Abstract
Following ischemia, the blood–brain barrier is compromised in the peri-infarct zone leading to secondary injury and dysfunction that can limit recovery. Currently, it is uncertain what structural changes could account for blood–brain barrier permeability, particularly with aging. Here we examined the ultrastructure of early and delayed changes (3 versus 72 h) to the blood–brain barrier in young adult and aged mice (3–4 versus 18 months) subjected to photothrombotic stroke. At both time points and ages, permeability was associated with a striking increase in endothelial caveolae and vacuoles. Tight junctions were generally intact although small spaces were detected in a few cases. In young mice, ischemia led to a significant increase in pericyte process area and vessel coverage whereas these changes were attenuated with aging. Stroke led to an expansion of the basement membrane region that peaked at 3 h and partially recovered by 72 h in both age groups. Astrocyte endfeet and their mitochondria were severely swollen at both times points and ages. Our results suggest that blood–brain barrier permeability in young and aged animals is mediated by transcellular pathways (caveolae/vacuoles), rather than tight junction loss. Further, our data indicate that the effects of ischemia on pericytes and basement membrane are affected by aging.
Keywords: Stroke, blood–brain barrier, ischemia, extravasation, endothelium, pericytes, astrocytes, basement membrane, transcytosis, aging
Introduction
The blood–brain barrier (BBB) is a specialized structure comprised of endothelial cells, basement membrane, pericytes, and astrocytes.1 This structure plays a crucial role in regulating the passage of ions, protein, and inflammatory cells between blood plasma and brain.2 Within hours and up to several days after an ischemic stroke, the function and integrity of the BBB is compromised in peri-infarct regions3 allowing an excess of water, protein (e.g. albumin), and other potentially toxic elements in the plasma to enter the brain parenchyma.4 BBB disruption correlates with astrocyte swelling, loss of synaptic structure, and normal brain function.5–8
Given the relatively long time window in which the BBB is disrupted after stroke and the deleterious consequences of this dysfunction, a greater understanding of the morphological changes that accompany BBB dysfunction in peri-infarct regions could stimulate new and inclusive stroke therapies. Over the past few decades, several theories have been proposed to account for BBB dysfunction. Particular attention has focused on endothelial tight junctions (TJ) and the proteins that comprise the TJ complex which regulate paracellular transport. Evidence supporting the role of TJ disassembly has come largely from light microscopic or biochemical experiments showing changes in TJ protein (e.g. claudin, occludin) immunofluoresence or protein/mRNA levels after stroke.9,10 Electron microscopic evidence of TJ disassembly has been more scant11 and seemingly contradictory with some studies reporting no evidence of TJ disruption early (<25 h) after stroke,12,13 while others have shown a partial disruption by 48 h.10 Another explanation for BBB permeability that has received renewed attention involves transcellular transport through the endothelium.14 After stroke, regulation of this transcytotic pathway can come into disarray, leading to aberrant transcellular movement of blood-borne constituents into the brain. This idea has been supported from ultrastructural investigations using experimental models of traumatic brain injury,14 global ischemia,15 ischemia–reperfusion,5,7,10,16 or embolic stroke.12 Beyond the endothelium, other cellular/structural elements such as pericytes, the basement membrane, and astrocytes likely influence the function and integrity of the BBB.1,8,17,18 For example, it is well established that ischemia can alter basement membrane integrity and induce persistent swelling of astrocyte endfeet.19–21 While the function of pericytes has remained somewhat enigmatic, pericyte swelling and degeneration has been described after ischemia.7,22 Collectively, these morphological studies show that each constituent of the BBB is affected by ischemia, although the degree to which they are affected is likely dependent on the timing and location (relative to the ischemic zone) studied.
One important and unresolved knowledge gap in the existing literature concerns the effect of aging on BBB ultrastructure after stroke. Aging is known to produce subtle morphological and functional changes to the BBB and is commonly associated with poorer functional recovery from stroke.23,24 Therefore, in the present study, we employed both light and electron microscopy to test the hypothesis that morphological changes to the BBB after stroke are affected by aging. We focused on the peri-infarct zone which previous studies have indicated is a hub for neuronal and vascular adaptations that are critical for the restoration of brain functions after focal ischemic stroke.25,26 Our data show that stroke-induced changes to the vascular endothelium and glial endfeet are remarkably similar between young adult and aged mice. However, aging did alter the nature of pericyte and basement membrane responses to stroke.
Materials and methods
Animals
Young adult and aged (3–4 versus 18 month old) male C57BL/6 mice were used in this study. Mice were housed under a 12 hour (h) light/dark cycle and had ad libitum access to water and laboratory diet. All experiments were conducted according to the guidelines set by the Canadian Council of Animal Care and approved by the University of Victoria Animal Care Committee. Reporting of this work complies with ARRIVE guidelines.
Induction of permanent focal ischemia
Focal ischemic stroke of the right forelimb somatosensory cortex was induced in mice using the photothrombotic method as previously described.26 Mice were anesthetized with 1–1.5% isoflurane in medical air (flow rate = 0.7 L/min). Each mouse was kept on a feedback-driven heating pad during surgery to stabilize body temperature at 37℃. The scalp was retracted and the skull overlying the forelimb cortex was thinned with a dental drill until the surface vessels became visible through the skull. Mice were then injected with 1% Rose Bengal (110 mg/kg i.p., Sigma) dissolved in 0.9% saline and photothrombosis was initiated by exposing the surface vessels over the forelimb cortex to a collimated green laser beam (532 nm beam at 17 mW, Beta Electronics) for 15 min. Sham operate mice received either Rose Bengal injection or light exposure, but not both. Mice were allowed to recover after surgery under a heating lamp before being returned to their cages. No mortality was encountered in stroke-affected mice.
Evans blue assay for visualizing BBB permeability
To visualize BBB permeability in the peri-infarct regions at the light microscopic level, mice were lightly anesthetized with 1% isoflurane and administered 2% Evans blue dye (0.15 ml i.v., Sigma) either 2.5 or 71.5 h after photothrombotic stroke (n = 3–4 mice per group) or sham surgery (n = 2 mice). Dye injection was administered slowly over a 10 min period. Thirty minutes after initiating dye injection, mice were transcardially perfused with heparinized PBS for ∼5 min until the liver cleared and then administered 2% glutaraldehyde and 2% paraformaldehyde in 0.15 M cacodylate buffer (pH 7.4) for ∼10 min. Brains were removed and fixed overnight in the same fixative at 4℃. Coronal brain sections were cut 100–150 µm thick and a series of sections were immediately mounted onto glass slides and imaged with a confocal microscope (Olympus with Fluoview software) using a 10X objective (NA = 0.40). Evans blue was excited with a 635 nm laser and emitted light between 665 and 735 nm was collected. High-resolution image stacks (1024 × 1024, 0.97 µm/pixel) of peri-infarct or contralateral cortex were taken in 4 µm Z steps. Images shown in Figure 1(b) represent 10 planar images projected (average intensity) in the Z plane. The brain from one young adult mouse (72 h recovery after stroke) that was not used for electron microscopic analysis was sectioned and stained for cresyl violet to illustrate the extent of the cerebral infarct (Supplementary Figure 1).
