Abstract
Normal brain homeostasis depends on the integrity of the blood–brain barrier that controls the access of nutrients, humoral factors, and immune cells to the CNS. The blood–brain barrier is composed mainly of brain endothelial cells. Forming the interface between two compartments, they are highly polarized. Apical/luminal and basolateral/abluminal membranes differ in their lipid and (glyco-)protein composition, allowing brain endothelial cells to secrete or transport soluble factors in a polarized manner and to maintain blood flow. Here, we summarize the basic concepts of apicobasal cell polarity in brain endothelial cells. To address potential molecular mechanisms underlying apicobasal polarity in brain endothelial cells, we draw on investigations in epithelial cells and discuss how polarity may go awry in neurological diseases.
Keywords: Polarized secretion, Par complex, cerebral cavernous malformations, Crumbs, scribble
During vertebrate evolution, the blood–brain barrier changed from being a mainly glial barrier and became an endothelial one.1 In mammals, astrocytes still play an essential part in the blood–brain barrier, for instance, by ensheathing capillaries with their endfeet (Figure 1), synthesizing a layer of the basement membrane, and promoting barrier properties in endothelial cells. The physical barrier, however, is composed of endothelial cells, which are firmly connected by tight junctions and for which the rate of transcytosis is lower than in other tissues. At the cellular level, tight junctions form the border between the apical/luminal and the basolateral/abluminal plasma membrane. Separation of the apical/luminal and the basolateral/abluminal side of endothelial cells is a prerequisite for apicobasal polarity and is at the core of the blood–brain barrier that prohibits polar compounds, pathogens, and immune cells from reaching the CNS.
Figure 1.
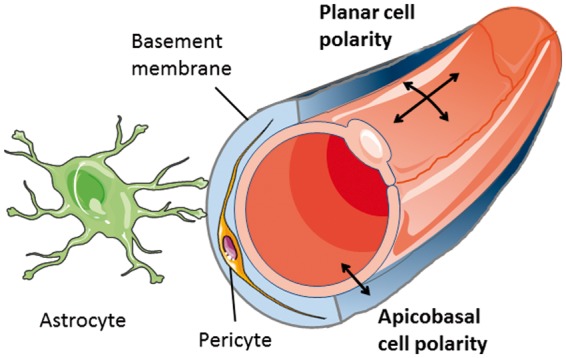
Key components of the blood-brain barrier. Apicobasal and planar cell polarity of endothelial cells differ in their orientation to the vessel axis.
Forms of endothelial polarity
Apicobasal polarity is not the only form of endothelial cell polarity. Geometrically speaking, apicobasal polarity is set apart from planar cell polarity and the polarity of endothelial cells migrating during angiogenesis (Figure 1). At the molecular level the distinction is more blurred as some forms of polarity share certain pathways but have different orientation. WNT signaling, for example, is essential for the planar cell polarity program but is also involved in apicobasal polarity of brain endothelial cells.2 The functional link between distinct polarity programs may facilitate the coordination of complex processes, such as angiogenesis, that involve both the polarity of migrating cells and apicobasal polarity.3
With respect to endothelial polarity, many studies have focused on the polarity of migrating endothelial cells. Apicobasal polarity has been investigated in the context of lumen formation during development or angiogenesis4,5 but less is known about the molecular basis of apicobasal polarity in quiescent brain endothelial cells, despite the likely importance of this program for the blood–brain barrier. Here we summarize some key findings with the aim to demonstrate that much remains to be learned about apicobasal polarity programs in brain endothelial cells.
Why does apicobasal polarity matter?
By maintaining apicobasal cell polarity, brain endothelial cells accomplish an amazing task in very small dimensions. Understanding the underlying mechanisms is of general biological interest and, in addition, may help to elucidate the pathophysiology of neurological diseases and to improve their treatment.
As a physical border between the apical/luminal and the basolateral/abluminal cell compartments, intercellular junctions play an essential role in maintaining apicobasal cell polarity.6 The complex relationship between intercellular junctions and apicobasal polarity involves two more aspects. Intercellular junctions provide docking sites for components of the apicobasal polarity program in the cell. Conversely, the polarity program regulates expression and function of intercellular junction proteins (see below). In many neurological diseases the continuous line of tight and adherens junctions is disrupted, leading to a loss of endothelial cell polarity and blood–brain barrier damage.7 In MS lesions destabilized adherens junctions were linked to a disturbed apicobasal polarity of brain endothelial cells with increased apical/luminal localization of CXCL12 that may attract immune cells to invade the CNS.8 Cerebral cavernous malformations seem to involve a primary defect in cell polarity. The genes that are mutated in familial cases, i.e. CCM1, CCM2, and CCM3, control cell polarity of brain endothelial cells9–11 (see below). Thus, correcting the polarity of brain endothelial cells may constitute a rational strategy for treating diverse neurological diseases.
On the other hand, the blood–brain barrier is an obstacle for treating brain diseases with macromolecules, such as recombinant proteins. Targeting brain endothelial cells with rAAV vectors or other tools may provide the means to express missing proteins in the CNS. Chen and colleagues12 successfully used this approach in a mouse model of a lysosomal storage disease. After gene transfer lysosomal enzymes are released from endothelial cells and then taken up by neural cells.13 To maximize the therapeutic potential of this approach, it is important to know how to direct transduced proteins to the correct side of brain endothelial cells and to understand polarized secretion.
Methods for studying apicobasal polarity of brain endothelial cells
In comparison to the situation in epithelium, endothelial polarity needs to occur in a miniaturized space. As brain endothelial cells are only about 200 nm thick,14 membrane protein sorting must be very efficient. The close vicinity of the apical/luminal and basolateral/abluminal plasma membranes has posed problems when studying the localization of membrane proteins. In several cases contradictory results were obtained as to whether membrane transporters were localized in the apical/luminal or the basolateral/abluminal membrane (Table 1). Such discrepancies may be partially explained by the species or experimental details of the study but they are probably more often due to technical problems in localizing membrane proteins. The following methods have been used to localize proteins in subcellular compartments of brain endothelial cells.
Functional assays
Table 1.
Localization of membrane proteins in brain endothelial cells.
| Name | Predominant localization | Method | Species | References | |
|---|---|---|---|---|---|
| Membrane transport proteins | PGP ( = MDR1, ABCB1) | Luminala,b | Immunostaining of brain tissue sections | Human, rat, mouse | 15–22 |
| Immunostaining of isolated brain microvessels | Human, rat, killfish | 23–26 | |||
| Measurement of transporter activity in isolated brain microvessels | Rat, pig, killfish | 25–27 | |||
| Western Blot analysis of plasma membrane fractions of isolated brain microvessels | Rat | 28,29 | |||
| Apicalc | Immunostaining (electron microscopy) of primary cultured brain endothelial cells and brain (microvessel) endothelial cell lines | Human, mouse | 30–32 | ||
| Immunostaining of brain microvessel endothelial cell line | Human | 2 | |||
| Western Blot analysis of plasma membrane fractions of primary cultured brain microvessel endothelial cells | Cow | 33 | |||
| MRP1 ( = ABCC1) | Abluminald | Immunostaining of brain tissue sections | Mouse | 16 | |
| Immunostaining of isolated brain microvessels | Rat | 23 | |||
| MRP2 ( = ABCC2) | Luminale | Immunostaining of isolated brain microvessels | Rat, killfish | 25,26,34 | |
| Measurement of transporter activity in isolated brain microvessels | Rat, pig, killfish | 25,26,34 | |||
| MRP4 ( = ABCC4) | Luminalf | Immunostaining of brain tissue sectionsg | Human, rat, mouse | 20,35,36 | |
| Immunostaining of isolated brain microvessels | Rat | 23 | |||
| MRP5 ( = ABCC5) | Luminalh | Immunostaining of brain tissue sectionsg | Human, mouse | 16,20,36 | |
| Apical | Immunostaining of primary cultured brain microvessel endothelial cells | Cow | 33 | ||
| Western Blot analysis of plasma membrane fractions of primary cultured brain microvessel endothelial cells | Cow | 33 | |||
| ABCG2 ( = BRCP) | Luminal | Immunostaining (electron microscopy) of brain tissue sections | Mouse | 37 | |
| Immunostaining of brain tissue sections | Human, rat, mouse | 37–40 | |||
| Immunostaining of isolated brain microvessels | Rat | 23,41 | |||
| Apical | Immunostaining of primary cultured brain microvessel endothelial cells | Pig | 42 | ||
| Na+-K+-ATPase | Abluminali | Cytochemical analysis (electron microscopy) of brain tissue sections | Rat | 43 | |
| Measurement of enzyme activity in plasma membrane fractions of isolated brain microvessels | Cow | 43 | |||
| Ca2+-ATPase | Luminal | Cytochemical analysis (electron microscopy) of brain tissue sections | Rat | 44 | |
| ASCT1 ( = SLC1A4, neutral amino acid transporter A)j | Luminal and abluminal | Immunostaining (electron microscopy) of brain tissue sections | Mouse | 45 | |
| ASCT2 ( = SLC1A5, neutral amino acid transporter B(0)) | Abluminal | Immunostaining of brain tissue sections | Rat | 46 | |
| GLUT1 ( = SLC2A1, facilitated glucose transporter member 1)k | Abluminal | Immunostaining (electron microscopy) of brain tissue sections | Rat, mouse, rabbit | 47–52 | |
| Luminal and abluminal | Immunostaining (electron microscopy) of brain tissue sections | Dog | 53 | ||
| Immunostaining of brain tissue sections | Rat, mouse | 37 40,54 | |||
| Immunostaining of isolated brain microvessels | Rat | 23 | |||
| Apical and basolateral | Immunostaining of brain microvessel endothelial cell line | Human | 2 | ||
| SGLT1 ( = SLC5A1, sodium/glucose cotransporter 1) | Luminal | Immunostaining of brain tissue sections | Rat | 55 | |
| ATB0,+ ( = SLC6A14, sodium- and chloride-dependent neutral and basic amino acid transporter) | Luminal | Immunostaining (electron microscopy) of brain tissue sections | Rat | 56 | |
| Lat1 ( = SLC7A5, large neutral amino acids transporter) | Luminal and abluminal | Immunostaining of brain tissue sections | Rat, mouse | 54,57 | |
| Immunostaining of isolated brain microvessels | Rat | 23 | |||
| NHE1 ( = SLC9A1, sodium/hydrogen exchanger) | Luminal | Immunostaining (electron microscopy) of brain tissue sections | Rat | 58 | |
| NHE2 ( = SLC9A2, sodium/hydrogen exchanger) | Luminal | Immunostaining (electron microscopy) of brain tissue sections | Rat | 58 | |
| NKCC1 ( = SLC12A2, Na-K-Cl symporter) | Luminal | Immunostaining (electron microscopy) of brain tissue sections | Rat | 59 | |
| Mct1 ( = SLC16A1, monocarboxylate transporter 1) | Luminal and abluminall | Immunostaining (electron microscopy) of brain tissue sections | Rat | 60–62 | |
| Immunostaining of isolated brain microvessels | Rat | 23 | |||
| OATP1A2 ( = OATP1, SLCO1A2) | Luminal | Immunostaining of brain tissue sectionsg | Human | 36 | |
| OATP1A4 ( = OATP2, SLCO1A4) | Luminal and abluminalm | Immunostaining of brain tissue sections | Rat | 63 | |
| Prostaglandin transporter ( = SLCO2A1, PGT) | Apicaln | Immunostaining of primary cultured brain endothelial cells | Rat | 64 | |
| OATP2B1 ( = SLCO2B1) | Luminal | Immunostaining of brain tissue sectionsg | Human | 36 | |
| OCTN1 ( = SLC22A4, organic cation transporter 1) | Apical | Immunostaining of primary cultured brain microvessel endothelial cells | Human, rat, mouse | 65 | |
| Western Blot analysis of plasma membrane fractions of primary cultured brain microvessel endothelial cells | Human, rat, mouse | 65 | |||
| OCTN2 ( = SLC22A5) | Apical | Immunostaining of primary cultured brain microvessel endothelial cells | Human, rat, mouse | 66 | |
