Abstract
Purpose of the Study:
Postmenopausal osteoporosis can impact quality-of-life even prefracture. To determine whether osteoporosis should be a greater concern in women Veterans versus non-Veterans, we compared fracture rates and bone mineral density (BMD) for Veterans and non-Veterans using Women’s Health Initiative data.
Design and Methods:
In this cohort study, participants were women aged 50–79 years. Outcomes were hip, central body, and limb fractures occurring during up to 19 years of follow-up and hip, spine, and whole body BMD collected three times over a 6-year period in a participant subsample. Covariates comprised risk factors for fracture, including fall history and other components of the World Health Organization Fracture Risk Assessment Tool (FRAX). Cox Proportional Hazards models were used to examine fracture rates for Veterans compared with non-Veterans.
Results:
Of 161,808 women, 145,521 self-identified as Veteran (n = 3,719) or non-Veteran (n = 141,802). Baseline FRAX scores showed that Veterans had higher 10-year probabilities for any major fracture (13.3 vs 10.2; p < .01) and hip fracture (4.1 vs 2.2; p < .01) compared with non-Veterans. The age-adjusted rate of hip fracture per 1,000 person-years for Veterans was 3.3 versus 2.4 for non-Veterans (p < .01). After adjustment, the hazards ratio for hip fracture was 1.24 (95% confidence interval 1.03–1.49) for Veterans versus non-Veterans. Hazards ratios at other anatomic sites did not differ by Veteran status. Mean BMD at baseline and at Years 3 and 6 also did not differ by Veteran status at any site.
Implications:
Women Veterans had an increased hip fracture rate not explained by differences in well-recognized fracture risk factors.
Key Words: Osteoporosis, Cohort, Risk factors, Fracture, Postmenopausal osteoporosis, Veterans
Purpose of the Study
It has been projected that the proportion of all living U.S. Veterans who are women will increase from 10.3% (2.5 million) in 2013 to 15% (13.5 million) by 2030 (U.S. Department of Veterans Affairs, 2014). In an effort to address this increase, the Veterans Health Administration (VHA) has created the Women Veterans Health Care program, whose strategic priorities include improving care to aging women Veterans, to “identify and address cardiovascular disease as well as advances in treatments for diabetes, osteoporosis, and menopause” (Department of Veterans Affairs, 2015). This sounds like good news for women Veterans who are at risk for osteoporosis. Postmenopausal osteoporosis can greatly impact quality of life, particularly in terms of pain, mobility, and activities of daily living, whether or not women have fractured (Baczyk, 2009; Hübscher, Vogt, Schmidt, Fink, & Banzer, 2010). In particular, hip fractures are associated with significant morbidity and mortality (Ohldin & Floyd, 2003).
In light of an aging and increasing women Veterans population as well as an increasing nationwide population of women with osteoporosis (a 29% increase from 2010 to 2020; Wright et al., 2014), we studied the rates of osteoporosis-related outcomes in women Veterans versus non-Veterans to see whether fractures should be a greater health concern in one population over the other. To-date, limited research has been conducted to address this question. Participants in studies conducted in the VHA are overwhelmingly men (Goldzweig, Balekian, Rolón, Yano, & Shekelle, 2006; Yano et al., 2010), and studies about osteoporosis have been no exception (Bass, French, Bradham, & Rubenstein, 2007; Shibli-Rahhal, Vaughan-Sarrazin, Richardson, & Cram, 2010), despite evidence that osteoporosis has greater prevalence in women compared with men (Willson, Nelson, Newbold, Nelson, & LaFleur, 2015). A study of a large, national cohort of postmenopausal Veterans in VHA databases (LaFleur et al., 2013) found lower fracture incidence rates than would be expected based on clinical trial data in a similar at-risk, non-Veteran population (Donaldson et al., 2012). However, there were no direct comparisons with non-Veteran women populations (LaFleur et al., 2013), leaving the question of whether Veteran women differ from non-Veteran women in fracture rates unanswered. In a recent study that used data from the Women’s Health Initiative (Weitlauf et al., 2015), investigators reported that Veterans were more than twice as likely as non-Veterans to have hip fractures in crude analyses, but it is not known whether those differences would remain when controlling for available known clinical risk factors for fracture.
The fracture rate differences between women Veterans and non-Veterans, however, remain largely unknown, as do the contributors to any risk differences. Numerous reasons might explain why women Veterans might differ from non-Veterans in some important risk factors for osteoporosis and fracture. For example, Veterans may generally differ from non-Veterans in the chronic conditions they have or the health care they require (e.g., mental health including posttraumatic stress disorder and dual coverage; Assari, 2014; Nelson, Starkebaum, & Reiber, 2007; Selim et al., 2004). To address this research gap, we examined the association between Veteran status and rate of fracture in the WHI cohort which, due to the large sample size and the completeness of the data on many risk factors for fracture, offers some unique opportunities to test whether fracture rates differ in women by veteran status.
Conceptual Framework
Our analysis is based on an adapted Biopsychosocial Model of Health and Aging, summarized and explained in the accompanying editorial (Reiber Editorial 1; 2016). The model describes the roles of several aspects of the social environment to health, including personal social relationships, socioeconomic status, and community characteristics (Seeman & Crimmins, 2001). The adapted model also incorporates the effects of change and adaptation (or allostatic load) on healthy aging (McEwen, 2003). We theorize that Veterans may differ from non-Veterans in many physical and sociocultural factors that might increase fracture rate, such as demographic characteristics, social challenges and stressors, and health behaviors. For example, Veterans from the postmenopausal age cohorts predominantly served as health care personnel in the armed forces during the World War II, Korean War, and Vietnam War eras. Thus, these women may have had higher education levels and higher socioeconomic status than non-Veterans, resulting in higher health literacy. Differences in health literacy could directly impact fracture rate because people with higher health literacy may have had diets higher in essential minerals and vitamins, such as calcium and vitamin D. These women may also have had different challenges and stressors such as sexual trauma, injuries, infections, and chemical exposures, which could impact fracture rate through changes in bone mineral density (BMD). Thus, our analysis focuses on Veteran status as the explanatory variable, and we control for available known clinical risk factors for fracture (Gray et al., 2010) as well as aggregate measures of fracture risk including 10-year fracture probabilities as estimated by the World Health Organization (WHO) Fracture Risk Assessment Tool (WHO FRAX) calculator (World Health Organization, 2011) in an effort to minimize the possibility that any differences in fracture rates between Veterans and non-Veterans are wholly a function of differences in these available known clinical risk factors.
Design and Methods
Study Population
The WHI program enrolled 161,808 women aged 50–79 years at baseline in 1993–1998 from 40 clinical centers across the United States. The main WHI study program, which included three clinical trials and an observational study, was completed in 2005. Two extension studies during the periods of 2005–2010 and 2010–2015 allowed for continued observation and outcomes assessment. Participants provided written informed consent at baseline and at enrollment in both extension studies. Among those initially enrolled in the WHI, 115,407 (71.3%) participants enrolled in Extension Study I, and 93,540 (57.8%) participants enrolled in Extension Study II. The study protocol was reviewed and approved by each clinical center’s institutional review board for the initial study and the first extension and by the Fred Hutchinson Cancer Research Center for the second extension.