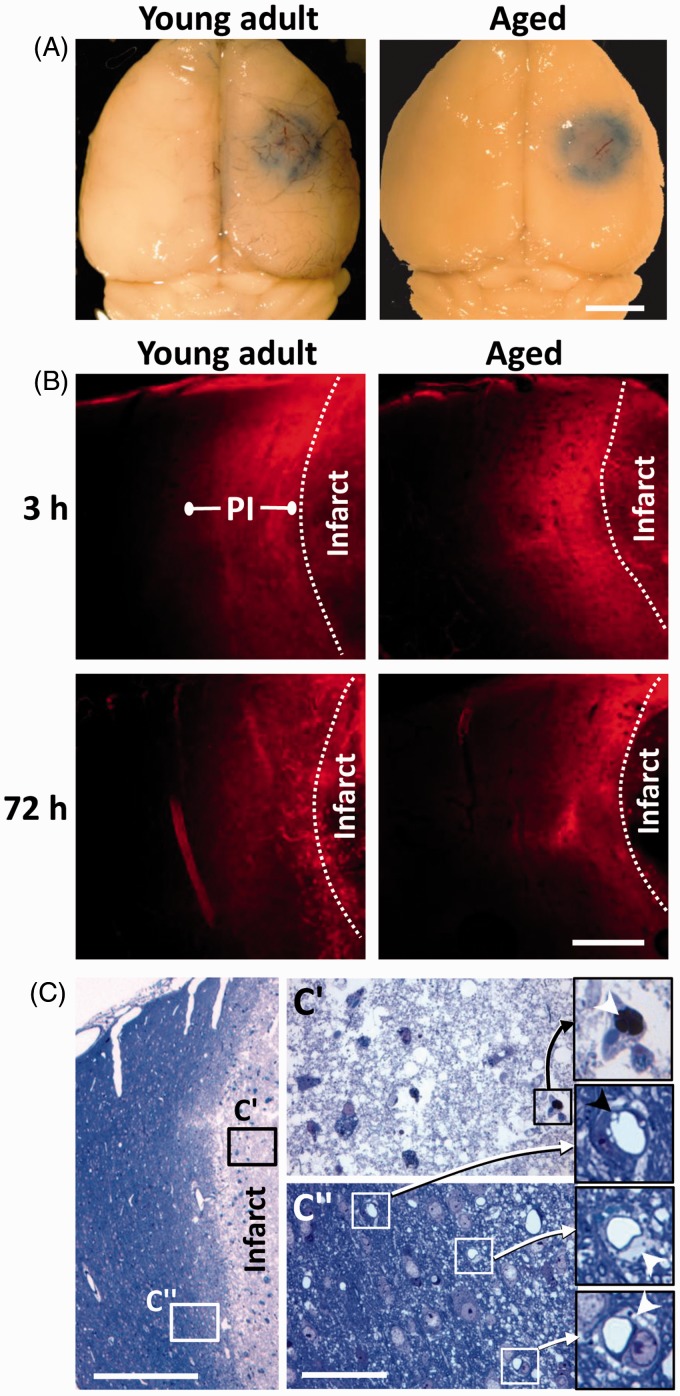
Figure 1.
Focal ischemic stroke leads to a long-lasting increase in vascular permeability in the peri-infarct cortex of young adult and aged mice. (a) Whole-brain brightfield images showing Evans blue dye extravasation around the cerebral infarct 3 h after initiating photothrombosis. Scale bar = 2 mm. (b) Representative confocal images (Z projection of 10 planar images taken in 4 µm steps) showing the halo of Evans blue fluorescence around the cerebral infarct. This halo of diffuse fluorescence usually extends ∼300–500 µm from the infarct border, which we refer to as “peri-infarct” (PI) cortex. Note that cortical areas more distant to the infarct exhibit little to no fluorescence. Scale bar = 200 µm. (c) A toluidine blue stained 0.5 µm thick section showing the infarct core (c′) and peri-infarct region (c″) 72 h after stroke in an aged mouse. The vessels inside the infarct core were either plugged (see arrowhead within inset from c′) or unrecognizable. Open perfused microvessels were evident in the peri-infarct zone (c″). Close inspection reveals vacuolated endothelium (black arrowhead) and swollen astrocyte endfeet (see white arrowheads within insets from c″). Scale bar = 400 µm for c and 50 µm for c″.
Transmission electron microscopy
Using Evans blue dye extravasation as a guide, peri-infarct zones (which also included a small portion of the necrotic infarct core for orientation) and contralateral regions of the somatosensory cortex were microdissected under a stereomicroscope. Tissue samples (1 × 1 mm) were postfixed in 1% osmium tetroxide and 1% potassium ferrocyanide in 0.15 M cacodylate buffer for 2 h, washed with distilled water, en bloc stained with 2% uranyl acetate (aq) for 2 h, dehydrated with ethanols, and embedded in Spurr’s resin. Semithin sections (0.5 µm) were cut with glass knives and stained with 1% toluidine blue (aq) for 30 s on a heating plate (60℃), rinsed with water, dried, and coverslipped with Cytoseal mounting medium. Toluidine blue-stained sections were used to identify tissue blocks for further imaging, specifically to confirm adequate perfusion and preservation of cytoarchitecture by evaluating the vasculature in the contralateral hemisphere. Signs of improper perfusion and preservation include stacking of red blood cells in arterioles, widespread swelling of astrocytes, and ruffling of endothelial cells. Brains exhibiting these characteristics were excluded from the analysis (one mouse was excluded from the young adult and aged experimental groups). Light microscopic imaging of toluidine blue-stained sections was performed on an Olympus BX51 brightfield microscope and images captured with a Q-color digital camera (Olympus). Ultrathin sections (50–80 nm) were cut on an Ultracut E ultramicrotome with a diamond knife (Diatome), collected on 200 hex copper mesh grids, poststained for 3–5 min with 0.5% lead citrate (aq), and observed on a JEOL JEM-1400 transmission electron microscope operated at 80 kV. Images were obtained with a Gatan SC-1000 digital camera. Calibration of images was performed by imaging a carbon replica grid (0.463 µm intervals) at the same magnifications. All chemicals for electron microscopy processing were obtained from Electron Microscopy Sciences.
