Abstract
To evaluate the effect of age on the response of brains to an ischemic challenge, we subjected young and aged mice to transient forebrain ischemia, and analyzed the heat shock response and unfolded protein response, ubiquitin conjugation and SUMO conjugation, and O-linked β-N-acetylglucosamine modification of proteins (O-GlcNAcylation). The most prominent age-related difference was an inability of aged mice to activate O-GlcNAcylation. Considering many reports on the protective role of O-GlcNAcylation in various stress conditions including myocardial ischemia, this pathway could be a promising target for therapeutic intervention to improve functional recovery of aged patients following brain ischemia.
Keywords: Aging, brain ischemia, O-GlcNAc modification, SUMO conjugation, ubiquitin conjugation, unfolded protein response
Introduction
Age is a key risk factor for ischemic stroke, and it has been predicted that by the year 2050, patients over 75 years of age will comprise more than 50% of new stroke cases.1 This will pose a tremendous burden on afflicted families and healthcare systems. Experimental stroke studies have shown that during vascular occlusion, the brain volume with critical perfusion deficits does not increase with age, whereas functional recovery is severely impaired in old animals.2 This suggests that the ability of brains to respond to an ischemic challenge declines with age.
To investigate the effects of age on the response of brains to ischemia, we subjected young and aged mice to transient forebrain ischemia and analyzed ischemia-induced activation of stress response pathways in the cortex and hippocampus. We used a model of transient forebrain ischemia rather than a stroke model to make sure that our results are not confounded by unrelated factors such as size of infarcts. Further, we chose 1 hour of reperfusion, when protective stress response pathways are activated that modulate gene expression and thereby provide a long-lasting effect on post-ischemic cells. These included the heat shock response, ubiquitin and small ubiquitin-like modifier (SUMO) conjugation, and the unfolded protein response (UPR), which is activated when endoplasmic reticulum (ER) function is impaired. We also analyzed changes in levels of O-linked β-N-acetylglucosamine (O-GlcNAc)-modified proteins, a modification that uses UDP-GlcNAc as substrate, the end product of the hexosamine biosynthetic pathway (HBP). Notably, many studies have reported that HBP is functionally connected with the O-GlcNAc modification pathway, and that activation of O-GlcNAcylation protects cells from a variety of stress conditions and hearts from ischemic stress.3,4
Materials and methods
Details of the experimental procedures are provided in the Supplementary Information. This study was approved by the Duke University Animal Care and Use Committee, and complies with the Guide for the Care and Use of Animals published by the National Institutes of Health. Experiments were reported according to the ARRIVE guidelines. Experiments were performed on anesthetized and temperature-controlled 2- and 22-month-old male C57Bl/6 mice. Animals were anesthetized with isoflurane, and forebrain ischemia was induced by 10 minutes bilateral common carotid artery occlusion and blood withdrawal to reduce mean arterial pressure to 30 mm Hg. After 60 minutes of reperfusion, animals were re-anesthetized, and brains were quickly removed, and the cortex and hippocampus were dissected and immediately frozen. Ischemia-induced changes in mRNA and protein levels were evaluated by quantitative PCR (qPCR) and Western blot analysis, respectively. Primers and antibodies used in this study are listed in Tables S1 and S2. All individual data were corrected for corresponding β-actin levels. Image analysis of Western blots was performed using the ImageJ program and a short exposure time to guarantee that optical densities were within the dynamic film range. Statistical analysis was performed using two-sample t-test or 2-way ANOVA to search for interactions between ischemia and age (GraphPad Prism 6). Data are reported as mean ± SD (n = 4–5 per group).
Results
Rotarod performance
Rotarod performance was evaluated before and 3 days after ischemia. Post-ischemic rotarod performance was significantly worse in aged than in young mice, implying age-related impairment of functional recovery (Figure S1).
Heat-shock response
Activation of Hsp70 expression is a global cellular response to a variety of stress conditions. Transient cerebral ischemia triggered a massive increase in Hsp70 mRNA levels in young mice, which was less pronounced in the hippocampus of old mice because the baseline level of Hsp70 mRNA is about 4.2 higher in aged mice compared to young animals (Table S3).
