Abstract
Background
Patients with angina-like symptoms without myocardial perfusion scintigram (MPS)-verified abnormality may still be at risk for cardiovascular events. We hypothesized that insulin resistance could play a role in this population even without diagnosed diabetes. We further explored physiological and blood biomarkers, as well as global gene expression patterns that could be closely related to impaired glucose homeostasis to deepen our mechanistic understanding.
Methods
A total of 365 non-diabetic patients with suspected myocardial ischemia referred to MPS were enrolled and followed up regarding event-free survival with a median time of 5.1 years. All patients underwent endothelial function assessment by reactive hyperemic index (RHI) using EndoPAT and extensive biomarker analysis. Whole blood global gene expression pathway analysis was performed in a subset of patients.
Results
Homeostasis model assessment of insulin resistance (HOMA-IR) added independent prognostic value in patients without myocardial perfusion defects. In a multivariable analysis, HOMA-IR was inversely associated with low RHI. Furthermore, elevated HOMA-IR was associated with decreased levels of vascular endothelial growth factor D, stem cell factor and endocan as well as to increased level of interleukin-6. Global gene expression pathway analysis of whole blood cells showed that high HOMA-IR and impaired endothelial function were associated with upregulated pro-inflammatory pathways and down-regulated eukaryotic initiation factor-2 pathway.
Conclusions
Insulin resistance measured by HOMA-IR is associated with endothelial dysfunction and confers independent prognostic information in non-diabetic patients with chest pain without myocardial perfusion defects. Increased systemic pro-inflammatory state and decreased levels of pro-angiogenic vascular growth factors may be important underlying molecular mechanisms.
Keywords: Non-obstructive coronary artery disease, Insulin resistance, Outcome, Endothelial function, Growth factors
Background
Myocardial ischemia has been shown to confer strong prognostic values in various patient populations [1, 2]. Traditionally obstructive, i.e., flow limiting coronary artery atherosclerosis has been in focus for coronary artery disease (CAD) management [3, 4]. However, the importance of non-obstructive CAD has gained increasing attention since approximately 40–50 % of patients with stable angina have normal or near normal coronary arteries on coronary angiography [5, 6]. Despite the lack of obvious obstructive CAD, many of these patients are still at increased risk for future cardiovascular events [5, 7]. Indeed, a recent study revealed high prevalence of microvascular dysfunction in patients with chest pain and non-obstructive CAD [8].
Stress myocardial perfusion scintigram (MPS) is of strong prognostic value identifying hemodynamically significant obstructive CAD in patients with suspected myocardial ischemia [2]. A recent study suggests MPS to better assess functionally significant CAD compared to coronary angiography in patients with intermediate pre-test probability [9]. However, since MPS provides relative flow distribution pattern rather than absolute flow, it might still be less sensitive to detect coronary microvascular disease, including endothelial-dependent as well as independent coronary microvascular abnormalities in patients with chest pain and non-obstructive CAD [10]. Recently, we reported a role of assessing peripheral endothelial function in risk stratification of patients with chest pain without myocardial perfusion defects. This further indicates that other pathophysiological features than hemodynamically significant macrovascular disease may be responsible for clinical outcome of patients with angina-like symptoms [11].
Endothelial dysfunction is a consequence of different mechanisms causing impaired vasodilation or increased vasoconstriction [12, 13] leading to microvascular dysfunction [14]. In addition to endothelial dysfunction, the involvement of vascular remodeling, rarefaction and collaterals are important mechanisms [13], emphasizing its complexity. Microvascular dysfunction in the absence of obstructive CAD may be an early sign of cardiovascular risk. Consequently, understanding of associated mechanisms could be of great importance in management of cardiometabolic diseases. Diabetic patients are known to be at high risk for development of premature cardiovascular complications [15]. In fact, type 2 diabetes diagnosis is preceded by years of impaired glucose homeostasis, which may contribute to early pathological alteration in the cardiovascular system. Pre-diabetes may cause functional [16, 17] as well as potentially structural vascular changes [18, 19], eventually leading to clinical manifestation of the underlying vascular diseases.
Cardiometabolic diseases may involve a complex chain of events including visceral adiposity, metabolic syndrome, Type 2 diabetes and CAD. In the present work we hypothesized that insulin resistance, assessed by homeostasis model assessment of insulin resistance (HOMA-IR), in non-diabetic patients is associated with peripheral endothelial dysfunction and worse clinical outcome in patients with chest pain without myocardial perfusion defects. Further, for deepened mechanistic understanding, we explored plasma protein and gene expression patterns in whole blood cells associated with HOMA-IR.
Methods
Subjects and study design
A total of 365 consecutive non-diabetic patients with chest pain referred to Sahlgrenska University Hospital in Gothenburg, Sweden due to suspected myocardial ischemia, were recruited to the study between 2006 and 2008 (Fig. 1). At a separate occasion within 2 weeks following clinical MPS examination, all patients underwent examinations of peripheral endothelial function. Overnight fasting blood samples were taken post examination of endothelial function. All patients underwent a standardized interview of medical history including history of diabetes, known CAD, smoking status and current cardiovascular medication. Patients with previous diabetes diagnosis, fasting plasma glucose ≥7.0 mmol/L or glycated haemoglobin A1c (HbA1c) level >48 mmol/mol were excluded from the study. Known CAD was defined as previous coronary artery bypass grafting, percutaneous coronary intervention or myocardial infarction (MI) and was collected from patients’ medical records. All participants provided written informed consent. The study complies with the declaration of Helsinki and was approved by the Local Ethics Committee at the University of Gothenburg.
Fig. 1.
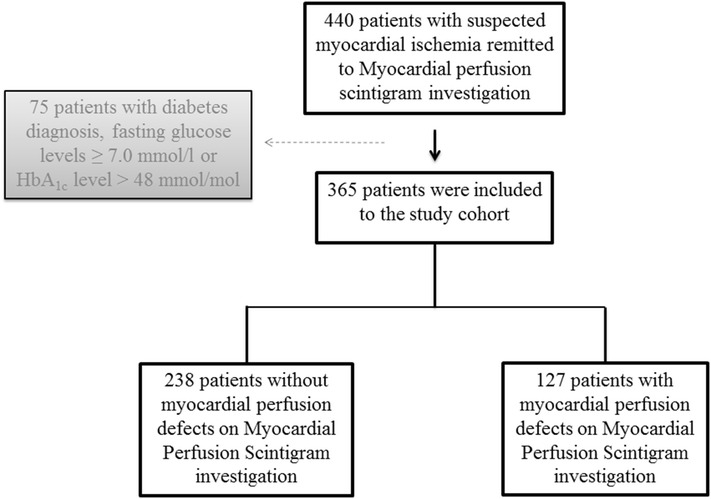
A flow-scheme illustrating the patient recruitment process
Myocardial perfusion scintigram examination
Gated-SPECT studies were performed with a 2-day standard clinical protocol using 99mTc-sestamibi. Images were obtained using dual-head SPECT cameras (Infinia or Millennium VG, GE Healthcare, Milwaukee, Wisconsin, USA). The severity of reversible myocardial ischemia was scored as no (score 0), mild (score 1), moderate (2) or severe (3). The extent was scored as none, small, medium or large (score 0 for no sign of ischemia, score 1 for <10 %, score 2 for 10–19 %, score 3 for >19 % of the entire myocardium being affected, respectively). No myocardial perfusion defects was defined when both extent and severity of ischemia were scored 0.