| Western Blot analysis of plasma membrane fractions of primary cultured brain microvessel endothelial cells | Human, rat, mouse | 66 | |||
| OAT3 ( = SLC22A8, organic anion transporter 3) | Abluminal | Immunostaining of brain tissue sections | Rat | 18,19 | |
| Immunostaining of isolated brain microvessels | Rat | 23 | |||
| Snat3 ( = SLC38A3, sodium-coupled neutral amino acid transporter 3) | Luminal and abluminal | Immunostaining of brain tissue sections | Mouse | 54 | |
| Mfsd2a (sodium-dependent lysophosphatidylcholine symporter 1) | Luminal | Immunostaining (electron microscopy) of brain tissue sections | Mouse | 66 | |
| Enzymes | Alkaline phosphatase (AP) | Luminal and abluminalo | Cytochemical analysis (electron microscopy) of brain tissue sections | Rat | 43 |
| Measurement of enzyme activity in plasma membrane fractions of isolated brain microvessels | Cow | 43 | |||
| Thiamine pyro-phosphatase | Luminalp | Cytochemical analysis (electron microscopy) of brain tissue sections | Mouse | 67 | |
| ecto-ATPase | Abluminal | Cytochemical analysis (electron microscopy) of brain tissue sections | Rat | 44 | |
| 5’-nucleotidase | Abluminalq | Cytochemical analysis (electron microscopy) of brain tissue sections | Mouse | 68 | |
| Measurement of enzyme activity in plasma membrane fractions of isolated brain microvessels | Cow | 43 | |||
| Gamma-glutamyl-transferase (γGT) | Luminalr | Immunostaining (electron microscopy) of brain tissue sections | Rat | 69 | |
| Glutathione-S-transferase (GST) | Luminal | Immunostaining of isolated brain microvessels | Rat | 34 | |
| Receptor proteins | Transferrin receptor | Luminals | Immunostaining of brain tissue sections | Mouse | 54 |
| Sphingosine-1-phosphate receptor 1 (S1PR1) | Luminal and abluminal | Immunostaining of isolated brain microvessels | Rat | 70 | |
| Sphingosine-1-phosphate receptor 3 (S1PR3) | Luminal | Immunostaining of isolated brain microvessels | Rat | 70 | |
| Apolipoprotein E receptor 2 (ApoER2 = low-density lipoprotein receptor-related protein 8 (LRP8)) | Abluminal | Immunostaining of brain tissue sections | Mouse | 71 | |
| Vascular endothelial growth factor receptor 1 (VEGFR1) | Luminal | Immunostaining (electron microscopy) of brain tissue sections | Mouse | 72 | |
| Apical | Immunostaining (electron microscopy) of primary cultured brain endothelial cells | Rat | 72 | ||
| Vascular endothelial growth factor receptor 2 (VEGFR2) | Abluminal | Immunostaining (electron microscopy) of brain tissue sections | Mouse | 72 | |
| Basolateral | Immunostaining (electron microscopy) of primary cultured brain endothelial cells | Rat | 72 | ||
| Adhesion molecules | Perlecan (part of heparin sulfate proteoglycan, HSPG) | Abluminal | Immunostaining of brain tissue sections | Mouse | 73 |
| Junctional adhesion molecule 1 (JAM-1) | Apicalt | Immunostaining of primary cultured brain endothelial cells, and of a brain endothelial cell line | Mouse | 74 | |
| Other | Nipah virus surface glycoproteins F and G | Apical and basolateral | Immunostaining of primary cultured brain microvessel endothelial cells | Pig | 75 |
| Caveolin-1 | Apical and at apical/basolateral border | Immunostaining of brain tissue sections | Human | 17,22 | |
| Podocalyxin | Apical/luminal | Immunostaining of brain tissue sections, of a brain microvessel endothelial cell line, and of HUVECs | Human, mouse | 2,9 |
Note: The above table shows predominant localization of transmembrane or membrane-associated proteins in brain microvessel endothelial cells.
Both luminal and abluminal localization of PGP have been reported76 in rat and human brain tissue sections using immunogold staining (electron microscopy).
Peripheral inflammatory hyperalgesia promotes translocation of PGP to the luminal membrane.28
The apical localization of PGP depends on PAR-32.
An apical localization of MRP1 has been reported in primary cultured bovine brain microvessel endothelial cells using immunostaining and Western Blot analysis of plasma membrane fractions.33
Both luminal and abluminal localization of MRP2 have been reported16 in mouse brain tissue sections using immunofluorescence staining.
Both luminal and abluminal localization of MRP4 have been reported34 in isolated rat brain microvessels using immunofluorescence staining. Both apical and basolateral localization of MRP4 have been reported in primary cultured bovine brain microvessel endothelial cells using immunostaining and Western Blot analysis of plasma membrane fractions.33
In Bronger et al.,36 sections of brain gliomas were analyzed.
An abluminal localization of MRP5 has been reported23 in isolated rat brain microvessels using immunofluorescence staining.
Both luminal and abluminal localization of Na+-K+-ATPase have been reported77,78 using cytochemical analysis (electron microscopy) of rat brain tissue sections.
ASCT1 expression is only detectable at embryonic and neonatal stages, not at adult stages.45
GLUT1 is predominantly expressed at the abluminal membrane; however, significant levels of GLUT1 can also be found at the luminal membrane.
MCT1 expression shifts to the abluminal membrane during ketogenic diet.62
An abluminal localization of OATP1A4 has been reported23 in isolated rat brain microvessels using immunofluorescence staining.
Polar localization of PGT at the apical membrane is lost after treatment with LPS.64
A luminal localization of alkaline phosphatase has been reported in mouse brain tissue sections using a cytochemical analysis (electron microscopy).67,79 Abluminal expression of alkaline phosphatase increases during scrapie infection.67
Abluminal expression of thiamine pyrophosphatase increases during scrapie infection as shown by a cytochemical analysis (electron microscopy) of mouse brain tissue sections.67
Abluminal expression of 5′-nucleotidase increases during scrapie infection as shown by a cytochemical analysis (electron microscopy) of mouse brain tissue sections.67
Both luminal and abluminal localization of γGT have been reported43 using measurement of enzyme activity in plasma membrane fractions of isolated cow brain microvessels.
Both luminal and abluminal localization of the transferrin receptor have been reported80 by measuring the uptake of fluorescent immunoliposomes into isolated rat brain microvessels.
CCL2 induces the translocation of JAM-1 from tight junctions to the apical membrane in a RHOA- and ROCK-dependent manner.74
When comparing the permeability of brain capillaries in vivo and in vitro, Betz and Goldstein81 noted marked differences. After intravenous administration, hydrophilic compounds only have access to the apical/luminal side of the endothelium, whereas in vitro they come in contact with both the apical/luminal and basolateral/abluminal side of endothelial cells. The dependency of permeability on the side of administration led them to propose that brain endothelial cells are polarized. Asymmetry of brain endothelial plasma membranes has also been predicted from mathematical modeling of glucose and radioactive deoxy-glucose distribution under basal and stimulated conditions.82
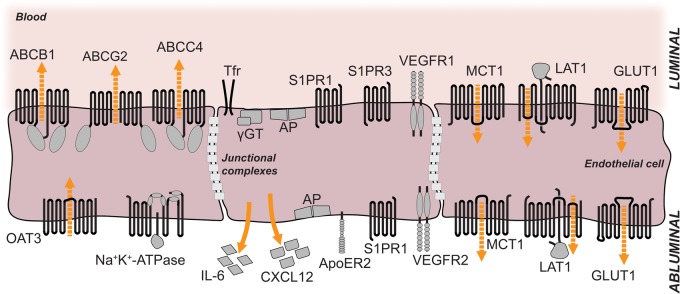
Importantly, the localization of transporters can be deduced from the accumulation of drugs in the brain of knockout mouse lines. The ABC transporter PGP (ABCB1, MDR1) pumps a wide range of compounds out of cells in an ATP-consuming manner (Figure 2). Thus, the direction of active transport depends on the localization in cell membranes. Localization only on the apical/luminal side of endothelial cells that links its activity to an outward transport of drugs across the blood–brain barrier explains accumulation of compounds in the brains of knockout mice.83 According to the same line of evidence, MRP4 (ABCC4) and BCRP (ABCG2) are also mainly localized in the apical/luminal membrane of brain endothelial cells (Figure 2).35,84
Density-gradient centrifugation of membranes
Figure 2.
Localization of selected membrane proteins at the luminal and abluminal sides of brain microvessel endothelial cells. The luminal and abluminal membrane domains are separated by tight junctions. The sizes of the receptors and their respective domains are not drawn in scale. For details see tables 1 and 2. Orange arrows indicate direction of secretion; dashed orange arrows indicate direction of transport. ABCB1, ABCC4, ABCG2: ABC-binding cassette transporters; OAT3: organic anion transporter 3; Tfr: transferrin receptor; γGT: gamma-glutamyl-transferase; AP: alkaline phosphatase; S1PR1: sphingosine-1-phosphate receptor 1; S1PR3: sphingosine-1-phosphate receptor 3; VEGFR1: vascular endothelial growth factor receptor 1; VEGFR2: vascular endothelial growth factor receptor 2; IL-6: interleukin-6; CXCL12: C-X-C motif chemokine 12 = stromal cell-derived factor 1 (SDF-1); ApoER2: apolipoprotein E receptor 2 = low-density lipoprotein receptor-related protein 8 (LRP8); MCT1: monocarboxylate transporter 1; LAT1: large neutral amino acids transporter; GLUT1: facilitated glucose transporter member 1.
By using density-gradient centrifugation brain capillary membranes can be fractionated to obtain purified preparations of apical/luminal and basolateral/abluminal membranes.85,86 The strength of this technique lies in the fact that biochemical assays can be performed and detection does not rely exclusively on the specificity of antibodies. Using this approach, Lee and colleagues87 found that the activity of GLUT1 was similar in the apical/luminal and basolateral/abluminal membranes of bovine brain endothelial cells although immunostains did detect it preferentially in the basolateral/abluminal membrane (Figure 2). Recently, a detergent-free technique was established.88 Protein levels can be quantitatively measured in various species independent of available antibodies.89,90 However, identifying fractions depends on the enrichment of marker proteins; therefore, allocating individual fractions to cellular compartments may be ambiguous.91 Often, the apical/luminal and basolateral/abluminal membranes can only be partially purified and glial or pericytic contamination is a concern.86
Microscopic techniques
The resolution of conventional light microscopy (about 200 nm) is limiting when attempting to reliably distinguish between apical/luminal and basolateral/abluminal plasma membranes of brain endothelial cells. In the perinuclear space, the endothelial plasma membranes are so far apart that they can be identified.2,15 With the help of high-resolution confocal microscopy and quantitative colocalization studies, membrane proteins could be localized in subcellular compartments of brain endothelial cells.15,23,29,46 In the future, super-resolution microscopy may bring critical progress. So far, spectral precision distance microscopy/spectral position determination microscopy (SPDM), a super-resolution technique, has been used to localize PGP within the apical/luminal membrane of brain endothelial cells.92 Up to now, subcellular localization of membrane proteins has largely relied on immunogold techniques in electron microscopy.91 Despite the high potential and the relative ease in quantifying results, practical limitations result from the fact that antibody binding is often impaired by tissue fixation and processing. Indeed, electron microscopical techniques have produced conflicting data about the localization of proteins, e.g. PGP and GLUT1.16,30,76,91
In vivo labeling of apical/luminal proteins
Proteins in the apical/luminal membrane of brain endothelial cells can be labeled independently of antibodies by perfusing animals with a biotinylation reagent.23 Biotinylated proteins in microvessel extracts are subsequently purified with the help of neutravidin-coated agarose beads. While this in vivo approach labels apical/luminal proteins, it does not detect proteins in the basolateral/abluminal membrane. Both basolateral/abluminal and intracellular proteins are included in the unlabeled fraction, which complicates the direct comparison of apical/luminal and basolateral/abluminal membranes.
