Abstract
Background
To explore the relationship between the heart-type fatty acid binding protein (H-FABP) gene and intramuscular fat (IMF), a polymorphism of the second exon of the H-FABP gene was investigated in 60 Three-yellow chickens (TYCs) and 60 Hetian-black chickens (HTBCs).
Results
The IMF contents of the cardiac, chest and leg muscles in HTBC were increased compared with TYC. Both TYC and HTBC populations exhibited Hardy-Weinberg Equilibrium (HWE) according to the χ2 test. Three variations of the two birds were detected, namely, G939A, G982A and C1014T. HTBCs with the TT genotypes exhibit increased IMF content in the chest muscles compared with the TC genotype. Thus, the G982A site could be considered a genetic marker for selecting increased IMF content in the chest muscles of HTBC. The correlation coefficients revealed that H-FABP mRNA expression was negatively correlated with the IMF content in the cardiac, chest and leg muscles of HTBC and in the cardiac and chest muscles of TYC. The relative mRNA expression of H-FABP was reduced in the cardiac and leg muscles of HTBC compared with TYC, but this difference was not observed at the protein level, as assessed by Western blot analysis.
Conclusions
These findings offer essential data that can be useful in the breeding program of HTBC and future research exploring the role of H-FABP in IMF deposition and regulation in chickens.
Keywords: Hetian-black Chicken, H-FABP, mRNA Expression, Polymorphism, Three-yellow Chicken
Background
Meat quality is one of the most important factors influencing the acceptability of meat [1]. However, the quality and flavor of chicken have decreased in the past decades as a result of genetic selection for faster growth velocity and increased feed conversion efficiency [2]. This phenomenon is particularly evident in China and many Southeast Asian countries and regions [3]. However, people prefer to consume the traditional slow-growing, meat-type, colored-feather chickens in many regions of the world [4]. These traditional chickens, which mainly include local varieties, are also popular in China, and the market share of these as meat birds is as high as 50 % [5].
The Hetian-black chicken (HTBC) is a type of slow-growing chicken with excellent meat taste and black feathers. Due to its rare genetic resources, it was included in the Directory of National Animal Genetic Resources in 2010. The HTBC has a history spanning more than 1750 years and is only distributed in the townships of Minfeng County of the Xinjiang Uygur Autonomous Region in China. Through attempts to improve the slow growth and low feed conversion rate, the HTBC was greatly hybridized with other chickens, and the pure breed is now in danger of extinction. In 2007, only 5,700 birds remained according to the Animal Genetic Resources of China. Three-yellow chicken (TYC), a fast-growing chicken, is widely farmed in China as a major meat-type broiler.
Intramuscular fat (IMF), which is the fat or lipid content extracted from muscle [5], has become one of the most important indicators of the quality of meat [2, 3, 6, 7]. Previous studies demonstrated that IMF influences the quality traits of meat [1, 2]. In addition, IMF is associated with the juiciness of beef and the flavor of pork [8, 9]. Moreover, moderate heritability and genetic selection for IMF have been utilized to improve meat quality in selection programs for swine [10, 11]. However, few reports have assessed the relationship between IMF deposition and the genes related to fat deposition in HTBC.
IMF content in muscle is related to the expression of lipogenic generation [1]. For example, the fatty acid binding protein (FABP) gene belongs to a supergene family of hydrophobic ligand-binding proteins [12, 13] and is composed of low-molecular-mass proteins that bind fatty acids [14]. FABPs have been isolated from several tissues of invertebrates and vertebrates [15, 16]. Nine types of FABPs have been identified in the mammalian FABP family [17–20]. Interestingly, the same types of FABP can be noted in more than one organ, and most tissues express various types of FABPs [21]. The FABPs have similar molecular weight [13] and similar molecular structure [18]. These proteins participate in transporting water insoluble fatty acids from the plasmalemma to the location of β-oxidation in the mitochondria as well as transporting other hydrophobic ligands [16, 21, 22]. Moreover, FABPs protect enzymes from the detergent-like effects of free fatty acids, modulate enzyme activity and gene transcription, and have signal transduction functions [12, 13, 15]. However, the precise functions of FABPs have not yet been fully elucidated [16, 22].
Of these genes, H-FABP was studied as a candidate gene to determine the IMF content for the evaluation of meat quality [2, 12, 15, 16, 20, 23]. Polymorphisms in the first exon and the first intron of the H-FABP gene in chickens are reportedly correlated with the IMF content [24]. A previous study demonstrated that H-FABP was the central gene involved in the fatty acid and fat metabolism of chickens [25]. H-FABP is related to the absorption of fatty acids and the promotion of effective fat storage and utilization [24, 26, 27]. H-FABP is also important in the development and adipogenic differentiation of stromal-vascular cells [1].
