia’, ‘vasoreactivity’ and ‘stroke’. Literature searches for column 3 in Tables 2–7 were conducted concurrently with those for column 2 (previous section).
Mild capillary dysfunction: CBF increases in conditions that represent risk factors for SVD
For mild capillary disturbances, the reduction in oxygen extraction efficacy is so small that it can be compensated for by elevated CBF. Observations of increased resting CBF or increased CBF responses early in the course of SVD precursors therefore suggest that capillary dysfunction (elevated CTH), rather than primary changes in the morphology or function of upstream arterioles, is involved in the early etiology of the condition. Meanwhile, the BOLD signal is often used to localize brain activity through its sensitivity to tissue deoxyhemoglobin concentrations [dHgb]. Mild capillary dysfunction is characterized by reduced OEF and proportionately higher CBF responses during functional activations, both of which reduce [dHgb] and thereby increase BOLD signal amplitudes before resting CBF and OEF become affected. In asymptomatic subjects presented with identical tasks, BOLD responses are therefore expected to be higher in those with mild capillary dysfunction than in controls, despite identical changes in metabolic activity. CBF and BOLD responses are recorded in grey matter and are hence sensitive to changes in capillary function in the cortex and subcortical nuclei. Subcortical lesions may result in secondary changes in cortical function, and, in theory, even compensatory hyperactivity in some regions.
In streptozotocin (STZ)-induced diabetes (Table 1) in rats, both total and cortical CBF values are indeed elevated compared to control animals early after induction of the disease,94–96 and cortical oxygen tension elevated.96 In early-stage hypertension (Table 2), elevated CBF and BOLD responses to hypercapnia have been reported in spontaneously hypertensive rats compared to age matched control animals.97 The apolipoprotein (APOE) ɛ4 allele is associated with both CAA and the development of AD98 (Table 2), and carriers of this allele have therefore been studied extensively. In asymptomatic APOE ɛ4 carriers aged 19–28, both resting- and activity-related CBF levels are elevated,99,100 and BOLD signal changes during memory encoding tasks are elevated in the asymptomatic APOE ɛ4 carriers,101–103 consistent with reduced OEF and compensatory hyperaemia. Similarly, BOLD responses are elevated in patients with systemic lupus erythematosus (SLE – Table 6) but little or no cognitive defects, compared to controls.104 In asymptomatic human immunodeficiency virus 1 (HIV-1) infected patients (Table 5), elevated BOLD signals can be observed105 in proportion to signs of glial activation.106 The notion that viral replication in brain parenchyma is associated with mild capillary dysfunction is consistent with findings that BOLD signal amplitudes are elevated in seropositive patients with low BBB penetration of combination antiretroviral therapy, but comparable to those found in controls and in patients with high penetrance and thereby virological control.107 It should be noted that relative BOLD signal changes depend on both resting and activation-related CBF and OEF levels. The interpretation of such changes in terms of the underlying microvascular pathology therefore requires detailed analysis of the underlying physiology and magnetic resonance signal mechanisms.17,108
Table 1.
Abbreviations.
| Aβ | Amyloid β |
| AD | Alzheimer’s disease |
| ANCA | Antineutrophilic cytoplasmic antibody |
| APOE | Apolipoprotein |
| AT2 | Angiotensin II |
| ATP | Adenoside triphosphate |
| BBB | Blood–brain barrier |
| BOLD | Blood oxygen level dependent |
| CAA | Cerebral amyloid angiopathy |
| CADASIL | Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy |
| CBF | Cerebral blood flow |
| CMRO2 | Cerebral metabolic rate of oxygen |
| CNS | Central nervous system |
| CTC | Concentration–time curve |
| CTH | Capillary transit-time heterogeneity |
| CTHmax | Maximum capillary transit-time heterogeneity |
| [dHgb] | Deoxyhemoglobin concentration |
| eNOS | endothelial nitric oxide synthase |
| FLAIR | Fluid attenuated inversion recovery |
| HCV | Hepatitis C virus |
| HIV-1 | Human immunodeficiency Virus 1 |
| MD | Mediterranean diet |
| MELAS | Mitochondrial encephalopathy with lactic acidosis and stroke-like episodes |
| MRI | Magnetic resonance imaging |
| MTT | Mean transit time |
| NCM | Nailfold capillary microscopy |
| NIHSS | National Institute of Health Stroke Scale |
| NO | Nitric oxide |
| NOTCH3 | Neurogenic locus notch homolog protein 3 |
| OEF | Oxygen extraction fraction |
| PDE | Phosphodisterease |
| PWI | Perfusion weighted imaging |
| ROS | Reactive oxygen species |
| STZ | Streptozotocin |
| SVD | Cerebral small vessel disease |
| VSMC | Vascular smooth muscle cell |
Moderate capillary dysfunction: Transition from hyperperfusion to CBF suppression
If capillary flow disturbances become more severe, then hyperemia fails as a means to compensate for reduced oxygen extraction efficacy. Instead, CBF responses – and ultimately resting CBF – must be attenuated in order to limit functional shunting. The transition from mild to moderate capillary dysfunction is therefore predicted to require dramatic, yet characteristic changes in CBF in order to maintain flow-metabolism coupling.
Such a transition, from hyper- to hypoperfusion, was indeed observed in a follow-up study of asymptomatic APOE ɛ4 carriers and controls.109 At the time of their initial examination, APOE ɛ4 carriers showed higher resting CBF values in vulnerable brain regions than did control subjects, while CBF reductions in these regions 8 years later were significantly larger in APOE ɛ4 carriers than in controls.109
If flow responses are limited by a physical, flow-limiting condition, then CBF cannot increase beyond a certain upper limit, irrespective of the metabolic requirements of brain tissue. If CBF suppression instead serves to maintain flow-metabolism coupling, then CBF, tissue metabolism and the extent of capillary dysfunction determine whether flow suppression is necessary. We briefly discuss how this phenomenon might have revealed itself in studies of SVD and its precursor states.
In a mouse genetic model of CADASIL, Joutel et al.50 examined CBF responses to functional activation, CO2 inhalation and reduced perfusion pressure in 5- to 6-month-old animals, at a time-point where no changes in arterial structure or signs of BBB breakdown could be observed. Animals with and without the CADASIL-causing NOTCH3 point-mutation had similar blood pressure, similar resting CBF and similar CBF responses to CO2 inhalation, a vasodilatory stimulus. CBF responses to functional activation, however, were attenuated in CADASIL mice.50 These findings are consistent with capillary dysfunction that only limits tissue oxygenation during functional activation – either by deficient pericyte-mediated capillary dilation (and CTH reduction) during functional activation,19 or because only the combination of elevated CBF and increased metabolic demands ‘unmasked’ capillary dysfunction at this early point in the development of characteristic disease signs.
In asymptomatic APOE ɛ4 carriers aged 50–65, elevated resting CBF values have been reported at a time where their CBF- and BOLD-responses to functional activation had been suppressed.110 Suppression of CBF-responses to sensory stimulation at a time where resting CBF is elevated has also been observed in spontaneously hypertensive rats, prior to any changes in their microvasculature.111 These findings are again consistent with the gradual transition from mild to moderate capillary dysfunction which first becomes apparent in states of high metabolic demand.
The unmasking of capillary dysfunction by combinations of high CBF and CTH that necessitate flow suppression was recently illustrated in a study by Suri et al.112 who observed flow suppression during hypercapnia (a strong vasodilator) in young APOE ɛ4 carriers compared to noncarriers. These young APOE ɛ4 carriers showed increased hippocampal BOLD responses to memory tasks, and the attenuation of their CBF responses during hypercapnia accounted for 70% of this increase,112 consistent with the prediction that mild (asymptomatic) capillary dysfunction links the two findings.
Attenuation of functional hyperemia can also be observed immediately after administration of AT2 and Aβ in animal models of hypertension and AD/amyloidosis, respectively (Table 2)113–115 and antedates any morphological changes in the vessel wall or brain parenchyma and even the development of high blood pressure in the model of hypertension.116 Although the effects of AT2 and Aβ on pericyte tone in living brain remain to be studied, these findings are consistent with capillary dysfunction caused by pericyte constrictions and compensatory increase in VSMC tone to limit flow responses.
Flow suppression and small vessel changes
The flow suppression observed in animal models of hypertension and AD/amyloidosis is caused by vascular production of ROS,117,118 which reacts with NO to form peroxynitrite.119 Both NO depletion and peroxynitrite cause vasoconstriction by impairing normal smooth muscle cell relaxation,120 but also long-term remodelling and thickening of the vessel walls and VSMC damage.121 By evoking flow suppression, capillary dysfunction may therefore contribute to – or even antedate – the wall damage observed in small arteries and arterioles before SVD becomes overt. Note that wall thickening narrows the vascular lumen and may reduce CBF and attenuate CBF responses. This ‘mechanical’ flow suppression would therefore be expected to make flow suppression by oxidative stress superfluous over time.
Oxygen diffuses through arteriolar walls to supply the capillary-free area around arterioles, and it can reach tissue with capillary supply as well. In fact, recent studies suggest that arterioles account for as much as 50% of the total oxygen extraction during rest, while capillaries serve as the primary site of oxygen extraction during functional hyperemia.122 Arteriolar oxygen extraction is insensitive to CTH downstream, and the increased arteriolar tortuosity and length observed in some SVD precursor states123 may therefore, paradoxically, increase arteriolar oxygen extraction capacity by increasing arteriolar surface area and transit time. The long, tortuous medullary arteries observed in SVD, however, are generally thought to cause hemodynamic insufficiency. Studies of retinal vessels in SVD patients suggest that venules are generally wide in SVD precursor states.124,125 While the restricted venular lumen observed in venous collagenosis123 (Table 7) would be expected to facilitate tissue oxygen extraction by prolonging blood’s transit time through the capillary bed, complete venous occlusions, instead, cause venous infarcts to develop.
Moderate capillary dysfunction: Failure to suppress CBF
If the microcirculation cannot evoke intrinsic mechanisms to limit CBF and CBF responses, then functional shunting can become so severe that stroke-like symptoms and hypoxic tissue injury may develop at CBF values well above the classical ischemic threshold.
Fabry’s Disease (Table 3) is associated with cerebral hyperperfusion,126–128 which is reversed or attenuated by therapies that remove the capillary deposits shown in Figure 2.126,127 This hyperperfusion might, in principle, represent flow metabolism coupling, with hyperaemia compensating for mild capillary dysfunction. The relative hyperemia is, however, observed in patients with severe neurological symptoms127 and seemingly antedates the development of white matter lesions that may occur in the disease,129 suggesting that tissue damage is the result of insufficient suppression of excessive vasodilation and functional shunting in this disease.