Endothelial function measured by reactive hyperemic index
To determine peripheral endothelial function in this patient group we performed peripheral arterial tonometry. The technician performing the examination was blinded to all clinical patient data including MPS results. Measurements were recorded using the EndoPAT2000 device (Itamar Medical, Caesarea, Israel). Endothelial function as assessed by reactive hyperemic index (RHI) has previously been described [20]. Briefly, a designed probe is placed bilaterally on each index finger. Following automatic expansion of the finger probe cuff, distal digit volume changes are calculated from pressure alterations through pressure transducers connected to the EndoPAT2000 device. RHI is calculated automatically as the ratio between the generated signals 5-min post forearm occlusion and at baseline, in relationship to the response in the contralateral arm.
Laboratory analyses
Blood samples were collected after an overnight fast. The biochemical analyses were performed using commercially available kits according to the manufacturers’ protocols; serum triglycerides and total cholesterol (Roche Diagnostics GMBH, Mannheim, Germany), direct high density lipoprotein (HDL) cholesterol (Horiba ABX, France), Apolipoprotein A1, Apolipoprotein B (DakoCytomation, Glostrup, Denmark) and serum insulin (Millipore Corporation, USA). Plasma glucose was measured using a photometric method and HbA1c by HPLC at the department of Clinical Chemistry, Sahlgrenska University Hospital, Gothenburg. Impaired glucose homeostasis, assessed by HOMA-IR was calculated to estimate insulin resistance using the formula: fasting serum insulin (mU/L) × fasting plasma glucose (mmol/L)/22.5 [21]. A number of cardiovascular associated biomarkers (stem cell factor, vascular endothelial growth factor D (VEGF-D), vascular endothelial growth factor A (VEGF-A), interleukin-6 (IL-6), and endothelial cell-specific molecule 1 also known as endocan were analyzed using the Olink Bioscience (Uppsala, Sweden) Proseek Multiplex CVD I 96 × 96 according to the manufacturer’s instructions [22]. All Proseek data are presented as arbitrary units (AU) in log2 values.
Follow-up and definitions of outcome measures
Follow-up was accomplished by a physician by telephone interviews and confirmed through patient’s medical record and/or the Swedish National Board of Health’s Registry and median follow-up time was 5.1 years (range: 4.4–6.2 years). Study endpoint was event-free survival and events were defined as the incidence of all-cause mortality, nonfatal stroke, nonfatal MI and coronary arterial revascularizations (either coronary artery bypass grafting or percutaneous coronary intervention). Time to most severe event was analyzed and ranged as follows death > MI/stroke > arterial revascularization. Definition of MI was clinically diagnosed by presence of persistent chest pain and confirmed by pathological troponin dynamics and/or electrocardiogram changes. The occurrence of stroke was defined as focal or global neurological deficits lasting for more than 24 h and verified clinically by a neurologist and/or by computed tomography brain scan.
Samples and gene expression analysis on patients without myocardial perfusion defects
Extraction of RNA was done on whole blood samples collected using Paxgene tubes from a subset of 54 consecutive patients without myocardial perfusion defects, in conjunction to the imaging procedure. Affymetrix Gene Arrays (Human Gene 1.0 ST array) was used to interrogate 28,869 transcript clusters and CEL files were imported to Partek Genomics Suite version 6.5 (Partek Inc., MO, USA). The data was log2-transformed and Robust Multi-array Average normalization was performed using ArrayStudio (Omicsoft, version 3.2.0 and 3.5.0). The normalized gene expression values were then fitted with a linear regression model using the Bioconductor R package ‘limma’ (linear models for microarray data) version 3.22.7. Using an empirical Bayesian approach, the ‘limma’ package infers differential expression in individual genes from the microarray data [23].
Partial least squares—discriminant analysis, functional and pathway analysis
The application of partial least squares—discriminant analysis (PLS-DA) to the microarray data of the 54 patient samples was carried out using the MATLAB® software v. 8.3.0.532 (R2012a, The MathWorks, Inc.) and the MATLAB® PLS Toolbox v. 7.5.2 (Eigenvector Research, Inc.). PLS-DA scores and weights plot were used to identify markers, from the ‘limma’ gene expression analysis results, correlated to patients with high HOMA-IR (cut-off value of median of HOMA-IR >3.1); and correlated to patients with decreased endothelial function (low RHI based on RHI median of <1.91). The PLS-DA scores plot based on the cut-offs for HOMA-IR and RHI levels showed discrimination of the patient samples into two classes; and the weights plot for the appropriate latent variables revealed which genes were important in separating the classes and hence association to the appropriate class (data not shown).
To define genes associated to high HOMA-IR, and low RHI; and identify the biological mechanisms, pathways and functions co-regulated by the genes, core analyses were performed using the ingenuity pathway analysis (IPA) software (Ingenuity Systems, Redwood City, CA). The significance of the connection between the expression data and the canonical pathway were calculated by ratio and/or Fisher’s exact test. Significant genes passing the test criterion (e.g., t test, correlation analysis p-value for ANOVA or fold change) were functionally categorized by gene ontology [24]. This resulted into associated genetic networks, canonical pathways, and biological functions enriched by the genes. Comparison analysis in IPA for the core analysis results was then carried out to identify co-regulated pathways.