Data analysis
For quantitative analysis, electron micrographs (4008 × 2672 pixels, 3.2 nm/pixel) of capillaries were analyzed using Image J software from control (young: n = 5 mice; aged n = 3 mice) or stroke-affected mice (n = 3 mice per group) using Image J software. Inclusion criteria for microvessel analysis were as follows: the diameter was <8 µm, the lumen was not occluded by blood cells or plasma, and the vessel was located in cortical layers 2–5 and within 300 µm of the infarct border. Images with an endothelial or pericyte nucleus were excluded from the analysis because they generated extreme measurements (e.g. for endothelial or pericyte area/coverage) and were too infrequent to adequately sample. Endothelial, pericyte, and astrocyte processes around the microvessel were manually traced by an experimenter blind to condition and thresholded to calculate area and perimeter. Vacuolation of the endothelium was quantified by normalizing the number of vacuoles to the circumference of the vascular endothelium. Pericyte coverage of endothelial cells (reported as percentage) was calculated based on the total length of inner pericyte processes around each vessel relative to the perimeter of the endothelium. The diameter of the capillary lumen, vacuoles, or caveolae, as well as basement membrane thickness, was measured in straight line segments at four cardinal points in the microvessel or organelle. Any endothelial TJ with a fluid space >50 nm wide was classified as a having an intercellular “space.”
Statistical comparisons were conducted with a two-way ANOVA followed by post hoc Bonferroni corrected t-tests comparing dependent measurements at 3 or 72 h relative to control microvessels. A summary of all statistical results for each two-way ANOVA is shown in Supplementary Table 1. All statistical tests were calculated based on means generated from the number of animals in each group. P values <0.05 were considered statistically significant. Data are expressed as the mean ± standard error of the mean.
Results
Light microscopic imaging of ischemic damage and BBB permeability
Since most of the sections containing part of the small infarct were microdissected for electron microscopic analysis, we were not able to use conventional stereological approaches to quantify infarct volume in young adult and aged mice. However, we did analyze brain surface images that showed Evans blue dye extravasation in the ischemic core/peri-infarct cortex at 3 h recovery (Figure 1(a)) and did not find any significant differences in damage-related surface area between young adult and aged mice (3.67 ± 1.21 versus 3.77 ± 0.83 mm2; t(6) = 0.06, p > 0.05; n = 4 mice per group). A representative series of coronal sections from a young adult mouse at 72 h recovery is shown in Supplementary Figure 1 to illustrate the normal extent of ischemic damage, which typically occupies a volume of 3–5 mm3 in the somatosensory cortex.27
Consistent with previous work,9,16 brains collected 3 or 72 h after stroke in young adult and aged mice reliably showed Evans blue dye fluorescence in cortical tissue within 300 µm of the infarct border (i.e. designated the “peri-infarct zone,” Figure 1(b)). Only perfused, unoccluded microvessels within this zone were selected for electron microscopic imaging and analyses (Figure 1(c) and (c″)), since these vessels would likely be a continual source of plasma (Evans blue) extravasation after stroke. Microvessels that were completely occluded or stalled were mostly found within the infarct core (Figure 1(c′)) and not selected for analysis because the cellular components of the BBB were difficult or impossible to identify due to severe necrosis by 72 h after ischemia (Supplementary Figure 2).
Ultrastructure of BBB breakdown
Sham operates versus contralateral hemisphere
The absence of Evans blue dye in the hemisphere contralateral to the stroke (see Figure 1(a)) suggested that BBB integrity was normal. However, since there is a report showing that transient middle cerebral artery occlusion can induce BBB dysfunction in distant brains regions,28 we compared BBB ultrastructure in young adult mice with sham stroke (25 capillaries from two mice) versus that obtained from the hemisphere contralateral to ischemic damage (30 capillaries from three mice). Our analysis revealed no significant differences between groups in any parameter tested (see Supplementary Table 2). Therefore, the BBB in the contralateral hemisphere could be used as a control group for subsequent analysis.