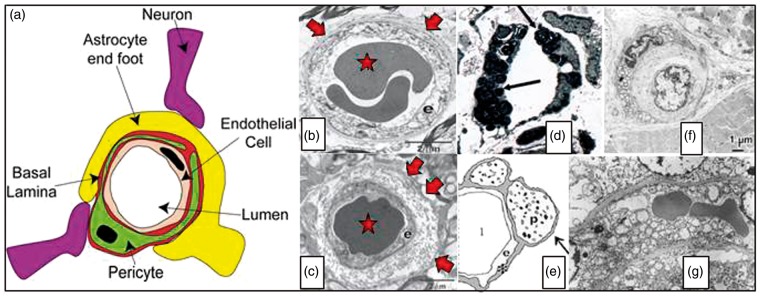
Figure 2.
Panel (a) illustrates the organization of endothelial cells, basement membrane and pericytes in the vessel wall. Capillaries are ensheathed by astrocytic endfeet, and neuronal terminals are closely apposed to capillaries and pericytes.66 Source: Reproduced from Hamilton et al.66 according to the Creative Commons terms. Panel (b) shows a cross section of normal capillary with a thin basement membrane (arrow) and normal appearing endothelial cell (e). In ageing (Panel (c)), thickened basement membranes (arrows), pericapillary fibrosis and pericyte loss are often found. Source: Panels (b) and (c) are reproduced from Farkas et al.188 Panel (d) shows a capillary cross section from the skin of a patient with Fabry’s disease. Note the lamellar sphingolipid inclusions in the capillary endothelium (arrow). These inclusions disappear upon enzyme replacement therapy. Source: Reproduced from Eng et al.189 Panel (e) shows typical cerebral capillary wall pathology in human AD. The arrow indicates pericyte degeneration. The symbols denote lumen (l), endothelial cell (e), basement membrane (*) and pericyte (p). Source: Reproduced from Farkas et al.190 Panel (f) shows a cross section of a muscle capillary from a patient with MELAS. Note the thickened basement membrane and increased number and size of mitochondria in the pericyte. Source: Reproduced from Sakuta and Nonaka.191 Panel (g) shows a cross section of a cerebral capillary from the motor cortex of a MELAS patient with accumulation of mitochondria in the endothelial cell. Source: Reproduced from Ohama et al.192 with permission from the publisher. AD: Alzheimer’s disease; MELAS: mitochondrial encephalopathy with lactic acidosis and stroke-like episodes.
Mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS – Table 3) is also associated with severe hyperperfusion, which seemingly contributes to tissue infarction. Studies have revealed globally elevated CBF, low OEF and reduced cerebral metabolic rate of oxygen (CMRO2) in MELAS patients both before130–133 and after131,132,134 stroke. In these patients, the mitochondrial enzymes needed for oxidative phosphorylation are deficient, and it is therefore unclear how tissue levels of oxygen, ATP and lactate affect neurovascular coupling. The lack of metabolic feedback mechanisms to limit excessive vasodilation in this condition is underscored by the finding that normal vasodilation is preserved, even in hyperperfused stroke lesions.135
Vascular endothelium is extremely vulnerable to radiation and post-radiation angiopathy (Table 7) is associated with capillary rarefaction and tissue hypoxia.136,137 Mineura et al.138 found hyperperfusion and low OEF early after radiation therapy in humans, and Hahn et al.139 demonstrated a dose-dependent CBF increase three months after irradiation. If this hyperaemia was a compensatory response to mild capillary dysfunction, then tissue oxygen tension would be elevated rather than reduced and hyperaemia limited by flow-metabolism coupling rather than radiation dose. Instead, these observations suggest that microvascular signalling is severely disturbed, possibly due to a loss of endothelial regulatory signalling. After radiation exposure, initial hyperperfusion is often followed by normalization of CBF,138 but OEF remains low,138 indicating reduced tissue metabolism as a result of tissue damage. Hahn et al.139 observed that neuropsychological performance deteriorated when the dose-dependent CBF increase three months after irradiation was followed by a reduction in CBF by six months. These findings suggest that uncontrolled hyperemia contributes to on-going tissue damage at least three months after radiation, but also lends hope to the notion that dose fractionation might ameliorate tissue damage and cognitive decline.
Severe capillary dysfunction
In severe capillary dysfunction, oxygen availability gradually approaches the metabolic needs of brain tissue. The parallel reduction in tissue oxygen tension, in turn, is highly conducive to BBB breakdown, Aβ deposition and loss of trophic support. See literature7,140 for a discussion of capillary dysfunction in dementia and preliminary data.
If capillary changes become so severe that CTH approaches CTHmax, then even increases in viscosity might reduce tissue oxygenation enough to trigger neurological symptoms or tissue injury. This suggests a mechanism by which dehydration or bacterial infections (during which WBC count is elevated) can cause neurological deterioration or stroke-like symptoms in patients with preexisting, capillary dysfunction.6 We discuss therapeutic implications of this pathophysiological process below.
In severe capillary dysfunction, resting CBF is predicted to approach the classical ischemic threshold at which oxygen extraction is optimal, even for high CTH values. This biophysical feature implies that, paradoxically, tissue oxygenation may be improved to meet the metabolic demands of functional activation by reducing CBF to reduce physiological shunting. Indeed, Ferraccioli et al.141 observed inverted CBF responses to painful stimuli (Raynaud’s phenomenon) in the sensorimotor cortex of patients with scleroderma or SLE vasculitis (Table 6).
What determines the course of tissue damage in SVD?
The analysis presented here suggests that progressive capillary dysfunction inevitably leads to tissue damage – either gradually, by loss of trophic support, amyloid pathology, hypoxic injury or inflow of toxic substances through a failing BBB – or suddenly, in relation to stroke-like episodes. The precise cause of the small subcortical infarcts, lacunes, white matter hyperintensities, microbleeds and cortical atrophy that accompany SVD has been the subject of intense scrutiny – see recent work by Wardlaw et al.3,4,142
Hassan et al.143 showed that the intron 4ab polymorphism of the endothelial NO synthase (eNOS) gene protects against the development of SVD in the form of symptomatic, small subcortical infarctions, but not of SVD with white matter hyperintensities, suggesting that insufficiency of NO may be associated with the development of small subcortical infarctions. Similarly, the intron 4aa genotype appears to be protective for lacunar infarctions.144 In mammals, dietary nitrite is a major source of NO,145 and eNOS may be involved in the conversion of nitrate to NO in tissue.146 The finding that blood nitrite levels depend on endothelial nitric oxide gene haplotype143 may indicate that interactions between genotype and dietary habits (see below) is one of many factors which determine whether SVD progresses along a ‘stroke-like’ clinical presentation or along one of white matter hyperintensities and cognitive decline.
Cognitive decline or stroke-like phenotype?
Both capillary dysfunction and arteriolar narrowing can lead to hypoxic tissue injury – but what determines whether a SVD precursor state develops along a ‘dementia-like’7 or a ‘stroke-like’6 pathway? Although ‘mixed’ cases exist, chronic spirochete infections by Treponema pallidum and Borrelia burgdorferi both come in a meningovascular form, which is dominated by lymphoplasmacytic infiltrates and intima-proliferation in the walls of leptomeningeal arteries, veins and vasa vasorum and associated with the development of multiple cerebral infarcts, that is, a stroke-like phenotype. In their parenchymal form, spirochetes invade brain tissue to aggregate around microvessels, and particularly capillaries.147 In this form, the infection is instead associated with cortical atrophy and dementia – and often amyloid deposits and neurofibrillary tangles. In severe neurosyphillis, antibacterial treatment in some cases leads to significant increases in CMRO2 without increases in CBF from its near-ischemic levels.147 Such an ‘isolated’ improvement of OEF and cerebral metabolism is difficult to reconcile with flow-limiting SVD, or the reversal of infection-specific suppression of neuronal function.147 We speculate that the pericapillary invasion of spirochetes may be associated with severe capillary dysfunction, causing CTH to exceed CTHmax in general paresis. Reduction of capillary flow disturbances by antibiotic therapy would then be expected to improve CMRO2 and neurological function, but only to normalize CBF if CTH was reduced considerably. In patients with neuroborreliosis and less severe neurological symptoms, cerebral hypoperfusion is partially reversed by antibiotic therapy.148
Similar correlates between microvascular histopathology and the progression along pathways dominated by stroke-like symptoms or cognitive decline may exist for other SVD risk factors and help elucidate what separates these etiologically related, but clinically separate, presentations.
SVD risk factors versus comorbidities
Many regard hypertension as the single strongest risk factor for SVD, but not all patients with SVD have hypertension or diabetes, and it has been debated whether hypertension might be secondary to impaired cerebral perfusion. Here we briefly discuss whether hypertension and type-2 diabetes are risk factors for SVD – or whether they themselves reflect evolving capillary dysfunction.
We argued above that the attenuation of functional hyperemia observed after administration of AT2 – a likely source of capillary dysfunction38 – may represent flow suppression to maintain flow-metabolism coupling in brain tissue. This phenomenon was shown to antedate the development of high blood pressure in AT2 animal models of hypertension.116 Is it conceivable that hypertension itself represents a systemic response to maintain tissue oxygenation in certain organs as their level of capillary dysfunction reaches the thresholds at which flow-suppression becomes necessary? If so, which organs? Dickinson proposed that essential hypertension serves to overcome increased vascular resistance in the brain’s large, feeding vessels.149 While studying large vessel resistance in hypertension, however, he points out that histopathological studies show early changes in capillaries and veins, rather than small arteries and arterioles, in brain tissue from hypertensive patients.149,150 These observations are consistent with capillary changes as a primary event in hypertension and with the notion that capillary dysfunction require proximal adjustments of vascular resistance to preserve brain oxygenation.
If hypertension represents an early sign of capillary dysfunction, either in brain or other in other organs, then antihypertensive therapy may be warranted at an early stage in patients at risk of SVD.151 Further studies should address whether the antihypertensive effects of AT2 inhibitors, angiotensin converting enzyme (ACE) inhibitors and Ca++ channel blockers are linked to inhibition of AT2 and Ca++-dependent pericyte constriction. Specific antihypertensive agents may also prove useful in targeting organ- and cell-specific aspects of capillary dysfunction.