Study Design and Measures
This study design was a historical cohort study of both clinical trial and cohort study WHI data. The explanatory variable was Veteran status, which participants self-reported at baseline, and was defined as having served in the U.S. armed forces for at least 180 days. A response was provided for 145,521 women, of whom 3,719 (2.6%) were characterized as Veterans. In 2005, 68.7% (n = 2,556) Veterans consented to participate in Extension Study I and 71.5% (n=101,367) of non-Veterans consented to participate. For Extension Study II, 51.6% (n = 1,918) Veterans and 58.2% (n = 82,527) non-Veterans enrolled.
The study outcomes were incident fracture and bone mineral density (BMD). Mean BMDs measured by dual energy x-ray absorptiometry at the hip, posterior–anterior spine, and whole body were examined in a subset of participants (i.e., participants enrolled at the Pittsburgh, PA; Phoenix and Tucson, AZ; and Birmingham, AL sites). Five percent (n = 186) of Veteran women and 5.4% (n = 7,611) of non-Veteran women had BMD measures at baseline. BMD measures from the same participants were collected again in Years 3 and 6 of study follow-up. In Year 3, 153 women were available for hip BMD, 155 women were available for total spine BMD, and 154 women were available for whole body BMD. In Year 6, 131 women were available for hip BMD, 126 women were available for total spine BMD, and 133 women were available for whole body BMD.
Throughout the follow-up period, including during the study extension periods, participants were asked annually whether a doctor told them for the first time they had broken a bone “since the date on the front of this form” (the date being the participant’s last study visit) and, if so, which bone had been broken. Based on these responses, fractures were grouped into four mutually exclusive sites: (1) hip; (2) central body (hip, pelvis, coccyx, or spine); (3) upper limb (wrist, elbow, hand, scapula, humerus, and lower or upper arm); and (4) lower limb (ankle, patella, foot, tibia or fibula, and lower or upper leg) (Crandall et al., 2015). When the occurrence of a broken bone was identified, the number of days from the time of the break since WHI enrollment was estimated. All hip fractures were centrally adjudicated by trained physicians because hip fracture was a primary outcome in the WHI program. Physicians in the Clinical Centers, the Clinical Coordinating Center, and the NIH classified outcomes. In the first stage, the local Clinical Center adjudicator reviewed the self-reported documents, as well as the radiologist’s written report, and assigned a diagnosis. Adjudicators also consulted hospital discharge summaries for hip fracture and consulted emergency room, clinic, and progress notes when a radiology report was not available for other nonspine fractures. Hip fractures were then centrally adjudicated using the same criteria and documentation as used at the local adjudication step, up to and including the radiograph if the hip fracture diagnosis was ambiguous. (Curb et al., 2003) Because a prior WHI report suggested higher prevalence of hip fractures for Veterans compared with non-Veterans (Weitlauf et al., 2015), central body fractures were examined both with and without the inclusion of hip fracture for this analysis. Fractures not occurring at the hip were self-reported and have been shown to have good to excellent agreement with adjudicated fractures (Chen et al., 2004).
Mean follow-up times for the capture and identification of incident fractures were similar for Veterans than for non-Veterans. In the main WHI program, Veterans’ mean and standard deviation (SD) years of follow-up was 7.8 (1.6), whereas for non-Veterans, it was 7.9 (1.5). For Extension Study I, the comparable means (SD) were 11.3 (3.5) and 11.6 (3.4) years for Veterans and non-Veterans, respectively. Similarly, for Extension Study II, mean (SD) years of follow-up were 13.0 (4.8) for Veterans and 13.6 (4.7) for non-Veteran women. In both groups, women were followed for up to 19 years.
At baseline, participants completed standardized questionnaires that captured demographic, lifestyle, and medical history information. Age was calculated using birth date and WHI enrollment date. Race/ethnicity was self-selected from a list of six possible categories: American Indian/Alaskan Native, Asian/Pacific Islander, Black/African-American, Hispanic/Latina, White, or Other. Because few Veterans were classified as America Indian/Alaskan Native and Asian/Pacific Islander, these racial/ethnic groups were combined with Other. Smoking behavior and alcohol intake were categorized into none, past, or current based on self-reported number of cigarettes smoked and number of alcoholic beverages consumed, respectively. Current drinkers were further categorized into three additional groups based on the amount consumed. Height and weight were clinically measured by trained staff and were used to calculate body mass index (BMI). BMI, in kilograms per meters squared, was categorized using WHO standard cut-points (National Institutes of Health, 1998). Physical activity was computed as metabolic equivalents per week using self-reported frequency and duration of recreational exercise (Ainsworth et al., 1993). Self-rated health and physical function scores were based on subscales from the RAND 36-Item Health Survey (Ware & Sherbourne, 1992). Depression was determined using scores from the Center for Epidemiologic Studies Depression Scale (Burnam, Wells, Leake, & Landsverk, 1988). Presence of diabetes, cardiovascular disease (CVD), rheumatoid arthritis, ulcer, and/or prior fracture was self-reported based on an affirmative response to a question that asked whether a doctor had ever diagnosed them with that condition. Participants also self-reported whether they had a procedure to remove their ovaries and/or uterus, the number of times they fell in the past 12 months, and whether their mother and/or father had ever broken a bone. All current medications were reviewed at baseline by clinic interviewers and inventoried into the WHI database. For this analysis, medications were identified based on their therapeutic class and included oral glucocorticosteroids, psychoactive medications (i.e., antianxiety agents, antidepressants, antipsychotics, hypnotics, and narcotic analgesics), hormone therapy (using random assignment for hormone use in women enrolled in the WHI Hormone Therapy Trial), and proton pump inhibitors. Therapeutic class codes for calcium and Vitamin D supplementation and self-reported use of supplements were used to identify calcium and Vitamin D intake. The WHO FRAX scores (not accounting for BMD at the femoral neck) for any major osteoporotic fracture and for hip fracture were estimated for all participants using data provided at baseline. The WHO FRAX, available online at https://www.shef.ac.uk/FRAX/, is an absolute risk assessment rule that estimates absolute 10-year risks for hip or any major fracture. The algorithm integrates the risks associated with individual clinical risk factors (age, race, sex, height, weight, personal and family history of fracture, smoking and alcohol status, glucocorticoid use, rheumatoid arthritis, and secondary osteoporosis; World Health Organization, 2011). Current guidelines from the National Osteoporosis Foundation recommend that, if the calculated 10-year risk for a given patient aged 50+ is above 3% for hip fracture or 20% for any major fracture, clinicians should consider initiating FDA-approved medical therapies in that patient. (National Osteoporosis Foundation, 2013) Analyses have validated the robustness of FRAX with BMI and the concordance of this test with BMD, showing that in the absence of BMD, FRAX with BMI preferentially identifies patients with low BMD. (Gadam, Schlauch, & Izuora, 2013; Kanis, McCloskey, Johansson, Oden, & Leslie, 2012) In a study by Johansson and colleagues (2004), using FRAX without BMD was found to result in misclassification (i.e., treat vs do not treat at 35% threshold) of 15% of women. In this analysis, our use of FRAX is a mechanism to control for baseline fracture risk in Veterans compared with non-Veterans. Our use of BMI instead of BMD allows us to also consider the influence of BMD as an important independent risk factor for fracture.