Endothelium
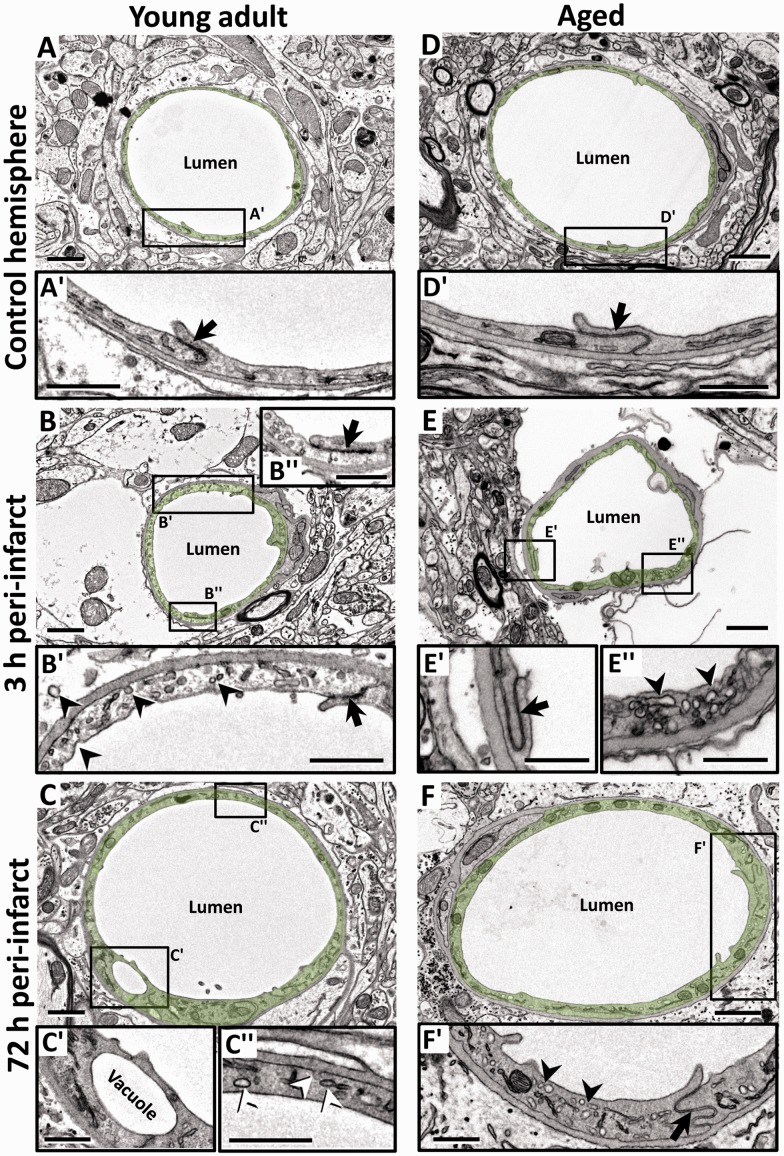
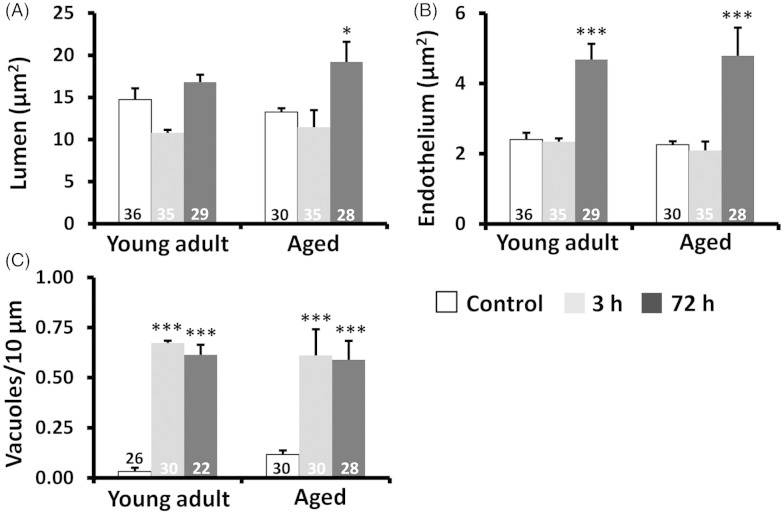
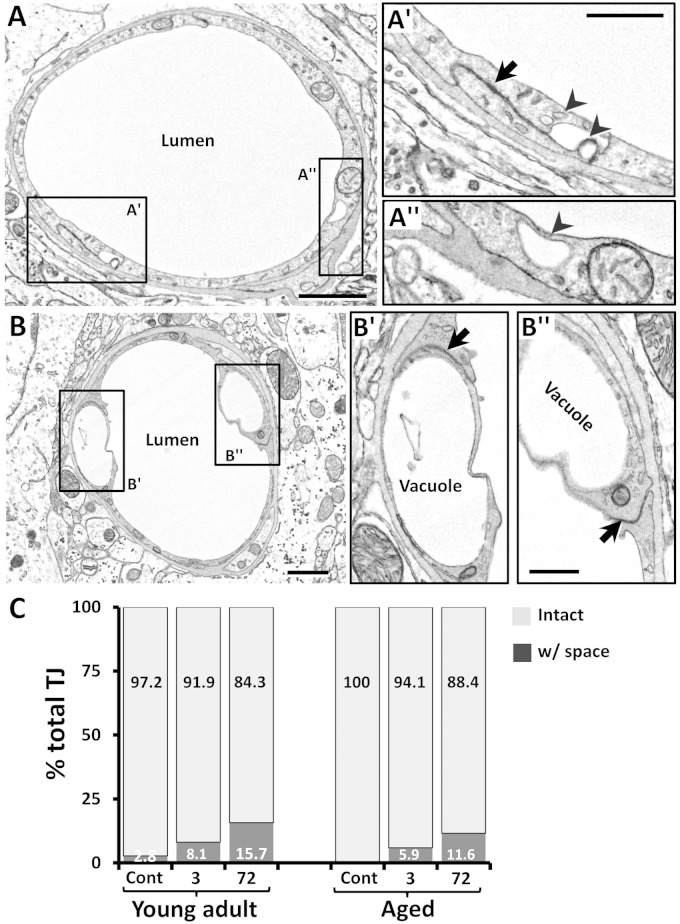
In nonischemic control microvessels at both ages, the endothelium was relatively thin (150 ± 50 nm thick in nonnuclear regions). Endothelial cells contained scattered small vesicles as well as TJs that appeared electron dense where the two endothelial cells interdigitated (Figure 2(a) and (d), arrows in 2(a′) and (d′)). Three hours after stroke, peri-infarct microvessels in both age groups were slightly constricted (Figure 2(b) and (e), also see histogram in Figure 3(a)). Notably, the endothelium was packed with small caveolae-like vesicles (diameter: 60 ± 17.5 nm) that lined both the luminal and abluminal sides of the endothelium (arrowheads in Figure 2(b′) and (e″)). By 72 h recovery from stroke, the microvessels in both age groups were more dilated than at 3 h recovery (Figure 2(c) and (f), also see histogram in Figure 3(a)). The endothelium was still filled with caveolae-like vesicles (Figure 2(c″) and (f′)) but now appeared quite swollen, as revealed by the significant increase in cross-sectional area of the endothelium (Figure 3(b)). In both age groups and recovery time points, larger fluid-filled organelles (>100 nm diameter) which we refer to as vacuoles formed in the endothelium (e.g. see Figure 2(c′) and 4(a) and (b)). After stroke, the number of vacuoles per 10 µm of vascular endothelium increased significantly for both ages and time points (Figure 3(c)). The location of a vacuole could occur at any site within the endothelium (Figure 4(a) and (a″)), although there was a tendency for vacuoles to appear adjacent to endothelial TJs (Figure 4(b) and (b″)). The mechanism that initiates large vacuole formation is not known but could be related to the long endothelial protrusions or marginal folds that we and others29 have observed (Supplementary Figure 3). Conceivably, these extensions could initiate the engulfment of debris from the vessel lumen, for example during microvessel recanalization.30 TJs, as a general rule, appeared intact in all groups examined (Figure 2(b″), (e′), and (f′)). However in a few instances, we observed a small fluid-filled space within the junction (Figure 4(a′)), which tended to increase in frequency over time after stroke (Figure 4(c)). These results suggest that ischemia-induced changes to the vascular endothelium are remarkably similar between young adult and aged mice.
Figure 2.
Focal ischemia induces endothelial swelling and upregulates caveolae and vacuole formation in peri-infarct capillaries in young adult and aged mice. (a, d) Electron micrographs (EMs) of capillaries in the nonischemic contralateral hemisphere in young adult (a) and aged (d) mice. Endothelium is lightly shaded green. Insets (a′ and d′) below each image show a relatively thin endothelial layer and intact TJs (arrow) at both ages. (b, e) Representative capillaries in peri-infarct cortex of young adult (b) and aged (e) mice 3 h after stroke. Insets (b′ and e″) show the slightly swollen endothelium that was densely packed with putative caveolae-like vesicles (arrowheads) on the luminal and abluminal sides. However, TJs (black arrows in b″ and e′) was intact at both time points. (c, f) EMs from young adult (c) and aged (f) mice showing dilated peri-infarct microvessels 72 h after stroke. At this time point, the endothelium appeared more swollen and was still laden with caveolae-like vesicles (c″ and f′) and the occasional vacuole (c′). TJs were generally intact (f′). Scale bar = 1 µm for a to f. Scale bar = 0.5 µm for insets a′ to f′.
Figure 3.
Analysis of endothelium, lumen area, and vacuolation in young adult and aged mice. (a and b) Histograms show that endothelial and microvessel lumen areas were significantly increased in both age groups 72 h after stroke. (c) In both young adult and aged mice, stroke significantly increased vacuole formation in the endothelium relative to control capillaries. Vacuoles are expressed as # vacuoles per 10 µm of the vascular endothelium circumference. The number in each histogram bar represents the number of capillaries analyzed per group, sampled from three to five mice per group. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 relative to controls.