Measurements of glucose-analogue extraction in the human brain suggest that capillary transit time heterogeneity limits glucose extraction.152 Although glucose extraction differs somewhat from that of oxygen,153 our analysis suggests that capillary dysfunction also limits the clearance of glucose from blood.5,154 In prediabetic rats, impaired glucose tolerance is indeed accompanied by reduced oxygen extraction efficacy,155 and the beneficial effects of Mediterranian Diet (MD) (see discussion below) extend to both ambulatory blood pressure and blood glucose levels.156 Our preliminary analysis indicates that capillary dysfunction favours the extraction of glucose over that of oxygen.157 As capillary dysfunction becomes severe, tissue is therefore predicted to reveal some degree of aerobic glycolysis.157 This property may contribute to findings of reduced respiratory coefficient in patients with severe hypertension.149
Therapeutic implications
Despite the heterogeneity and complexity of the conditions known to contribute to the development of SVD and to subsequent stroke or cognitive decline, we speculate that capillary flow disturbances may be a shared feature of some if not most of these conditions. Below, we therefore discuss aspects of capillary dysfunction that can be prevented or alleviated and therefore might be of general benefit to patients at risk of SVD, stroke or cognitive decline.
Blood viscosity
Dehydration and infection-induced leukocytosis increase blood viscosity. These common occurrences can therefore reduce the brain’s oxygen supply by increasing CTH. Influenza vaccinations are offered to the elderly in order to reduce the incidence of influenza and secondary infections, and this is known to reduce the number of stroke deaths.158–160 Infections may trigger thromboembolism by destabilizing atheromatous plaques in the walls of cerebral vessels, but we speculate that increases in blood viscosity may elicit stroke-like symptoms in patients with preexisting, severe capillary dysfunction as well. Therefore, influenza vaccinations may be beneficial for SVD patients, irrespective of age. The impact of bacterial infections on cerebral oxygenation and thereby cognition may also be reflected in the observation that eradication of chronic helicobacter pylori infections in AD patients dramatically improves their cognitive scores and overall survival.161,162
CTH may be reduced by lowering blood viscosity, and aggressive management of hyperlipidaemia may therefore be warranted in SVD patients. Similarly, high homocysteine levels increase blood viscosity, increase the adhesion of monocytes to the capillary wall and cause oxidation of low-density lipoproteins163 and could therefore represent a source of capillary dysfunction. Indeed, Hassan et al.164 showed that elevated homocysteine levels represent an independent risk factor of SVD. A meta-analysis has suggested that homocysteine-lowering therapy may reduce stroke risk in regions where folate levels are low.165
Phosphodiesterase (PDE) inhibitors reduce platelet aggregation,166 decrease blood viscosity167 and increase the flexibility of erythrocytes.167 While reduced platelet aggregation might increase bleeding risk, the haemorheologic PDE inhibitor effects would be expected to reduce CTH and thereby improve tissue oxygenation. For a recent review of pharmacological approaches to SVD management, see literature.168
Nicotine up-regulates the expression of adhesion molecules in the capillary endothelium169 and increases leukocyte rolling,170 keeping in mind that the latter is observed mainly in post-capillary venules where selectin density and glycocalyx properties provide optimal adhesion for leukocyte recruitment.171 In additions to nicotine’s effects on larger vessels and large vessel atheromatous plaques, smoking would therefore be expected to worsen capillary flow disturbances, to accelerate the development of SVD from its risk factors and to increase the risk of an ischemia-like event. Indeed, pack years of smoking are associated with an increased risk of stroke in CADASIL172 and with a higher burden of SVD lesions in patients with sporadic lacunar stroke.173 Patients with SVD precursor states should therefore receive help to reduce not only smoking, but also nicotine consumption in general, in order to reduce their risk of later cognitive decline or stroke.
Hyperaemia, anaemia and poor oxygen saturation
Hyperaemia is predicted to be harmful in patients with capillary dysfunction.
Obstructive sleep apnea (OSA) is associated with periods of severe nocturnal hypercapnia and hypoxemia, both of which cause dramatic increases in CBF in the normal brain. In addition, reductions in oxygen saturation cause a proportionate reduction in the net oxygen metabolism that can be supported for a given level of capillary dysfunction – increasing the risk of neurological symptoms. The risk that hyperaemia poses to patients who suffer from capillary dysfunction may in part be reflected in the observation that continuous positive airway pressure (CPAP) treatment reduces the incidence of strokes.174
The vulnerability of patients with capillary dysfunction to increased blood viscosity and reduced arterial oxygen content may contribute to the strong association between delirium and dementia:175 declining haemoglobin levels, dehydration and minor infections are known to herald delirium in the elderly. In patients with severe capillary dysfunction, small reductions in haemoglobin concentration or blood saturation, as well as increases in blood viscosity, may trigger regional cerebral hypoxia and thus, in principle, contribute to delirium symptoms. For patients with SVD precursor states, stricter definitions of anaemia and special attention to pulmonary function may therefore be warranted to reduce the risk of delirium and to alleviate the long-term consequences of chronic brain tissue hypoxia.
NO depletion
Capillary NO depletion due to oxidative stress and tissue hypoxia may represent a modifiable aspect of capillary dysfunction. Mediterranean diet is rich in green-leafed vegetables, a major source of dietary nitrate (see above). One might expect this diet to offer some protection towards NO depletion in patients with capillary dysfunction, and hence the development of SVD pathology. Preference for MD is indeed associated with a lower burden of white matter hyperintensities176 and, more generally, with a lower risk of ischemic stroke.177,178 Keeping in mind that this protective effect may be attributable to other MD constituents, these observations may warrant further studies of dietary interventions in SVD and its risk factors. See also the study by Bath and Wardlaw.168
Stroke management in SVD patients
Given the deleterious effects of dehydration and poor saturation on tissue oxygenation, pre-hospital rehydration and efforts to reach full blood saturation may be warranted in patients with capillary dysfunction. While recanalization therapy clearly limits infarct size if large vessel occlusion is the cause of tissue hypoxia, it is important to keep in mind that means of restoring capillary flow patterns should also be explored.19,24,179 Since SVD risk factors are generally prevalent in the stroke population irrespective of mechanisms of individual events, these interventions may be of general benefit.
Diagnostic considerations
Flow-limiting conditions and moderate capillary dysfunction are both predicted to reduce cerebrovascular reserve capacity and increase OEF. We showed recently that elevated CTH can contribute significantly to the elevated OEF observed in patients with carotid stenosis140 and proposed that concurrent capillary dysfunction may contribute to the non-superiority of bypass surgery over aggressive cardiovascular risk factor management for stroke prevention in patients with severe carotid disease.180 Assessment of CTH and thus the ‘capillary contribution’ to elevated OEF may therefore be warranted when revascularization therapy is considered in patients with carotid stenosis and SVD progenitor states such as diabetes and hypertension.
The detection of capillary dysfunction may also prove important in the management of acute stroke in SVD patients. In acute ischemic stroke, perfusion weighted imaging (PWI) is widely used in the identification of salvageable tissue prior to recanalization therapy. Based on dynamic computerized tomography (CT) or magnetic resonance imaging (MRI) during bolus injection of an intravascular contrast agent, concentration–time curves (CTC) can be estimated in each image voxel and corrected for arterial contrast profile in each patient. The resulting transit time metrics, such as the mean transit time (MTT) and time-to-maximum (Tmax), can then be compared to thresholds above or below which literature studies have shown high likelihood of infarction in the absence of recanalization. Such studies of the relation between perfusion metrics and tissue outcome have found inconsistent transit time thresholds.181 We speculate that this finding relates to the fact that tissue outcome depends on the extent of capillary dysfunction in ‘hypoperfused’ tissue: first, the tissue CTC recorded during PWI depends on both CBF and CTH, but the PWI algorithms used in studies so far cannot disentangle the two.182 The success of current recanalization therapies, however, clearly hinges on whether hypoperfusion is the result of a vascular occlusion, exacerbated capillary dysfunction, or both. Differences in the proportion of stroke subtypes and the incidental dependence of preferred transit time metric on oxygen extraction efficacy6 may therefore explain the difficulty in establishing ‘universal’ transit time thresholds to define tissue-at-risk in acute stroke patients from cohort studies.
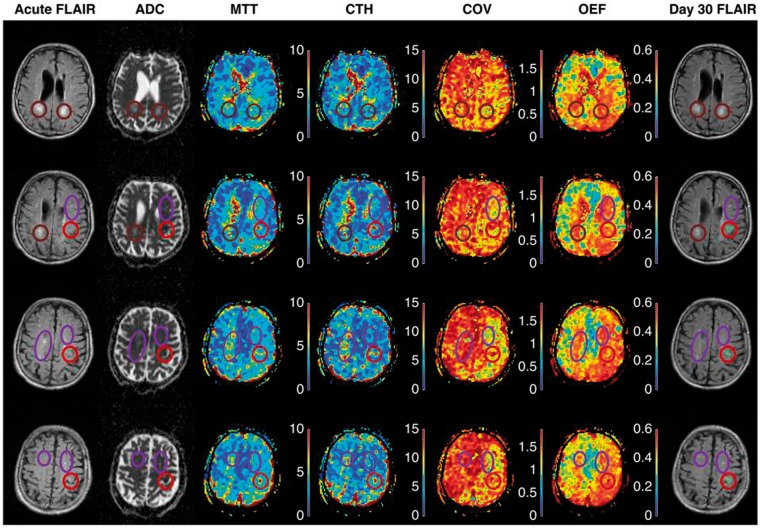
Figure 3 shows acute PWI maps from an 84-year-old man with a history of hypertension and smoking who presented with mild (NIHSS 4) stroke symptoms. We note that MTT was prolonged and OEF predicted to be high in relation to tissue that subsequently went on to infarction (red circle). Prolonged MTT and high OEF are indeed central characteristic of penumbral tissue,22 yet areas with white matter hyperintensities showed similar changes in this patient. Our preliminary data suggest that knowledge of CTH is necessary to predict OEF as obtained by positron emission tomography (PET).140 Needless to say, further studies are needed to disentangle the effects of limited flow (low CBF) and capillary dysfunction (high CTH relative to MTT) on oxygen extraction in SVD and SVD-related strokes.
Figure 3.