Statistics
Deviations in sample size for the various statistical analyses were due to differences in the availability of RHI, as well as missing values in some analyzed biomarkers. All analyses were performed in SPSS, (version 21.0, Chicago Inc, USA). P-values of less than 0.05 were considered significant (2-tailed). Due to there is no present “golden standard” cut of value for HOMA-IR, we used the median value. We calculated the sample size based on the Cox PH one-sided superiority formula. With an overall event rate 20 %, an alpha level 5 and 80 % power, we need approximately 260 patients to estimate a hazard ratio (HR) of 2. Sub-analyses were performed in patients with and without myocardial perfusion defects, defined as above. Values are displayed as mean ± SD for continuous variables and frequency and percentages for categorical variables. The test of skewness was used to assess normal distribution. Non-normally distributed variables are presented with their median and interquartile range. Differences among continuous variables were analyzed using unpaired t test or Mann–Whitney U test, as appropriate. Categorical data was analyzed by Pearson Chi square test. Spearman’s correlation coefficients were used to examine relationships between continuous variables. Continuous and categorical HOMA-IR, divided by the median value was used in a linear regression model, predicting continuous RHI in patients without myocardial perfusion defects. Possible co-linearity between the independent variables was tested using Spearman correlation coefficient test and a coefficient >0.7 was considered significant. High co-linearity was found between fasting glucose levels and pre-diabetes diagnosis (correlation coefficient 0.8). The latter was added to the multivariable model investigating factors of importance for outcome. The multivariable linear regression model was adjusted for categorical HOMA-IR median and other relevant independent parameters associated to the dependent parameter with a p < 0.25 (gender, previously known CAD, body mass index and HDL). Results are displayed as β-values and 95 % confidence intervals (CI). Kaplan–Meier curves are used to display survival rates. HR and 95 % CIs were analyzed by univariate Cox regression with continuous and categorical HOMA-IR, divided by the median value in the whole population as well as in patients with and without myocardial perfusion defects. Also multivariable COX regression analyses were performed with HOMA-IR as a categorical variable divided by the median value, adjusted for age, body mass index, gender, systolic blood pressure, HDL and previously known CAD, selected based on their observed relevance to the dependent parameter (univariate Cox regression, p < 0.25) in the current study.
Results
Demographic and clinical characteristics
Demographic data of the 365 non-diabetic patients with suspected myocardial ischemia are shown in Table 1. HbA1c levels were normally distributed within the range 4.3-6.5 % (24–48 mmol/mol), of which 4 subjects are above the 95:th percentile, normal range 4.6–6.4 % (27–46 mmol/mol) as by local definition (Department of Clinical Chemistry, Sahlgrenska University Hospital, Gothenburg, Sweden). Fasting serum insulin levels showed a right skewed distribution with the range 28–493 pmol/L. Fasting plasma glucose levels were normally distributed with the range 4.0–6.8 mmol/L. Also HOMA-IR showed a right skewed distribution with the range 1.1–14.0. Among the 365 patients, 116 subjects (32 %) were found to have impaired fasting glucose levels (100–125 mg/dL; 5.6–6.9 mmol/L) as defined by American Diabetes Association. Impaired fasting glucose levels (≥5.6 mmol/L) was used to define patients with pre-diabetes in subsequent analyses.
Table 1.
Baseline characteristics of study cohort
| Whole study population (n = 365) | Without perfusion defects (n = 238) | |
|---|---|---|
| Age (years) | 62 ± 9 | 61 ± 9 |
| Women | 202 (55 %) | 150 (63 %) |
| Body mass index | 25.7 ± 3.5 | 25.4 ± 3.5 |
| Current smoker | 47 (13 %) | 31 (13 %) |
| Family history of CAD | 142 (39 %) | 89 (37 %) |
| ACE-inhibitors | 69 (19 %) | 32 (13 %) |
| Beta-blockers | 173 (47 %) | 96 (40 %) |
| Statins | 146 (40 %) | 83 (35 %) |
| Aspirin | 178 (49 %) | 102 (43 %) |
| Systolic blood pressure (mmHg) | 144 ± 23 | 144 ± 22 |
| Known CAD | 94 (26 %) | 44 (18 %) |
| Previous MI | 51 (14 %) | 19 (8 %) |
| Triglycerides (mmol/L) (n = 362) | 1.2 (0.8–1.6) | 1.1 (0.8–1.3) (n = 235) |
| Cholesterol (mmol/L) (n = 360) | 5.4 ± 1.3 | 5.4 ± 1.1 (n = 234) |
| HDL (mmol/L) | 1.47 (1.24–1.72) | 1.49 (1.29–1.75) |
| ApoB/ApoA1 | 0.64 (0.53–0.79) | 0.62 (0.52–0.76) |
| Fasting glucose (mmol/L) | 5.3 ± 0.5 | 5.3 ± 0.5 |
| Insulin (pmol/L) | 94 (69–125) | 92 (69–122) |
| HbA1c (mmol/mol, %) | 36.6 ± 3.8 (5.5 ± 0.3 %) | 36.2 ± 3.5 (5.5 ± 0.32 %) |
| HOMA-IR | 3.1 (2.3–4.3) | 3.1 (2.3–4.1) |
| RHI (n = 345) | 1.89 (1.55–2.47) | 1.91 (1.59–2.48) (n = 225) |
Values are displayed as mean ± SD or median and interquartile range for continuous variables and frequency and percentages for categorical variables
ApoA apolipoprotein A, ApoB apolipoprotein B, HbA 1c glycosylated hemoglobin, HDL high density lipoprotein cholesterol. MI myocardial infarction, Known CAD previously known coronary artery disease, RHI reactive hyperemic index
HOMA-IR as predictor of clinical outcome
The median follow-up time in our study was 62 months (5.1 years) with a range of 53–74 months. There was no loss to follow-up. The occurrence of events during the follow-up time in the study population was 21 % (death n = 15, MI/stroke n = 15, coronary revascularization n = 52). In a univariate analysis continuous HOMA-IR (HR: 1.13, CI: 1.04–1.24, p = 0.005, χ2: 7.9) predicted event-free survival. Furthermore, in a univariate survival analysis (Table 2) HOMA-IR (χ2: 17.1) above median value significantly predicted outcome together with age (χ2: 15.3), previously known CAD (χ2: 30.6), gender (χ2: 23.6), HDL (χ2: 7.7) and systolic blood pressure (χ2: 4.4). In a multivariable model of survival analysis (Table 2) adjusting for relevant risk factors (p < 0.001, χ2: 55.0), HOMA-IR above median independently predicted outcome (Fig. 2a) together with age, previously known CAD and gender. Also when adding pre-diabetes (univariate survival analysis: HR: 1.7, CI: 1.1–2.7, p = 0.020, χ2: 5.5) to the multivariable model, HOMA-IR above median (HR: 1.8, CI: 1.1–3.2, p = 0.042, χ2: 55.1) but not pre-diabetes (HR: 1.05, CI: 1.7, p = 0.854, χ2: 55.1) remained an independent predictor of outcome.
Table 2.