Statistical Analysis
Demographic characteristics, lifestyle and behavioral factors, comorbid conditions, health status, and medication use were compared between Veterans and non-Veterans. Frequencies were reported for categorical variables, and statistically significant differences between Veterans and non-Veterans were examined using the chi-Square test. Means and standard deviations were shown for continuous variables and evaluated using Student’s t tests. Using the entire WHI cohort in age decades as the standard population, the crude and age-adjusted fracture rates per 1,000 person-years were calculated for Veterans compared with non-Veterans. Mean hip, spine, and whole body BMD measures were taken at baseline and Years 3 and 6 follow-up visits by Veterans status included adjustment for WHI study membership to account for hormone therapy use at baseline.
To determine whether time to hip fracture differed at varying risk levels, Kaplan–Meier curves were generated comparing Veterans and non-Veterans across tertiles of WHO FRAX scores for 10-year hip fracture risk. Cox Proportional Hazards regression models were used to estimate the fracture rate for Veteran women relative to non-Veteran women. Three separate models were examined. The first model adjusted for age and race/ethnicity. The second model included the addition of BMI and a history of fracture. The third and maximally adjusted model included the addition of smoking status, alcohol use, physical activity, physical functioning, history of falls, CVD, bilateral oophorectomy or hysterectomy, depression, parental history of fracture, use of hormone therapy, corticosteroids, or calcium, and WHI study membership. Hazard rate ratios (HRs) and 95% confidence intervals (CIs) were reported. Model assumptions were tested graphically using Kaplan–Meier curves. Event times were characterized from time of enrollment to first fracture at a given site, with censoring at the time of last known follow-up. All analyses were completed using SAS v9.3 software (SAS Institute Inc., Cary, NC).
Results
Of the 161,808 women enrolled in the WHI, a total of 145,521 women (89.9%) provided information about service in the U.S. armed forces, 3,719 (2.6%) of whom were characterized as Veterans. The mean (SD) age of all participants at baseline was 63.3 (7.2) years. The cohort was predominantly White (82.6%) or Black (9.1%). The mean (SD) follow-up time was 13.5 (4.7) years.
Veterans differed from non-Veterans in a number of characteristics related to higher fracture rate (Table 1). Veterans were older [mean (SD) age in years: 67.1 (7.9) vs 63.3 (7.2), p < .001], more likely to be White (87.4% vs 82.5%, p < .001), more likely to be a past or current smoker (55.2% vs 48.8%, p < .001), slightly more likely to consume alcohol (72.0% vs 70.0%, p < .001), and more likely to have had one or more falls in the 12 months prior to entering the WHI study (33.7% vs 31.8%, p < .001). The median and interquartile range for Veterans was 69 (13) and for non-Veterans was 63 (12); a greater percentage of female Veterans than non-Veterans was aged 70–79 years at baseline. The WHO FRAX scores showed that the 10-year probability of any fracture was higher for Veterans, with a mean (SD) probability of 13.3% (8.4) compared with non-Veterans whose mean (SD) was 10.2% (6.8) (p < .001). The mean (SD) BMD of the total hip at baseline was 0.83 (0.13) g/cm2 for Veteran women compared with 0.85 (0.14) g/cm2 for non-Veteran women (p = .05). However, comparisons of baseline BMD measures of total spine or of the whole body did not differ by Veteran status (Table 1). Significantly more Veterans experienced personal history of fracture (44.5%) compared with non-Veterans (38.5%, p < .0001). However, significantly more non-Veterans use hormone therapy (65.8%) compared with Veterans (59.3%, p < .0001). Calcium supplementation was slightly more common in Veterans compared with non-Veterans (27.2% vs 25.7%, p = .04), whereas vitamin D supplementation was not significantly different.
Table 1.
Baseline Characteristics of N = 145,521 WHI Participants by Veteran Status
| Characteristic | Veteran n = 3,719 | Non-Veteran n = 141,802 | p Value |
|---|---|---|---|
| Demographic | |||
| Age, years, mean (SD) | 67.1 (7.9) | 63.3 (7.2) | <.0001 |
| Age group, n (%) | <.0001 | ||
| 50–59 years | 785 (21.1) | 46,367 (32.7) | |
| 60–69 years | 1,081 (29.1) | 64,643 (45.6) | |
| 70–79 years | 1,853 (49.8) | 30,792 (21.7) | |
| Race/ethnicity, n (%) | <.0001 | ||
| White | 3,239 (87.4) | 116,617 (82.5) | |
| Black | 263 (7.1) | 12,874 (9.1) | |
| Hispanic or Latina | 86 (2.3) | 5,671 (4.0) | |
| Other | 118 (3.2) | 6,267 (4.4) | |
| Lifestyle | |||
| Smoking status, n (%) | <.0001 | ||
| Nonsmoker | 1,631 (44.8) | 71,573 (51.1) | |
| Past smoker | 1,693 (46.5) | 58,838 (42.0) | |
| Current smoker | 317 (8.7) | 9,552 (6.8) | |
| Alcohol intake, n (%) | <.0001 | ||
| Lifetime nondrinker | 250 (6.8) | 15,521 (11.0) | |
| Former drinker | 769 (20.9) | 26,357 (18.7) | |
| <1 drink per week | 1,213 (32.9) | 46,207 (32.8) | |
| 1–6 drinks per week | 977 (26.3) | 36,129 (25.5) | |
| ≥7 drinks per week | 476 (12.8) | 16,577 (11.7) | |
| Body mass index, kg/m2, mean (SD) | 27.9 (5.8) | 27.9 (6.0) | .39 |
| Body mass index categories | .03 | ||
| Underweight | 44 (1.2) | 1,248 (0.9) | |
| Normal weight | 1,247 (33.9) | 48,639 (34.6) | |