The two leftmost columns in Figure 3 show acute FLAIR and ADC at four identical slice-positions in an 84-year-old patient who presented with acute stroke symptoms three hours earlier. Acute ischemic changes are visible as areas of low ADC (red circles), consistent with reduced extracellular water diffusion and often ascribed to anoxic depolarizations. The FLAIR images show discrete (purple ellipses) and confluent (brown circles) white matter hyperintensities. The rightmost column show FLAIR images in the same slice positions 30 days later. The green overlays on bright tissue lesions (within the red circles) indicate tissue that infarcted in relation to the stroke episode. Note that areas of elevated CTH and MTT are observed in relation to the area of low ADC.
The COV is relatively independent of CBF in normal microvascular network, and this map therefore helps visualize areas where CTH are higher or lower than expected.17 Note that COV is elevated in the tissue areas with elevated ADC, indicating that microvascular flow patterns are disturbed beyond what would be expected based on reduced CBF alone. It should be kept in mind that PWI is sensitive to the tracer retention in a large tissue volume, in which small arteries/arterioles, capillaries and venules/small veins each take up roughly one-third of the blood volume. The gradient-echo pulse sequence used in this study is equally sensitive to tracer in these vessels, irrespective of their size, while PWI by spin-echo MRI is weighted towards capillary-size vessels.193 Our preliminary experience shows that disease may alter COV, as determined by gradient- and spin-echo PWI, respectively, in opposing directions (results not shown). We speculate that areas of reduced COV in this patient may reflect that flow through small arteries and arterioles become more uniform as their walls undergo morphological changes in chronic SVD. The OEF as determined by our biophysical model5 is also shown. Widespread areas of elevated white matter OEF are noted, especially in the hemisphere affected by the stroke. Detailed studies of well-characterized SVD patients are clearly needed to understand how changes in capillary morphology and local tissue oedema (elevated ADC) affect CTH values determined by PWI methods. ADC: apparent diffusion coefficient; CBF: cerebral blood flow; MTT: Mean Transit Time; COV: CTH/MTT ratio; CTH: capillary transit-time heterogeneity; FLAIR: fluid attenuated inversion recovery; MRI: magnetic resonance imaging; OEF: oxygen extraction fraction; PWI: perfusion weighted imaging; SVD: small vessel disease.
Conclusion
The morphology and function of cerebral capillaries in conditions considered risk factors for SVD remains relatively understudied. This review suggests that capillary dysfunction may be an early and shared feature of SVD risk factors, and a source of neurodegeneration, stroke and cognitive decline, despite considerable differences in the aetiologies and clinical presentations of these syndromes. We propose that the study of parallel changes in capillary and arteriolar morphology and function may represent an important area of preclinical SVD research. Except for studies in animal models of hypertension, diabetes and CADASIL and in human APOE-ɛ4 carriers, we identified few reports of neurovascular coupling in the early or presymptomatic phase of SVD risk factors. Given that altered neurovascular coupling may reveal capillary dysfunction before symptoms are predicted to arise, studies using BOLD contrast or CBF-sensitive methods might provide new insights into the aetiopathogenesis of SVD. Similarly, direct measurements of CTH as an index of capillary dysfunction should be applied to SVD risk factors to test the sensitivity of PWI as an investigative or diagnostic tool.
In this review, the pericyte emerged as a critical determinant of several aspects of capillary function. Recent breakthroughs in the understanding of this cell19,24,64,67,183–185 have already contributed to our understanding of neurodegeneration and stroke. Studies in stroke179 and diabetic retinopathy186 lend hope to the notion that pericytes and other components of the neurovascular unit that are affected in SVD may represent targets in future efforts to prevent capillary dysfunction.
Acknowledgments
The authors wish to thank Marie-Germaine Bousser, Anne Joutel, Richard Buxton, David Brooks, Simon Fristed Eskildsen, Rikke Beese Dalby, Kim Mouridsen, and Sune Nørhøj Jespersen for inspiration and helpful comments to our manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: LØ, TSE and MBH were supported by the Danish National Research Foundation (CFIN), The Danish Ministry of Science, Innovation, and Education (MINDLab) and the VELUX Foundation (ARCADIA). HSM is supported by an NIHR Senior Investigator award and by the Cambridge University Hospitals NIHR Comprehensive Biomedical Research Centre. FM was supported by a Chief Scientist Office of Scotland project grant. JW was supported by the Scottish Funding Council and Chief Scientist Office through the Scottish Imaging Network A Platform for Scientific Excellence (SINAPSE), the Wellcome Trust and the Medical Research Council through the Centre for Cognitive Ageing and Cognitive Epidemiology (CCACE).
Declaration of conflicting interests
MBH owns stock in COMBAT Stroke.
References
- 1.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010; 9: 689–701. [DOI] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wardlaw JM, Doubal FN, Valdes-Hernandez M, et al. Blood–brain barrier permeability and long-term clinical and imaging outcomes in cerebral small vessel disease. Stroke 2013; 44: 525–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013; 12: 483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jespersen SN, Østergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab 2012; 32: 264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Østergaard L, Jespersen SN, Mouridsen K, et al. The role of the cerebral capillaries in acute ischemic stroke: the extended penumbra model. J Cereb Blood Flow Metab 2013; 33: 635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Østergaard L, Aamand R, Gutierrez-Jimenez E, et al. The capillary dysfunction hypothesis of Alzheimer’s disease. Neurobiol Aging 2013; 34: 1018–1031. [DOI] [PubMed] [Google Scholar]
- 8.Baron JC, Bousser MG, Comar D, et al. Noninvasive tomographic study of cerebral blood flow and oxygen metabolism in vivo. Potentials, limitations, and clinical applications in cerebral ischemic disorders. Eur Neurol 1981; 20: 273–284. [DOI] [PubMed] [Google Scholar]
- 9.Renkin EM. Regulation of the microcirculation. Microvasc Res 1985; 30: 251–263. B. W. Zweifach Award lecture. [DOI] [PubMed] [Google Scholar]
- 10.Kleinfeld D, Mitra PP, Helmchen F, et al. Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc Natl Acad Sci U S A 1998; 95: 15741–15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villringer A, Them A, Lindauer U, et al. Capillary perfusion of the rat brain cortex. An in vivo confocal microscopy study. Circ Res 1994; 75: 55–62. [DOI] [PubMed] [Google Scholar]
- 12.Stefanovic B, Hutchinson E, Yakovleva V, et al. Functional reactivity of cerebral capillaries. J Cereb Blood Flow Metab 2008; 28: 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Østergaard L, Chesler DA, Weisskoff RM, et al. Modeling cerebral blood flow and flow heterogeneity from magnetic resonance residue data. J Cereb Blood Flow Metab 1999; 19: 690–699. [DOI] [PubMed] [Google Scholar]
- 14.Østergaard L, Sorensen AG, Chesler DA, et al. Combined diffusion-weighted and perfusion-weighted flow heterogeneity magnetic resonance imaging in acute stroke. Stroke 2000; 31: 1097–1103. [DOI] [PubMed] [Google Scholar]
- 15.Schulte ML, Wood JD, Hudetz AG. Cortical electrical stimulation alters erythrocyte perfusion pattern in the cerebral capillary network of the rat. Brain Res 2003; 963: 81–92. [DOI] [PubMed] [Google Scholar]
- 16.Vogel J, Kuschinsky W. Decreased heterogeneity of capillary plasma flow in the rat whisker-barrel cortex during functional hyperemia. J Cereb Blood Flow Metab 1996; 16: 1300–1306. [DOI] [PubMed] [Google Scholar]
- 17.Rasmussen PM, Jespersen SN, Østergaard L. The effects of transit time heterogeneity on brain oxygenation during rest and functional activation. J Cereb Blood Flow Metab 2015; 35: 432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peppiatt CM, Howarth C, Mobbs P, et al. Bidirectional control of CNS capillary diameter by pericytes. Nature 2006; 443: 700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014; 508: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Østergaard L. Cerebral perfusion imaging by bolus tracking. Top Magn Reson Imaging 2004; 15: 3–9. [DOI] [PubMed] [Google Scholar]
- 21.Angleys H, Østergaard L, Jespersen SN. The effects of capillary transit time heterogeneity (cth) on brain oxygenation. J Cereb Blood Flow Metab 2015; 35: 806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donnan GA, Baron JC, Davis SM, et al. The ischemic penumbra: overview, definition, and criteria. In: Donnan GA, Baron JC, Davis SM, et al. (eds). The Ischemic Penumbra, New York: Informa Healthcare USA, 2007, pp. 7–20. [Google Scholar]
- 23.Lassen NA. The luxury-perfusion syndrome and its possible relation to acute metabolic acidosis localised within the brain. Lancet 1966; 2: 1113–1115. [DOI] [PubMed] [Google Scholar]
- 24.Yemisci M, Gursoy-Ozdemir Y, Vural A, et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med 2009; 15: 1031–1037. [DOI] [PubMed] [Google Scholar]
- 25.Jennette JC, Falk RJ, Bacon PA, et al. 2012 Revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum 2013; 65: 1–11. [DOI] [PubMed] [Google Scholar]
- 26.Younger DS. Vasculitis of the nervous system. Curr Opin Neurol 2004; 17: 317–336. [DOI] [PubMed] [Google Scholar]
- 27.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res 2005; 97: 512–523. [DOI] [PubMed] [Google Scholar]
- 28.Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature 2010; 468: 557–561. [DOI] [PubMed] [Google Scholar]
- 29.Thomas WE. Brain macrophages: on the role of pericytes and perivascular cells. Brain Res Brain Res Rev 1999; 31: 42–57. [DOI] [PubMed] [Google Scholar]
- 30.Dore-Duffy P, LaManna JC. Physiologic angiodynamics in the brain. Antioxid Redox Signal 2007; 9: 1363–1371. [DOI] [PubMed] [Google Scholar]
- 31.Domenga V, Fardoux P, Lacombe P, et al. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev 2004; 18: 2730–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrow D, Sweeney C, Birney YA, et al. Cyclic strain inhibits Notch receptor signaling in vascular smooth muscle cells in vitro. Circ Res 2005; 96: 567–575. [DOI] [PubMed] [Google Scholar]
- 33.Boulos N, Helle F, Dussaule JC, et al. Notch3 is essential for regulation of the renal vascular tone. Hypertension 2011; 57: 1176–1182. [DOI] [PubMed] [Google Scholar]
- 34.Hill RA, Tong L, Yuan P, et al. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 2015; 87: 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Klett F, Offenhauser N, Dirnagl U, et al. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A 2010; 107: 22290–22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Attwell D. Comments to: smooth muscle cells, not pericytes, control brain blood flow. http://www.alzforum org/news/research-news/smooth-muscle-cells-not-pericytes-control-brain-blood-flow (2015, accessed 19 July 2015).