In vitro studies of cell polarity
Multiple in vitro models of the blood–brain barrier have been developed that differ in how endothelial cells are cultured.93 All of these models have in common a lack of some of the decisive external factors that induce polarity in the endothelial cells, including blood flow, the basement membrane, astrocytic endfeet, or pericytes (see below). These shortcomings lower the barrier quality of in vitro models compared to the in vivo situation.93 Nevertheless, endothelial polarity is at least partially preserved in vitro, thus enabling analytical techniques that would be out of reach for in vivo studies. For example, culturing porcine brain capillary endothelial cells revealed that the concentration of phosphatidylcholines is higher and the concentration of sphingomyelins and glucosylceramides lower in the apical/luminal plasma membrane than in the basolateral/abluminal membrane.94 In vitro studies also provide information about human cells. The immortalized human brain endothelial cell line hCMEC/D3 retains some degree of cell polarity, such as polarized localization of PGP predominantly in the apical/luminal membrane.95 Importantly, in vitro models offer the opportunity to investigate polarized secretion13,96,97 (Table 2). To study polarized secretion, brain endothelial cells are plated on a permeable, porous membrane in a cell culture insert that has been coated with collagen IV or other basement membrane components. Once the endothelial layer has reached confluency, as shown by a high transendothelial resistance or a low permeability of tracers, e.g. luciferin yellow, concentrations of secreted factors in the two compartments are compared. The upper compartment corresponds to blood while the lower represents the brain parenchyma.
Table 2.
Factors released from endothelial cells in a polarized manner.
| Compound | Side of release or localization | Experimental conditions | Modulation of release or localization | References |
|---|---|---|---|---|
| Basement membrane components: collagen IV, fibronectin, metalloproteinases | Basolateral | Bovine aortic arch endothelial cells | 98 | |
| IL-1α, IL-6, IL-10, TNF, GM-CSF | Apical > basolateral | Mouse brain endothelial cells | LPS stimulates apical IL-6 release more when added to the apical than to the basolateral side | 96 |
| IL-6 | Basolateral | Rat brain microvascular endothelial cells cocultured with astrocytes after flow cessation | Reperfusion | 99 |
| IL-8, GM-CSF | Apical | Human dermal microvascular endothelial cells | 100 | |
| MIC-1 (GDF15) | Basolateral | |||
| von Willebrand factor (vWF) | Basolateral | Human umbilical vein endothelial cells (HUVEC) | Calcium ionophore or phorbol myristate stimulate basolateral release (constitutive secretion was not polarized) | 101 |
| Platelet-derived growth factor (PDGF) | Basolateral | Bovine aortic endothelial cells on nitrocellulose membranes | 102 | |
| Cholesterol | Basolateral | Porcine brain capillary endothelial cells | Cholesterol acceptors | 97 |
| 24(S)-OH cholesterol | Apical | HDL3 | ||
| Endothelin-1 | Basolateral > apical | HUVEC on amniotic membranes; bovine brain capillary endothelial cells on collagen-coated Millicell CM inserts | Dexamethasone reduces apical secretion | 103,104 |
| Glial cell-derived neurotrophic factor (GDNF) | Basolateral | Mouse brain endothelial cell line MBEC4 transfected with expression vector | 105 | |
| CCL2 | Apical > basolateral | Human brain microvessel endothelial cells | TNF + Interferon γ enhance binding to basolateral membrane | 106 |
| CCL3 | Basolateral > apical | TNF + Interferon γ enhance binding to apical membrane | ||
| SDF1 (CXCL12) | Basolateral/abluminal | Human hCMEC/D3 cells, mice in vivo | Inflammation, S1PR2 signaling, female gender are associated with apical/luminal localization | 107 |
| Beta-hexosaminidase | Basolateral | Human hCMEC/D3 cells | 13 |
The apicobasal polarity of brain endothelial cells
As interface between blood and the vessel wall, endothelial cells are polarized in an apicobasal orientation. In the CNS, apicobasal polarity seems to be more pronounced than in other vascular territories in order to form the blood–brain barrier and to meet the metabolic demands of this highly active tissue. Apicobasal polarity of brain endothelial cells presents in several ways.
Differences in lipid and (glyco-)protein composition of the apical/luminal and basolateral/abluminal plasma membranes
Electron microscopy demonstrates that the apical/luminal side of brain endothelial cells is covered by a layer of polysaccharides, the glycocalyx, that is attached to glycoproteins and proteoglycans, such as syndecan-1, -2, -4, and glypican.108 The relative contribution of proteoglycans for anchoring the glycocalyx is still unknown.109 In addition, the lipid composition differs in the apical/luminal and basolateral/abluminal membranes.94 The basolateral/abluminal membrane of brain endothelial cells has been reported to contain more caveolae, a membrane microdomain that is rich in sphingomyelin and glycosphingolipids.76 Most conspicuous are differences in the protein composition of the apical/luminal and basolateral/abluminal membranes, although in many cases consensus has not been reached about the preferential localization of proteins (Table 1). The metabolic demands of the CNS require that numerous compounds be bidirectionally transported across the blood–brain barrier. As in kidney and gastrointestinal epithelium, the directed transport over barriers is facilitated by endothelial cell polarity.
Directed transcytosis
Transcytosis involves different mechanisms of endocytosis. Best understood is the clathrin-mediated pathway that is responsible for transferrin transport through the blood–brain barrier.110,111 Among the clathrin-independent pathways, caveolae mediate the transport of albumin and other factors. Disruption of caveolae-dependent transcytosis seems to contribute to juvenile neuronal ceroid lipofuscinosis caused by mutations in ceroid lipofuscinosis neuronal-3 (CLN3).112 Another only recently identified form of clathrin-independent endocytosis relies on endophilin, a protein that is expressed in brain endothelial cells.113,114 Target selectivity of transcytosis results from the involvement of membrane receptors that bind targets, such as insulin, leptin, cytokines, transferrin, and lipoproteins, and that are included in the endocytotic vescicle. The distribution of target receptors between the apical/luminal and basolateral/abluminal membranes represents an important determinant of the direction of transcytosis. In the case of transferrin, its receptor is found on the apical/luminal side of brain endothelial cells, largely limiting endocytosis to the blood side and transport in a blood-to-brain direction.111 For bidirectional transport two receptors may be needed, as in the case of Aβ, which is transported out of the brain by LRP1 and into the brain by RAGE.115,116
Polarized release
For the differential interaction with blood and the parenchyma, endothelial cells secrete proteins and other factors in a polarized manner either to the apical/luminal or the basolateral/abluminal side (Table 2). In some cases, external stimuli were reported to control the side of secretion. As in epithelial cells, some factors, including von Willebrand factor (vWF), are packaged in clathrin-coated vesicles and released at the basolateral/abluminal side117 (Table 2). However, the signals that direct secretion to the apical/luminal side are less well understood. Apparently, N-linked glycosylation is not involved, in contrast to what has been reported for epithelial cells.100
Polarized response to stimuli
Cell polarity also influences the response of brain endothelial cells to external stimuli. The cytokines IL-6 and GM-CSF increase neuroinvasion of HIV-1 when applied to the apical/luminal, but not to the basolateral/abluminal side.118 Vascular endothelial growth factor (VEGF), histamine, and insulin-like growth factor-binding protein 3 (IGFBP3) increase the transendothelial permeability of the blood–brain barrier when administered to the basolateral/abluminal, but not to the apical/luminal side.72 In contrast, thrombin and bradykinin are effective on both sides and lysophosphatidic acid (LPA) mostly on the apical/luminal side and less on the basolateral/abluminal side. Further investigations demonstrated that VEGF binds to VEGFR2 on the basolateral/abluminal membrane and increases the permeability through p38 kinase, whereas on the apical/luminal side VEGFR1 stimulates Akt and facilitates cytoprotection. Intriguingly, the polarized response to VEGF is found exclusively in brain and retinal, but not in peripheral endothelial cells, highlighting the special status of endothelial cells in the CNS.
Epithelial polarity program
While the molecular mechanisms governing endothelial apicobasal polarity are just beginning to emerge, a wealth of literature has reported on epithelial apicobasal polarity regulation. Epithelial apicobasal polarity is established and maintained by a molecular network of polarity protein complexes, adhesion complexes, small GTPases, and lipids. Fundamental concepts of epithelial polarity were initially discovered in flies and worms; albeit more complex, many of these concepts also apply to vertebrate cells. In the following paragraph, we will introduce important molecular determinants of epithelial polarity and their interplay. For more detailed information on this topic, we refer to several excellent recent reviews.119–124
Polarity complexes in epithelial cells
Par complex
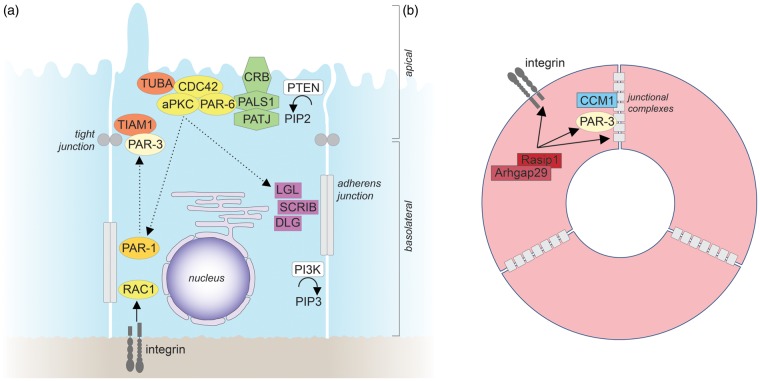
The Par proteins comprise a family of structurally and functionally distinct cytoplasmic proteins, which were identified as genes essential for the polarity of the early C. elegans embryo.125 They are evolutionarily conserved in other animals and widely expressed. Whereas all Par proteins are encoded by single genes in Drosophila and C. elegans, some proteins have several isoforms in vertebrates, which might carry out specific functions in particular cell types. Par proteins have been shown to function as central regulators not only of epithelial polarity, but also of endothelial polarity, particularly in angiogenesis.126,127 They comprise the following members: the serine/threonine protein kinases, PAR-1 ( = microtubule affinity-regulating kinase 2, MARK2, in vertebrates), and PAR-4 ( = Liver kinase B1, LKB1 or Serine/threonine-protein kinase 11, STK11, in vertebrates); the scaffold and adapter proteins PAR-3 (two isoforms, PAR-3 and PAR-3 L, also known as PAR-3B, in vertebrates) and PAR-6 (three isoforms, PAR-6 A = PAR-6 C, PAR-6B, and PAR-6D = PAR-6 G in vertebrates); the adapter protein PAR-5 (a 14-3-3 isoform). The serine/threonine protein kinase atypical PKC (two isoforms, aPKCζ and aPKCι/λ in vertebrates) and the small GTPase CDC42 (see also below) are closely linked to Par proteins both physically and functionally; together with PAR-3 and PAR-6, they are therefore referred to as the “Par complex”. The membrane localization of Par proteins is established and maintained by a system of reciprocal exclusion119,120,128 (Figure 3(a)): aPKC, which forms a complex with PAR-6 and CDC42 at the apical membrane, phosphorylates PAR-1 and other basolateral proteins to exclude them from the apical membrane, while at the basolateral membrane PAR-1 phosphorylates PAR-3 and apical proteins to exclude them from the basolateral membrane. The adapter protein PAR-5 binds proteins phosphorylated by aPKC and PAR-1 to detach them from the plasma membrane into the cytosol, where they are dephosphorylated, and thereby allows shuttling to the correct membrane domain. Par proteins form a highly interconnected network with other polarity proteins. For example, aPKC also phosphorylates LGL2, a member of the Scribble family of polarity proteins (see below), to restrict it to the basolateral membrane.129 PAR-6 binds to CRB3 and PALS1, members of the Crumbs family of polarity proteins (see below), which confer the anchoring of PAR-6 to the apical membrane.130 Moreover, PAR-4 phosphorylates and activates AMPK to control cell metabolism and growth.131
Figure 3.