Univariate and multivariable adjustment of HOMA-IR as predictor of clinical outcome
| Univariate model | Multivariable model | |||
|---|---|---|---|---|
| HR (95 % CI) | p value | HR (95 % CI) | p value | |
| Age | 1.05 (1.03–1.08) | <0.001 | 1.04 (1.01–1.07) | 0.007 |
| Known CAD | 3.27 (2.10–5.10) | <0.001 | 1.87 (1.15–3.05) | 0.012 |
| Gender | 3.10 (1.92–5.01) | <0.001 | 1.92 (1.11–3.35) | 0.020 |
| HOMA-IR above median | 2.72 (1.70–4.46) | <0.001 | 1.88 (1.09–3.26) | 0.023 |
| HDL | 0.38 (0.19–0.75) | 0.005 | 0.82 (0.41–1.63) | 0.570 |
| Systolic blood pressure | 1.01 (1.00–1.02) | 0.036 | 1.00 (0.99–1.01) | 0.693 |
| Body mass index | 1.05 (0.99–1.12) | 0.120 | 1.00 (0.92–1.08) | 0.915 |
Survival analyses on the whole study population of non-diabetic patients with suspected myocardial ischemia. Data are presented with hazard ratio and 95 % CI, (n = 365)
CI confidence interval, HDL high density lipoprotein cholesterol, HR hazard ratio Known CAD previously known coronary artery disease
Fig. 2.
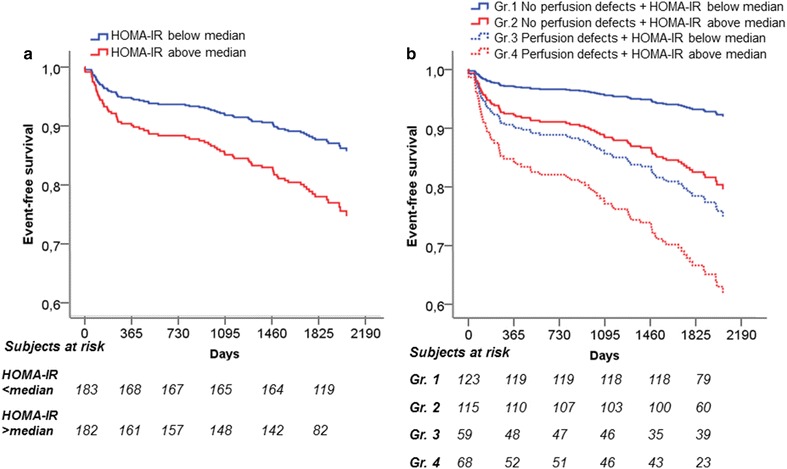
COX regression analyses between HOMA-IR below and above median on non-diabetic patients with suspected myocardial ischemia (n = 365). In a COX regression analysis a HOMA-IR above median provides an independent prognostic value predicting long-term events in the whole study population. In a COX regression analysis b HOMA-IR provides independent prognostic information in non-diabetic patients with no myocardial perfusion defects (HR: 2.7, p = 0.02, n = 238), but not in patients with myocardial perfusion defects (HR: 1.7, p = 0.14, n = 127). Statistics are presented as Chi square values and HR. Gr group, HR hazard ratio
Independent prognostic value of HOMA-IR in patients without myocardial perfusion defects
Impaired glucose homeostasis in the non-diabetic state may be of importance in progression of microvascular dysfunction, i.e., in patients with chest pain without obstructive CAD. We therefore investigated the additive value of HOMA-IR to MPS results. In our cohort, the 238 patients without myocardial perfusion defects had an event rate of 13 %, while 127 patients displayed myocardial perfusion defects with an event rate of 35 %. In patients without myocardial perfusion defects the difference in event rate in patients with HOMA-IR below versus above median was 15 % (p = 0.001), and in patients with myocardial perfusion defects, the difference was 22 % (p = 0.008). Furthermore, HOMA-IR showed additive value for outcome when combined with MPS results. High versus low HOMA-IR had significant prognostic value in patients without (HR: 3.4, CI: 1.5–7.5, p = 0.003, χ2: 45.0) and with myocardial perfusion defects (HR: 2.1, CI: 1.4–4.0, p = 0.018, χ2: 45.0). In the multivariable model including conventional risk factors (age, gender, systolic blood pressure, body mass index, HDL and previously known CAD (p < 0.001, χ2: 71.1), HOMA-IR showed independent prognostic value in patients without myocardial perfusion defects (HR: 2.7, CI: 1.2–6.3, p = 0.02), but not in patients with myocardial perfusion defects (HR: 1.7, CI: 0.3–1.2, p = 0.14) (Fig. 2b). Importantly, HOMA-IR above median remained an independent predictor of outcome in patients without myocardial perfusion defects also when adding pre-diabetes (HOMA-IR HR: 2.7 CI: 1.1–6.3, p = 0.02) to the multivariable model (χ2: 71.2). Moreover, when using the first multivariable model and excluding the 94 patients with previously known CAD, HOMA-IR above median increasingly predicted outcome in patients without myocardial perfusion defects (HR: 5.0, CI: 1.4–18.3, p = 0.014) alongside age (HR: 1.05, CI: 1.01–1.09, p = 0.008). However, HOMA-IR had no independent prognostic value in patients with myocardial perfusion defects (HR: 1.5, CI: 0.59–3.9, p = 0.388) when patients with previously known CAD were excluded.
HOMA-IR is associated with decreased peripheral endothelial function in patients without myocardial perfusion defects
Insulin resistance and endothelial dysfunction are known to coexist [14]. Therefore, we investigated the association of HOMA-IR to peripheral endothelial function in non-diabetic patients with chest pain without myocardial perfusion defects. Continuous HOMA-IR displayed a significant negative association with RHI (β: −0.014, (CI: −0.023–0.004), p = 0.004). Also, patients with HOMA-IR in the upper compared to lower median value displayed a significant inverse association with RHI (Table 3) and we observed a significantly higher RHI value in patients above compared to below median value (2.2 ± 0.7 and 1.9 ± 0.7, respectively, p = 0.001). Additionally, in a multivariable linear regression analysis (Table 3) adjusting for risk factors associated with RHI in our study, HOMA-IR above median independently predicted decreased RHI (R square = 0.05).
Table 3.