UPR
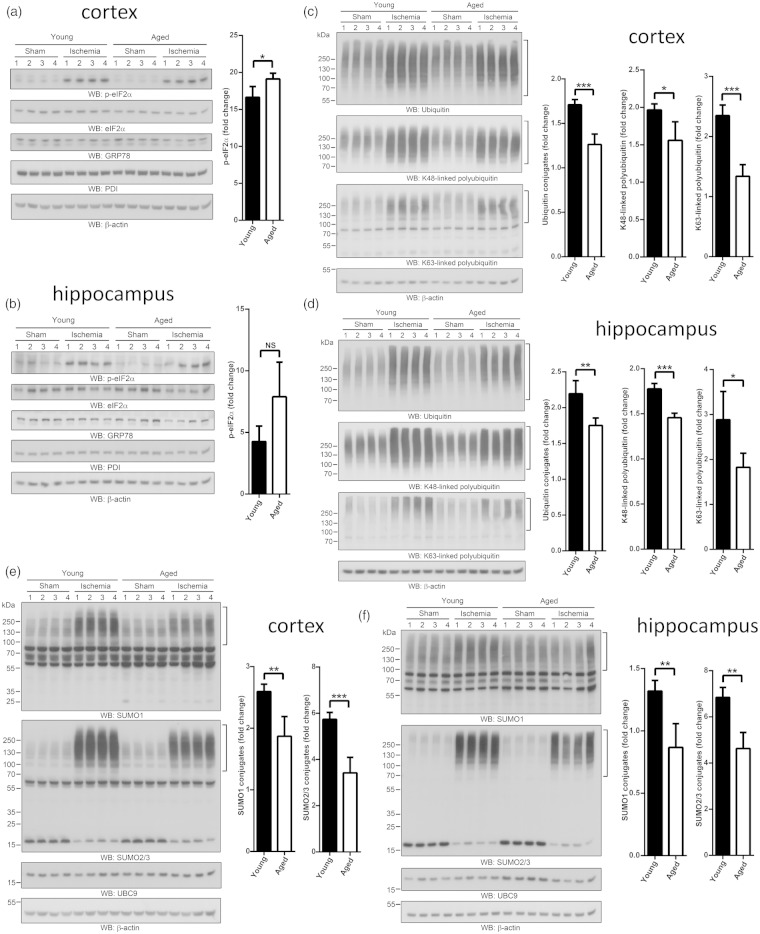
It is well documented that transient cerebral ischemia impairs ER functions and consequently activates UPR.5,6 We analyzed protein levels of eIF2α, phosphorylated eIF2α, GRP78, and PDI, and mRNA levels of spliced Xbp1 (Xbp1s), as measures of activation of UPR. In line with previous studies, phosphorylation of eIF2α and Xbp1 mRNA splicing were activated after transient forebrain ischemia in young and old mice, but we found only minor age-related differences (Figure 1a and b, Figure S2, Table S3).
Figure 1.
Activation of stress response pathways in post-ischemic brains of young and aged mice. Two- and 22-month-old male mice were subjected to 10 minutes forebrain ischemia and 1 hour of reperfusion. Sham-operated mice served as controls. (a and b) Effects of age on ischemia-induced changes in levels of phosphorylated eIF2α, total eIF2α, GRP78, and PDI as measures of UPR induction. Ischemia-induced phosphorylation of eIF2α was significantly more pronounced in cortex of aged than in young mice (a). (c and d) Effects of age on ischemia-induced changes in total ubiquitin conjugation and levels of polyubiquitin with K48 and K63 linkage. In cortex and hippocampus of aged mice the post-ischemic increase in levels of ubiquitin-conjugated proteins was significantly suppressed, particularly polyubiquitin with K63 linkage. (e and f) Effects of age on ischemia-induced activation of SUMO1 and SUMO2/3 conjugation and changes in Ubc9 levels. In aged mice, the post-ischemic activation of SUMO1 and SUMO2/3 conjugation was markedly suppressed, both in the cortex (e) and hippocampus (f). The high-molecular-weight regions marked by brackets were used to quantify levels of conjugates. All individual data were normalized to β-actin. For calculation of fold change, mean values of sham groups were set to 1.0. Data are presented as means ± SD (n = 4 per group). *p < 0.05; **p < 0.01; ***p < 0.001.