Epithelial and endothelial apicobasal polarity. (a) Molecular mechanisms of epithelial apicobasal polarity. For details see text. The straight arrow indicates activation, straight dashed arrows indicate phosphorylation, and curved arrows indicate enzymatic reactions. (b) Molecular mechanisms that establish endothelial apicobasal polarity during vascular lumen formation. Only molecules for which functional roles have been demonstrated in vivo are depicted. For further details see text. Similar to epithelial cells, β1 integrin-matrix interactions provide an initial cue to establish endothelial apicobasal polarity.60 β1 integrin is required for proper PAR-3 expression levels and for the correct localization of VE-cadherin at lateral cell-cell contacts.60 VE-cadherin, which can bind directly to PAR-3,202 forms a complex with CCM1.9 CCM1 stabilizes VE-cadherin at adherens junctions to further establish and maintain endothelial apicobasal polarity.9,203 Rasip1 and its binding partner Arhgap29 suppress the activity of the small GTPase RHOA to promote integrin-mediated adhesion, and to regulate the correct localization of PAR-3, as well as of intercellular junctions.204 In contrast to epithelial cells, the organization of tight and adherens junctions in endothelial cells is less clearly defined, with tight and adherens junctions frequently being intermingled. CCM1: cerebral cavernous malformations 1; Rasip1: Ras interacting protein 1.
Crumbs complex
Crumbs proteins are apically localized, single-pass transmembrane proteins that were initially identified in Drosophila.132 In vertebrates, three different Crumbs isoforms (CRB1, CRB2, and CRB3) with largely non-overlapping expression patterns are found, CRB3 being the major isoform in epithelial cells.133 Via their C-terminus, Crumbs proteins bind PALS1 (protein associated with Lin-7 1), an adapter protein that forms a complex with the multi-PDZ domain protein PATJ (PALS1-associated tight junction protein) (Figure 3(a)). Crumbs proteins are important determinants of apical membrane identity and play a central role in tight junction assembly and maintenance.119,133 Very little is known about the role of Crumbs proteins in endothelial cell polarity. Recent evidence in primary endothelial cells in vitro suggests that Crumbs proteins might be involved in regulating endothelial cell junctions.134
Scribble complex
The Scribble family of polarity proteins was first identified in Drosophila as important regulator of apicobasal cell polarity and tissue growth. Loss of any of these genes in Drosophila results in the mistargeting of apical proteins to the basolateral membrane, the disorganization of adherens junctions, and tumor formation.135 The Scribble protein complex consists of Scribble (SCRIB), Discs Large (DLG; several isoforms in vertebrates), and Lethal Giant Larvae (LGL; several isoforms in vertebrates), which all localize to the basolateral membrane (Figure 3(a)). The basolateral localization of LGL depends on its phosphorylation and exclusion from the apical membrane by aPKC.136 In vertebrate epithelial cells, SCRIB is required for E-cadherin–mediated cell–cell adhesion.137 While the role of the Scribble complex in endothelial cell polarity is largely unexplored, one recent study shows that SCRIB is expressed in primary endothelial cells and involved in endothelial cell migration.138
RhoGTPases in epithelial polarity
Small GTPases of the Rho family have been extensively studied for their role as master regulators of the actin cytoskeleton. A large body of evidence indicates that these GTPases, particularly CDC42 (see also above) and RAC1, are centrally involved in epithelial apicobasal polarity.139 CDC42, a master regulator of epithelial cell polarity, is activated by TUBA, an apically localized guanine nucleotide exchange factor (GEF) for CDC42, and positively regulates the activity of aPKC to promote apical membrane identity and the formation of adherens junctions, and to control vesicle trafficking6,119,129,140,141 (Figure 3(a)). RAC1, which often cooperates functionally with CDC42, is enriched basolaterally, is required for the assembly of the basement membrane, and is crucially involved in the formation and maintenance of E-cadherin-based adherens junctions.119 Activation of RAC1 is triggered by β1 integrin, E-cadherin, or the RAC GEF TIAM1 that interacts directly with PAR-3.119,142,143 Moreover, CDC42 and RAC1 activity can be regulated by SCRIB, which binds and activates the RAC/CDC42 GEF βPix;144 however, the significance of the SCRIB/βPix interaction for epithelial polarity is still unclear. RHOA, which frequently acts antagonistically to RAC, controls actomyosin contractility through its downstream effector kinase ROCK.139 Furthermore, ROCK can phosphorylate PAR-3 to disrupt its association with aPKC and PAR-6.145 Conversely, aPKC phosphorylates ROCK to exclude it from apical junctional complexes in order to prevent excessive apical constriction.146 CDC42, RAC1, and RHOA are also crucial for endothelial apicobasal polarity and formation of endothelial junctions (see below).
Lipids in epithelial polarity
Phosphoinositides have been shown to play a central role in epithelial cell polarity by determining the identities of apical and basolateral plasma membranes.147 Whereas phosphatidylinositol 4,5-bisphosphate (PIP2) localizes to the apical membrane, phosphatidylinositol 3,4,5-trisphosphate (PIP3) is enriched at the basolateral membrane6,148 (Figure 3(a)). Insertion of exogenous PIP2 into the basolateral membrane of epithelial cells causes the mistargeting of apical proteins to the basolateral membrane, while, conversely, insertion of exogenous PIP3 into the apical membrane results in the formation of membrane protrusions, which contain basolateral proteins.6,148 Via an adapter protein, PIP2 binds to CDC42 and recruits it to the apical membrane.6 The polar distribution of PIP2 and PIP3 is established and maintained by two enzymes, phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase (PTEN) and phosphatidylinositol 3-kinase (PI3K), which localize to the apical and basolateral membrane, respectively.6,149 At the apical membrane, PTEN catalyzes the formation of PIP2 and the removal of PIP3.6 At the basolateral membrane, the activity of PI3K results in the conversion of PIP2 into PIP3.149 Like epithelial cells, endothelial cells – including cultured brain capillary endothelial cells – show an asymmetric distribution of lipids between the luminal and the abluminal membrane.94 However, whether lipids play a role in establishing endothelial polarity is so far unknown.
Generation and maintenance of epithelial polarity
The initial cues for the generation of epithelial polarity are provided by epithelial cell-cell contacts and extracellular matrix proteins, which directly activate receptor-mediated signal transduction pathways and, in addition, act through mechanical forces and cell confinement.122 Early cell-cell contacts induce the formation of intercellular junctions, which recruit and activate polarity proteins. In turn, in a complex interplay, polarity proteins regulate the further maturation of adherens and tight junctions.120 The formation and maintenance of junctions is tightly coupled to the reorganization of the cytoskeleton and depends in particular on actin dynamics.119 At the same time, activation of β1 integrin by collagen results in RAC1 activation, which leads to the secretion of laminin and assembly of the basement membrane. These processes promote the establishment of apical and basal membrane domains.120 The identities of the apical and basolateral membrane further critically rely on a polarized trafficking machinery that drives the targeted transport of vesicles carrying distinct lipids and proteins.122 The delivery of these lipids and proteins to the correct plasma membrane is ensured by diverse apical and basolateral sorting signals and mechanisms.122 Tight junctions that prevent diffusion of proteins and lipids from one membrane domain to the other are required to maintain membrane identity.120 Finally, the polarization of individual cells is translated into the arrangement of higher-order cell structures to build the proper epithelial tissue architecture.
Mechanisms of endothelial polarity
Studies in epithelial cells have largely formed our current concepts of apicobasal cell polarity. However, how polarity is achieved may differ among cell types. MRP4, for example, is localized in the basolateral membrane of epithelial cells in the choroid plexus, but in the apical/luminal membrane of brain capillaries.35 Endothelial polarity has often been studied in the context of lumen formation of blood vessels, some key findings of which are depicted in Figure 3(b). In the following we will summarize what is known about the mechanisms that control polarity in endothelial cells, with a special focus on the brain.
Par complex in endothelial polarity
The Par complex (PAR-3, PAR-6, aPKC, and CDC42) is involved in endothelial polarity during lumen formation126 (Figure 3(b)). Components of this complex are expressed in brain endothelial cells.2,150,151 The Par complex is localized to tight junctions of endothelial cells by binding PAR-3 to JAM proteins.2,152 In addition, PAR-3 binds to VE-cadherin, explaining why PAR-3, phosphorylated aPKCζ, and TIAM localize to intercellular junctions, depending on the presence of VE-cadherin.9 When meningococci adhere to brain endothelial cells, the Par complex moves away from intercellular junctions to the site of meningococcal adhesion, disturbing tight junction function and increasing paracellular permeability.150 Knockdown of PAR-3 interferes with the morphology and function of tight junctions as well as with apicobasal polarity and lumen formation.2,9 Interestingly, WNT5a, a growth factor expressed by astrocytes, seems to enhance PAR-3 levels in intercellular junctions and to increase apicobasal polarity,2,153 suggesting that astrocytes promote endothelial polarity via this pathway.
Crumbs complex in endothelial polarity
Components of the Crumbs complex (PALS1, PATJ) are expressed in endothelial cells.134 The complex is localized to tight junctions of endothelial cells. By binding SYX, a RHOA-specific GEF that activates RHOA, members of the Crumbs complex stabilize intercellular junctions and decrease paracellular permeability.134 SYX deficiency impairs normal vascular development.154 The Crumbs complex also interacts with angiomotin (Amot) and Angiomotin-like Protein 1 (AmotL1).155 Both proteins are localized in tight junctions. Knockdown of Amot and AmotL1 increases paracellular permeability, suggesting that Amot and AmotL1 are required for tight junctions to function properly. They are also essential for the polarity of migrating endothelial cells.155 Amot knockout mice have a high embryonic lethality and develop dilated vessels in the brain.156 Less is known about the role of Amot or AmotL1 in apicobasal polarity beyond formation of tight junctions.
Scribble complex in endothelial polarity
Members of the Scribble complex are found in brain endothelial cells. DLG1 is expressed in endothelial cells during mouse development and in the human brain endothelial cell line hCMEC.157,158 In brain endothelial cells, DLG1 is not colocalized with SCRIB, suggesting that the two factors do not form a complex as in Drosophila epithelial cells.117 By binding to GPR124 as a cofactor, DLG1 enhances canonical WNT signaling in response to WNT7a.157 The latter is released by neuroepithelial cells and has a profound effect on the development of the blood–brain barrier.159,160 Other interaction partners of DLG1 include clathrin, AP-1, and von Willebrand factor.117 Knockdown of DLG1 inhibited the formation of Weibel-Palade bodies and induced disassembly of the trans-Golgi network. SCRIB mediates planar cell polarity and migration of endothelial cells.138 Mice with a frameshift mutation in SCRIB (circle tail mice) or zebrafish treated with SCRIB morpholinos developed vascular malformations in the brain and hemorrhages138 that are reminiscent of the phenotype of mice deficient in LGL1.161 However, it is still unclear whether endothelial SCRIB or LGL1 affects CNS development. Thus, despite evidence that mammalian homologues of SCRIB, DLG, and LGL play a role in endothelial cells, how they contribute to apicobasal polarity is still largely unclear.