Figure 4.
Vacuole and vesicle formation in relation to the tight junction. (a) EM of a peri-infarct capillary 3 h after stroke in a young adult mouse. On occasion, a membrane-bound space was evident at the TJ (black arrow in a′), perhaps due to the pooling and fusion of transcytotic vesicles (arrowheads in a′). However, these vacuoles could be found in parts of the endothelium not associated with TJ (a″). (b) Of note, vacuoles often formed in endothelium adjacent to the TJ (b′–b″). (c) Histogram shows the percentage of TJs that were completely intact or had a space present in young adult and aged mice. Although there were slightly more TJs with spaces after stroke (e.g. at 72 h), they were relatively infrequent. Percentages were based on the analysis of 34–43 TJs per group. Three mice were analyzed in each group. Scale bar = 1 µm for a and b and 0.5 µm for a′–b″.
Pericytes
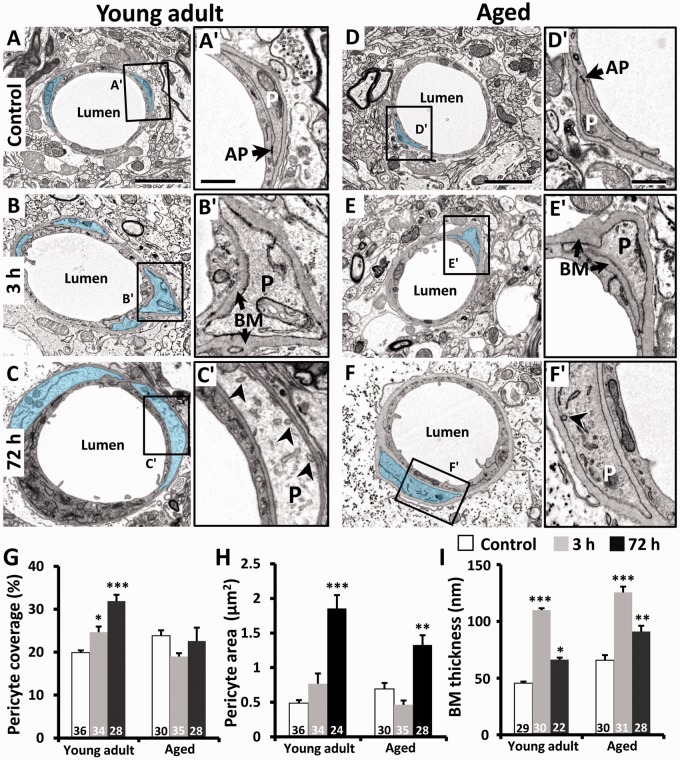
Endothelial cells were enwrapped by a discontinuous layer of pericytes whose processes are mostly ensheathed in basement membrane. In electron micrographs from young adult and aged mice (Figure 5(a) and (d)), pericyte processes could be distinguished by the granulated appearance of the cytoplasm and the presence of occasional electron-dense adhesion plaques with endothelial cells (Figure 5(a′) and (d′)). In agreement with previous studies,31,32 pericytes normally covered 19–24% of the outer perimeter of the endothelium in young adult and aged mice (Figure 5(g)). Interestingly, pericyte coverage of endothelial cells in young adult mice increased progressively and significantly over 72 h postischemia (Figure 5(g)). By contrast in aged animals, pericyte coverage was slightly reduced or unchanged at 3 and 72 h recovery (Figure 5(g)). Pericyte process area was also significantly increased at 72 h in both age groups (Figure 5(c) and (f)), albeit to a lesser extent in aged animals (Figure 5(h)). The thickness of the basement membrane region was greater in aged animals, even in control microvessels (Figure 5(i)), which is consistent with previous studies.23 However in both age groups, ischemia led to a pronounced increase in basement membrane region thickness at 3 h (Figure 5(b′) and (e′)), which declined by 72 h (Figure 5(c′) and (f′)). It should also be noted that caveolae-like vesicles and vacuoles, similar to those found in the endothelium were also present in pericytes at 3 and 72 h recovery (see arrowheads in Figure 5(c′) and Supplementary Figure 4), albeit to a lesser extent.
Figure 5.
Effects of ischemia on pericyte area, basement membrane thickness, and pericyte coverage of the vascular endothelium. (a to f) EMs of microvessels in young adult (a to c) and aged mice (d to f) in the contralateral control hemisphere or peri-infarct cortex 3 and 72 h after stroke. Pericytes are highlighted in blue. (a′ and d′) In control microvessels, pericyte processes (P) were encapsulated with a thin basement membrane (BM) and showed typical features such as an adhesion plaque (AP) at endothelial cell contact points. Three hours after stroke (b, e), the basement membrane thickened considerably and pericyte processes appeared slightly swollen (b′, e′). At 72 h, pericyte swelling increased along with its coverage of the endothelium in young adult mice (c′). However in aged mice, pericytes appeared swollen (f′) but do not show any change in their coverage of the endothelium (g). Quantitative analysis indicated that pericyte coverage of the endothelium (g) and cross-sectional area (h) increased significantly in peri-infarct cortex at 72 h in young adult mice. (i) Thickness of the basement membrane region increased significantly in both age groups at 3 h recovery and remained elevated in aged mice. The number in each histogram bar represents the number of capillaries analyzed per group, sampled from three to five mice per group. Scale bar = 2 µm for a to f. Scale bar for insets a′–f′ = 0.5 µm. *p ≤ 0.05, ***p ≤ 0.001 relative to controls.