Univariate and multivariable parameters associated to reactive hyperemic index in patients without myocardial perfusion defects
| Univariate | Multivariable model | |||
|---|---|---|---|---|
| β (95 % CI) | p | β (95 % CI) | p | |
| HOMA-IR above median | −0.273 (−0.443–0.102) | 0.002 | −0.230 (−0.426–0.034) | 0.022 |
| Body mass index | −0.020 (−0.046–0.006) | 0.129 | −0.009 (−0.038–0.020) | 0.548 |
| HDL | 0.160 (−0.068–0.388) | 0.167 | 0.079 (−0.176–0.335) | 0.541 |
| Gender | 0.124 (−0.057–0.305) | 0.177 | −0.028 (−0.241–0.185) | 0.795 |
| Known CAD | 0.139 (−0.083–0.362) | 0.219 | 0.091 (−0.143–0.324) | 0.444 |
Linear regression analyses on non-diabetic patients without myocardial perfusion defects (n = 225)
CI confidence interval, HDL high density lipoprotein cholesterol, Known CAD previously known coronary artery disease
HOMA-IR and cardiovascular biomarkers in patients without myocardial perfusion defects
Furthermore, we aimed to investigate the relation of increased HOMA-IR to inflammatory and vascular growth factor associated biomarkers in patients without myocardial perfusion defects (n = 238). The systemic low degree inflammation marker IL-6 showed positive correlation to HOMA-IR (corr.coeff = 0.200, p = 0.002) and a significantly higher value in patients with HOMA-IR above compared to below median value (3.8 ± 1.1 and 3.4 ± 0.9 AU, respectively, p = 0.008). The growth factor VEGF-D was inversely correlated to HOMA-IR (corr.coeff = −0.213, p = 0.001) and was significantly lower in patients with HOMA-IR above compared to lower median value (6.3 ± 0.3 and 6.1 ± 0.4 AU, below versus above median, respectively, p = 0.04). Reduced levels of VEGF related factor endocan also called the endothelial cell-specific molecule 1 was further associated with high HOMA-IR (corr.coeff = −0.249, p < 0.001) and lower in patients with HOMA-IR above median (2.5 ± 0.3 and 2.3 ± 0.3 AU, below versus above median, respectively, p = 0.006). We observed positive correlation between VEGF-D and endocan (corr.coeff = 0.375, p < 0.001), which confirmed the relationship suggested in previous studies [25]. However, no significant correlation was found between VEGF-A and HOMA-IR (corr.coeff = 0.062, p = 0.34) and no difference was found between VEGF-A in patients with HOMA-IR above compared to below median (9.0 ± 0.4 and 9.0 ± 0.3, respectively). Furthermore, high HOMA-IR was inversely correlated with Stem cell factor (corr.coeff = −0.219, p = 0.001) and patients with HOMA-IR above median had reduced level of Stem cell factor (8.1 ± 0.4 and 8.0 ± 0.3 AU, below versus above median, respectively, p = 0.001).
Gene Expression, PLS-DA and pathway analyses in patients with high HOMA-IR and low RHI
The outcome of ‘limma’ gene expression analysis is moderated t-statistics with Bayesian-adjusted denominators that incorporate information across all genes [26]. The ‘limma’ result consisting of the probeset IDs and the log-fold expression values of gene transcripts were then analyzed using the IPA software. Differential expressed genes were characterized having an estimated fold-change >1.5, and the Benjamini and Hochberg’s method was used to control false discovery rate smaller than 0.05 [27]. Further analysis by PLS-DA produced lists of gene transcripts associated to HOMA-IR above median and RHI below median. Thereafter, we examined the canonical pathways enriched. IPA comparison analysis gene transcripts predict patients with high HOMA-IR and low RHI co-regulated signaling pathways. Worth mentioning is the activated pro-inflammatory pathways, dendritic cell maturation signaling, nuclear factor kappa B signaling and toll-like receptor signaling as well as inhibited eukaryotic initiation factor 2 (EIF2) signaling (Fig. 3).
Fig. 3.

Global gene expression pathway analysis on patients without myocardial perfusion defects (n = 54). Red bars predict an overall increase in the activity of the pathway (activation) while blue bars indicate a prediction of an overall decrease in activity (deactivation/inhibition). White bars are those with a z-score which is zero or very close to zero. The overall activation/inhibition (deactivation) states of the pathways are predicted based on a Z-score algorithm. Z-score gives a statistical measure of the relationship of up and downregulated gene transcripts in the microarray data set with-respect-to a particular pathway. This Z-score is used to mathematically compare the microarray data set with the canonical pathway patterns. A pathway is predicted as activated or inhibited by comparing the expected pattern (up/downregulation of key genes in the pathway) if the pathway is activated against the actual pattern (up/downregulated key genes) in the microarray data set. If the actual pattern matches the expected pattern, the Z-score is positive (Z-score > ~2 = activated pathway) otherwise negative (Z-score <2 = inhibited pathway)
Discussion
We show how elevated HOMA-IR, a marker of insulin resistance [28], independently predicts outcome in non-diabetic patients with chest pain symptoms and suspected myocardial ischemia. Importantly, HOMA-IR has independent prognostic value in patients without myocardial perfusion defects and in this subgroup increased HOMA-IR was associated with decreased endothelial function. Furthermore, in patients without myocardial perfusion defects increased HOMA-IR was associated with increased circulating levels of IL-6 and decreased levels of vascular growth factors, suggesting systemic inflammation and impaired angiogenic potential. In support to the protein level data, global gene expression analysis revealed that co-regulated pro-inflammatory pathways and down-regulated EIF2 signaling pathway were associated with high HOMA-IR and impaired endothelial function.
HOMA-IR as predictor of clinical outcome
MPS is a well-established tool in detecting hemodynamically significant obstructive CAD and has strong prognostic value [2]. However MPS may not be the optimal modality to detect global microvascular dysfunction, which typically requires absolute flow reserve quantification [29]. In the current non-diabetic population, increased insulin resistance as measured by HOMA-IR predicted future events. Interestingly, an independent prognostic value of HOMA-IR was only observed in patients without myocardial perfusion defects. In line with previous reports, patients with perfusion defects may already be at high risk for cardiovascular events, so testing HOMA-IR may not add independent values for prognostic evaluation [11]. The fact that an independent prognostic value of HOMA-IR was observed in non-diabetic patients without myocardial perfusion defects independently of pre-diabetes, supported our hypothesis that insulin resistance could be one of the main mechanisms associated with increased cardiovascular risk in these patients. Further, assessing HOMA-IR may add clinical value also in patients with long standing type 2 diabetes. Srinivasan et al. recently showed that HOMA-IR below 2.5 is associated with normal coronary angiogram indicating importance of assessing insulin resistance also to help to predict outcome in type 2 diabetes patients [30].