CCM proteins in endothelial polarity
An important regulatory pathway for endothelial polarity was uncovered through the study of cerebral cavernous malformations. These enlarged, thin-walled, and leaky vascular structures consist mainly of endothelial cells with only few glia or pericytes. Three structurally unrelated genes are mutated in familiar cases of the disease, CCM1 (Krit1), CCM2 (OSM), and CCM3 (PDCD10). CCM1 is a binding partner and effector of the small G protein RAP1 that is involved in epithelial polarity programs and tightens endothelial barriers.162–164 Knockdown of RAP1 impairs apicobasal polarization and lumen formation of endothelial cells.9 Upon activation RAP1 mediates the translocation of CCM1 from microtubule to adherens junctions, where CCM1 associates with β-catenin and VE-cadherin.164,165 Loss of CCM1 causes β-catenin to dissociate from VE-cadherin.9,166 Consequently, the released β-catenin translocates to the nucleus and stimulates gene transcription, whereas adherens junctions are disrupted.166 In CCM1-deficient endothelial cells, the lack of junctional VE-cadherin that normally recruits the Par complex (see above) results in a defect in apicobasal polarity and lumen formation.9
CCM1 forms a complex with CCM2 and CCM3. CCM2 deletion in endothelial cells causes a defect in lumen formation of branchial arch arteries during development but does not affect apicobasal polarity in endothelial cells.11,167 In addition, deletion of CCM3 interrupts lumen formation but not the development of branchial arch arteries.168,169 At the biochemical level, CCM2 is required for maintaining the cytoskeleton and limiting RHOA activation.10 CCM2 deficiency leads to low levels of CDC42, a component of the Par complex.11 The effect of CCM3 on RHOA activation is the subject of controversy.169,170 Instead, CCM3 is located on the Golgi apparatus and stabilizes the germinal center kinase III (GCKIII).171 In support of a pathogenic role of GCKIII in CCMs, overexpression of the GCKIII kinase STK25b rescued the vascular defects in zebrafish deficient in CCM3.168 Furthermore, deletion of STK25b mimicked the effect of CCM3 deletion. The presence of CCM3 in the Golgi apparatus seems to determine Golgi orientation,171 which itself has implications for cell polarity and polarized secretion, at least in the epithelial HeLa cell line.172 So far, very little is known about the mechanisms underlying polarized secretion in endothelial cells. As epithelial and endothelial cells secrete some proteins in different directions,111,173,174 concepts that have been established to explain the mechanisms underlying polarized secretions in epithelial cells may not be true for endothelial cells.
RhoGTPases and shear stress in endothelial polarity
The small GTPases RHOA, RAC, and CDC42 control brain endothelial polarity in a complex manner. A physiological activator of all three is shear stress, as reviewed previously.175 The spatial distribution of activity in response to flow is polarized in the cell plane with CDC42 and RAC1 activation at the downstream edge of the cell. RHOA is first inhibited and then activated by flow.176,177 Other stimuli of RHOA include inflammatory mediators that increase the permeability of the barrier, such as LPS,178 thrombin,179 and CCL2.180 In contrast, inhibitors of RHOA, such as ANXA1 which acts via G protein-coupled FPR2 receptors178,181 and CCM1 or CCM2, stabilize barrier function.10,11 During shear stress RHOA is involved in cell alignment and stress fiber formation in endothelial cells.177 The RHOA-mediated increase in blood–brain barrier permeability has been attributed to increased phosphorylation of the myosin light chain and contraction of actomyosin, pulling intercellular adhesions apart.182 Additionally, RHOA enhances the phosphorylation of tight junction proteins, which leads to their disintegration.183 RHOA modulates the apicobasal polarity of brain endothelial cells by redirecting JAM-1 from tight junctions to the apical/luminal surface, where it functions as a leukocyte adhesion molecule.74 Through this mechanism RHOA stimulates the transendothelial migration of neutrophils and monocytes. RHOA is inhibited by CCM1, CCM2, and CCM3 (see above).184 Thus, a deficiency in any of the CCM proteins leads to RHOA overactivation. Interestingly, the increased endothelial permeability in CCM1- or CCM2-deficient mice could be ameliorated by fasudil, a pharmacological blocker of the RHOA effector ROCK.10
In contrast to the destabilizing effects of RHOA on the barrier, CDC42 enhances cell polarity and barrier properties.185 As part of the Par complex, it is normally located at intercellular junctions. Adhesion of meningococci to the apical membrane of brain endothelial cells relocates the Par complex to the site of adhesion and CDC42 plays an active part in this process.150 CDC42 also promotes endothelial lumen formation, a process that depends on other members of the Par complex.186
External stimuli of endothelial polarity in the blood–brain barrier
External cues provide spatial orientation for brain endothelial cells to maintain their apicobasal polarity. On the basolateral/abluminal side, astrocytes and pericytes are found in close proximity. Astrocytes induce barrier properties and promote the apicobasal polarity of brain endothelial cells.187,188 Sonic hedgehog is one of the factors released by astrocytes; it activates the receptor Patched-1 on brain endothelial cells and stimulates the expression of tight junction components, such as claudin-5 and occludin.189 Other factors that are produced by astrocytes and stabilize the barrier include angiotensin-II, GDNF, angiopoietin 1,188,190 and possibly WNT5a.2,153
More recently, an important influence of pericytes on the basolateral/abluminal side of brain endothelial cells was recognized.191–193 Pericytes are not only essential for establishing the blood–brain barrier, but also for maintaining it in adulthood. They induce the expression of the tight junction proteins ZO-1 and occludin in brain endothelial cells and thus reduce paracellular permeability.192 Moreover, pericytes control the expression of MFSD2a in neighboring endothelial cells.194 MFSD2a is a transmembrane protein localized in the apical/luminal membrane of brain endothelial cells. It functions as a transporter for the ω3 fatty acid docosahexaenoic acid that is esterified with lysophosphatidylcholines195 and at the same time reduces transcytosis across the blood–brain barrier.194 Pericytes and endothelial cells are in direct cell-cell contact at so-called peg-and-socket junctions that contain N-cadherin.196 These specialized junctions may be the site where membrane receptors interact directly, e.g., Notch3 on pericytes and DLL4 on endothelial cells.197
Outside of peg-and-socket junctions, pericytes and endothelial cells are separated by the endothelial sheath of the basement membrane, consisting of collagen type IV, perlecan, the laminins 411 and 511, and other proteins. Collagen IV binds to β1 integrins (α1β1, α2β1), which play an essential role in endothelial polarity and arterial lumen formation and in the development of intercellular junctions (Figure 3(b)).126,198 β1 integrin seems to be located upstream of PAR-3 because PAR-3 levels were lower in endothelial cells deficient in β1 integrin and their phenotype could be partially rescued by replacing PAR-3.126
On the apical/luminal side brain endothelial cells are continuously exposed to a shear stress of 1 – 10 N/cm2 in capillaries.199 Applying this level of shear stress to brain endothelial cells in vitro increases their barrier properties and the expression of tight junction components.93,200,201
Conclusion
Apicobasal polarity seems to be a cellular hallmark of the blood–brain barrier. So far the integrity of intercellular junctions as an isolated aspect of cell polarity has received the most attention. Other important aspects, including the polar distribution of membrane proteins, were less frequently investigated in a functional or pathophysiological context. Largely unclear are the molecular mechanisms that determine cell polarity in adult brain endothelial cells. However, important clues come from the study of endothelial lumen formation during development or the analogy of epithelial cells, where apicobasal polarity has been extensively studied. An in-depth investigation of apicobasal polarity could open new windows to understanding the blood–brain barrier. Progress will likely depend on improved genetic tools to manipulate brain endothelial cells in vivo and on high resolution fluorescence microscopy.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Bundgaard M, Abbott NJ. All vertebrates started out with a glial blood-brain barrier 4-500 million years ago. Glia 2008; 56: 699–708. [DOI] [PubMed] [Google Scholar]
- 2.Artus C, Glacial F, Ganeshamoorthy K, et al. The Wnt/planar cell polarity signaling pathway contributes to the integrity of tight junctions in brain endothelial cells. J Cereb Blood Flow Metabol 2014; 34: 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lizama CO, Zovein AC. Polarizing pathways: balancing endothelial polarity, permeability, and lumen formation. Exp Cell Res 2013; 319: 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sigurbjornsdottir S, Mathew R, Leptin M. Molecular mechanisms of de novo lumen formation. Nat Rev Mol Cell Biol 2014; 15: 665–676. [DOI] [PubMed] [Google Scholar]
- 5.Charpentier MS, Conlon FL. Cellular and molecular mechanisms underlying blood vessel lumen formation. Bioessays 2014; 36: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin-Belmonte F, Gassama A, Datta A, et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 2007; 128: 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luissint A-C, Artus C, Glacial F, et al. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluid Barriers CNS 2012; 9: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz-Orengo L, Daniels BP, Dorsey D, et al. Enhanced sphingosine-1-phosphate receptor 2 expression underlies female CNS autoimmunity susceptibility. J Clin Invest 2014; 124: 2571–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lampugnani MG, Orsenigo F, Rudini N, et al. CCM1 regulates vascular-lumen organization by inducing endothelial polarity. J Cell Sci 2010; 123: 1073–1080. [DOI] [PubMed] [Google Scholar]
- 10.Stockton RA, Shenkar R, Awad IA, et al. Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. J Exp Med 2010; 207: 881–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitehead KJ, Chan AC, Navankasattusas S, et al. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nat Med 2009; 15: 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YH, Chang M, Davidson BL. Molecular signatures of disease brain endothelia provide new sites for CNS-directed enzyme therapy. Nat Med 2009; 15: 1215–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batista L, Miller F, Clave C, et al. Induced secretion of [beta]-hexosaminidase by human brain endothelial cells: A novel approach in Sandhoff disease? Neurobiol Dis 2009; 37: 656–660. [DOI] [PubMed]
- 14.Wolff J, Chao TI. Cytoarchitectonics of non-neuronal cells in the central nervous system. Adv Mol Cell Biol 2004; 31: 1–51. [Google Scholar]
- 15.Stewart PA, Beliveau R, Rogers KA. Cellular localization of P-glycoprotein in brain versus gonadal capillaries. J Histochem Cytochem 1996; 44: 679–685. [DOI] [PubMed] [Google Scholar]
- 16.Soontornmalai A, Vlaming ML, Fritschy JM. Differential, strain-specific cellular and subcellular distribution of multidrug transporters in murine choroid plexus and blood-brain barrier. Neuroscience 2006; 138: 159–169. [DOI] [PubMed] [Google Scholar]
- 17.Guo Z, Zhu J, Zhao L, et al. Expression and clinical significance of multidrug resistance proteins in brain tumors. J Exp Clin Cancer Res 2010; 29: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kikuchi R, Kusuhara H, Sugiyama D, et al. Contribution of organic anion transporter 3 (Slc22a8) to the elimination of p-aminohippuric acid and benzylpenicillin across the blood-brain barrier. J Pharmacol Exper Therapeut 2003; 306: 51–58. [DOI] [PubMed] [Google Scholar]