In addition, the relationship between H-FABP polymorphisms and expression and IMF has not been demonstrated in TYCs and HTBCs. Therefore, the aim of the current study was to explore the association of IMF and H-FABP gene polymorphisms and expression levels in these two chicken breeds. These findings will offer essential molecular information that can be used to explore the role of H-FABP in IMF deposition and regulation in chickens.
Materials and methods
Animals
The protocol for the animals in the current study was approved by the Tarim University Institutional Animal Care and Use Committee (TARU -ACUC-2012-051). All of the breeding HTBC specimens were collected from the breed’s sole provenance, Minfeng County. All of the TYC and HTBC specimens used in our experiment were maintained under the same environmental conditions at the Tarim University experimental station for animals, including ad libitum access to food and water. The commercial diets used in the current study met all National Research Council (NRC) requirements [28]. All treatments for the animals were in accordance with the Institute for Laboratory Animal Research (ILAR) Guide for the Care and Use of Laboratory Animals. A total of 120 birds divided into two groups were hatched and reared from 1 d until their slaughtering ages (70 d for TYC and 120 d for HTBC). 60 TYC specimens and 60 HTBC specimens with a 1:1 sex ratio were selected randomly and then anesthetized and sacrificed by exsanguination.
IMF content
The IMF contents in the cardiac, chest and leg muscles were measured using the Soxhlet petroleum-ether extraction method according to Chinese National Standards GB/T 5009.6.2004, and the IMF content was determined as a weight percentage.
Polymerase chain reaction-single-strand conformation polymorphism (PCR-SSCP)
Blood samples taken from the wing vein were anticoagulated with acid citrate dextrose (ACD) and stored at −20 °C for DNA extraction. The PCR was performed using a typical 20 μL system containing 10 μL 2 × SG PCR MasterMix (Beijing SinoGene Scientific Co. Ltd., China), 1 μL DNA, 8 μL dd H2O, and 0.5 μL primers (10 μmol/L) (F: 5’-CGACAAGGCGACGGTGAA-3’; R: 5’-TGGGGCAGGAAGGAGTTT-3’) (accession number: AY648562). The amplification conditions were as follows: pre-denaturation at 94 °C for 3 min; 35 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s and extension at 72 °C for 30 s; and a final extension at 72 °C for 10 min. The PCR products were detected on 1 % agarose gel. A 50 μL expansion system was used to recover the products.
The polymorphism of the H-FABP gene second exon was detected via PCR-SSCP. The PCR products were combined with PCR–SSCP buffer containing 0.1 % bromophenol blue and 0.1 % xylene cyanol in formamide. Then, the mixtures were degenerated for 10 min at 98 °C and maintained on ice for 5 min. Each sample was transferred to a 12 % polyacrylamide gel with 10 × TBE buffer. The gels were run at 4 °C under the following conditions: 250 V for 10 min and 56 V for 16 h. The gels were stained according to a standard protocol [24]. Homozygotic type fragments were cut under an ultraviolet lamp and then purified with a DNA purification kit. The recovered DNA fragments were linked with pMD18-T Simple Vector and then transformed into a DH5α strain. The positive clones were selected and identified by PCR and then sequenced using TaKaRa (TaKaRa Biotechnology Inc., Dalian, China).
Quantitative real-time PCR (qPCR)
Total RNA was extracted from the cardiac, chest and leg muscle tissue samples using TRIzol reagent (Invitrogen, Carlsbad, CA), as previously described [29]. The integrity of the RNA extracted from each sample was confirmed by agarose gel electrophoresis with ethidium bromide staining and visualization under ultraviolet (UV) light. A NanoDrop® ND-2000C spectrophotometer (Thermo Fisher Scientific, Wilmington, DE) was used to determine the amount of RNA extracted and verify its purity (OD260/OD280 absorption ratio > 1.9). Next, 1 μg of total RNA was reverse transcribed into first-strand cDNA using the GoScript reverse transcription system (Promega, Madison, WI). To control for DNA contamination, a negative control (without enzyme) was included. The synthesized cDNA was stored at –20 °C prior to real-time PCR analysis.