| Overweight | 1,321 (35.9) | 48,524 (34.5) | |
| Obese I | 689 (18.7) | 25,830 (18.4) | |
| Obese II | 237 (6.4) | 10,544 (7.5) | |
| Obese III | 142 (3.9) | 5,709 (4.1) | |
| Physical activity, MET-hours per week, mean (SD) | 13.2 (14.1) | 12.5 (13.7) | .003 |
| Self-rated health | .91 | ||
| Excellent | 625 (16.9) | 24,064 (17.1) | |
| Very good or good | 2,727 (73.8) | 1,04,119 (73.8) | |
| Fair or poor | 343 (9.3) | 12,824 (9.1) | |
| Health status | |||
| Number of falls in the past 12 months | .0004 | ||
| 0 | 2,450 (66.3) | 96,259 (68.2) | |
| 1 | 717 (19.4) | 27,729 (19.7) | |
| 2 or 3 | 528 (14.3) | 17,116 (12.1) | |
| Diabetes, n (%) | 218 (5.9) | 8,493 (6.0) | .75 |
| Cardiovascular disease, n (%) | 801 (21.9) | 24,848 (17.7) | <.0001 |
| Rheumatoid arthritis, n (%) | 205 (10.4) | 7,169 (10.8) | .54 |
| Stomach or duodenal ulcer, n (%) | 256 (7.0) | 8,911 (6.4) | .14 |
| Bilateral oophorectomy, n (%) | 793 (21.8) | 27,822 (20.1) | .01 |
| Hysterectomy, n (%) | 1,636 (44.0) | 59,243 (41.8) | .007 |
| CES-D score suggestive of depression, n (%) | 341 (9.4) | 15,335 (11.1) | .001 |
| RAND-36 physical function score, mean (SD) | 78.4 (21.2) | 81.1 (20.1) | <.0001 |
| Parental history of fracture, n (%) | 1,291 (38.2) | 51,881 (39.8) | .07 |
| Personal history of fracture, n (%) | 1,639 (44.5) | 54,285 (38.5) | <.0001 |
| WHO FRAX 10-year probability (any), mean (SD) | 13.3 (8.4) | 10.2 (6.8) | <.0001 |
| WHO FRAX 10-year probability (hip), mean (SD) | 4.08 (5.39) | 2.20 (3.59) | <.0001 |
| BMD of total hip, g/cm2, mean (SD)a | 0.83 (0.13) | 0.85 (0.14) | .05 |
| BMD of total spine, g/cm2, mean (SD)a | 0.98 (0.17) | 0.98 (0.17) | .73 |
| BMD of whole body, g/cm2, mean (SD)a | 1.01 (0.11) | 1.01 (0.11) | .54 |
| Medications | |||
| Using hormone therapy, n (%) | 1,470 (59.3) | 61,854 (65.8) | <.0001 |
| Using psychoactive medications, n (%) | 491 (13.2) | 18,847 (13.3) | .88 |
| Using oral corticosteroids daily, n (%) | 44 (1.2) | 1,016 (0.7) | .001 |
| Using proton pump inhibitor, n (%) | 86 (2.3) | 3,130 (2.2) | .67 |
| Taking calcium, n (%) | 1,013 (27.2) | 36,505 (25.7) | .04 |
| Taking vitamin D, n (%) | 147 (4.0) | 5,954 (4.2) | .46 |
Notes: BMD = bone mineral density; CES-D = Center for Epidemiologic Studies Depression Scale; MET = metabolic equivalents; SD = standard deviation; WHI = Women’s Health Initiative; WHO FRAX = World Health Organization Fracture Risk Assessment Tool.
aAvailable only for a subsample of the cohort.
The crude rate of hip fractures per 1,000 person-years was 4.7 for Veterans, whereas for non-Veterans it was 2.4 (Table 2). The age-standardized rates narrowed the difference, but the rates were still higher for Veterans (3.3 vs 2.4, p < .001). Central body fractures that included hip fractures also had age-standardized rates that were different for Veterans and non-Veterans (8.5 per 1,000 person-years vs 7.3 per 1,000 person-years, respectively; p < .01); however, central body fracture rates that excluded hip fractures were no longer statistically different. Likewise, no differences in the age-standardized rates were observed by Veterans status for upper or lower limb fractures (Table 2).
Table 2.
Crude and Age-adjusted Incident Fracture Rates in N = 145,521 WHI Participants, by Veteran Status
| Events | Total PY | Crude rate (per 1,000 PY) | Age-standardized estimates | ||
|---|---|---|---|---|---|
| Rateb (per 1,000 PY) | 95% CI | ||||
| Veterans | |||||
| Hip fracturea | 223 | 47,277.3 | 4.72 | 3.25 | 2.75–3.76 |
| Central body fracture, incl. hipa | 526 | 45,619.3 | 11.53 | 8.51 | 7.66–9.37 |
| Central body fracture, excl. hip | 350 | 48,214.6 | 7.26 | 5.52 | 4.83–6.20 |
| Upper limb fracture | 486 | 44,971.1 | 10.81 | 9.64 | 8.65–10.63 |
| Lower limb fracture | 456 | 45,297.5 | 10.07 | 9.79 | 8.77–10.81 |
| Non-Veterans | |||||
| Hip fracturea | 4,476 | 18,98,638.7 | 2.36 | 2.40 | 2.33–2.47 |
| Central body fracture, incl. hipa | 13,308 | 18,51,552.9 | 7.19 | 7.30 | 7.18–7.43 |
| Central body fracture, excl. hip | 9,801 | 19,18,281.4 | 5.11 | 5.14 | 5.04–5.24 |
| Upper limb fracture | 16,688 | 18,06,252.6 | 9.24 | 9.28 | 9.14–9.42 |
| Lower limb fracture | 17,319 | 17,97,291.1 | 9.64 | 9.64 | 9.49–9.78 |
Notes: CI = confidence interval; PY = person-years; WHI = Women’s Health Initiative.
a p Value for rate difference <.01.
bStandard population is whole WHI population, in age decades.
Figure 1 summarizes mean BMD at baseline, Year 3, and Year 6 for (A) total hip, (B) total spine, and (C) whole body for the subset of women with these measures available. Although all three mean measures for hip BMD were lower for Veterans (0.836g/cm2 at baseline, 0.856g/cm2 at Year 3, 0.849g/cm2 at Year 6) compared with non-Veterans (0.851g/cm2 at baseline, 0.865g/cm2 at Year 3, 0.855g/cm2 at Year 6), overlapping error bars, which represent 95% CIs, suggest that the differences observed were not statistically significant. Mean BMD measures at all times for both total spine (0.973g/cm2 at baseline, 1.012g/cm2 at Year 3, 1.036g/cm2 at Year 6 for Veterans vs 0.975g/cm2 at baseline, 1.004g/cm2 at Year 3, 1.012g/cm2 at Year 6 for non-Veterans) and whole body (1.006g/cm2 at baseline, 1.036g/cm2 at Year 3, 1.047g/cm2 at Year 6 for Veterans vs 1.009g/cm2 at baseline, 1.029g/cm2 at Year 3, 1.038g/cm2 at Year 6 for non-Veterans) also were not significantly different between Veteran women and non-Veteran women.
Figure 1.