- 19.Mori S, Takanaga H, Ohtsuki S, et al. Rat organic anion transporter 3 (rOAT3) is responsible for brain-to-blood efflux of homovanillic acid at the abluminal membrane of brain capillary endothelial cells. J Cereb Blood Flow Metabol 2003; 23: 432–440. [DOI] [PubMed] [Google Scholar]
- 20.Nies AT, Jedlitschky G, Konig J, et al. Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6), in human brain. Neuroscience 2004; 129: 349–360. [DOI] [PubMed] [Google Scholar]
- 21.Seetharaman S, Barrand MA, Maskell L, et al. Multidrug resistance-related transport proteins in isolated human brain microvessels and in cells cultured from these isolates. J Neurochem 1998; 70: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 22.Virgintino D, Robertson D, Errede M, et al. Expression of P-glycoprotein in human cerebral cortex microvessels. J Histochem Cytochem 2002; 50: 1671–1676. [DOI] [PubMed] [Google Scholar]
- 23.Roberts LM, Black DS, Raman C, et al. Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience 2008; 155: 423–438. [DOI] [PubMed] [Google Scholar]
- 24.Fellner S, Bauer B, Miller DS, et al. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest 2002; 110: 1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller DS, Nobmann SN, Gutmann H, et al. Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol Pharmacol 2000; 58: 1357–1367. [DOI] [PubMed] [Google Scholar]
- 26.Miller DS, Graeff C, Droulle L, et al. Xenobiotic efflux pumps in isolated fish brain capillaries. Am J Physiol Regulat Integrat Compar Physiol 2002; 282: R191–198. [DOI] [PubMed] [Google Scholar]
- 27.Bauer B, Hartz AM, Pekcec A, et al. Seizure-induced up-regulation of P-glycoprotein at the blood-brain barrier through glutamate and cyclooxygenase-2 signaling. Mol Pharmacol 2008; 73: 1444–1453. [DOI] [PubMed] [Google Scholar]
- 28.McCaffrey G, Staatz WD, Sanchez-Covarrubias L, et al. P-glycoprotein trafficking at the blood-brain barrier altered by peripheral inflammatory hyperalgesia. J Neurochem 2012; 122: 962–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beaulieu E, Demeule M, Ghitescu L, et al. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem J 1997; 326: 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tai LM, Reddy PS, Lopez-Ramirez MA, et al. Polarized P-glycoprotein expression by the immortalised human brain endothelial cell line, hCMEC/D3, restricts apical-to-basolateral permeability to rhodamine 123. Brain Res 2009; 1292: 14–24. [DOI] [PubMed] [Google Scholar]
- 31.Biegel D, Spencer DD, Pachter JS. Isolation and culture of human brain microvessel endothelial cells for the study of blood-brain barrier properties in vitro. Brain Res 1995; 692: 183–189. [DOI] [PubMed] [Google Scholar]
- 32.Tatsuta T, Naito M, Oh-hara T, et al. Functional involvement of P-glycoprotein in blood-brain barrier. J Biol Chem 1992; 267: 20383–20391. [PubMed] [Google Scholar]
- 33.Zhang Y, Schuetz JD, Elmquist WF, et al. Plasma membrane localization of multidrug resistance-associated protein homologs in brain capillary endothelial cells. J Pharmacol Exper Therapeut 2004; 311: 449–455. [DOI] [PubMed] [Google Scholar]
- 34.Bauer B, Hartz AM, Lucking JR, et al. Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase-II metabolizing enzyme, GSTpi, at the blood-brain barrier. J Cereb Blood Flow Metabol 2008; 28: 1222–1234. [DOI] [PubMed] [Google Scholar]
- 35.Leggas M, Adachi M, Scheffer GL, et al. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol 2004; 24: 7612–7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bronger H, Konig J, Kopplow K, et al. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Res 2005; 65: 11419–11428. [DOI] [PubMed] [Google Scholar]
- 37.Tachikawa M, Watanabe M, Hori S, et al. Distinct spatio-temporal expression of ABCA and ABCG transporters in the developing and adult mouse brain. J Neurochem 2005; 95: 294–304. [DOI] [PubMed] [Google Scholar]
- 38.Aronica E, Gorter JA, Redeker S, et al. Localization of breast cancer resistance protein (BCRP) in microvessel endothelium of human control and epileptic brain. Epilepsia 2005; 46: 849–857. [DOI] [PubMed] [Google Scholar]
- 39.Cooray HC, Blackmore CG, Maskell L, et al. Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport 2002; 13: 2059–2063. [DOI] [PubMed] [Google Scholar]
- 40.Hori S, Ohtsuki S, Tachikawa M, et al. Functional expression of rat ABCG2 on the luminal side of brain capillaries and its enhancement by astrocyte-derived soluble factor(s). J Neurochem 2004; 90: 526–536. [DOI] [PubMed] [Google Scholar]
- 41.Hartz AM, Mahringer A, Miller DS, et al. 17-beta-Estradiol: a powerful modulator of blood-brain barrier BCRP activity. Blood Flow and Metabolism 2010; 30: 1742–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisenblatter T, Huwel S, Galla HJ. Characterisation of the brain multidrug resistance protein (BMDP/ABCG2/BCRP) expressed at the blood-brain barrier. Brain Res 2003; 971: 221–231. [DOI] [PubMed] [Google Scholar]
- 43.Betz AL, Firth JA, Goldstein GW. Polarity of the blood-brain barrier: distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res 1980; 192: 17–28. [DOI] [PubMed] [Google Scholar]
- 44.Manoonkitiwongsa PS, Whitter EF, Wareesangtip W, et al. Calcium-dependent ATPase unlike ecto-ATPase is located primarily on the luminal surface of brain endothelial cells. Histochem J 2000; 32: 313–324. [DOI] [PubMed] [Google Scholar]
- 45.Sakai K, Shimizu H, Koike T, et al. Neutral amino acid transporter ASCT1 is preferentially expressed in L-Ser-synthetic/storing glial cells in the mouse brain with transient expression in developing capillaries. J Neurosci 2003; 23: 550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tetsuka K, Takanaga H, Ohtsuki S, et al. The l-isomer-selective transport of aspartic acid is mediated by ASCT2 at the blood-brain barrier. J Neurochem 2003; 87: 891–901. [DOI] [PubMed] [Google Scholar]
- 47.Bolz S, Farrell CL, Dietz K, et al. Subcellular distribution of glucose transporter (GLUT-1) during development of the blood-brain barrier in rats. Cell Tissue Res 1996; 284: 355–365. [DOI] [PubMed] [Google Scholar]
- 48.Cornford EM, Hyman Sa, Pardridge WM. An electron microscopic immunogold analysis of developmental up-regulation of the blood-brain barrier GLUT1 glucose transporter. J Cereb Blood Flow Metabol 1993; 13: 841–854. [DOI] [PubMed] [Google Scholar]
- 49.Dobrogowska DH, Vorbrodt AW. Quantitative immunocytochemical study of blood-brain barrier glucose transporter (GLUT-1) in four regions of mouse brain. J Histochem Cytochem 1999; 47: 1021–1030. [DOI] [PubMed] [Google Scholar]
- 50.Farrell C, Pardridge WM. Blood-brain barrier glucose transporter is asymmetrically distributed on brain capillary endothelial lumenal and ablumenal membranes: an electron microscopic immunogold study. Proc Natl Acad Sci U S A 1991; 88: 5779–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lippoldt A, Kniesel U, Liebner S, et al. Structural alterations of tight junctions are associated with loss of polarity in stroke-prone spontaneously hypertensive rat blood-brain barrier endothelial cells. Brain Res 2000; 885: 251–261. [DOI] [PubMed] [Google Scholar]
- 52.Vorbrodt AW, Dobrogowska DH, Tarnawski M. Immunogold study of interendothelial junction-associated and glucose transporter proteins during postnatal maturation of the mouse blood-brain barrier. J Neurocytol 2001; 30: 705–716. [DOI] [PubMed] [Google Scholar]
- 53.Gerhart DZ, LeVasseur RJ, Broderius MA, et al. Glucose transporter localization in brain using light and electron immunocytochemistry. J Neurosci Res 1989; 22: 464–472. [DOI] [PubMed] [Google Scholar]
- 54.Ruderisch N, Virgintino D, Makrides V, et al. Differential axial localization along the mouse brain vascular tree of luminal sodium-dependent glutamine transporters Snat1 and Snat3. J Cereb Blood Flow Metabol 2011; 31: 1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elfeber K, Kohler A, Lutzenburg M, et al. Localization of the Na+-D-glucose cotransporter SGLT1 in the blood-brain barrier. Histochem Cell Biol 2004; 121: 201–207. [DOI] [PubMed] [Google Scholar]
- 56.Michalec K, Mysiorek C, Kuntz M, et al. Protein kinase C restricts transport of carnitine by amino acid transporter ATB(0,+) apically localized in the blood-brain barrier. Arch Biochem Biophys 2014; 554: 28–35. [DOI] [PubMed] [Google Scholar]
- 57.Duelli R, Enerson BE, Gerhart DZ, et al. Expression of large amino acid transporter LAT1 in rat brain endothelium. J Cereb Blood Flow Metabol 2000; 20: 1557–1562. [DOI] [PubMed] [Google Scholar]
- 58.Lam TI, Wise PM, O'Donnell ME. Cerebral microvascular endothelial cell Na/H exchange: evidence for the presence of NHE1 and NHE2 isoforms and regulation by arginine vasopressin. Am J Physiol. Cell physiology 2009; 297: C278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Donnell ME, Tran L, Lam TI, et al. Bumetanide inhibition of the blood-brain barrier Na-K-Cl cotransporter reduces edema formation in the rat middle cerebral artery occlusion model of stroke. J Cereb Blood Flow Metabol 2004; 24: 1046–1056. [DOI] [PubMed] [Google Scholar]
- 60.Gerhart DZ, Enerson BE, Zhdankina OY, et al. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am J Physiol 1997; 273: E207–213. [DOI] [PubMed] [Google Scholar]
- 61.Leino RL, Gerhart DZ, Drewes LR. Monocarboxylate transporter (MCT1) abundance in brains of suckling and adult rats: a quantitative electron microscopic immunogold study. Brain Res Develop Brain Res 1999; 113: 47–54. [DOI] [PubMed] [Google Scholar]
- 62.Leino RL, Gerhart DZ, Duelli R, et al. Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem Int 2001; 38: 519–527. [DOI] [PubMed] [Google Scholar]
- 63.Gao B, Stieger B, Noe B, et al. Localization of the organic anion transporting polypeptide 2 (Oatp2) in capillary endothelium and choroid plexus epithelium of rat brain. J Histochem Cytochem 1999; 47: 1255–1264. [DOI] [PubMed] [Google Scholar]
- 64.Kis B, Isse T, Snipes JA, et al. Effects of LPS stimulation on the expression of prostaglandin carriers in the cells of the blood-brain and blood-cerebrospinal fluid barriers. J Appl Physiol 2006; 100: 1392–1399. [DOI] [PubMed] [Google Scholar]
- 65.Lin CJ, Tai Y, Huang MT, et al. Cellular localization of the organic cation transporters, OCT1 and OCT2, in brain microvessel endothelial cells and its implication for MPTP transport across the blood-brain barrier and MPTP-induced dopaminergic toxicity in rodents. J Neurochem 2010; 114: 717–727. [DOI] [PubMed] [Google Scholar]
- 66.Ben-Zvi A, Lacoste B, Kur E, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014; 509: 507–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vorbrodt AW, Lossinsky AS, Wisniewski HM, et al. Ultrastructural cytochemical studies of cerebral microvasculature in scrapie infected mice. Acta Neuropathologica 1981; 53: 203–211. [DOI] [PubMed] [Google Scholar]
- 68.Vorbrodt AW, Lossinsky AS, Wisniewski HM. Ultrastructural studies of concanavalin A receptors and 5'-nucleotidase localization in normal and injured mouse cerebral microvasculature. Acta Neuropathologica 1984; 63: 210–217. [DOI] [PubMed] [Google Scholar]
- 69.Ghandour MS, Langley OK, Varga V. Immunohistological localization of gamma-glutamyltranspeptidase in cerebellum at light and electron microscope levels. Neurosci Lett 1980; 20: 125–129. [DOI] [PubMed] [Google Scholar]
- 70.Cannon RE, Peart JC, Hawkins BT, et al. Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci U S A 2012; 109: 15930–15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.ElAli A, Hermann DM. Apolipoprotein E controls ATP-binding cassette transporters in the ischemic brain. Sci Signal 2010; 3: ra72. [DOI] [PubMed] [Google Scholar]
- 72.Hudson N, Powner MB, Sarker MH, et al. Differential apicobasal VEGF signaling at vascular blood-neural barriers. Dev Cell 2014; 30: 541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deguchi Y, Okutsu H, Okura T, et al. Internalization of basic fibroblast growth factor at the mouse blood-brain barrier involves perlecan, a heparan sulfate proteoglycan. J Neurochem 2002; 83: 381–389. [DOI] [PubMed] [Google Scholar]