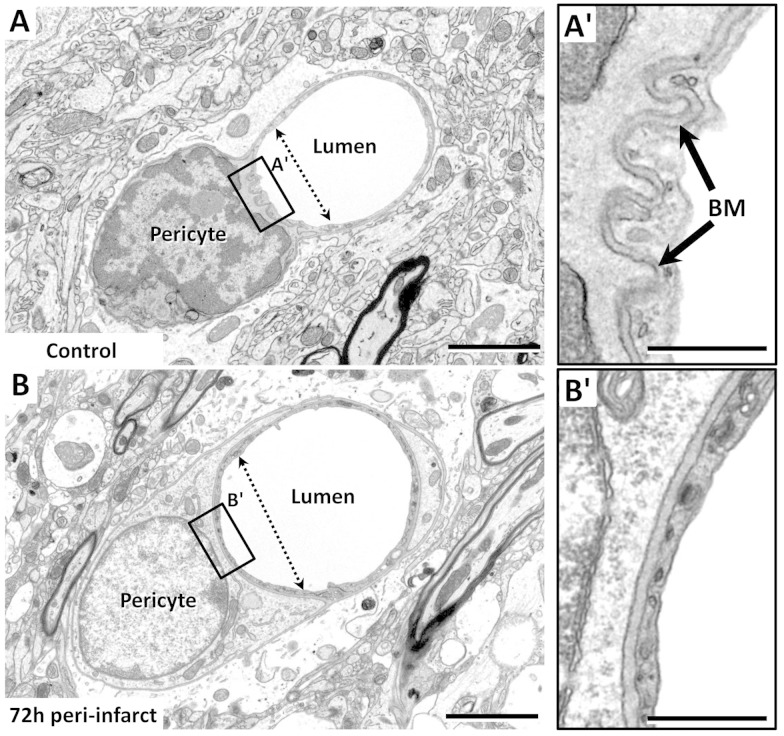
In a few capillaries, we encountered the pericyte soma and nucleus (Figure 6). We noted that the nucleus of pericytes appeared more electron dense (heterochromatic) in the control hemisphere relative to that observed in the peri-infarct zone in which nuclei appeared more euchromatic (compare Figure 6(a) and (b)). Additionally, in some examples, the vessel wall adjacent to the pericyte soma appeared constricted (Figure 6(a)) and was accompanied by a corrugated basement membrane (Figure 6(a′)). This contrasted with pericytes in the peri-infarct cortex (at 72 h recovery) that possessed a wider vessel lumen (Figure 6(b)) without a corrugated appearance (Figure 6(b′)).
Figure 6.
Examples of a pericyte wrapping around a microvessel. (a and b) EMs of a pericyte in the control hemisphere (a) or in peri-infarct cortex 72 h after stroke (b). Note that in the control microvessel, the endothelium and basement membrane were corrugated next to the pericyte soma (a′), and the lumen proximal to the soma was narrower than that distally, suggestive of a contractile function. Seventy-two hours after stroke when microvessels tended to be dilated, the corrugated appearance of the basement membrane was absent (b′), and the lumen of the vessel was larger. Scale bar = 2 µm for (a)–(b). Scale bar for insets a′–b′ = 0.5 µm.
Astrocytes
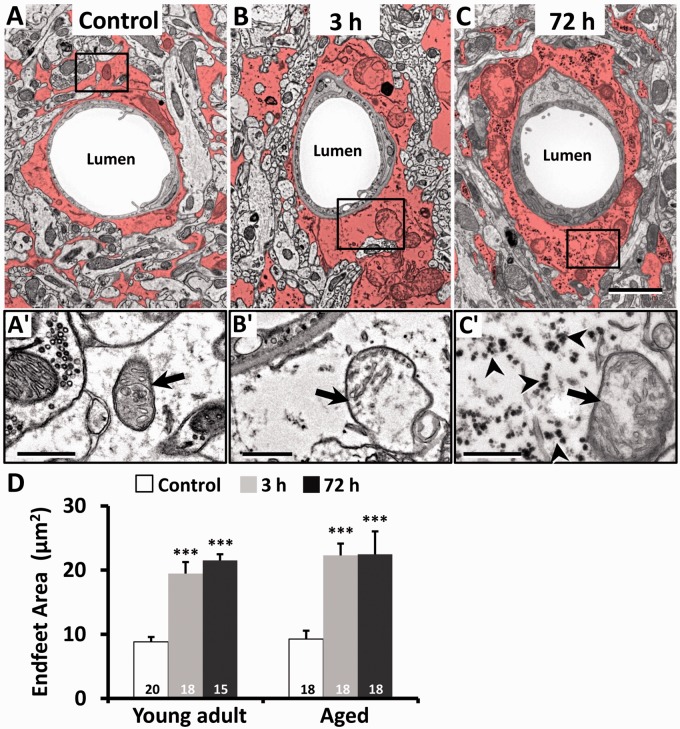
Consistent with previous work,19,33 astrocytes could be distinguished by the relative sparseness of electron-dense material in the cytoplasm (Figure 7(a) to (c)). In addition, we could identify astrocytes based on the fact that their mitochondrial membranes were less electron dense than those found in neurons, endothelium, and pericytes (Figure 7(a′)). Following stroke, perivascular astrocytes underwent large-scale changes in area by 3 h and continued to remain swollen at 72 h (see red shaded area in Figure 7(b) and (c)). Quantitatively, the cross-sectional area of astrocyte endfeet increased significantly after stroke in both young adult and aged mice (Figure 7(d)). Mitochondria in astrocytes of young adult and aged mice were also disrupted after stroke, appearing swollen with partially intact cristae (see arrows in Figure 7(b′) and (c′)). In agreement with a previous ultrastructure study,34 electron-dense glycogen granules (15–25 nm diameter) and rosettes (40–60 nm diameter) became abundant in the cytoplasm of astrocytes 72 h after stroke (see arrowheads in Figure 7(c′)).
Figure 7.
Astrocytic swelling and mitochondrial disruption in the peri-infarct zone. Representative EMs of perivascular astrocytes (shaded in red) in the nonischemic contralateral hemisphere (a) or peri-infarct cortex at 3 (b) and 72 h (c) after stroke in a young adult mouse. Astrocyte endfoot area increased considerably after stroke. Bottom row of images (a′ to c′) shows normal appearing mitochondria (arrow) in control astrocytes or swollen mitochondria with disorganized or absent cristae in the peri-infarct cortex at both 3 and 72 h recovery. Of note, astrocytes at 72 h were conspicuously packed with glycogen granules (see arrowheads in c′). (d) Histogram shows the average area of perivascular astrocyte endfeet in young adult and aged mice. The number in each histogram bar represents the number of capillaries analyzed per group, sampled from three to five mice per group. Scale bar = 2 µm for (a) to (c). Scale bar = 0.5 µm for a′ to c′ and D. **p < 0.01 or ***p < 0.001 compared to control.