HOMA-IR and peripheral endothelial function
The results of the present study indicate that insulin resistance is associated with peripheral endothelial dysfunction and predicts clinical outcome also in patients without myocardial perfusion defects. It was recently shown that the prevalence of microvascular dysfunction in patients with chest pain and non-obstructive CAD is high in both men and women and that conventional risk factors play a minor role [8]. In the present study, endothelial function was assessed by Endo-PAT, which has been shown to predict coronary endothelial dysfunction [20] as well as having additive prognostic value to the Framingham risk score [11]. HOMA-IR is a surrogate measure of insulin resistance that has been shown to be more strongly associated with cardiovascular disease than glucose or insulin concentrations alone in non-diabetic patients [31]. Endothelial dysfunction is a consequence of different mechanisms associated with insulin resistance causing impaired vasodilation or increased vasoconstriction [12–14]. Normal insulin action results in vasodilation at the arterial, venous, and microcirculatory levels [32] via increased production of endothelial nitric oxide [14, 17, 32]. In accordance, insulin resistance has been shown to reduce the expression and function of the endothelial nitric oxide-synthase gene in endothelial cells and microvessels in insulin resistant rats [16]. Interestingly, insulin resistance in apparently healthy adolescents is associated with endothelial dysfunction [33]. Furthermore, microalbuminuria is known to correlate with endothelial dysfunction and has been independently associated with insulin resistance in type 2 diabetes and it was recently shown that HOMA-IR below 2.5 and absence of microalbuminuria is associated with beneficial CAD profile [30].
HOMA-IR and angiogenic factors
The pathophysiological mechanisms underlying microvascular dysfunction is multifactorial [13]. In addition to e.g., endothelial dysfunction, structural vascular adaption, including vascular remodeling, rarefaction, and collateralization could also play roles [13]. Ischemia is known to stimulate angiogenesis, and conditions of only minimal ischemia stimulate development of collateral vessels [34]. The growth factor Stem cell factor is important in mobilization and recruitment of vascular angiogenic endothelial progenitor cells in response to ischemia [35]. Interestingly, decreased Stem cell factor levels were recently found in diabetic patients and were related to incidence of cardiovascular events [19]. In our study, decreased Stem cell factor correlated significantly to high HOMA-IR and decreased VEGF-D levels (data not shown). VEGF-D has been shown to be important for vascular angiogenesis and lymphangiogenesis, as well as stimulating endothelial production of nitric oxide [36, 37]. In the current study increased HOMA-IR was inversely correlated to VEGF-D at a protein level. We further demonstrate that VEGF-D correlates positively to endocan, which is known to be induced by VEGF and involved in tumor angiogenesis [25]. In line with our data, He et al. showed that reduced PI3K/Akt signaling is likely responsible for the reduction in VEGF induced vascularization in the myocardium at both basal and ischemic states [18]. Furthermore, Bonner et al. showed that deletion of VEGF in murine cardiac muscle induced capillary rarefaction and promoted insulin resistance [38]. Taken together, these studies indicate that insulin resistance might promote downregulation of VEGF, causing vascular rarefaction which may accelerate further development of insulin resistance due to insufficient delivery of insulin. Ischemia-induced hypoxia is also a trigger for increased transcription and translation of vascular endothelial growth factors [34] ultimately leading to angiogenesis. The current study displayed impaired EIF2 pathway to be associated with insulin resistance and endothelial dysfunction. Since VEGF mRNA translation is dependent on the EIF2 pathway, especially during hypoxia [39], an impaired EIF2 pathway could at least in part be responsible for the low VEGF-D protein levels associated with high HOMA-IR. In addition, it was recently reported a novel VEGF signaling mechanism involving the EIF2 pathway to activate the unfolded protein response in endothelial cells [40], which seems to be important for angiogenesis and cell survival. Thus, a down-regulated EIF2 pathway may further diminish the action of VEGF on the vascular wall. Taken together, the reduced levels of VEGF-D and Stem cell factor could be mechanisms underlying endothelial dysfunction associated with insulin resistance in this patient population.
HOMA-IR and subclinical inflammation
A large proportion of the study population was pre-diabetes patients. The pre-diabetic state is associated with increased systemic inflammation [41] and IL-6 has been shown to be related to coronary microvascular dysfunction [42]. We demonstrated that high HOMA-IR was associated with increased levels of IL-6 also in this cohort, which is in line with previous findings that obese, non-diabetic insulin resistant patients have elevated IL-6 levels [43]. Interestingly, Antoniades et al. showed increased IL-6 levels in healthy subjects to be associated with impaired peripheral flow-mediated vasodilation and to increased levels of asymmetrical dimethylarginine, an endogenous inhibitor of endothelial nitric oxide synthase [44]. In line with these results, IL-6 was demonstrated to inhibit endothelium-dependent nitric oxide-mediated relaxation and enhance contraction in an experimental model [45], which suggests a direct role of IL-6 in microvascular dysfunction associated with insulin resistance. In accordance, gene expression and pathway analysis indicated upregulation of multiple pro-inflammatory pathways including dendritic cell maturation signaling pathway and pattern recognition receptors signaling pathway, which both could result in e.g., increased systemic levels of IL-6.
Study limitations
Despite extensive biomarker analysis several other important markers for cardiovascular risk may still be missing, such as adiponectin [46–48] and microalbuminuria [30]. Global gene expression pathway analysis was performed on RNA extracted from whole blood cells, used as a surrogate cell compartment mirroring similar biological processes in other relevant tissues. Caution should be taken when extrapolating the finding to other target tissues, such as endothelial cells. Further, more studies are warranted to investigate mechanistic relevance in other populations. Finally, the current study is still of limited size and therefore caution should be taken when interpreting the data.
Conclusions
Our study shows that pre-diabetes as indicated by impaired glucose homeostasis is prevalent in non-diabetic patients with chest pain and suspected myocardial ischemia. Elevated HOMA-IR is associated with decreased endothelial function, and adds independent prognostic information in patients without myocardial perfusion defects. Furthermore, our results also indicate that increased systemic low degree inflammation and decreased vascular growth factor production may be underlying mechanisms connecting insulin resistance with microvascular dysfunction in this patient population.
Authors’ contributions
HW and RM performed data analysis, interpretation of data and writing of the manuscript. SS participated in data collection, data analysis, data interpretation as well as drafted and critically revised the manuscript. JB and KW contributed to interpretation of data and critically revised the manuscript. ER and PG performed data analysis and revised the manuscript critically for important intellectual content. RFD participated in inception and design of submitted manuscript as well as revised the manuscript. JO participated in inception and design of submitted manuscript, interpretation of metabolic parameters, drafting and revising the manuscript critically for important intellectual content as well as important clinical input. LMG is the principal investigator of the conducted work and hold specific contribution in conception and design of the study as well as interpretation of data. LMG has also drafted and critically revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We gratefully acknowledge excellent technical assistance from Charlotte Eklund, statistical input from Marianne Månsson and the performance of the biochemical analysis at AstraZeneca R&D Gothenburg. This work was supported by agreement concerning research and education of doctors (ALF/LUA grant), Swedish federal government.