- 37.Yamanishi S, Katsumura K, Kobayashi T, et al. Extracellular lactate as a dynamic vasoactive signal in the rat retinal microvasculature. Am J Physiol Heart Circ Physiol 2006; 290: H925–H934. [DOI] [PubMed] [Google Scholar]
- 38.Kawamura H, Kobayashi M, Li Q, et al. Effects of angiotensin II on the pericyte-containing microvasculature of the rat retina. J Physiol 2004; 561: 671–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schonfelder U, Hofer A, Paul M, et al. In situ observation of living pericytes in rat retinal capillaries. Microvasc Res 1998; 56: 22–29. [DOI] [PubMed] [Google Scholar]
- 40.Diaz-Flores L, Gutierrez R, Madrid JF, et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol 2009; 24: 909–969. [DOI] [PubMed] [Google Scholar]
- 41.Matsugi T, Chen Q, Anderson DR. Adenosine-induced relaxation of cultured bovine retinal pericytes. Invest Ophthalmol Vis Sci 1997; 38: 2695–2701. [PubMed] [Google Scholar]
- 42.Kawamura H, Sugiyama T, Wu DM, et al. ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J Physiol 2003; 551: 787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haefliger IO, Zschauer A, Anderson DR. Relaxation of retinal pericyte contractile tone through the nitric oxide-cyclic guanosine monophosphate pathway. Invest Ophthalmol Vis Sci 1994; 35: 991–997. [PubMed] [Google Scholar]
- 44.Haefliger IO, Anderson DR. Oxygen modulation of guanylate cyclase-mediated retinal pericyte relaxations with 3-morpholino-sydnonimine and atrial natriuretic peptide. Invest Ophthalmol Vis Sci 1997; 38: 1563–1568. [PubMed] [Google Scholar]
- 45.Wu DM, Kawamura H, Sakagami K, et al. Cholinergic regulation of pericyte-containing retinal microvessels. Am J Physiol Heart Circ Physiol 2003; 284: H2083–H2090. [DOI] [PubMed] [Google Scholar]
- 46.Attwell D, Buchan AM, Charpak S, et al. Glial and neuronal control of brain blood flow. Nature 2010; 468: 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joutel A, Corpechot C, Ducros A, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 1996; 383: 707–710. [DOI] [PubMed] [Google Scholar]
- 48.Joutel A, Andreux F, Gaulis S, et al. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest 2000; 105: 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dziewulska D, Lewandowska E. Pericytes as a new target for pathological processes in CADASIL. Neuropathology 2012; 32: 515–521. [DOI] [PubMed] [Google Scholar]
- 50.Joutel A, Monet-Lepretre M, Gosele C, et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest 2010; 120: 433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gu X, Liu XY, Fagan A, et al. Ultrastructural changes in cerebral capillary pericytes in aged Notch3 mutant transgenic mice. Ultrastruct Pathol 2012; 36: 48–55. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Pan L, Moens CB, et al. Notch3 establishes brain vascular integrity by regulating pericyte number. Development 2014; 141: 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hill J, Rom S, Ramirez SH, et al. Emerging roles of pericytes in the regulation of the neurovascular unit in health and disease. J Neuroimmune Pharmacol 2014; 9: 591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang S, Cao C, Chen Z, et al. Pericytes regulate vascular basement membrane remodeling and govern neutrophil extravasation during inflammation. PLoS One 2012; 7: e45499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dore-Duffy P, Owen C, Balabanov R, et al. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc Res 2000; 60: 55–69. [DOI] [PubMed] [Google Scholar]
- 56.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012; 4: 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carare RO, Bernardes-Silva M, Newman TA, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol 2008; 34: 131–144. [DOI] [PubMed] [Google Scholar]
- 58.Kress BT, Iliff JJ, Xia M, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 2014; 76: 845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weller RO, Subash M, Preston SD, et al. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol 2008; 18: 253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shibata M, Yamada S, Kumar SR, et al. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 2000; 106: 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ball KK, Cruz NF, Mrak RE, et al. Trafficking of glucose, lactate, and amyloid-beta from the inferior colliculus through perivascular routes. J Cereb Blood Flow Metab 2010; 30: 162–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verbeek MM, de Waal RM, Schipper JJ, et al. Rapid degeneration of cultured human brain pericytes by amyloid beta protein. J Neurochem 1997; 68: 1135–1141. [DOI] [PubMed] [Google Scholar]
- 63.Wilhelmus MMM, Otte-Höller I, van Triel JJJ, et al. Lipoprotein receptor-related protein-1 mediates amyloid-β-mediated cell death of cerebrovascular cells. Am J Pathol 2007; 171: 1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012; 485: 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010; 68: 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Frontiers in Neuroenergetics 2010; 2: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci 2011; 14: 1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Macdonald RL, Weir BK. A review of hemoglobin and the pathogenesis of cerebral vasospasm. Stroke 1991; 22: 971–982. [DOI] [PubMed] [Google Scholar]
- 69.Pluta RM. Delayed cerebral vasospasm and nitric oxide: review, new hypothesis, and proposed treatment. Pharmacol Ther 2005; 105: 23–56. [DOI] [PubMed] [Google Scholar]
- 70.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 1986; 320: 454–456. [DOI] [PubMed] [Google Scholar]
- 71.Rey FE, Li XC, Carretero OA, et al. Perivascular superoxide anion contributes to impairment of endothelium-dependent relaxation: role of gp91(phox). Circulation 2002; 106: 2497–2502. [DOI] [PubMed] [Google Scholar]
- 72.Sasaki T, Wakai S, Asano T, et al. The effect of a lipid hydroperoxide of arachidonic acid on the canine basilar artery. An experimental study on cerebral vasospasm. J Neurosurg 1981; 54: 357–365. [DOI] [PubMed] [Google Scholar]
- 73.Pyne-Geithman GJ, Nair SG, Stamper DN, et al. Role of bilirubin oxidation products in the pathophysiology of DIND following SAH. Acta Neurochir Suppl 2013; 115: 267–273. [DOI] [PubMed] [Google Scholar]
- 74.Isakson BE, Damon DN, Day KH, et al. Connexin40 and connexin43 in mouse aortic endothelium: evidence for coordinated regulation. Am J Physiol Heart Circ Physiol 2006; 290: H1199–H1205. [DOI] [PubMed] [Google Scholar]
- 75.Segal SS, Duling BR. Conduction of vasomotor responses in arterioles: a role for cell-to-cell coupling? Am J Physiol 1989; 256: H838–H845. [DOI] [PubMed] [Google Scholar]
- 76.Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science 1986; 234: 868–870. [DOI] [PubMed] [Google Scholar]
- 77.Duling BR, Berne RM. Propagated vasodilation in the microcirculation of the hamster cheek pouch. Circ Res 1970; 26: 163–170. [DOI] [PubMed] [Google Scholar]
- 78.Pries AR, Hopfner M, le Noble F, et al. The shunt problem: control of functional shunting in normal and tumour vasculature. Nat Rev Cancer 2010; 10: 587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woywodt A, Streiber F, de Groot K, et al. Circulating endothelial cells as markers for ANCA-associated small-vessel vasculitis. Lancet 2003; 361: 206–210. [DOI] [PubMed] [Google Scholar]
- 80.Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res 1996; 79: 581–589. [DOI] [PubMed] [Google Scholar]
- 81.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch 2000; 440: 653–666. [DOI] [PubMed] [Google Scholar]
- 82.Secomb TW, Hsu R, Pries AR. A model for red blood cell motion in glycocalyx-lined capillaries. Am J Physiol 1998; 274: H1016–H1022. [DOI] [PubMed] [Google Scholar]
- 83.Constantinescu AA, Vink H, Spaan JA. Elevated capillary tube hematocrit reflects degradation of endothelial cell glycocalyx by oxidized LDL. Am J Physiol Heart Circ Physiol 2001; 280: H1051–H1057. [DOI] [PubMed] [Google Scholar]
- 84.Desjardins C, Duling BR. Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am J Physiol 1990; 258: H647–H654. [DOI] [PubMed] [Google Scholar]
- 85.van Haaren PM, VanBavel E, Vink H, et al. Localization of the permeability barrier to solutes in isolated arteries by confocal microscopy. Am J Physiol Heart Circ Physiol 2003; 285: H2848–H2856. [DOI] [PubMed] [Google Scholar]
- 86.van den Berg BM, Vink H, Spaan JA. The endothelial glycocalyx protects against myocardial edema. Circ Res 2003; 92: 592–594. [DOI] [PubMed] [Google Scholar]
- 87.Vink H, Constantinescu AA, Spaan JA. Oxidized lipoproteins degrade the endothelial surface layer: implications for platelet-endothelial cell adhesion. Circulation 2000; 101: 1500–1502. [DOI] [PubMed] [Google Scholar]
- 88.Czarnowska E, Karwatowska-Prokopczuk E. Ultrastructural demonstration of endothelial glycocalyx disruption in the reperfused rat heart. Involvement of oxygen free radicals. Basic Res Cardiol 1995; 90: 357–364. [DOI] [PubMed] [Google Scholar]
- 89.Nieuwdorp M, Mooij HL, Kroon J, et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 2006; 55: 1127–1132. [DOI] [PubMed] [Google Scholar]
- 90.Ishiharajima S, Aida T, Nakagawa R, et al. Early membrane damage during ischemia in rat heart. Exp Mol Pathol 1986; 44: 1–6. [DOI] [PubMed] [Google Scholar]
- 91.Mazzoni MC, Schmid-Schonbein GW. Mechanisms and consequences of cell activation in the microcirculation. Cardiovasc Res 1996; 32: 709–719. [PubMed] [Google Scholar]
- 92.Barnes AJ, Locke P, Scudder PR. Is hyperviscosity a treatable component of diabetic microcirculatory disease? Lancet 1977; 2: 789–791. [DOI] [PubMed] [Google Scholar]
- 93.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 2004; 5: 347–360. [DOI] [PubMed] [Google Scholar]