- 74.Stamatovic SM, Sladojevic N, Keep RF, et al. Relocalization of junctional adhesion molecule A during inflammatory stimulation of brain endothelial cells. Mol Cell Biol 2012; 32: 3414–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Erbar S, Maisner A. Nipah virus infection and glycoprotein targeting in endothelial cells. Virol J 2010; 7: 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bendayan R, Ronaldson PT, Gingras D, et al. In situ localization of P-glycoprotein (ABCB1) in human and rat brain. J Histochem Cytochem 2006; 54: 1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manoonkitiwongsa PS, Schultz RL, Wareesangtip W, et al. Luminal localization of blood-brain barrier sodium, potassium adenosine triphosphatase is dependent on fixation. J Histochem Cytochem 2000; 48: 859–865. [DOI] [PubMed] [Google Scholar]
- 78.Manoonkitiwongsa PS, Whitter EF, Schultz RL. An in situ cytochemical evaluation of blood-brain barrier sodium, potassium-activated adenosine triphosphatase polarity. Brain Res 1998; 798: 261–270. [DOI] [PubMed] [Google Scholar]
- 79.Vorbrodt AW, Lossinsky AS, Wisniewski HM. Localization of alkaline phosphatase activity in endothelia of developing and mature mouse blood-brain barrier. Develop Neurosci 1986; 8: 1–13. [DOI] [PubMed] [Google Scholar]
- 80.Huwyler J, Pardridge WM. Examination of blood-brain barrier transferrin receptor by confocal fluorescent microscopy of unfixed isolated rat brain capillaries. J Neurochem 1998; 70: 883–886. [DOI] [PubMed] [Google Scholar]
- 81.Betz A, Goldstein G. Polarity of the blood-brain barrier: neutral amino acid transport into isolated brain capillaries. Science 1978; 202: 225–227. [DOI] [PubMed] [Google Scholar]
- 82.Cremer JE, Seville MP, Cunningham VJ. Tracer 2-deoxyglucose kinetics in brain regions of rats given kainic acid. J Cereb Blood Flow Metab 1988; 8: 244–53. [DOI] [PubMed] [Google Scholar]
- 83.Schinkel A, Smit J, van Tellingen O, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 1994; 77: 491–502. [DOI] [PubMed] [Google Scholar]
- 84.Tang SC, Lankheet NAG, Poller B, et al. P-Glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) restrict brain accumulation of the active sunitinib metabolite N-desethyl sunitinib. J Pharmacol Exper Therapeut 2012; 341: 164–173. [DOI] [PubMed] [Google Scholar]
- 85.Betz AL, Firth JA, Goldstein GW. Polarity of the blood-brain barrier: distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res 1980; 192: 17–28. [DOI] [PubMed] [Google Scholar]
- 86.del Pino MMS, Hawkins RA, Peterson DR. Biochemical discrimination between luminal and abluminal enzyme and transport activities of the blood-brain barrier. J Biol Chem 1995; 270: 14907–14912. [DOI] [PubMed] [Google Scholar]
- 87.Lee WJ, Peterson DR, Sukowski EJ, et al. Glucose transport by isolated plasma membranes of the bovine blood-brain barrier. Am J Physiol 1997; 272: C1552–1557. [DOI] [PubMed] [Google Scholar]
- 88.McCaffrey G, Staatz WD, Quigley CA, et al. Tight junctions contain oligomeric protein assembly critical for maintaining blood-brain barrier integrity in vivo. J Neurochem 2007; 103: 2540–2555. [DOI] [PubMed] [Google Scholar]
- 89.Hoshi Y, Uchida Y, Tachikawa M, et al. Quantitative atlas of blood-brain barrier transporters, receptors, and tight junction proteins in rats and common marmoset. J Pharm Sci 2013; 102: 3343–3455. [DOI] [PubMed] [Google Scholar]
- 90.Kubo Y, Ohtsuki S, Uchida Y, et al. Quantitative Determination of Luminal and Abluminal Membrane Distributions of Transporters in Porcine Brain Capillaries by Plasma Membrane Fractionation and Quantitative Targeted Proteomics. J Pharm Sci 2015; 104: 3060–3068. [DOI] [PubMed]
- 91.Cornford EM, Hyman S. Localization of Brain Endothelial Luminal and Abluminal Transporters with Immunogold Electron Microscopy. NeuroRx 2005; 2: 27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huber O, Brunner A, Maier P, et al. Localization Microscopy (SPDM) Reveals Clustered Formations of P-Glycoprotein in a Human Blood-Brain Barrier Model. PLoS ONE 2012; 7: e44776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cecchelli R. Modelling the blood-brain barrier in drug discovery and development. Nat Rev Drug Discov 2007; 6: 650–661. [DOI] [PubMed] [Google Scholar]
- 94.Tewes BJ, Galla HJ. Lipid polarity in brain capillary endothelial cells. Endothelium 2001; 8: 207–220. [DOI] [PubMed] [Google Scholar]
- 95.Tai LM, Reddy PS, Lopez-Ramirez MA, et al. Polarized P-glycoprotein expression by the immortalised human brain endothelial cell line, hCMEC/D3, restricts apical-to-basolateral permeability to rhodamine 123. Brain Res 2009; 1292: 14–24. [DOI] [PubMed] [Google Scholar]
- 96.Verma S, Nakaoke R, Dohgu S, et al. Release of cytokines by brain endothelial cells: A polarized response to lipopolysaccharide. Brain Behav Immun 2006; 20: 449–455. [DOI] [PubMed] [Google Scholar]
- 97.Panzenboeck U, Balazs Z, Sovic A, et al. ABCA1 and Scavenger Receptor Class B, Type I, are modulators of reverse sterol transport at an in vitro blood-brain barrier constituted of porcine brain capillary endothelial cells. J Biol Chem 2002; 277: 42781–42789. [DOI] [PubMed] [Google Scholar]
- 98.Unemori EN, Bouhana KS, Werb Z. Vectorial secretion of extracellular matrix proteins, matrix-degrading proteinases, and tissue inhibitor of metalloproteinases by endothelial cells. J Biol Chem 1990; 265: 445–451. [PubMed] [Google Scholar]
- 99.Krizanac-Bengez L, Kapural M, Parkinson F, et al. Effects of transient loss of shear stress on blood–brain barrier endothelium: role of nitric oxide and IL-6. Brain Res 2003; 977: 239–246. [DOI] [PubMed] [Google Scholar]
- 100.Jurczyluk J, Brown D, Stanley KK. Polarised secretion of cytokines in primary human microvascular endothelial cells is not dependent on N-linked glycosylation. Cell Biol Int 2003; 27: 997–1003. [DOI] [PubMed] [Google Scholar]
- 101.Sporn LA, Marder VJ, Wagner DD. Differing polarity of the constitutive and regulated secretory pathways for von Willebrand factor in endothelial cells. J Cell Biol 1989; 108: 1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zerwes HG, Risau W. Polarized secretion of a platelet-derived growth factor-like chemotactic factor by endothelial cells in vitro. J Cell Biol 1987; 105: 2037–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wagner OF, Christ G, Wojta J, et al. Polar secretion of endothelin-1 by cultured endothelial cells. J Biol Chem 1992; 267: 16066–16068. [PubMed] [Google Scholar]
- 104.Dehouck M-P, Vigne P, Torpier G, et al. Endothelin-1 as a mediator of endothelial cell-pericyte interactions in bovine brain capillaries. J Cereb Blood Flow Metab 1997; 17: 464–469. [DOI] [PubMed] [Google Scholar]
- 105.Jiang C, Koyabu N, Yonemitsu Y, et al. In vivo delivery of glial cell-derived neurotrophic factor across the blood-brain barrier by gene transfer into brain capillary endothelial cells. Human Gene Ther 2003; 14: 1181–1191. [DOI] [PubMed] [Google Scholar]
- 106.Chui R, Dorovini-Zis K. Regulation of CCL2 and CCL3 expression in human brain endothelial cells by cytokines and lipopolysaccharide. J Neuroinflammation 2010; 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cruz-Orengo L, Daniels BP, Dorsey D, et al. Enhanced sphingosine-1-phosphate receptor 2 expression underlies female CNS autoimmunity susceptibility. J Clin Invest 2014; 124: 2571–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reitsma S, Slaaf DW, Vink H, et al. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 2007; 454: 345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Savery MD, Jiang JX, Park PW, et al. The endothelial glycocalyx in syndecan-1 deficient mice. Microvasc Res 2013; 87: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 2011; 12: 517–533. [DOI] [PubMed] [Google Scholar]
- 111.Roberts RL, Fine RE, Sandra A. Receptor-mediated endocytosis of transferrin at the blood-brain barrier. J Cell Sci 1993; 104: 521–532. [DOI] [PubMed] [Google Scholar]
- 112.Tecedor L, Stein CS, Schultz ML, et al. CLN3 loss disturbs membrane microdomain properties and protein transport in brain endothelial cells. J Neurosci 2013; 33: 18065–18079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boucrot E, Ferreira AP, Almeida-Souza L, et al. Endophilin marks and controls a clathrin-independent endocytic pathway. Nature 2015; 517: 460–465. [DOI] [PubMed] [Google Scholar]
- 114.Liu W, Wang P, Shang C, et al. Endophilin-1 regulates blood–brain barrier permeability by controlling ZO-1 and occludin expression via the EGFR–ERK1/2 pathway. Brain Res 2014; 1573: 17–26. [DOI] [PubMed] [Google Scholar]
- 115.Deane R, Du Yan S, Submamaryan RK, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med 2003; 9: 907–913. [DOI] [PubMed] [Google Scholar]
- 116.Shibata M, Yamada S, Kumar SR, et al. Clearance of Alzheimer's amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 2000; 106: 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Philippe M, Léger T, Desvaux R, et al. Discs Large 1 (Dlg1) scaffolding protein participates with clathrin and adaptator protein complex 1 (AP-1) in forming Weibel-Palade bodies of endothelial cells. J Biol Chem 2013; 288: 13046–13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dohgu S, Fleegal-DeMotta MA, Banks WA. Lipopolysaccharide-enhanced transcellular transport of HIV-1 across the blood-brain barrier is mediated by luminal microvessel IL-6 and GM-CSF. J Neuroinflammation 2011; 8: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rodriguez-Boulan E, Macara IG. Organization and execution of the epithelial polarity programme. Nature reviews. Molecular cell biology 2014; 15: 225–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roignot J, Peng X, Mostov K. Polarity in mammalian epithelial morphogenesis. Cold Spring Harbor Perspect Biol 2013; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rodriguez-Fraticelli AE, Martin-Belmonte F. Picking up the threads: extracellular matrix signals in epithelial morphogenesis. Curr Opin Cell Biol 2014; 30: 83–90. [DOI] [PubMed] [Google Scholar]
- 122.Eaton S, Martin-Belmonte F. Cargo sorting in the endocytic pathway: a key regulator of cell polarity and tissue dynamics. Cold Spring Harbor Perspect Biol 2014; 6: a016899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.St Johnston D, Sanson B. Epithelial polarity and morphogenesis. Curr Opin Cell Biol 2011; 23: 540–546. [DOI] [PubMed] [Google Scholar]
- 124.Tepass U. The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu Rev Cell Dev Biol 2012; 28: 655–685. [DOI] [PubMed] [Google Scholar]
- 125.Kemphues KJ, Priess JR, Morton DG, et al. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell 1988; 52: 311–320. [DOI] [PubMed] [Google Scholar]
- 126.Zovein AC, Luque A, Turlo KA, et al. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev Cell 2010; 18: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakayama M, Nakayama A, van Lessen M, et al. Spatial regulation of VEGF receptor endocytosis in angiogenesis. Nat Cell Biol 2013; 15: 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 2003; 115: 691–704. [DOI] [PubMed] [Google Scholar]
- 129.Yamanaka T, Horikoshi Y, Sugiyama Y, et al. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr Biol 2003; 13: 734–743. [DOI] [PubMed] [Google Scholar]
- 130.Halaoui R, McCaffrey L. Rewiring cell polarity signaling in cancer. Oncogene 2015; 34: 939–950. [DOI] [PubMed] [Google Scholar]
- 131.Jansen M, Ten Klooster JP, Offerhaus GJ, et al. LKB1 and AMPK family signaling: the intimate link between cell polarity and energy metabolism. Physiol Rev 2009; 89: 777–798. [DOI] [PubMed] [Google Scholar]
- 132.Pocha SM, Knust E. Complexities of Crumbs function and regulation in tissue morphogenesis. Curr Biol 2013; 23: R289–293. [DOI] [PubMed] [Google Scholar]
- 133.Pieczynski J, Margolis B. Protein complexes that control renal epithelial polarity. Am J Physiol. Renal physiology 2011; 300: F589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ngok SP, Geyer R, Liu M, et al. VEGF and Angiopoietin-1 exert opposing effects on cell junctions by regulating the Rho GEF Syx. J Cell Biol 2012; 199: 1103–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Humbert PO, Grzeschik NA, Brumby AM, et al. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene 2008; 27: 6888–6907. [DOI] [PubMed] [Google Scholar]
- 136.Betschinger J, Mechtler K, Knoblich JA. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature 2003; 422: 326–330. [DOI] [PubMed] [Google Scholar]