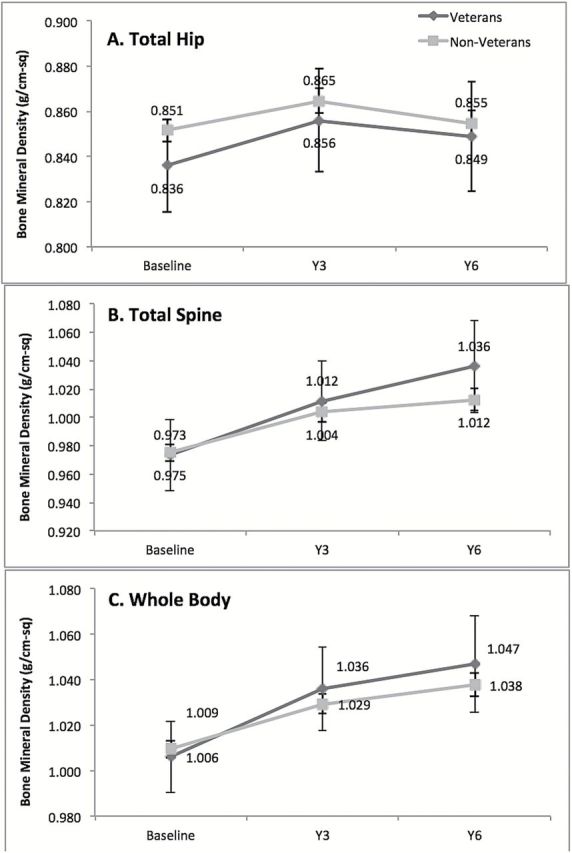
Mean bone mineral density at baseline, Year 3, and Year 6 for (A) total hip; (B) total spine; and (C) whole body for the subset of women with bone mineral density measures.
The mean (SD) WHO FRAX score for hip fracture for the total study population was 10.3 (6.9), with the lowest risk score at 0.7 and the highest at 83.9. Kaplan–Meier curves for the probability of hip fracture, by Veteran status and tertile of WHO FRAX score for hip fracture, are displayed in Figure 2. Tertile cut-off values were largely driven by the non-Veteran sample because they represented more than 97% of the total study population. Examination of the distribution of women Veterans across the tertile cutoffs showed a disproportionate number of Veterans in highest tertile: 21.7% and 24.6% of Veterans were in the lowest and middle tertile, respectively, but 53.7% of Veterans were in the highest tertile. Referring to Figure 2, rates of hip fracture were higher among Veterans than non-Veterans in all three tertiles of WHO FRAX hip score, and the stratum-specific differences were statistically significant in both the lowest and highest tertiles of WHO FRAX hip score, at p < .01.
Figure 2.
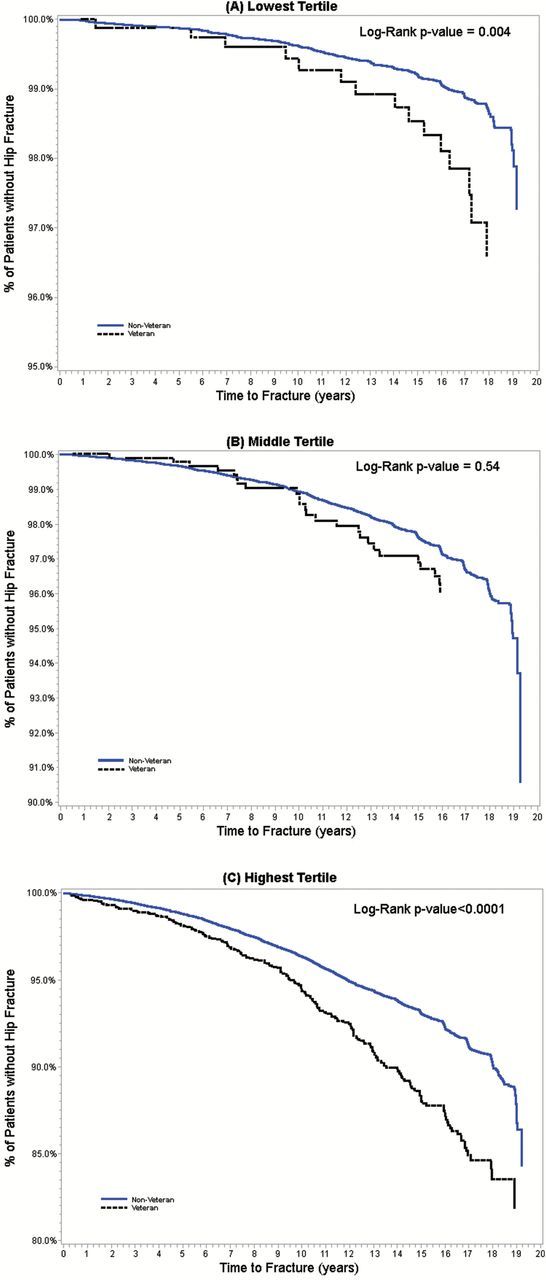
Kaplan–Meier curves for probabilities of hip fracture, by Veteran status, across WHO FRAX tertiles: (A) lowest tertile (FRAX scores of 0.7%–6.5%); (B) middle tertile (FRAX scores of >6.5%–11.0%); (C) highest tertile (FRAX scores of >11.0%–83.9%).
After adjusting for age and race/ethnicity, the HRs for hip fracture for Veteran women was 1.24 (95% CI: 1.08–1.42) relative to non-Veteran women. This rate ratio did not change and remained statistically significant after full adjustment of a variety of other risk factors for fracture, including BMI, history of fracture, smoking status, alcohol use, physical activity, physical function, history of falls, CVD, bilateral oophorectomy and hysterectomy, depression, parental history of fracture, use of hormone therapy, corticosteroids, calcium, and WHI study membership. The HR for fractures at all other sites did not differ by Veteran status (Table 3).
Table 3.
Cox Proportional Hazards Models (and 95% confidence intervals) for Fracture in Veterans Compared With Non-Veterans Among N = 145,521 WHI Participants
| Hip fracture | Central body fractures, including hip | Central body fractures, excluding hip | Upper limb fractures | Lower limb fractures | |
|---|---|---|---|---|---|
| Minimally adjusteda | 1.24 (1.08–1.42) | 1.16 (1.06–1.27) | 1.03 (0.93–1.15) | 1.02 (0.93–1.12) | 1.03 (0.94–1.13) |
| Moderately adjustedb | 1.23 (1.07–1.41) | 1.15 (1.05–1.25) | 1.01 (0.90–1.12) | 1.01 (0.92–1.10) | 1.01 (0.91–1.11) |
| Maximally adjustedc | 1.24 (1.03–1.49) | 1.21 (1.08–1.36) | 1.11 (0.97–1.28) | 0.93 (0.82–1.06) | 1.00 (0.88–1.13) |
| Adjustment for WHO FRAX risk for hip fracture | |||||
| WHO FRAX only | 1.53 (1.33–1.75) | 1.31 (1.20–1.43) | 1.19 (1.07–1.33) | 1.04 (0.95–1.14) | 0.99 (0.90–1.09) |
| WHO FRAX and age | 1.22 (1.06–1.39) | 1.14 (1.05–1.25) | 1.01 (0.91–1.13) | 1.01 (0.92–1.10) | 1.01 (0.92–1.11) |
| WHO FRAX, age, and race/ ethnicity | 1.21 (1.06–1.39) | 1.14 (1.04–1.24) | 1.01 (0.91–1.13) | 1.01 (0.92–1.10) | 1.01 (0.92–1.11) |
| Adjustment for WHO FRAX risk for any major fracture | |||||
| WHO FRAX only | 1.61 (1.41–1.84) | 1.37 (1.25–1.49) | 1.23 (1.10–1.37) | 1.06 (0.97–1.17) | 1.01 (0.92–1.11) |
| WHO FRAX and age | 1.22 (1.07–1.40) | 1.14 (1.05–1.25) | 1.01 (0.91–1.12) | 1.01 (0.92–1.10) | 1.01 (0.92–1.11) |
Notes: BMI = body mass index; BMD = bone mineral density; CVD = cardiovascular disease; WHO FRAX = World Health Organization Fracture Risk Assessment Tool; WHI = Women’s Health Initiative.