Ubiquitin conjugation
Global ubiquitin conjugation is induced by transient brain ischemia, and accumulation of ubiquitin-conjugated proteins in Triton-insoluble aggregates is massively increased in post-ischemic brains.7–9 We evaluated the effect of age on ischemia-induced changes in global ubiquitin conjugation and polyubiquitin with K48- and K63-linkage (Figure 1c and d, Figure S3). Global ubiquitin conjugation and polyubiquitin with K48 and K63 linkage were significantly increased after ischemia in young mice but this stress response was significantly suppressed in aged mice. This age-related effect was particularly pronounced for polyubiquitin with K63 linkage (Figure 1c and d; Figure S3), i.e. ischemia-induced activation of polyubiquitin with K48 linkage was 20% and 18% less in the cortex and hippocampus of aged mice as compared to young animals, while polyubiquitin with K63 linkage was 43% and 37% less in the cortex and hippocampus of aged animals as compared to young animals, respectively (Figure 1c and d).
SUMO conjugation
SUMO1 and SUMO2/3 conjugation is markedly activated after brain ischemia, and evidence suggests that this is a neuroprotective stress response.10–12 We analyzed levels of SUMO1- and SUMO2/3-conjugated proteins and protein levels of the conjugating enzyme Ubc9. Interestingly, we found that levels of SUMO1- and SUMO2/3-conjugated proteins were markedly increased in brains of young mice, but this stress response was significantly suppressed in the cortex and hippocampus of aged mice (Figure 1e and f, Figure S4). For Ubc9, age-related changes were confined to the hippocampus in which Ubc9 levels were higher in sham and decreased in post-ischemic brains in aged mice as compared to young animals (Figure S4).
O-GlcNAc modification
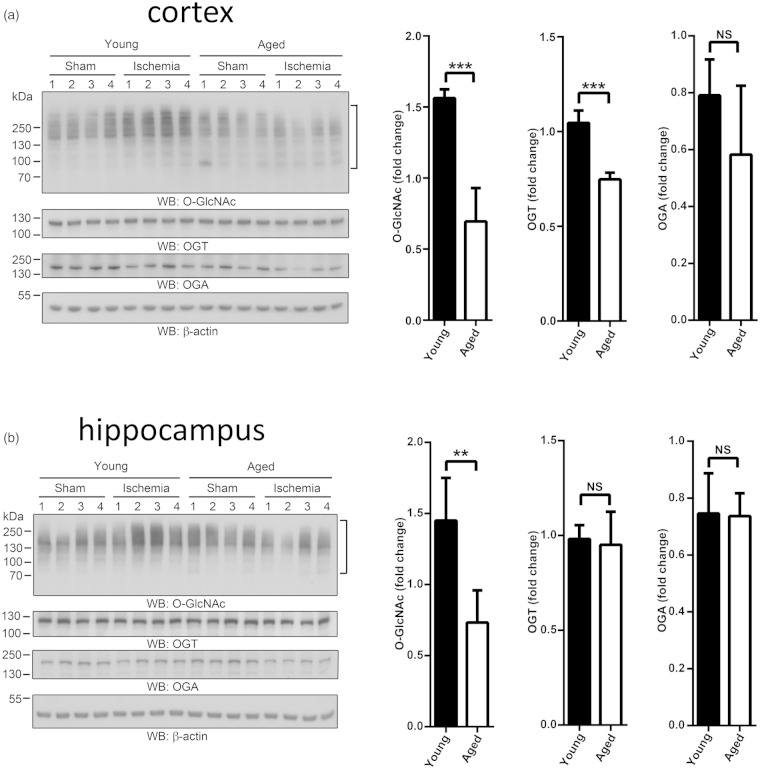
O-GlcNAcylation has been extensively studied in a variety of stress conditions and in models of myocardial ischemia but not yet in models of brain ischemia. We, therefore, decided to compare levels of O-GlcNAcylated proteins in post-ischemic brains of young and old mice. Notably, O-GlcNAc modification was significantly activated in the young mice, both in the cortex and hippocampus, but this stress response was completely absent in brains of aged animals (Figure 2a and b). As a first attempt to uncover the mechanisms underlying the inability of aged mice to activate O-GlcNAc modification of proteins after an ischemic challenge, we analyzed protein levels of O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), as well as mRNA levels of OGT, OGA, and glutamine fructose-6-phosphate aminotransferase 1 (GFAT1). O-GlcNAc is added and removed from target proteins by OGT and OGA, respectively, while GFAT1 is the rate-limiting enzyme of the HBP. In the cerebral cortex, transient ischemia induced a significant decrease of OGT levels in aged animals (Figure S5). Furthermore, in post-ischemic brains of aged mice, GFAT1 mRNA levels were significantly lower than in young animals (Table S3).
Figure 2.