- 137.Qin Y, Capaldo C, Gumbiner BM, et al. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol 2005; 171: 1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Michaelis UR, Chavakis E, Kruse C, et al. The polarity protein Scrib is essential for directed endothelial cell migration. Circ Res 2013; 112: 924–934. [DOI] [PubMed] [Google Scholar]
- 139.Mack NA, Georgiou M. The interdependence of the Rho GTPases and apicobasal cell polarity. Small GTPases 2014; 5: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Qin Y, Meisen WH, Hao Y, et al. Tuba, a Cdc42 GEF, is required for polarized spindle orientation during epithelial cyst formation. J Cell Biol 2010; 189: 661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lin D, Edwards AS, Fawcett JP, et al. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol 2000; 2: 540–547. [DOI] [PubMed] [Google Scholar]
- 142.Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol 2005; 7: 262–269. [DOI] [PubMed] [Google Scholar]
- 143.Nishimura T, Yamaguchi T, Kato K, et al. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol 2005; 7: 270–277. [DOI] [PubMed] [Google Scholar]
- 144.Audebert S, Navarro C, Nourry C, et al. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr Biol 2004; 14: 987–995. [DOI] [PubMed] [Google Scholar]
- 145.Nakayama M, Goto TM, Sugimoto M, et al. Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev Cell 2008; 14: 205–215. [DOI] [PubMed] [Google Scholar]
- 146.Ishiuchi T, Takeichi M. Willin and Par3 cooperatively regulate epithelial apical constriction through aPKC-mediated ROCK phosphorylation. Nat Cell Biol 2011; 13: 860–866. [DOI] [PubMed] [Google Scholar]
- 147.Shewan A, Eastburn DJ, Mostov K. Phosphoinositides in cell architecture. Cold Spring Harbor Perspect Biol 2011; 3: a004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gassama-Diagne A, Yu W, ter Beest M, et al. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol 2006; 8: 963–970. [DOI] [PubMed] [Google Scholar]
- 149.Peng J, Awad A, Sar S, et al. Phosphoinositide 3-kinase p110delta promotes lumen formation through the enhancement of apico-basal polarity and basal membrane organization. Nature Commun 2015; 6: 5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Coureuil M, Mikaty G, Miller F, et al. Meningococcal type IV Pili recruit the polarity complex to cross the brain endothelium. Science 2009; 325: 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Daneman R, Zhou L, Agalliu D, et al. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS ONE 2010; 5: e13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ebnet K, Aurrand-Lions M, Kuhn A, et al. The junctional adhesion molecule (JAM) family members JAM-2 and JAM-3 associate with the cell polarity protein PAR-3: a possible role for JAMs in endothelial cell polarity. J Cell Sci 2003; 116: 3879–3891. [DOI] [PubMed] [Google Scholar]
- 153.Li X, Guan Y, Chen Y, et al. Expression of Wnt5a and its receptor Fzd2 is changed in the spinal cord of adult amyotrophic lateral sclerosis transgenic mice. Int J Clin Exper Pathol 2013; 6: 1245–1260. [PMC free article] [PubMed] [Google Scholar]
- 154.Garnaas MK, Moodie KL, Liu M-l, et al. Syx, a RhoA guanine exchange factor, is essential for angiogenesis in vivo. Circ Res 2008; 103: 710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zheng Y, Vertuani S, Nyström S, et al. Angiomotin-like protein 1 controls endothelial polarity and junction stability during sprouting angiogenesis. Circ Res 2009; 105: 260–270. [DOI] [PubMed] [Google Scholar]
- 156.Aase K, Ernkvist M, Ebarasi L, et al. Angiomotin regulates endothelial cell migration during embryonic angiogenesis. Genes Develop 2007; 21: 2055–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Posokhova E, Shukla A, Seaman S, et al. GPR124 functions as a WNT7-specific coactivator of canonical β-catenin signaling. Cell Rep 2015; 10: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Caruana G, Bernstein A. Craniofacial dysmorphogenesis including cleft palate in mice with an insertional mutation in the discs large gene. Mol Cell Biol 2001; 21: 1475–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stenman JM, Rajagopal J, Carroll TJ, et al. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 2008; 322: 1247–1250. [DOI] [PubMed] [Google Scholar]
- 160.Daneman R, Agalliu D, Zhou L, et al. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A 2009; 106: 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Klezovitch O, Fernandez TE, Tapscott SJ, et al. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Develop 2004; 18: 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pannekoek W-J, Post A, Bos JL. Rap1 signaling in endothelial barrier control. Cell Adhes Migration 2014; 8: 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Itoh M, Nelson CM, Myers CA, et al. Rap1 integrates tissue polarity, lumen formation, and tumorigenic potential in human breast epithelial cells. Cancer Res 2007; 67: 4759–4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Glading A, Han J, Stockton RA, et al. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J Cell Biol 2007; 179: 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Beraud-Dufour S, Gautier R, Albiges-Rizo C, et al. Krit 1 interactions with microtubules and membranes are regulated by Rap1 and integrin cytoplasmic domain associated protein-1. FEBS J 2007; 274: 5518–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Glading AJ, Ginsberg MH. Rap1 and its effector KRIT1/CCM1 regulate beta-catenin signaling. Dis Model Mech 2010; 3: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Boulday G, Rudini N, Maddaluno L, et al. Developmental timing of CCM2 loss influences cerebral cavernous malformations in mice. J Exp Med 2011; 208: 1835–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yoruk B, Gillers BS, Chi NC, et al. Ccm3 functions in a manner distinct from Ccm1 and Ccm2 in a zebrafish model of CCM vascular disease. Develop Biol 2012; 362: 121–131. [DOI] [PubMed] [Google Scholar]
- 169.Chan AC, Drakos SG, Ruiz OE, et al. Mutations in 2 distinct genetic pathways result in cerebral cavernous malformations in mice. J Clin Invest 2011; 121: 1871–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zheng X, Xu C, Di Lorenzo A, et al. CCM3 signaling through sterile 20-like kinases plays an essential role during zebrafish cardiovascular development and cerebral cavernous malformations. J Clin Invest 2010; 120: 2795–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fidalgo M, Fraile M, Pires A, et al. CCM3/PDCD10 stabilizes GCKIII proteins to promote Golgi assembly and cell orientation. J Cell Sci 2010; 123: 1274–1284. [DOI] [PubMed] [Google Scholar]
- 172.Yadav S, Puri S, Linstedt AD. A primary role for golgi positioning in directed secretion, cell polarity, and wound healing. Mol Biol Cell 2009; 20: 1728–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Camerer E, Pringle S, Skartlien AH, et al. Opposite sorting of tissue factor in human umbilical vein endothelial cells and Madin-Darby canine kidney epithelial cells. Blood 1996; 88: 1339–1349. [PubMed] [Google Scholar]
- 174.Jefferies WA, Brandon MR, Hunt SV, et al. Transferrin receptor on endothelium of brain capillaries. Nature 1984; 312: 162–163. [DOI] [PubMed] [Google Scholar]
- 175.Collins C, Tzima E. Rac[e] to the pole: setting up polarity in endothelial cells. Small GTPases 2014; 5: e28650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu B, Lu S, Hu YL, et al. RhoA and membrane fluidity mediates the spatially polarized Src/FAK activation in response to shear stress. Scientific Rep 2014; 4: 7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ Res 2006; 98: 176–185. [DOI] [PubMed] [Google Scholar]
- 178.He F, Peng J, Deng XL, et al. RhoA and NF-κB are involved in lipopolysaccharide-induced brain microvascular cell line hyperpermeability. Neuroscience 2011; 188: 35–47. [DOI] [PubMed] [Google Scholar]
- 179.Birukova AA, Birukov KG, Smurova K, et al. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J 2004; 18: 1879–1890. [DOI] [PubMed] [Google Scholar]
- 180.Stamatovic SM, Dimitrijevic OB, Keep RF, et al. Protein kinase Calpha-RhoA cross-talk in CCL2-induced alterations in brain endothelial permeability. J Biol Chem 2006; 281: 8379–8388. [DOI] [PubMed] [Google Scholar]
- 181.Cristante E, McArthur S, Mauro C, et al. Identification of an essential endogenous regulator of blood-brain barrier integrity, and its pathological and therapeutic implications. Proc Natl Acad Sci U S A 2013; 110: 832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shen Q, Rigor RR, Pivetti CD, et al. Myosin light chain kinase in microvascular endothelial barrier function. Cardiovasc Res 2010; 87: 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Persidsky Y, Heilman D, Haorah J, et al. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE). Blood 2006; 107: 4770–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Draheim KM, Fisher OS, Boggon TJ, et al. Cerebral cavernous malformation proteins at a glance. J Cell Sci 2014; 127: 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Broman MT, Kouklis P, Gao X, et al. Cdc42 regulates adherens junction stability and endothelial permeability by inducing alpha-catenin interaction with the vascular endothelial cadherin complex. Circ Res 2006; 98: 73–80. [DOI] [PubMed] [Google Scholar]
- 186.Koh W, Mahan RD, Davis GE. Cdc42- and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. J Cell Sci 2008; 121: 989–1001. [DOI] [PubMed] [Google Scholar]
- 187.Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 1987; 325: 253–257. [DOI] [PubMed] [Google Scholar]
- 188.Lee SW, Kim WJ, Choi YK, et al. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med 2003; 9: 900–906. [DOI] [PubMed] [Google Scholar]
- 189.Alvarez JI, Dodelet-Devillers A, Kebir H, et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 2011; 334: 1727–1731. [DOI] [PubMed] [Google Scholar]
- 190.Alvarez JI, Katayama T, Prat A. Glial influence on the blood brain barrier. Glia 2013; 61: 1939–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Armulik A, Genove G, Mae M, et al. Pericytes regulate the blood-brain barrier. Nature 2010; 468: 557–561. [DOI] [PubMed] [Google Scholar]
- 192.Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010; 68: 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Daneman R, Zhou L, Kebede AA, et al. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010; 468: 562–566. [DOI] [PMC free article] [PubMed]
- 194.Ben-Zvi A, Lacoste B, Kur E, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014; 509: 507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nguyen LN, Ma D, Shui G, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014; 509: 503–506. [DOI] [PubMed] [Google Scholar]
- 196.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res 2005; 97: 512–523. [DOI] [PubMed] [Google Scholar]
- 197.Schulz GB, Wieland E, Wüstehube-Lausch J, et al. Cerebral cavernous malformation-1 protein controls DLL4-notch3 signaling between the endothelium and pericytes. Stroke 2015; 46: 1337–1343. [DOI] [PubMed] [Google Scholar]
- 198.Yamamoto H, Ehling M, Kato K, et al. Integrin beta1 controls VE-cadherin localization and blood vessel stability. Nat Commun 2015; 6: 6429. [DOI] [PubMed] [Google Scholar]
- 199.Koutsiaris AG, Tachmitzi SV, Batis N, et al. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology 2007; 44: 375–386. [PubMed] [Google Scholar]
- 200.Cucullo L, Hossain M, Puvenna V, et al. The role of shear stress in blood-brain barrier endothelial physiology. BMC Neurosci 2011; 12: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Booth R, Kim H. Permeability analysis of neuroactive drugs through a dynamic microfluidic in vitro blood–brain barrier model. Ann Biomed Eng 2014; 42: 2379–2391. [DOI] [PubMed] [Google Scholar]
- 202.Iden S, Rehder D, August B, et al. A distinct PAR complex associates physically with VE-cadherin in vertebrate endothelial cells. EMBO Rep 2006; 7: 1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Strilic B, Kucera T, Eglinger J, et al. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev Cell 2009; 17: 505–515. [DOI] [PubMed] [Google Scholar]
- 204.Xu K, Sacharidou A, Fu S, et al. Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev Cell 2011; 20: 526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]