Discussion
In the absence of ischemia, we did not observe Evans blue dye in extravascular spaces in young or aged animals. This is perhaps not surprising since mice in both age groups were generally healthy, and we did not examine very old mice (>2 years of age) where subtle changes to barrier function have been reported, although usually when other conditions such as hypertension or Alzheimer’s disease are present.23 At the ultrastructural level, our analysis suggested that endothelial and astrocytic components in control microvessels were morphologically similar between young and aged mice. For example, analysis of vessel lumen or endothelial area, endothelial vacuoles, TJ integrity, and astrocyte endfoot area in control microvessels was equivalent between age groups. These findings are in agreement with previous electron microscopy studies in aging human gray matter35 and 24-month-old rodent brain.36 However, our study did find that aging was associated with increased pericyte process area and basement membrane region thickness. While reports of pericyte area changes have been mixed,35–37 thickening of the basement membrane with aging is well appreciated.23,36,37
Based on our survey of the literature, there is a paucity of data comparing age-related changes in BBB ultrastructure after stroke. Given this limitation, our discussion will focus primarily on how our data compares to previous electron microscopy studies that subjected young adult animals to stroke. In general, ultrastructural studies have indicated that vessel permeability induced by ischemia, seizures, or other forms of brain injury is mediated primarily by a transcellular conduit system.12–14,33 It should be noted that BBB permeability could also be reliant on paracellular transport through TJ that normally bind endothelial cells together.2 Ischemia-induced disruption of TJ has been reported by several studies which show a loss of TJ protein levels or immunofluorescence in ischemic tissues.9,10,38 Reconciling these competing views, a recent study by Knowland et al.10 suggested that the initial phases of BBB permeability following ischemia and reperfusion in young animals are dependent on a caveolin-based transcytosis in the first 24 h which is accompanied by a disruption of TJs 2–3 days poststroke. In the present study, our ultrastructural analysis in young adult and aged mice provided strong evidence that BBB permeability at both early and late recovery time points (3 and 72 h) was associated with an increase in caveolae and vacuoles in the endothelium. While we are cognizant of the perils of assuming the direction of vesicular movement (and hence permeability) based on static images, our previous nanogold tracer experiments as well as others3,5,7,10,12 suggest that vesicles/vacuoles were likely moving blood-borne constituents from the luminal to abluminal side of the vascular endothelium. With respect to TJs, our data indicate that subtle perturbations can occur as we did see small spaces at these interfaces which is consistent with previous studies10,33 and may help explain previous reports of TJ protein loss. Pooling of fluid-filled spaces at the TJ would seem likely given the constant movement of vesicles/vacuoles through the endothelium (see Figure 4(a)). However, the incidence of these partial disruptions was relatively low (≤15.7%) as the majority of TJs were contiguous. It should be noted that complete uncoupling of the TJ from the luminal to abluminal side of the endothelium was never observed in the peri-infarct vessels we sampled from. Even stalled or occluded vessels at the edge of the infarct core still contained intact TJs (see Supplementary Figure 2(a)), suggesting TJs are quite resilient to the effects of ischemia.12 Given the paucity of even partial TJ disruption and the near ubiquitous increase in vesicles and vacuoles in the endothelium, our data suggest that BBB permeability (at the level of the endothelium) in peri-infarct cortex of young adult and aged mice is likely based on transcellular mechanisms. The functional consequences of increased transcytotic activity are not entirely clear. One prediction is that increased transport of proteins and ions into endothelial cells, astrocytes, and pericytes may disrupt osmotic gradients and promote water influx and perivascular swelling.
Once the endothelial barrier has been breached, the basement membrane and pericytes can serve as another protective layer between blood and nervous tissue. Expansion of the basement membrane has been frequently described with cerebral ischemia and aging.23 In our study, the expansion of the basement membrane region was strongly related to the degree of ischemia, since the basement membrane region in stalled/occluded vessels in the infarct core appeared much thicker than that found in peri-infarct cortex (compare Figure 5(b) and (e) to Supplementary Figure 2(a)), while those in peri-infarct were on average twice as thick as control vessels. The cause or functional significance of this expansion is unclear. Given the degree to which the basement membrane region expands after stroke and how quickly it occurs (3 h), it is presumably not caused by protein synthesis-dependent thickening of the fibrous basal lamina sheet, but instead could reflect pooling of electron dense plasma in this perivascular space.39
Pericytes have been implicated as important players in the maintenance of BBB integrity given that pericyte loss in mutant mice leads to micro-aneurysms and increased vascular permeability.8,18 Further, pericytes can secrete enzymes such as matrix metalloproteinases that degrade the basement membrane and thus regulate BBB permeability. One of the primary age-dependent effects in our study was that ischemia-induced swelling of pericytes and resultant increases in their coverage of the vascular endothelium were blunted in aged animals. It is possible that the progressive increase in pericyte area and coverage after ischemia in young animals represents a compensatory mechanism to limit further BBB permeability. Another possibility that cannot be discounted is that pericytes in the aged brain may be more susceptible to ischemic cell death. Therefore, a drop in pericyte coverage despite some swelling7 could be related to cell death. Unfortunately, we could not sample enough pericyte cell bodies in electron micrographs to definitively answer this question, although future studies examining age-related changes in cellular susceptibility to ischemia are warranted. Another interesting finding of our study was the increased presence of vesicles/vacuoles in pericytes. Transcellular movement of blood-borne protein through pericytes has been reported in nonischemic vessels.40 Our study extends this observation to suggest that stroke can upregulate constitutive activity of this transcellular pathway in pericytes.
Astrocyte endfeet are known to undergo significant swelling under ischemic conditions.13,19 Our data corroborate this effect and show its persistence after permanent focal ischemia induced by photothrombosis. Mitochondria in astrocytes were also severely swollen and disrupted, and exhibited disorganized cristae.19 Interestingly, these enlarged astrocytes became highly enriched with glycogen granules by 72 h recovery. This observation is consistent with previous electron microscopic studies showing that glycogen granules are present at 2–5 days, but not at seven days, or acutely (within few hours) after ischemia.34 While the functional significance of these glycogen granules is not known, it may reflect a reduction in the enzymes that degrade glycogen, or increased synthesis of glycogen due to lower blood flow and elevated levels of glucose uptake/utilization in peri-infarct areas.41