- 94.Al-Saeedi FJ. Perfusion scanning using 99mTc-HMPAO detects early cerebrovascular changes in the diabetic rat. BMC Med Phys 2008; 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Simpson RE, III, Phillis JW, Buchannan J. A comparison of cerebral blood flow during basal, hypotensive, hypoxic and hypercapnic conditions between normal and streptozotocin diabetic rats. Brain Res 1990; 531: 136–142. [DOI] [PubMed] [Google Scholar]
- 96.Rubin MJ, Bohlen HG. Cerebral vascular autoregulation of blood flow and tissue PO2 in diabetic rats. Am J Physiol 1985; 249: H540–H546. [DOI] [PubMed] [Google Scholar]
- 97.Kim T, Richard Jennings J, Kim SG. Regional cerebral blood flow and arterial blood volume and their reactivity to hypercapnia in hypertensive and normotensive rats. J Cereb Blood Flow Metab 2014; 34: 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thal DR, Papassotiropoulos A, Saido TC, et al. Capillary cerebral amyloid angiopathy identifies a distinct APOE epsilon4-associated subtype of sporadic Alzheimer’s disease. Acta Neuropathol 2010; 120: 169–183. [DOI] [PubMed] [Google Scholar]
- 99.Scarmeas N, Habeck CG, Stern Y, et al. APOE genotype and cerebral blood flow in healthy young individuals. JAMA 2003; 290: 1581–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Scarmeas N, Habeck CG, Hilton J, et al. APOE related alterations in cerebral activation even at college age. J Neurol Neurosurg Psychiatry 2005; 76: 1440–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bookheimer SY, Strojwas MH, Cohen MS, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med 2000; 343: 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Filippini N, MacIntosh BJ, Hough MG, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A 2009; 106: 7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fleisher AS, Houston WS, Eyler LT, et al. Identification of Alzheimer disease risk by functional magnetic resonance imaging. Arch Neurol 2005; 62: 1881–1888. [DOI] [PubMed] [Google Scholar]
- 104.DiFrancesco MW, Holland SK, Ris MD, et al. Functional magnetic resonance imaging assessment of cognitive function in childhood-onset systemic lupus erythematosus: a pilot study. Arthritis Rheum 2007; 56: 4151–4163. [DOI] [PubMed] [Google Scholar]
- 105.Ernst T, Chang L, Jovicich J, et al. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology 2002; 59: 1343–1349. [DOI] [PubMed] [Google Scholar]
- 106.Ernst T, Chang L, Arnold S. Increased glial metabolites predict increased working memory network activation in HIV brain injury. Neuroimage 2003; 19: 1686–1693. [DOI] [PubMed] [Google Scholar]
- 107.Ances BM, Roc AC, Korczykowski M, et al. Combination antiretroviral therapy modulates the blood oxygen level-dependent amplitude in human immunodeficiency virus-seropositive patients. J Neurovirol 2008; 14: 418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Buxton RB. Dynamic models of BOLD contrast. Neuroimage 2012; 62: 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thambisetty M, Beason-Held L, An Y, et al. APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch Neurol 2010; 67: 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fleisher AS, Podraza KM, Bangen KJ, et al. Cerebral perfusion and oxygenation differences in Alzheimer’s disease risk. Neurobiol Aging 2009; 30: 1737–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Calcinaghi N, Wyss MT, Jolivet R, et al. Multimodal imaging in rats reveals impaired neurovascular coupling in sustained hypertension. Stroke 2013; 44: 1957–1964. [DOI] [PubMed] [Google Scholar]
- 112.Suri S, Mackay CE, Kelly ME, et al. Reduced cerebrovascular reactivity in young adults carrying the APOE epsilon4 allele. Alzheimers Dement 2015; 11: 648–657. [DOI] [PubMed] [Google Scholar]
- 113.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 2006; 100: 328–335. [DOI] [PubMed] [Google Scholar]
- 114.Kazama K, Wang G, Frys K, et al. Angiotensin II attenuates functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol 2003; 285: H1890–H1899. [DOI] [PubMed] [Google Scholar]
- 115.Niwa K, Younkin L, Ebeling C, et al. Abeta 1-40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci U S A 2000; 97: 9735–9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Capone C, Faraco G, Park L, et al. The cerebrovascular dysfunction induced by slow pressor doses of angiotensin II precedes the development of hypertension. Am J Physiol Heart Circ Physiol 2011; 300: H397–H407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kazama K, Anrather J, Zhou P, et al. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res 2004; 95: 1019–1026. [DOI] [PubMed] [Google Scholar]
- 118.Girouard H, Park L, Anrather J, et al. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler Thromb Vasc Biol 2007; 27: 303–309. [DOI] [PubMed] [Google Scholar]
- 119.Dawson VL, Dawson TM. Nitric oxide neurotoxicity. J Chem Neuroanat 1996; 10: 179–190. [DOI] [PubMed] [Google Scholar]
- 120.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007; 87: 315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol 2004; 122: 339–352. [DOI] [PubMed] [Google Scholar]
- 122.Sakadzic S, Mandeville ET, Gagnon L, et al. Large arteriolar component of oxygen delivery implies a safe margin of oxygen supply to cerebral tissue. Nat Commun 2014; 5: 5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Moody DM, Brown WR, Challa VR, et al. Cerebral microvascular alterations in aging, leukoaraiosis, and Alzheimer’s disease. Ann N Y Acad Sci 1997; 826: 103–116. [DOI] [PubMed] [Google Scholar]
- 124.Doubal FN, MacGillivray TJ, Hokke PE, et al. Differences in retinal vessels support a distinct vasculopathy causing lacunar stroke. Neurology 2009; 72: 1773–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lindley RI, Wang JJ, Wong MC, et al. Retinal microvasculature in acute lacunar stroke: a cross-sectional study. Lancet Neurol 2009; 8: 628–634. [DOI] [PubMed] [Google Scholar]
- 126.Moore DF, Altarescu G, Ling GS, et al. Elevated cerebral blood flow velocities in Fabry disease with reversal after enzyme replacement. Stroke 2002; 33: 525–531. [DOI] [PubMed] [Google Scholar]
- 127.Moore DF, Scott LT, Gladwin MT, et al. Regional cerebral hyperperfusion and nitric oxide pathway dysregulation in Fabry disease: reversal by enzyme replacement therapy. Circulation 2001; 104: 1506–1512. [DOI] [PubMed] [Google Scholar]
- 128.Moore DF, Herscovitch P, Schiffmann R. Selective arterial distribution of cerebral hyperperfusion in Fabry disease. J Neuroimaging 2001; 11: 303–307. [DOI] [PubMed] [Google Scholar]
- 129.Moore DF, Altarescu G, Barker WC, et al. White matter lesions in Fabry disease occur in “prior” selectively hypometabolic and hyperperfused brain regions. Brain Res Bull 2003; 62: 231–240. [DOI] [PubMed] [Google Scholar]
- 130.Sano M, Ishii K, Momose Y, et al. Cerebral metabolism of oxygen and glucose in a patient with MELAS syndrome. Acta Neurol Scand 1995; 92: 497–502. [DOI] [PubMed] [Google Scholar]
- 131.Takahashi S, Tohgi H, Yonezawa H, et al. Cerebral blood flow and oxygen metabolism before and after a stroke-like episode in patients with mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS). J Neurol Sci 1998; 158: 58–64. [DOI] [PubMed] [Google Scholar]
- 132.Wang Z, Xiao J, Xie S, et al. MR evaluation of cerebral oxygen metabolism and blood flow in stroke-like episodes of MELAS. J Neurol Sci 2012; 323: 173–177. [DOI] [PubMed] [Google Scholar]
- 133.Yu L, Xie S, Xiao J, et al. Quantitative measurement of cerebral oxygen extraction fraction using MRI in patients with MELAS. PLoS One 2013; 8: e79859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gropen TI, Prohovnik I, Tatemichi TK, et al. Cerebral hyperemia in MELAS. Stroke 1994; 25: 1873–1876. [DOI] [PubMed] [Google Scholar]
- 135.Amagasaki K, Shimizu T, Suzuki Y, et al. Focal hyperperfusion in a patient with mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Case report. J Neurosurg 2001; 94: 133–136. [DOI] [PubMed] [Google Scholar]
- 136.Warrington JP, Ashpole N, Csiszar A, et al. Whole brain radiation-induced vascular cognitive impairment: mechanisms and implications. J Vasc Res 2013; 50: 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Warrington JP, Csiszar A, Johnson DA, et al. Cerebral microvascular rarefaction induced by whole brain radiation is reversible by systemic hypoxia in mice. Am J Physiol Heart Circ Physiol 2011; 300: H736–H744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mineura K, Suda Y, Yasuda T, et al. Early and late stage positron emission tomography (PET) studies on the haemocirculation and metabolism of seemingly normal brain tissue in patients with gliomas following radiochemotherapy. Acta Neurochir (Wien) 1988; 93: 110–115. [DOI] [PubMed] [Google Scholar]
- 139.Hahn CA, Zhou SM, Raynor R, et al. Dose-dependent effects of radiation therapy on cerebral blood flow, metabolism, and neurocognitive dysfunction. Int J Radiat Oncol Biol Phys 2009; 73: 1082–1087. [DOI] [PubMed] [Google Scholar]
- 140.Østergaard L, Jespersen SN, Engedal TS, et al. Capillary dysfunction: its detection and causative role in dementias and stroke. Curr Neurol Neurosci Rep 2015; 15: 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ferraccioli G, Di Poi E, Di Gregorio F, et al. Changes in regional cerebral blood flow after a cold hand test in systemic lupus erythematosus patients with Raynaud’s syndrome. Lancet 1999; 354: 2135–2136. [DOI] [PubMed] [Google Scholar]
- 142.Wardlaw JM. What causes lacunar stroke? J Neurol Neurosurg Psychiatry 2005; 76: 617–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hassan A, Gormley K, O’Sullivan M, et al. Endothelial nitric oxide gene haplotypes and risk of cerebral small-vessel disease. Stroke 2004; 35: 654–659. [DOI] [PubMed] [Google Scholar]