BMI was used in WHO FRAX instead of BMD.
aAdjusted for age and race/ethnicity.
bAdjusted for age, race/ethnicity, BMI, and history of fracture.
cAdjusted for age, race/ethnicity, BMI, history of fracture, smoking status, alcohol use, physical activity, physical function, history of falls, CVD, bilateral oophorectomy, and hysterectomy, depression, parental history of fracture, use of hormone therapy, corticosteroids, and calcium, and WHI study membership.
When WHO FRAX-adjusted hip fracture HR for Veteran women compared with non-Veteran women remained significant, at 1.53 (1.33–1.75). Adding age to this adjustment decreased the elevated risk to 1.22 (1.06–1.39), and adding race/ethnicity to age and the WHO FRAX made little difference. The classification of hip fractures as central body fractures accounts for most of that increased risk (1.31, 95% CI 1.20–1.43), which was reduced to 1.19 (1.07–1.33) once hip fractures were excluded from central body fractures.
WHO-FRAX-adjusted any major fractures followed a similar trend. Adjusting any major fracture with WHO FRAX returned a significant hip fracture HR of 1.61 (1.41–1.84), which was reduced to 1.22 (1.07–1.40) with the adjustment for age. The classification of hip fractures as central body fractures accounts for most of that increased risk (1.37, 95% CI 1.25–1.49), which was reduced to 1.23 (1.10–1.37) once hip fractures were excluded from central body fractures.
Discussion
In this comparison of fracture rates among Veteran and non-Veteran women, Veterans were found to have a 24% higher rate of hip fracture, even after controlling for available known clinical risk factors such as age, BMI, history of prior fracture, and relevant drug exposures. In contrast, the rate of fracture at nonhip sites did not differ between Veterans and non-Veterans. Mean BMD at the hip in Veterans was lower compared with non-Veterans (0.83 vs 0.85), but that difference was small and was not statistically significant. In addition, differences in mean BMDs at the spine and for the whole body were also small and nonsignificant by a similar magnitude (0.98 and 1.01, respectively). However, Veterans also had significantly higher WHO FRAX scores based on BMI (not BMD), indicating a higher absolute fracture risk, for both hip (4.08 vs 2.20) and any major fracture (13.3 vs 10.2). Although these FRAX scores align with the observed difference in hip fracture rates, they do not align with the lack of difference in nonhip fractures or with BMD. Certain contributors to these FRAX scores, particularly age, smoking, alcohol intake, and personal history of fracture, were also significantly higher in Veterans than non-Veterans. However, controlling for these factors, or any other factors beyond age and race, did not attenuate the difference in hip fracture rates we observed in the crude model. Therefore, we must conclude that Veteran status appears to be associated with an increased rate of hip fracture, independent of adjustment for available known clinical risk factors. This may be explained partially by residual confounding by known and unknown clinical risk factors. However, it is also possible that Veteran status is a marker for other exposures that increase rate of hip fractures but not other osteoporotic fractures.
One theory for our observed difference in hip fracture rates is that Veteran women experience a higher degree of frailty and disability compared with non-Veterans, (C. Colón-Emeric et al., 2007; C. Colón-Emeric et al., 2015; C. S. Colón-Emeric, 2013; Lyles, Schenck, & Colón-Emeric, 2008) which may translate into higher hip fracture rate. Although our observed difference in hip fracture rates remained even after controlling for components of frailty at baseline (functional status, falls, and BMI; Xue, 2011), it is possible that frailty increased in Veterans at a greater rate than in non-Veterans as they aged. This is suggested by LaCroix and colleagues, who reported functional status outcomes in a survival analysis of the subcohort of women Veterans from the WHI who reached ≥80 years. According to their study, among women aged 80 and older, Veterans were significantly less likely to report having good perceived health, were more likely to reside in a nursing home or assisted living facility, and had significantly lower mean physical functioning scores compared with non-Veterans in the same age cohort. Another prior analysis of the WHI data revealed that functional impairment was associated with fracture rate even after adjusting for falls (Lee, Pieper, Lyles, Weber, & Colón-Emeric, 2015). Veterans in our study had a slightly higher self-reported history of falls at baseline, and although adjusting for baseline falls did not impact the hazards ratio, an increasing difference in fall rates in follow-up data could be a mediator of hip fractures. Falls are also not included in the calculation of FRAX, which may be a contributor to the discrepancies among FRAX, BMD, and rate of any fracture. Women Veterans in WHI generally had substantially higher rates of functional impairment despite higher competing mortality (Reiber Editorial 1; 2016).
Another way that frailty could have influenced our findings is by incomplete adjustment for this multidimensional phenotype. For example, we were unable to adjust for the main indicators of sarcopenia (low grip strength and low lean body mass) or unintended weight loss. Sarcopenia in particular, a hallmark of frailty, is associated with osteopenia and falls and may mediate this association (Sjöblom et al., 2013). These unmeasured mechanisms reveal targets for future research and prevention strategies. A time-varying study of functional status and falls in Veterans versus non-Veterans of the WHI cohort might provide additional insights into the hip fracture rate differences found here but was beyond the scope of this analysis. Older women Veterans may also be good candidates for fall prevention and physical function preservation programs.
If frailty syndrome, in total and over time, is the underlying mediator of the higher hip fracture rates in Veterans compared with non-Veterans, the next question may be why Veterans might have a greater tendency toward frailty. One line of inquiry could be activities or exposures related to military service, particularly for pre Vietnam-era women Veterans, who would be the oldest in the cohort and most likely to be frail. For example, women who served as nurses could have experienced stress fractures due to heavy lifting. However, no data regarding military occupational exposures are available in WHI. A recent study by Washington and colleagues (2015) that addresses Veteran mortality, stratified by military generation, found that the higher mortality experienced by pre-Vietnam era women Veterans (compared with same-aged non-Veterans) was largely explained by increased comorbidities, whereas the higher mortality experienced by post-Vietnam era women Veterans was marked by increased trauma.
Current health care practices may have a greater influence on frailty and fracture rates than past military occupational exposures. Veterans may differ fundamentally from non-Veterans in how they utilize healthcare, or health systems may vary in how they screen and treat osteoporosis. Veterans are unique in their access to health care at the VHA, but within the WHI cohort, only a very small proportion of Veterans report ever receiving care in the VHA (<15%). Given that small percentage, there is a low likelihood that VHA utilization could explain results of the magnitude we observed. Osteoporosis medication prescribing and adherence rates may differ between Veteran and non-Veteran women, as they do for men. Adherence rates are lower among male Veterans compared with non-Veterans (Hansen et al., 2008), and while 7%–12% of all postmenopausal women receive prescriptions for bisphosphonates (Wysowski & Greene, 2013), postmenopausal Veteran women are given prescriptions at a much lower rate; a recent study found that only 2.6% received prescriptions for a bisphosphonate (LaFleur et al., 2015). Although such health service factors deserve further investigation, they are unlikely to fully explain the present results, because a strong association between Veteran status and osteoporosis treatment would likely influence all fracture types, rather than just hip fractures.