In summary, our imaging data reveal that BBB disruption in the peri-infarct cortex is associated with: (1) a striking increase in putative transcytotic vesicles and vacuoles in the endothelium of both age groups, (2) sparse evidence for disruption of interendothelial TJs, (3) progressive swelling of pericytes with increased endothelial coverage in young adult but not aged mice, (4) transient expansion of the basement membrane region, (5) astrocyte swelling and mitochondrial disorganization, and (6) progressive accumulation of glycogen granules in astrocytes at 72 h. These observations at the ultrastructural level should help guide future studies that seek to ameliorate BBB dysfunction after focal ischemia.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by operating, salary, and equipment grants to C.E.B from Heart and Stroke Foundation of BC and Yukon, CIHR, MSFHR, and CFI. An NSERC Discovery Grant and CFI award supported work by P.C.N.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
PCN and CEB conceived the study and wrote the manuscript. PCN, PRR, and CEB collected and analyzed data.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 2004; 16: 1–13. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 2005; 57: 173–185. [DOI] [PubMed] [Google Scholar]
- 3.Reeson P, Tennant KA, Gerrow K, et al. Delayed inhibition of VEGF signaling after stroke attenuates blood-brain barrier breakdown and improves functional recovery in a comorbidity-dependent manner. J Neurosci 2015; 35: 5128–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab 2003; 23: 879–894. [DOI] [PubMed] [Google Scholar]
- 5.Chen B, Friedman B, Cheng Q, et al. Severe blood-brain barrier disruption and surrounding tissue injury. Stroke 2009; 40: e666–e674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomkins O, Friedman O, Ivens S, et al. Blood-brain barrier disruption results in delayed functional and structural alterations in the rat neocortex. Neurobiol Dis 2007; 25: 367–377. [DOI] [PubMed] [Google Scholar]
- 7.Shinnou M, Ueno M, Sakamoto H, et al. Blood-brain barrier damage in reperfusion following ischemia in the hippocampus of the Mongolian gerbil brain. Acta Neurol Scand 1998; 98: 406–411. [DOI] [PubMed] [Google Scholar]
- 8.Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010; 68: 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Lopez D, Faustino J, Daneman R, et al. Blood-brain barrier permeability is increased after acute adult stroke but not neonatal stroke in the rat. J Neurosci 2012; 32: 9588–9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowland D, Arac A, Sekiguchi KJ, et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron 2014; 82: 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience 1998; 86: 1245–1257. [DOI] [PubMed] [Google Scholar]
- 12.Krueger M, Hartig W, Reichenbach A, et al. Blood-brain barrier breakdown after embolic stroke in rats occurs without ultrastructural evidence for disrupting tight junctions. PLoS One 2013; 8: e56419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westergaard E, Go G, Klatzo I, et al. Increased permeability of cerebral vessels to horseradish peroxidase induced by ischemia in Mongolian Gerbils. Acta Neuropathol 1976; 35: 307–325. [PubMed] [Google Scholar]
- 14.Lossinsky AS, Shivers RR. Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Histol Histopathol 2004; 19: 535–564. [DOI] [PubMed] [Google Scholar]
- 15.Dietrich WD, Busto R, Halley M, et al. The importance of brain temperature in alterations of the blood-brain barrier following cerebral ischemia. J Neuropathol Exp Neurol 1990; 49: 486–497. [DOI] [PubMed] [Google Scholar]
- 16.Jackman K, Kahles T, Lane D, et al. Progranulin deficiency promotes post-ischemic blood-brain barrier disruption. J Neurosci 2013; 33: 19579–19589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sagare AP, Bell RD, Zhao Z, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun 2013; 4: 2932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature 2010; 468: 557–561. [DOI] [PubMed] [Google Scholar]
- 19.Ito U, Hakamata Y, Kawakami E, et al. Temporary [corrected] cerebral ischemia results in swollen astrocytic end-feet that compress microvessels and lead to delayed [corrected] focal cortical infarction. J Cereb Blood Flow Metab 2011; 31: 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Risher WC, Croom D, Kirov SA. Persistent astroglial swelling accompanies rapid reversible dendritic injury during stroke-induced spreading depolarizations. Glia 2012; 60: 1709–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon I, Kim EH, del Zoppo GJ, et al. Ultrastructural and temporal changes of the microvascular basement membrane and astrocyte interface following focal cerebral ischemia. J Neurosci Res 2009; 87: 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueno M, Tomimoto H, Akiguchi I, et al. Blood-brain barrier disruption in white matter lesions in a rat model of chronic cerebral hypoperfusion. J Cereb Blood Flow Metab 2002; 22: 97–104. [DOI] [PubMed] [Google Scholar]
- 23.Mooradian AD. Effect of aging on the blood-brain barrier. Neurobiol Aging 1988; 9: 31–39. [DOI] [PubMed] [Google Scholar]
- 24.DiNapoli VA, Huber JD, Houser K, et al. Early disruptions of the blood-brain barrier may contribute to exacerbated neuronal damage and prolonged functional recovery following stroke in aged rats. Neurobiol Aging 2008; 29: 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol 2006; 59: 735–742. [DOI] [PubMed] [Google Scholar]
- 26.Brown CE, Li P, Boyd JD, et al. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J Neurosci 2007; 27: 4101–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seto A, Taylor S, Trudeau D, et al. Induction of ischemic stroke in awake freely moving mice reveals that isoflurane anesthesia can mask the benefits of a neuroprotection therapy. Front Neuroenergetics 2014; 6: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garbuzova-Davis S, Rodrigues MC, Hernandez-Ontiveros DG, et al. Blood-brain barrier alterations provide evidence of subacute diaschisis in an ischemic stroke rat model. PLoS One 2013; 8: e63553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hazama F, Ozaki T, Amano S. Scanning electron microscopic study of endothelial cells of cerebral arteries from spontaneously hypertensive rats. Stroke 1979; 10: 245–252. [DOI] [PubMed] [Google Scholar]
- 30.Lam CK, Yoo T, Hiner B, et al. Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature 2010; 465: 478–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011; 21: 193–215. [DOI] [PubMed] [Google Scholar]
- 32.Sims DE. The pericyte—a review. Tissue Cell 1986; 18: 153–174. [DOI] [PubMed] [Google Scholar]
- 33.Pluta R, Lossinsky AS, Wisniewski HM, et al. Early blood-brain barrier changes in the rat following transient complete cerebral ischemia induced by cardiac arrest. Brain Res 1994; 633: 41–52. [DOI] [PubMed] [Google Scholar]
- 34.Kajihara H, Tsutsumi E, Kinoshita A, et al. Activated astrocytes with glycogen accumulation in ischemic penumbra during the early stage of brain infarction: immunohistochemical and electron microscopic studies. Brain Res 2001; 909: 92–101. [DOI] [PubMed] [Google Scholar]
- 35.Stewart PA, Magliocco M, Hayakawa K, et al. A quantitative analysis of blood-brain barrier ultrastructure in the aging human. Microvasc Res 1987; 33: 270–282. [DOI] [PubMed] [Google Scholar]
- 36.Alba C, Vidal L, Diaz F, et al. Ultrastructural and quantitative age-related changes in capillaries of the dorsal lateral geniculate nucleus. Brain Res Bull 2004; 64: 145–153. [DOI] [PubMed] [Google Scholar]
- 37.Hicks P, Rolsten C, Brizzee D, et al. Age-related changes in rat brain capillaries. Neurobiol Aging 1983; 4: 69–75. [DOI] [PubMed] [Google Scholar]
- 38.Witt KA, Mark KS, Hom S, et al. Effects of hypoxia-reoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol 2003; 285: H2820–H2831. [DOI] [PubMed] [Google Scholar]
- 39.Ueno M, Tomita S, Nakagawa T, et al. Effects of aging and HIF-1alpha deficiency on permeability of hippocampal vessels. Microsc Res Tech 2006; 69: 29–35. [DOI] [PubMed] [Google Scholar]
- 40.Broadwell RD, Balin BJ, Salcman M. Transcytotic pathway for blood-borne protein through the blood-brain barrier. Proc Natl Acad Sci USA 1988; 85: 632–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nedergaard M, Jakobsen J, Diemer NH. Autoradiographic determination of cerebral glucose content, blood flow, and glucose utilization in focal ischemia of the rat brain: influence of the plasma glucose concentration. J Cereb Blood Flow Metab 1988; 8: 100–108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.