Activation of O-GlcNAc modification in brains of young and aged mice. Two- and 22-month-old male mice were subjected to 10 minutes forebrain ischemia and 1 hour of reperfusion. Sham-operated mice served as controls. (a) Ischemia-induced changes in O-GlcNAc-modified proteins and levels of OGT and OGA in cortex of young and aged mice. (b) Ischemia-induced changes in O-GlcNAc-modified proteins and levels of OGT and OGA in hippocampus of young and aged mice. The high-molecular-weight regions marked by brackets were used to quantify levels of O-GlcNAc-modified proteins. All individual data were normalized to β-actin. For calculation of fold change, the mean values of sham groups were set to 1.0. Data are presented as means ± SD (n = 4 per group). *p < 0.05; **p < 0.01; ***p < 0.001.
Interactions between age and ischemia
Two-way ANOVA identified the following significant interactions: Ubiquitin (cortex, p = 0.0023; hippocampus, p = 0.0042); polyubiquitin K48 linkage (hippocampus, p = 0.0006); polyubiquitin K63 linkage (cortex, p = 0.0009); p-eIF2α (cortex, p = 0.0049); SUMO1 (cortex, p = 0.0042; hippocampus, p = 0.003); SUMO2/3 (cortex, p = 0.0021; hippocampus, p = 0.0119); Ubc9 (hippocampus, p = 0.0133); O-GlcNAc (cortex, p = 0.0007; hippocampus, p = 0.0103); OGT (cortex, p = 0.0003).
Discussion
Here, we have reported results from an experimental study designed to evaluate the effects of age on the response of brains to an ischemic challenge. Our long-term goal is to uncover the mechanisms underlying impaired functional recovery of elderly stroke patients. However, we used a model of transient global brain ischemia for our studies to guarantee that our results would reflect only the ability of young and aged brains to activate stress response pathways and not be confounded by unrelated factors such as size of infarcts. Further, since we were particularly interested in brain protection, we focused our studies on the cortex, a brain region resistant to a short period of transient forebrain ischemia. To determine whether age-related changes were limited to the cortex, or indicative of a global age-related change in the response of brains to a severe form of stress, we also analyzed hippocampal samples. Considering the wealth of information on the critical role of stress response pathways in recovery of cells after an insult, it is conceivable that the ability of cells to activate these pathways critically defines outcome. We, therefore, conclude that a key determinant of impaired functional recovery could be the inability of post-ischemic brains of old mice to activate key stress pathways to the same extent as do brains of young mice.
We have reported here that activation of ubiquitin, SUMO2/3, and O-GlcNAc modification was particularly suppressed in post-ischemic brains of old mice. In earlier studies, we have demonstrated that SUMO2/3 conjugation is massively activated after brain ischemia, and we provided evidence that this is a neuroprotective stress response.11,13 We have also characterized the SUMO3-modified proteome regulated by brain ischemia to identify proteins and pathways with putative neuroprotective functions.10 These include glucocorticoid receptor signaling, RNA processing, and SUMOylation-dependent ubiquitin conjugation, a process that plays a key role in DNA damage repair. Results presented here suggest that post-ischemic activation of the DNA damage repair pathway may be particularly impaired in old mice, since activation of both SUMO2/3 and ubiquitin conjugation was markedly less than in young animals (Figure 1). This notion is further supported by our observation that the effect of age on ischemia-induced activation of ubiquitin conjugation was particularly pronounced for polyubiquitin with K63 linkage, which is involved in DNA damage repair.
For several reasons, we believe that our results regarding O-GlcNAc modification are potentially the most relevant to deciphering the molecular mechanisms underlying the age-related decline of the brain capacity for functional recovery from a transient interruption of blood supply. (1) Ample evidence has been presented to support the notion that activation of O-GlcNAcylation protects hearts from ischemic damage.3,4 Specifically, increasing global O-GlcNAcylation is cardioprotective and improves functional recovery from ischemia, while decreasing global O-GlcNAc modification sensitizes cardiomyocytes to re-oxygenation injury. Further, activation of O-GlcNAcylation attenuates ischemia-induced oxidative stress and calcium overload, and suppresses mitochondrial transition pore opening triggered by oxidative stress. It is, therefore, reasonable to conclude that the activation of O-GlcNAc protein modification reported here is also a protective stress response shielding neurons from ischemic damage. (2) We show that O-GlcNAc modification is activated early after ischemia. Since UDP-GlcNAc, the substrate for O-GlcNAcylation, is the end product of the hexosamine biosynthetic pathway (HBP), and both pathways are functionally connected,14 it would be interesting to determine whether more glucose is channeled through HBP during early reperfusion when glucose is needed to replenish ATP stores. (3) Brains of old mice had completely lost their ability to activate O-GlcNAcylation in response to ischemia. Since brain levels of O-GlcNAc-modified proteins are particularly enriched at synapses, and since this pathway plays a key role in synaptic plasticity,15 the age-related dysfunctional HBP/O-GlcNAc axis could be a critical factor that limits functional recovery of aged brains from a period of non-sufficient blood supply. Of note, our conclusion is currently based only on correlative evidence. Considering the many reports on the protective role of the stress response pathway under investigation, we expect to report causality in the near future.