Our findings should be interpreted in the context of the strengths and weaknesses of the study design. The WHI had detailed clinical, functional status, and medication information, allowing for adjustment of a wide variety of fracture risk factors as well as the composite WHO FRAX (without BMD) scores. Large sample size and excellent follow-up rates are also strengths. However, in any cohort study, the potential for unmeasured explanatory variables remains. Selection factors associated with initially volunteering for participation in WHI would be similar among Veterans and non-Veterans, and thus would not change the magnitude of the HRs, but such factors could result in lower fracture rates than expected for all participants. A previous analysis of the WHI revealed higher mortality rates among the Veteran participants (Weitlauf et al., 2015), and we did not account for this competing mortality in our analyses. This would tend to make our HR appear lower than it actually is (i.e., the hip fracture rate in Veterans would be even greater if competing mortality was considered). Furthermore, just as epidemiology study results from subpopulations may not be generalizable to more inclusive populations, findings for women Veterans participating in WHI may not be generalizable to all women Veterans.
Implications
In summary, we identified a clinically important increased rate of hip fractures among women Veterans that is not explained by differences in many important available known underlying fracture risk factors. Future examinations of factors not measured in this study are needed to understand this increased rate. Programs may be needed to improve screening and treatment of osteoporosis among women Veterans and decrease their higher rates of falls and functional decline.
Funding
This research was supported by the Office of Women’s Health and by the Agency for Healthcare Research and Quality (K Award K08HS018582 to J. L.). The Women’s Health Initiative (WHI) program is funded by the National Heart, Lung, and Blood Institute, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201 100003C, HHSN268201100004C, and HHSN271201100004C. Wyeth Pharmaceuticals provided the study drug and the placebo to the WHI trial. This research was also supported by Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service FOP14-439 and the VA Office of Women’s Health.
Acknowledgments
The authors wish to thank Carrie Edlund for her editorial assistance. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
References
- Ainsworth B. E., Haskell W. L., Leon A. S., Jacobs D. R., Jr., Montoye H. J., Sallis J. F., Paffenbarger R. S., Jr (1993). Compendium of physical activities: Classification of energy costs of human physical activities. Medicine and Science in Sports and Exercise, 25, 71–80. doi:10.1249/00005768-199301000-00011 [DOI] [PubMed] [Google Scholar]
- Assari S. (2014). Veterans and risk of heart disease in the United States: A cohort with 20 years of follow up. International Journal of Preventive Medicine, 5, 703–709. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25013689 [PMC free article] [PubMed] [Google Scholar]
- Baczyk G. (2009). Quality of life of women with osteoporosis—Review of literature. Ortopedia, Traumatologia, Rehabilitacja, 11, 291–303. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19828911 [PubMed] [Google Scholar]
- Bass E., French D. D., Bradham D. D., Rubenstein L. Z. (2007). Risk-adjusted mortality rates of elderly veterans with hip fractures. Annals of Epidemiology, 17, 514–519. doi:10.1016/j.annepidem.2006.12.004 [DOI] [PubMed] [Google Scholar]
- Burnam M. A., Wells K. B., Leake B., Landsverk J. (1988). Development of a brief screening instrument for detecting depressive disorders. Medical Care, 26, 775–789. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3398606 [DOI] [PubMed] [Google Scholar]
- Chen Z., Kooperberg C., Pettinger M. B., Bassford T., Cauley J. A., LaCroix A. Z., Jackson R. D. (2004). Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: Results from the Women’s Health Initiative observational study and clinical trials. Menopause, 11, 264–274. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15167305 [DOI] [PubMed] [Google Scholar]
- Colón-Emeric C., Lyles K. W., Levine D. A., House P., Schenck A., Gorospe J., Saag K. (2007). Prevalence and predictors of osteoporosis treatment in nursing home residents with known osteoporosis or recent fracture. Osteoporosis International, 18, 553–559. 10.1007/s00198-006-0260-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón-Emeric C., Pieper C. F., Grubber J., Van Scoyoc L., Schnell M. L., Van Houtven C. H., Adler R. A. (2015). Correlation of hip fracture with other fracture types: Toward a rational composite hip fracture endpoint. Bone, 81, 67–71. doi:10.1016/j.bone.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón-Emeric C. S. (2013). Recent advances: Osteoporosis in the “oldest old”. Current Osteoporosis Reports, 11, 270–275. doi:10.1007/s11914-013-0158-z [DOI] [PubMed] [Google Scholar]
- Crandall C. J., Yildiz V. O., Wactawski-Wende J., Johnson K. C., Chen Z., Going S. B., Cauley J. A. (2015). Postmenopausal weight change and incidence of fracture: Post hoc findings from Women’s Health Initiative Observational Study and Clinical Trials. British Medical Journal, 350, h25 doi:10.1136/bmj. h25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curb J. D., McTiernan A., Heckbert S. R., Kooperberg C., Stanford J., Nevitt M., Mortality C. (2003). Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Annals of Epidemiology, 13(9 Suppl), S122–S128. doi:10.1016/S1047-2797(03)00048-6 [DOI] [PubMed] [Google Scholar]
- Department of Veterans Affairs (2015, June 3). Special Populations—Strategic Priorities—Women Veterans Health Care. Retrieved from http://www.womenshealth.va.gov/WOMENSHEALTH/programoverview/strategic_priorities.asp
- Donaldson M. G., Palermo L., Ensrud K. E., Hochberg M. C., Schousboe J. T., Cummings S. R. (2012). Effect of alendronate for reducing fracture by FRAX score and femoral neck bone mineral density: The Fracture Intervention Trial. Journal of Bone and Mineral Research, 27, 1804–1810. doi:10.1002/jbmr. 1625 [DOI] [PubMed] [Google Scholar]
- Gadam R. K., Schlauch K., Izuora K. E. (2013). Frax prediction without BMD for assessment of osteoporotic fracture risk. Endocrine Practice, 19, 780–784. doi:10.4158/EP12416.OR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldzweig C. L., Balekian T. M., Rolón C., Yano E. M., Shekelle P. G. (2006). The state of women veterans’ health research. Results of a systematic literature review. Journal of General Internal Medicine, 21(Suppl. 3), S82–S92. doi:10.1111/j.1525-1497.2006.00380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S. L., LaCroix A. Z., Larson J., Robbins J., Cauley J. A., Manson J. E., Chen Z. (2010). Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: Results from the Women’s Health Initiative. Archives of Internal Medicine, 170, 765–771. doi:10.1001/archinternmed.2010.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K. E., Swenson E. D., Baltz B., Schuna A. A., Jones A. N., Elliott M. E., Elliott M. E. (2008). Adherence to alendronate in male veterans. Osteoporosis International, 19, 349–356. Retrieved from http://link.springer.com/article/10.1007%2Fs00198-007-0471-4 [DOI] [PubMed] [Google Scholar]