Competing interests
Co-authors Remi A Momo, Juuso I Blomster, Karin Wåhlander, Erika Rehnström, Peter J Greasley, Regina Fritsche-Danielson, Jan Oscarsson and Li-Ming Gan are employed by AstraZeneca R&D Gothenburg. AstraZeneca provided support in the form of biochemical analysis and salaries for authors RAM, JIB, KW, ER, PJG, RFD, JO and LMG. AstraZeneca did not have any additional role in the study design, data collection and analysis, or preparation of the manuscript. First author Helena U Westergren and co-author Sara Svedlund are supported by the Agreement concerning research and education of doctors (ALF/LUA grant), Swedish federal government. The decision to publish was made based on a consensus from all authors followed by an internal AstraZeneca review of the manuscript.
Abbreviations
- ApoA
apolipoprotein A
- ApoB
apolipoprotein B
- AU
arbitrary units
- CAD
coronary artery disease
- CI
confidence interval
- EIF2
eukaryotic initiation factor 2
- HbA1c
glycated haemoglobin A1c
- HDL
high density lipoprotein cholesterol
- HOMA-IR
homeostasis model assessment of insulin resistance
- HR
hazard ratio
- IL-6
interleukin-6
- IPA
ingenuity pathway analysis
- MI
myocardial infarction
- MPS
myocardial perfusion scintigram
- PLS-DA
partial least squares—discriminant analysis
- RHI
reactive hyperemic index
- VEGF-A
vascular endothelial growth factor A
- VEGF-D
vascular endothelial growth factor D
Contributor Information
Helena U. Westergren, Email: helena.westergren@gu.se
Sara Svedlund, Email: Sara.svedlund@gu.se.
Remi A. Momo, Email: Remi.momo@astrazeneca.com
Juuso I. Blomster, Email: Juuso.blomster@gmail.com
Karin Wåhlander, Email: karin.fc.wahlander@telia.com.
Erika Rehnström, Email: Erika.Rehnstrom@astrazeneca.com.
Peter J. Greasley, Email: Peter.Greasley@astrazeneca.com
Regina Fritsche-Danielson, Email: Regina.Fritsche-Danielson@astrazeneca.com.
Jan Oscarsson, Email: Jan.oscarsson@astrazeneca.com.
Li-Ming Gan, Phone: +46 31 342 10 00, Email: li-ming.gan@gu.se.
References
- 1.Kang X, Berman DS, Lewin HC, Cohen I, Friedman JD, Germano G, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography in patients with diabetes mellitus. Am Heart J. 1999;138(6 Pt 1):1025–1032. doi: 10.1016/S0002-8703(99)70066-9. [DOI] [PubMed] [Google Scholar]
- 2.Hachamovitch R, Berman DS, Kiat H, Cohen I, Cabico JA, Friedman J, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation. 1996;93(5):905–914. doi: 10.1161/01.CIR.93.5.905. [DOI] [PubMed] [Google Scholar]
- 3.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines, and the American College of Physicians, American Association for thoracic surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Task Force M, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 5.Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33(6):734–744. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 6.Ong P, Athanasiadis A, Borgulya G, Mahrholdt H, Kaski JC, Sechtem U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries) J Am Coll Cardiol. 2012;59(7):655–662. doi: 10.1016/j.jacc.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Steg PG, Greenlaw N, Tendera M, Tardif JC, Ferrari R, Al-Zaibag M, et al. Prevalence of anginal symptoms and myocardial ischemia and their effect on clinical outcomes in outpatients with stable coronary artery disease: data from the International Observational CLARIFY Registry. JAMA Intern Med. 2014;174(10):1651–1659. doi: 10.1001/jamainternmed.2014.3773. [DOI] [PubMed] [Google Scholar]
- 8.Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8(11):1445–1453. doi: 10.1016/j.jcin.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Ponte M, Bettencourt N, Pereira E, Ferreira ND, Chiribiri A, Schuster A, et al. Anatomical versus functional assessment of coronary artery disease: direct comparison of computed tomography coronary angiography and magnetic resonance myocardial perfusion imaging in patients with intermediate pre-test probability. Int J Cardiovasc Imaging. 2014;30(8):1589–1597. doi: 10.1007/s10554-014-0492-y. [DOI] [PubMed] [Google Scholar]
- 10.Cassar A, Chareonthaitawee P, Rihal CS, Prasad A, Lennon RJ, Lerman LO, et al. Lack of correlation between noninvasive stress tests and invasive coronary vasomotor dysfunction in patients with nonobstructive coronary artery disease. Circ Cardiovasc Interv. 2009;2(3):237–244. doi: 10.1161/CIRCINTERVENTIONS.108.841056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuzawa Y, Svedlund S, Aoki T, Guddeti RR, Kwon TG, Cilluffo R, et al. Utility of both carotid intima-media thickness and endothelial function for cardiovascular risk stratification in patients with angina-like symptoms. Int J Cardiol. 2015;190:90–98. doi: 10.1016/j.ijcard.2015.04.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camici PG, d’Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol. 2015;12(1):48–62. doi: 10.1038/nrcardio.2014.160. [DOI] [PubMed] [Google Scholar]
- 13.Pries AR, Badimon L, Bugiardini R, Camici PG, Dorobantu M, Duncker DJ, et al. Coronary vascular regulation, remodelling, and collateralization: mechanisms and clinical implications on behalf of the working group on coronary pathophysiology and microcirculation. Eur Heart J. 2015 doi: 10.1093/eurheartj/ehv100. [DOI] [PubMed] [Google Scholar]
- 14.Cersosimo E, DeFronzo RA. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab Res Rev. 2006;22(6):423–436. doi: 10.1002/dmrr.634. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 16.Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, et al. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation. 2000;101(6):676–681. doi: 10.1161/01.CIR.101.6.676. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94(3):1172–1179. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Z, Opland DM, Way KJ, Ueki K, Bodyak N, Kang PM, et al. Regulation of vascular endothelial growth factor expression and vascularization in the myocardium by insulin receptor and PI3K/Akt pathways in insulin resistance and ischemia. Arterioscler Thromb Vasc Biol. 2006;26(4):787–793. doi: 10.1161/01.ATV.0000209500.15801.4e. [DOI] [PubMed] [Google Scholar]
- 19.Wigren M, Rattik S, Hultman K, Bjorkbacka H, Nordin-Fredrikson G, Bengtsson E, et al. Decreased levels of stem cell factor in subjects with incident coronary events. J Intern Med. 2015 doi: 10.1111/joim.12443. [DOI] [PubMed] [Google Scholar]