Supplementary Material
Acknowledgements
The authors thank Pei Miao for her excellent technical assistance, Kathy Gage, research development associate, for her excellent editorial contribution in the preparation of this manuscript, and Dr Yi-Ju Ji, Duke Department of Biostatistics & Bioinformatics, for excellent statistical advice.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by NIH R01 grant NS081299 and R03 grant NS078590 (to WP) and by AHA scientist development grant 12SDG11950003 (to WY).
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
SL, HS, ZY, and WY performed experiments and data analysis; WP and WY conceived the study; WP and WY wrote the manuscript
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Hallstrom B, Jonsson AC, Nerbrand C, et al. Stroke incidence and survival in the beginning of the 21st century in southern Sweden: comparisons with the late 20th century and projections into the future. Stroke 2008; 391: 10–15. [DOI] [PubMed] [Google Scholar]
- 2.Manwani B, Friedler B, Verma R, et al. Perfusion of ischemic brain in young and aged animals: a laser speckle flowmetry study. Stroke 2014; 45: 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ngoh GA, Jones SP. New insights into metabolic signaling and cell survival: the role of beta-O-linkage of N-acetylglucosamine. J Pharmacol Exp Ther 2008; 327: 602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatham JC, Marchase RB. The role of protein O-linked beta-N-acetylglucosamine in mediating cardiac stress responses. Biochim Biophys Acta 2010; 1800: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar R, Krause GS, Yoshida H, et al. Dysfunction of the unfolded protein response during global brain ischemia and reperfusion. J Cereb Blood Flow Metabol 2003; 23: 462–71. [DOI] [PubMed] [Google Scholar]
- 6.Paschen W, Aufenberg C, Hotop S, et al. Transient cerebral ischemia activates processing of xbp1 messenger RNA indicative of endoplasmic reticulum stress. J Cereb Blood Flow Metabol 2003; 23: 449–461. [DOI] [PubMed] [Google Scholar]
- 7.Hu BR, Janelidze S, Ginsberg MD, et al. Protein aggregation after focal brain ischemia and reperfusion. J Cereb Blood Flow Metabol 2001; 21: 865–875. [DOI] [PubMed] [Google Scholar]
- 8.Hochrainer K, Jackman K, Anrather J, et al. Reperfusion rather than ischemia drives the formation of ubiquitin aggregates after middle cerebral artery occlusion. Stroke 2012; 43: 2229–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwabuchi M, Sheng H, Thompson JW, et al. Characterization of the ubiquitin-modified proteome regulated by transient forebrain ischemia. J Cereb Blood Flow Metabol 2014; 34: 425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang W, Sheng H, Thompson JW, et al. Small ubiquitin-like modifier 3-modified proteome regulated by brain ischemia in novel small ubiquitin-like modifier transgenic mice: putative protective proteins/pathways. Stroke 2014; 45: 1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datwyler AL, Lattig-Tunnemann G, Yang W, et al. SUMO2/3 conjugation is an endogenous neuroprotective mechanism. J Cereb Blood Flow Metabol 2011; 31: 2152–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YJ, Mou Y, Maric D, et al. Elevated global SUMOylation in Ubc9 transgenic mice protects their brains against focal cerebral ischemic damage. PloS One 2011; 6: e25852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang W, Sheng H, Warner DS, et al. Transient global cerebral ischemia induces a massive increase in protein sumoylation. J Cereb Blood Flow Metabol 2008; 28: 269–279. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Pang Y, Chang T, et al. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol 2006; 40: 303–312. [DOI] [PubMed] [Google Scholar]
- 15.Tallent MK, Varghis N, Skorobogatko Y, et al. In vivo modulation of O-GlcNAc levels regulates hippocampal synaptic plasticity through interplay with phosphorylation. J Biol Chem 2009; 284: 174–181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.