- Hübscher M., Vogt L., Schmidt K., Fink M., Banzer W. (2010). Perceived pain, fear of falling and physical function in women with osteoporosis. Gait & Posture, 32, 383–385. 10.1016/j.gaitpost.2010.06.018 [DOI] [PubMed] [Google Scholar]
- Johansson H., Oden A., Johnell O., Jonsson B., de Laet C., Oglesby A., Kanis J. A. (2004). Optimization of BMD measurements to identify high risk groups for treatment—A test analysis. Journal of Bone and Mineral Research, 19, 906–913. doi:10.1359/jbmr.2004.19.6.906 [DOI] [PubMed] [Google Scholar]
- Kanis J. A., McCloskey E., Johansson H., Oden A., Leslie W. D. (2012). FRAX((R)) with and without bone mineral density. Calcified Tissue International, 90, 1–13. doi:10.1007/s00223-011-9544-7 [DOI] [PubMed] [Google Scholar]
- LaFleur J., DuVall S., Curtis J., Adler R., Willson T., Agodoa I., Nelson R. (2013). Association between bisphosphonate switching behavior and cost outcomes in postmenopausal United States Veterans. Paper presented at the Annual Meeting of the American College of Rheumatology (ACR), San Diego, California: Poster retrieved from http://pharmacy.utah.edu/PORC/pdf/2013/Association_between.pdf [Google Scholar]
- LaFleur, J., DuVall, S. L., Willson, T., Ginter, T., Patterson, O., Cheng, Y., . . . Nelson, R. E. (2015). Analysis of osteoporosis treatment patterns with bisphosphonates and outcomes among postmenopausal veterans. Bone, 78, 174–185. doi:10.1016/j.bone.2015.04.022 doi: 10.1016/j.bone.2015.04.022. [DOI] [PubMed] [Google Scholar]
- Lee, R.H., Pieper, C.F., & Colón-Emeric, C. (2015). Functional impairments mediate association between clinical fracture risk and type 2 diabetes mellitus in older women. Journal of the American Geriatrics Society, 63, 1546–1551. 10.1111/jgs.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles K. W., Schenck A. P., Colón-Emeric C. S. (2008). Hip and other osteoporotic fractures increase the risk of subsequent fractures in nursing home residents. Osteoporosis International, 19, 1225–1233. doi:10.1007/s00198-008-0569-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S. (2003). Interacting mediators of allostasis and allostatic load: Towards an understanding of resilience in aging. Metabolism: Clinical and Experimental, 52(10 Suppl. 2), 10–16. doi:10.1016/S0026-0495(03)00295-6 [DOI] [PubMed] [Google Scholar]
- National Institutes of Health (1998). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. Obes Res, 6(Suppl. 2), S51–S209. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9813653 [PubMed] [Google Scholar]
- National Osteoporosis Foundation (2013). Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; Retrieved from http://www.nof.org/professionals/clinical-guidelines [Google Scholar]
- Nelson K., Starkebaum G., Reiber G. (2007). Veterans using and uninsured veterans not using Veterans Affairs (VA) Health Care. Public Health Reports, 122, 93–100. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1802114/pdf/phr122000093.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohldin A., Floyd J. (2003). Unrecognized risks among Veterans with hip fractures: Opportunities for improvements. Journal of the Southern Orthopaedic Association, 12, 18–22. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12735620 [PubMed] [Google Scholar]
- Seeman T. E., Crimmins E. (2001). Social environment effects on health and aging: Integrating epidemiologic and demographic approaches and perspectives. Annals of the New York Academy of Sciences, 954, 88–117. [DOI] [PubMed] [Google Scholar]
- Selim A. J., Berlowitz D. R., Fincke G., Cong Z., Rogers W., Haffer S. C., Kazis L. E. (2004). The health status of elderly veteran enrollees in the Veterans Health Administration. Journal of the American Geriatrics Society, 52, 1271–1276. 10.1111/j.1532-5415.2004.52355.x [DOI] [PubMed] [Google Scholar]
- Shibli-Rahhal A., Vaughan-Sarrazin M. S., Richardson K., Cram P. (2010). Testing and treatment for osteoporosis following hip fracture in an integrated U.S. healthcare delivery system. Osteoporosis International. doi:10.1007/s00198-011-1536-y [DOI] [PubMed] [Google Scholar]
- Sjöblom S., Suuronen J., Rikkonen T., Honkanen R., Kröger H., Sirola J. (2013). Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas, 75, 175–180. doi:10.1016/j.maturitas.2013.03.016 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Veterans Affairs (2014, November 17). Veteran Population Projection Model 2014 National Table by Age and Gender. Retrieved from http://www.va.gov/vetdata/veteran_population.asp
- Ware J. E., Jr., Sherbourne C. D. (1992). The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care, 30, 473–483. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1593914 [PubMed] [Google Scholar]
- Washington, D.L., Bastian, L.A., Bird, C.E., Gass, M., Goldstein, K.M., LaMonte, M.J., . . . Weitlauf, J.C. (2015). Military generation and its relationship to mortality in women Veterans in the Women’s Health Initiative. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitlauf J. C., Lacroix A. Z., Bird C. E., Woods N. F., Washington D. L., Katon J. G., Stefanick M. L. (2015). Prospective analysis of health and mortality risk in veteran and non-veteran participants in the Women’s Health Initiative. Women’s Health Issues, 25, 648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson T., Nelson S. D., Newbold J., Nelson R. E., LaFleur J. (2015). The clinical epidemiology of male osteoporosis: A review of the recent literature. Clinical Epidemiology, 7, 65–76. doi:10.2147/CLEP.S40966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2011). FRAX: WHO Fracture Risk Assessment Tool (Version 3.9) [Online application]. Sheffield, UK: World Health Organization Collaborating Centre for Metabolic Bone Diseases; Retrieved from https://www.shef.ac.uk/FRAX/ [Google Scholar]
- Wright N. C., Looker A. C., Saag K. G., Curtis J. R., Delzell E. S., Randall S., Dawson-Hughes B. (2014). The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. Journal of Bone and Mineral Research, 29, 2520–2526. doi:10.1002/jbmr.2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysowski D. K., Greene P. (2013). Trends in osteoporosis treatment with oral and intravenous bisphosphonates in the United States, 2002–2012. Bone, 57, 423–428. 10.1016/j.bone.2013.09.008 [DOI] [PubMed] [Google Scholar]
- Xue Q. L. (2011). The frailty syndrome: Definition and natural history. Clinics in Geriatric Medicine, 27, 1–15. doi:10.1016/j.cger.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano E. M., Hayes P., Wright S., Schnurr P. P., Lipson L., Bean-Mayberry B., Washington D. L. (2010). Integration of women veterans into VA quality improvement research efforts: What researchers need to know. Journal of General Internal Medicine, 25(Suppl. 1), 56–61. doi:10.1007/s11606-009-1116-4 [DOI] [PMC free article] [PubMed] [Google Scholar]