- 144.Yemisci M, Sinici I, Ozkara HA, et al. Protective role of 27bp repeat polymorphism in intron 4 of eNOS gene in lacunar infarction. Free Radic Res 2009; 43: 272–279. [DOI] [PubMed] [Google Scholar]
- 145.van Faassen EE, Bahrami S, Feelisch M, et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev 2009; 29: 683–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aamand R, Dalsgaard T, Ho YC, et al. A NO way to BOLD? Dietary nitrate alters the hemodynamic response to visual stimulation. Neuroimage 2013; 83: 397–407. [DOI] [PubMed] [Google Scholar]
- 147.Patterson JL, Jr, Heyman A, Nichols FT., Jr Cerebral blood flow and oxygen consumption in neurosyphilis. J Clin Invest 1950; 29: 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Logigian EL, Johnson KA, Kijewski MF, et al. Reversible cerebral hypoperfusion in Lyme encephalopathy. Neurology 1997; 49: 1661–1670. [DOI] [PubMed] [Google Scholar]
- 149.Dickinson J, Paton JFR. Cerebrovascular hypertension, Brighton, UK: Book Guild Publishing, 2012. [Google Scholar]
- 150.Scheinker IM. Alterations of cerebral capillaries in the early stage of arterial hypertension. Am J Pathol 1948; 24: 211–221. [PMC free article] [PubMed] [Google Scholar]
- 151.Soros P, Whitehead S, Spence JD, et al. Antihypertensive treatment can prevent stroke and cognitive decline. Nat Rev Neurol 2013; 9: 174–178. [DOI] [PubMed] [Google Scholar]
- 152.Knudsen GM, Pettigrew KD, Paulson OB, et al. Kinetic analysis of blood-brain barrier transport of D-glucose in man: quantitative evaluation in the presence of tracer backflux and capillary heterogeneity. Microvasc Res 1990; 39: 28–49. [DOI] [PubMed] [Google Scholar]
- 153.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 2007; 27: 1766–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Østergaard L. Principles of cerebral perfusion imaging by bolus tracking. J Magn Reson Imaging 2005; 22: 710–717. [DOI] [PubMed] [Google Scholar]
- 155.Ellis CG, Goldman D, Hanson M, et al. Defects in oxygen supply to skeletal muscle of prediabetic ZDF rats. Am J Physiol Heart Circ Physiol 2010; 298: H1661–H1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Domenech M, Roman P, Lapetra J, et al. Mediterranean diet reduces 24-hour ambulatory blood pressure, blood glucose, and lipids: one-year randomized, clinical trial. Hypertension 2014; 64: 69–76. [DOI] [PubMed] [Google Scholar]
- 157.Østergaard L, Tietze A, Nielsen T, et al. The relationship between tumor blood flow, angiogenesis, tumor hypoxia, and aerobic glycolysis. Cancer Res 2013; 73: 5618–5624. [DOI] [PubMed] [Google Scholar]
- 158.Lavallee P, Perchaud V, Gautier-Bertrand M, et al. Association between influenza vaccination and reduced risk of brain infarction. Stroke 2002; 33: 513–518. [DOI] [PubMed] [Google Scholar]
- 159.Nichol KL, Nordin J, Mullooly J, et al. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med 2003; 348: 1322–1332. [DOI] [PubMed] [Google Scholar]
- 160.Grau AJ, Fischer B, Barth C, et al. Influenza vaccination is associated with a reduced risk of stroke. Stroke 2005; 36: 1501–1506. [DOI] [PubMed] [Google Scholar]
- 161.Kountouras J, Boziki M, Gavalas E, et al. Eradication of Helicobacter pylori may be beneficial in the management of Alzheimer’s disease. J Neurol 2009; 256: 758–767. [DOI] [PubMed] [Google Scholar]
- 162.Kountouras J, Boziki M, Gavalas E, et al. Five-year survival after Helicobacter pylori eradication in Alzheimer disease patients. Cogn Behav Neurol 2010; 23: 199–204. [DOI] [PubMed] [Google Scholar]
- 163.Nappo F, De Rosa N, Marfella R, et al. Impairment of endothelial functions by acute hyperhomocysteinemia and reversal by antioxidant vitamins. JAMA 1999; 281: 2113–2118. [DOI] [PubMed] [Google Scholar]
- 164.Hassan A, Hunt BJ, O’Sullivan M, et al. Homocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunction. Brain 2004; 127: 212–219. [DOI] [PubMed] [Google Scholar]
- 165.Holmes MV, Newcombe P, Hubacek JA, et al. Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: a meta-analysis of genetic studies and randomised trials. Lancet 2011; 378: 584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Horlington M, Watson PA. Inhibition of 3′5′-cyclic-AMP phosphodiesterase by some platelet aggregation inhibitors. Biochem Pharmacol 1970; 19: 955–956. [DOI] [PubMed] [Google Scholar]
- 167.Jacoby D, Mohler ER., III Drug treatment of intermittent claudication. Drugs 2004; 64: 1657–1670. [DOI] [PubMed] [Google Scholar]
- 168.Bath PM, Wardlaw JM. Pharmacological treatment and prevention of cerebral small vessel disease: a review of potential interventions. Int J Stroke 2015; 10: 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Albaugh G, Bellavance E, Strande L, et al. Nicotine induces mononuclear leukocyte adhesion and expression of adhesion molecules, VCAM and ICAM, in endothelial cells in vitro. Ann Vasc Surg 2004; 18: 302–307. [DOI] [PubMed] [Google Scholar]
- 170.Yong T, Zheng MQ, Linthicum DS. Nicotine induces leukocyte rolling and adhesion in the cerebral microcirculation of the mouse. J Neuroimmunol 1997; 80: 158–164. [DOI] [PubMed] [Google Scholar]
- 171.Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. J Immunol 2008; 180: 6439–6446. [DOI] [PubMed] [Google Scholar]
- 172.Adib-Samii P, Brice G, Martin RJ, et al. Clinical spectrum of CADASIL and the effect of cardiovascular risk factors on phenotype: study in 200 consecutively recruited individuals. Stroke 2010; 41: 630–634. [DOI] [PubMed] [Google Scholar]
- 173.Staals J, Makin SD, Doubal FN, et al. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014; 83: 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365: 1046–1053. [DOI] [PubMed] [Google Scholar]
- 175.Davis DH, Muniz Terrera G, Keage H, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain 2012; 135: 2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gardener H, Scarmeas N, Gu Y, et al. Mediterranean diet and white matter hyperintensity volume in the Northern Manhattan Study. Arch Neurol 2012; 69: 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gardener H, Wright CB, Gu Y, et al. Mediterranean-style diet and risk of ischemic stroke, myocardial infarction, and vascular death: the Northern Manhattan Study. Am J Clin Nutr 2011; 94: 1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013; 368: 1279–1290. [DOI] [PubMed] [Google Scholar]
- 179.Gaudin A, Yemisci M, Eroglu H, et al. Squalenoyl adenosine nanoparticles provide neuroprotection after stroke and spinal cord injury. Nat Nanotechnol 2014; 9: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Powers WJ, Clarke WR, Grubb RL, Jr, et al. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the Carotid Occlusion Surgery Study randomized trial. JAMA 2011; 306: 1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dani KA, Thomas RG, Chappell FM, et al. Computed tomography and magnetic resonance perfusion imaging in ischemic stroke: definitions and thresholds. Ann Neurol 2011; 70: 384–401. [DOI] [PubMed] [Google Scholar]
- 182.Mouridsen K, Hansen MB, Østergaard L, et al. Reliable estimation of capillary transit time distributions using DSC-MRI. J Cereb Blood Flow Metab 2014; 34: 1511–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zlokovic BV, Deane R, Sagare AP, et al. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid beta-peptide elimination from the brain. J Neurochem 2010; 115: 1077–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 2011; 12: 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dalkara T, Gursoy-Ozdemir Y, Yemisci M. Brain microvascular pericytes in health and disease. Acta Neuropathol 2011; 122: 1–9. [DOI] [PubMed] [Google Scholar]
- 186.Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, et al. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med 2009; 15: 1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sette G, Baron JC, Mazoyer B, et al. Local brain haemodynamics and oxygen metabolism in cerebrovascular disease. Positron emission tomography. Brain 1989; 112 (Pt 4): 931–951. [DOI] [PubMed] [Google Scholar]
- 188.Farkas E, de Vos RA, Donka G, et al. Age-related microvascular degeneration in the human cerebral periventricular white matter. Acta Neuropathol 2006; 111: 150–157. [DOI] [PubMed] [Google Scholar]
- 189.Eng CM, Banikazemi M, Gordon RE, et al. A phase 1/2 clinical trial of enzyme replacement in fabry disease: pharmacokinetic, substrate clearance, and safety studies. Am J Hum Genet 2001; 68: 711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol 2001; 64: 575–611. [DOI] [PubMed] [Google Scholar]
- 191.Sakuta R, Nonaka I. Vascular involvement in mitochondrial myopathy. Ann Neurol 1989; 25: 594–601. [DOI] [PubMed] [Google Scholar]
- 192.Ohama E, Ohara S, Ikuta F, et al. Mitochondrial angiopathy in cerebral blood vessels of mitochondrial encephalomyopathy. Acta Neuropathol 1987; 74: 226–233. [DOI] [PubMed] [Google Scholar]
- 193.Weisskoff RM, Zuo CS, Boxerman JL, et al. Microscopic susceptibility variation and transverse relaxation: theory and experiment. Magn Reson Med 1994; 31: 601–610. [DOI] [PubMed] [Google Scholar]
- 194.Kalaria RN. Cerebral vessels in ageing and Alzheimer’s disease. Pharmacol Ther 1996; 72: 193–214. [DOI] [PubMed] [Google Scholar]
- 195.Bell MA, Ball MJ. Morphometric comparison of hippocampal microvasculature in ageing and demented people: diameters and densities. Acta Neuropathol 1981; 53: 299–318. [DOI] [PubMed] [Google Scholar]
- 196.Junker U, Jaggi C, Bestetti G, et al. Basement membrane of hypothalamus and cortex capillaries from normotensive and spontaneously hypertensive rats with streptozotocin-induced diabetes. Acta Neuropathol 1985; 65: 202–208. [DOI] [PubMed] [Google Scholar]
- 197.Tagami M, Nara Y, Kubota A, et al. Ultrastructural changes in cerebral pericytes and astrocytes of stroke-prone spontaneously hypertensive rats. Stroke 1990; 21: 1064–1071. [DOI] [PubMed] [Google Scholar]