- 20.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44(11):2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.Assarsson E, Lundberg M, Holmquist G, Bjorkesten J, Thorsen SB, Ekman D, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9(4):e95192. doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wettenhall JM, Smyth GK. limmaGUI: a graphical user interface for linear modeling of microarray data. Bioinformatics. 2004;20(18):3705–3706. doi: 10.1093/bioinformatics/bth449. [DOI] [PubMed] [Google Scholar]
- 24.Mirza Z, Kamal MA, Buzenadah AM, Al-Qahtani MH, Karim S. Establishing genomic/transcriptomic links between Alzheimer’s disease and type 2 diabetes mellitus by meta-analysis approach. CNS Neurol Disord Drug Targets. 2014;13(3):501–516. doi: 10.2174/18715273113126660154. [DOI] [PubMed] [Google Scholar]
- 25.Rennel E, Mellberg S, Dimberg A, Petersson L, Botling J, Ameur A, et al. Endocan is a VEGF-A and PI3K regulated gene with increased expression in human renal cancer. Exp Cell Res. 2007;313(7):1285–1294. doi: 10.1016/j.yexcr.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 27.Groppe DM, Urbach TP, Kutas M. Mass univariate analysis of event-related brain potentials/fields I: a critical tutorial review. Psychophysiology. 2011;48(12):1711–1725. doi: 10.1111/j.1469-8986.2011.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 29.Johnson NP, Gould KL. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. JACC Cardiovasc Imaging. 2012;5(4):430–440. doi: 10.1016/j.jcmg.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan MP, Kamath PK, Bhat NM, Pai ND, Manjrekar PA, Mahabala C. Factors associated with no apparent coronary artery disease in patients with type 2 diabetes mellitus for more than 10 years of duration: a case control study. Cardiovasc Diabetol. 2015;14:146. doi: 10.1186/s12933-015-0307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLoS One. 2012;7(12):e52036. doi: 10.1371/journal.pone.0052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grover A, Padginton C, Wilson MF, Sung BH, Izzo JL, Jr, Dandona P. Insulin attenuates norepinephrine-induced venoconstriction. An ultrasonographic study. Hypertension. 1995;25(4 Pt 2):779–784. doi: 10.1161/01.HYP.25.4.779. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Osika W, Dangardt F, Gan LM, Strandvik B, Friberg P. High levels of soluble intercellular adhesion molecule-1, insulin resistance and saturated fatty acids are associated with endothelial dysfunction in healthy adolescents. Atherosclerosis. 2010;211(2):638–642. doi: 10.1016/j.atherosclerosis.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Schaper W. Collateral circulation: past and present. Basic Res Cardiol. 2009;104(1):5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim KL, Meng Y, Kim JY, Baek EJ, Suh W. Direct and differential effects of stem cell factor on the neovascularization activity of endothelial progenitor cells. Cardiovasc Res. 2011;92(1):132–140. doi: 10.1093/cvr/cvr161. [DOI] [PubMed] [Google Scholar]
- 36.Rissanen TT, Markkanen JE, Gruchala M, Heikura T, Puranen A, Kettunen MI, et al. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ Res. 2003;92(10):1098–1106. doi: 10.1161/01.RES.0000073584.46059.E3. [DOI] [PubMed] [Google Scholar]
- 37.Govers R, Rabelink TJ. Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol. 2001;280(2):F193–F206. doi: 10.1152/ajprenal.2001.280.2.F193. [DOI] [PubMed] [Google Scholar]
- 38.Bonner JS, Lantier L, Hasenour CM, James FD, Bracy DP, Wasserman DH. Muscle-specific vascular endothelial growth factor deletion induces muscle capillary rarefaction creating muscle insulin resistance. Diabetes. 2013;62(2):572–580. doi: 10.2337/db12-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein I, Itin A, Einat P, Skaliter R, Grossman Z, Keshet E. Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Mol Cell Biol. 1998;18(6):3112–3119. doi: 10.1128/MCB.18.6.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karali E, Bellou S, Stellas D, Klinakis A, Murphy C, Fotsis T. VEGF Signals through ATF6 and PERK to promote endothelial cell survival and angiogenesis in the absence of ER stress. Mol Cell. 2014;54(4):559–572. doi: 10.1016/j.molcel.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 41.Festa A, Hanley AJ, Tracy RP, D’Agostino R, Jr, Haffner SM. Inflammation in the prediabetic state is related to increased insulin resistance rather than decreased insulin secretion. Circulation. 2003;108(15):1822–1830. doi: 10.1161/01.CIR.0000091339.70120.53. [DOI] [PubMed] [Google Scholar]
- 42.Vaccarino V, Khan D, Votaw J, Faber T, Veledar E, Jones DP, et al. Inflammation is related to coronary flow reserve detected by positron emission tomography in asymptomatic male twins. J Am Coll Cardiol. 2011;57(11):1271–1279. doi: 10.1016/j.jacc.2010.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Filippo G, Rendina D, Moccia F, Rocco V, Campanozzi A. Interleukin-6, soluble interleukin-6 receptor/interleukin-6 complex and insulin resistance in obese children and adolescents. J Endocrinol Invest. 2015;38(3):339–343. doi: 10.1007/s40618-014-0176-4. [DOI] [PubMed] [Google Scholar]
- 44.Antoniades C, Demosthenous M, Tousoulis D, Antonopoulos AS, Vlachopoulos C, Toutouza M, et al. Role of asymmetrical dimethylarginine in inflammation-induced endothelial dysfunction in human atherosclerosis. Hypertension. 2011;58(1):93–98. doi: 10.1161/HYPERTENSIONAHA.110.168245. [DOI] [PubMed] [Google Scholar]
- 45.Orshal JM, Khalil RA. Interleukin-6 impairs endothelium-dependent NO-cGMP-mediated relaxation and enhances contraction in systemic vessels of pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2004;286(6):R1013–R1023. doi: 10.1152/ajpregu.00729.2003. [DOI] [PubMed] [Google Scholar]
- 46.Ortega Moreno L, Copetti M, Fontana A, De Bonis C, Salvemini L, Trischitta V, et al. Evidence of a causal relationship between high serum adiponectin levels and increased cardiovascular mortality rate in patients with type 2 diabetes. Cardiovasc Diabetol. 2016;15(1):17. doi: 10.1186/s12933-016-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medina-Urrutia A, Posadas-Romero C, Posadas-Sanchez R, Jorge-Galarza E, Villarreal-Molina T, Gonzalez-Salazar Mdel C, et al. Role of adiponectin and free fatty acids on the association between abdominal visceral fat and insulin resistance. Cardiovasc Diabetol. 2015;14:20. doi: 10.1186/s12933-015-0184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding YS, Guo SX, Ma RL, Li SG, Guo H, Zhang JY, et al. Association of metabolic syndrome with the adiponectin to homeostasis model assessment of insulin resistance ratio. Mediators Inflamm. 2015;2015:607364. doi: 10.1155/2015/607364. [DOI] [PMC free article] [PubMed] [Google Scholar]