- 198.Johnson PC, Brendel K, Meezan E. Thickened cerebral cortical capillary basement membranes in diabetics. Arch Pathol Lab Med 1982; 106: 214–217. [PubMed] [Google Scholar]
- 199.Reske-Nielsen E, Lundbæk K, Rafaelsen OJ. Pathological changes in the central and peripheral nervous system of young long-term diabetics. I. Diabetic encephalopathy. Diabetologia 1965; 1: 233–241. [DOI] [PubMed] [Google Scholar]
- 200.McCuskey PA, McCuskey RS. In vivo and electron microscopic study of the development of cerebral diabetic microangiography. Microcirc Endothelium Lymphatics 1984; 1: 221–244. [PubMed] [Google Scholar]
- 201.Price TO, Eranki V, Banks WA, et al. Topiramate treatment protects blood-brain barrier pericytes from hyperglycemia-induced oxidative damage in diabetic mice. Endocrinology 2012; 153: 362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Shah GN, Price TO, Banks WA, et al. Pharmacological inhibition of mitochondrial carbonic anhydrases protects mouse cerebral pericytes from high glucose-induced oxidative stress and apoptosis. J Pharmacol Exp Ther 2013; 344: 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Low WC, Junna M, Borjesson-Hanson A, et al. Hereditary multi-infarct dementia of the Swedish type is a novel disorder different from NOTCH3 causing CADASIL. Brain 2007; 130: 357–367. [DOI] [PubMed] [Google Scholar]
- 204.Tikka S, Mykkanen K, Ruchoux MM, et al. Congruence between NOTCH3 mutations and GOM in 131 CADASIL patients. Brain 2009; 132: 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yamamoto Y, Craggs LJ, Watanabe A, et al. Brain microvascular accumulation and distribution of the NOTCH3 ectodomain and granular osmiophilic material in CADASIL. J Neuropathol Exp Neurol 2013; 72: 416–431. [DOI] [PubMed] [Google Scholar]
- 206.Mizukami K, Sasaki M, Suzuki T, et al. Central nervous system changes in mitochondrial encephalomyopathy: light and electron microscopic study. Acta Neuropathol 1992; 83: 449–452. [DOI] [PubMed] [Google Scholar]
- 207.Koo B, Becker LE, Chuang S, et al. Mitochondrial encephalomyopathy, lactic acidosis, stroke-like episodes (MELAS): clinical, radiological, pathological, and genetic observations. Ann Neurol 1993; 34: 25–32. [DOI] [PubMed] [Google Scholar]
- 208.Lou HO, Reske-Nielsen E. the central nervous system in Fabry’s disease. A clinical, pathological, and biochemical investigation. Arch Neurol 1971; 25: 351–359. [DOI] [PubMed] [Google Scholar]
- 209.Grunnet ML, Spilsbury PR. The central nervous system in Fabry’s disease. An ultrastructural study. Arch Neurol 1973; 28: 231–234. [DOI] [PubMed] [Google Scholar]
- 210.Itoh Y, Esaki T, Cook M, et al. Local and global cerebral blood flow and glucose utilization in the alpha-galactosidase A knockout mouse model of Fabry disease. J Neurochem 2001; 79: 1217–1224. [DOI] [PubMed] [Google Scholar]
- 211.Weil S, Reifenberger G, Dudel C, et al. Cerebroretinal vasculopathy mimicking a brain tumor: a case of a rare hereditary syndrome. Neurology 1999; 53: 629–631. [DOI] [PubMed] [Google Scholar]
- 212.Altarescu G, Moore DF, Pursley R, et al. Enhanced endothelium-dependent vasodilation in Fabry disease. Stroke 2001; 32: 1559–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cohn AC, Kotschet K, Veitch A, et al. Novel ophthalmological features in hereditary endotheliopathy with retinopathy, nephropathy and stroke syndrome. Clin Experiment Ophthalmol 2005; 33: 181–183. [DOI] [PubMed] [Google Scholar]
- 214.Jen J, Cohen AH, Yue Q, et al. Hereditary endotheliopathy with retinopathy, nephropathy, and stroke (HERNS). Neurology 1997; 49: 1322–1330. [DOI] [PubMed] [Google Scholar]
- 215.Gould DB, Phalan FC, van Mil SE, et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med 2006; 354: 1489–1496. [DOI] [PubMed] [Google Scholar]
- 216.Poschl E, Schlotzer-Schrehardt U, Brachvogel B, et al. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 2004; 131: 1619–1628. [DOI] [PubMed] [Google Scholar]
- 217.Hagel C, Groden C, Niemeyer R, et al. Subcortical angiopathic encephalopathy in a German kindred suggests an autosomal dominant disorder distinct from CADASIL. Acta Neuropathol 2004; 108: 231–240. [DOI] [PubMed] [Google Scholar]
- 218.Craggs LJ, Hagel C, Kuhlenbaeumer G, et al. Quantitative vascular pathology and phenotyping familial and sporadic cerebral small vessel diseases. Brain Pathol 2013; 23: 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Halbwachs L, Lesavre P. Endothelium-neutrophil interactions in ANCA-associated diseases. J Am Soc Nephrol 2012; 23: 1449–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rodriguez-Pla A, Monach PA. Primary angiitis of the central nervous system in adults and children. Rheum Dis Clin North Am 2015; 41: 47–62. viii. [DOI] [PubMed] [Google Scholar]
- 221.SNEDDON IB. Cerebro-vascular lesions and livedo reticularis. Br J Dermatol 1965; 77: 180–185. [DOI] [PubMed] [Google Scholar]
- 222.Lewandowska E, Wierzba-Bobrowicz T, Wagner T, et al. Sneddon’s syndrome as a disorder of small arteries with endothelial cells proliferation: ultrastructural and neuroimaging study. Folia Neuropathol 2005; 43: 345–354. [PubMed] [Google Scholar]
- 223.Gobert A. Sneddon’s syndrome with bilateral peripheral retinal neovascularization. Bull Soc Belge Ophtalmol 1995; 255: 85–90. [PubMed] [Google Scholar]
- 224.Rehany U, Kassif Y, Rumelt S. Sneddon’s syndrome: neuro-ophthalmologic manifestations in a possible autosomal recessive pattern. Neurology 1998; 51: 1185–1187. [DOI] [PubMed] [Google Scholar]
- 225.Basu N, Murray AD, Jones GT, et al. Neural correlates of fatigue in granulomatosis with polyangiitis: a functional magnetic resonance imaging study. Rheumatology (Oxford) 2014; 53: 2080–2087. [DOI] [PubMed] [Google Scholar]
- 226.Nagel MA, Gilden D. Update on varicella zoster virus vasculopathy. Curr Infect Dis Rep 2014; 16: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology 2012; 142: 634–643.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chetty R. Vasculitides associated with HIV infection. J Clin Pathol 2001; 54: 275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sen S, Rabinstein AA, Elkind MS, et al. Recent developments regarding human immunodeficiency virus infection and stroke. Cerebrovasc Dis 2012; 33: 209–218. [DOI] [PubMed] [Google Scholar]
- 230.Ortiz G, Koch S, Romano JG, et al. Mechanisms of ischemic stroke in HIV-infected patients. Neurology 2007; 68: 1257–1261. [DOI] [PubMed] [Google Scholar]
- 231.Morgello S, Murray J, Van Der Elst S, et al. HCV, but not HIV, is a risk factor for cerebral small vessel disease. Neurol Neuroimmunol Neuroinflamm 2014; 1: e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Benjamin LA, Bryer A, Emsley HC, et al. HIV infection and stroke: current perspectives and future directions. Lancet Neurol 2012; 11: 878–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol 2005; 5: 69–81. [DOI] [PubMed] [Google Scholar]
- 234.Moses AV, Bloom FE, Pauza CD, et al. Human immunodeficiency virus infection of human brain capillary endothelial cells occurs via a CD4/galactosylceramide-independent mechanism. Proc Natl Acad Sci USA 1993; 90: 10474–10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kim TA, Avraham HK, Koh YH, et al. HIV-1 Tat-mediated apoptosis in human brain microvascular endothelial cells. J Immunol 2003; 170: 2629–2637. [DOI] [PubMed] [Google Scholar]
- 236.Niu F, Yao H, Zhang W, et al. Tat 101-mediated enhancement of brain pericyte migration involves platelet-derived growth factor subunit B homodimer: implications for human immunodeficiency virus-associated neurocognitive disorders. J Neurosci 2014; 34: 11812–11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Miklossy J. Biology and neuropathology of dementia in syphilis and Lyme disease. Handb Clin Neurol 2008; 89: 825–844. [DOI] [PubMed] [Google Scholar]
- 238.May EF, Jabbari B. Stroke in neuroborreliosis. Stroke 1990; 21: 1232–1235. [DOI] [PubMed] [Google Scholar]
- 239.Silva J, Polesskaya O, Knight W, et al. Transient hypercapnia reveals an underlying cerebrovascular pathology in a murine model for HIV-1 associated neuroinflammation: role of NO-cGMP signaling and normalization by inhibition of cyclic nucleotide phosphodiesterase-5. J Neuroinflamm 2012; 9: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Fallon BA, Lipkin RB, Corbera KM, et al. Regional cerebral blood flow and metabolic rate in persistent Lyme encephalopathy. Arch Gen Psychiatry 2009; 66: 554–563. [DOI] [PubMed] [Google Scholar]
- 241.Nagy Z, Czirjak L. Nailfold digital capillaroscopy in 447 patients with connective tissue disease and Raynaud’s disease. J Eur Acad Dermatol Venereol 2004; 18: 62–68. [DOI] [PubMed] [Google Scholar]
- 242.Koenig M, Joyal F, Fritzler MJ, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: a twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum 2008; 58: 3902–3912. [DOI] [PubMed] [Google Scholar]
- 243.Amaral TN, Peres FA, Lapa AT, et al. Neurologic involvement in scleroderma: a systematic review. Semin Arthritis Rheum 2013; 43: 335–347. [DOI] [PubMed] [Google Scholar]
- 244.Heron E, Fornes P, Rance A, et al. Brain involvement in scleroderma: two autopsy cases. Stroke 1998; 29: 719–721. [DOI] [PubMed] [Google Scholar]
- 245.Nobili F, Cutolo M, Sulli A, et al. Impaired quantitative cerebral blood flow in scleroderma patients. J Neurol Sci 1997; 152: 63–71. [DOI] [PubMed] [Google Scholar]
- 246.Cutolo M, Nobili F, Sulli A, et al. Evidence of cerebral hypoperfusion in scleroderma patients. Rheumatology (Oxford) 2000; 39: 1366–1373. [DOI] [PubMed] [Google Scholar]
- 247.Jimenez C, Rowe PC, Keene D. Cardiac and central nervous system vasculitis in a child with dermatomyositis. J Child Neurol 1994; 9: 297–300. [DOI] [PubMed] [Google Scholar]