An ABI 7500 Real-time PCR System (Applied Biosystems, Foster City, CA) was used for qPCR analyses. The sequences of the primers used are listed below: H-FABP, F: 5’-CAGAAGTGGGATGGGAAGGAGA-3’, R: 5’-TCATAGGTGCGGGTGGAGAC-3’ (accession number: NM204290); β-actin (housekeeping gene), F: 5’-AACACCCACACCCCTGTGAT-3’, R: 5’-TGAGTCAAGCGCCAAAAGAA-3’ (accession number: L08165). The cDNA was amplified with SYBR® Premix DimerEraserTM (TaKaRa Biotechnology Inc., Dalian, China) containing 2 μL of cDNA, 1.0 μmol/L primers, 10 μL of 2 × SYBR Premix DimerEraser and 0.4 μL of ROX (passive reference dye). A non-template control of nuclease-free water was included in each run. All reactions were conducted in triplicate. The reaction was performed as follows: 1 cycle of 95 °C for 30 s; 39 cycles of 95 °C for 5 s, 60 °C for 30 s, and 72 °C for 60 s; and 1 cycle of 95 °C for 15 s, 60 °C for 60 s, 95 °C for 30 s, and 60 °C for 15 s. To quantify the relative mRNA expression, the cycle threshold (CT) values of the target genes were normalized to the CT value of the housekeeping gene, and the results are presented as the fold change using the 2−ΔΔCT method. The relative expression of the target gene mRNA in each group was calculated using the following equations: ΔCT = CT target gene ‐ CT housekeeping gene and ΔΔCT = ΔCTtreated group ‐ ΔCTcontrol group.
Western blotting
Frozen tissue samples (0.1 g) were ground with protease inhibitors (1 μg/mL leupeptin, 1 μg/mL pepstatin A, and 2 μg/mL aprotinin) using a glass grinder on ice. The lysates were centrifuged to remove the insoluble material. The supernatant was collected, and the protein concentration was measured using a protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (10 mmol/L Tris-HCl, pH 6.8, 2 % SDS, 10 % glycerol, 0.2 mol/L dithiothreitol (DTT)) was added to the lysates. The mixture was heated at 100 °C for 5 min, followed by centrifugation at 15,000 × g for 15 min at 4 °C to remove the insoluble debris. The supernatant was used for Western blot analysis. A total of 50 μg of protein was loaded into each well in a 10 % SDS-PAGE gel. The resolved proteins were transferred onto nitrocellulose and blocked with 5 % non-fat milk. An anti-cardiac FABP primary antibody (Abcam, Cambridge, UK) was used at a dilution of 1:500 at 4 °C overnight. The blots were thoroughly washed and then exposed to goat anti-rabbit IgG HRP (M21002; Abmart) at a dilution of 1:1,000 for 1 h at room temperature. Finally, the signal was detected using an enhanced chemiluminescence (ECL) kit. β-actin (4970; Cell Signal) in each sample was amplified as a housekeeping control as presented in the lower panel.
Statistical analysis
The frequencies of alleles and genotypes were analyzed using the POPGENE software package (v.1.31), and the PowerMaker software package (v.3.25) was used to analyze the polymorphic information content (PIC).
The correlation between the H-FABP genotypes and IMF content was performed using the SAS statistical software package, version 9.0 (SAS Institute, Inc., Cary, NC, USA) using the SAS software PROC GLM procedures. The following statistical model was applied: Y = μ + G + S + f + h + e, where Y = the dependent variable, μ = the population mean, G = fixed effects of the breed, S = fixed effects of sex, f = family, h = random effects, and e = random error. The G × S interaction was not significant for any trait and therefore was not included in the model.
Statistical analyses of the mRNA differential expression were conducted with the SPSS statistical software package, version 17.0 (SPSS Inc., Chicago, IL, USA) using the independent samples t-test. The correlation between the mRNA expression (2−ΔCt) [10] and the IMF content was assessed by Pearson’s correlation coefficient [30]. The difference was considered significant at P < 0.05 unless otherwise specified.
Statistical analysis of the H-FABP protein expression in different chickens was performed with Quantity One (v. 4.62). SPSS (17.0) was utilized to analyze the significant difference between two birds through the independent-samples t-test.
Results
IMF content
Contents of IMF in two chicken populations are shown in Fig. 1. IMF contents in the cardiac, chest and leg muscles of HTBC were increased compared with those in TYC (P = 0.028, P = 0.047, P = 0.016, respectively).
Fig. 1.

IMF content in cardiac muscle, chest and leg muscles of TYC and HTBC. Cardiac, chest and leg muscles were collected from TYC at 70 d and HTBC at 120 d. The IMF contents of cardiac (a), chest (b) and leg (c) muscles were measured via the Soxhlet petroleum-ether extraction method according to Chinese National Standards GB/T 5009.6.2004. The data are presented as the mean ± standard error of the mean (SEM) for each tissue (n = 60 per group). * P < 0.05. IMF, intramuscular fat; TYC, Three-yellow chicken; HTBC, Hetian-black chicken
H-FABP gene polymorphism
The results of PCR-SSCP indicated that the H-FABP gene had three types of single-strand conformation polymorphism (SSCP) bands: TT, TC and CC (Fig. 2). Genotypic frequency and gene frequency analyses revealed that TT was the dominant genotype and that T was the dominant allele of both TYC and HTBC in natural selection (Table 1). The sequences of TT and CC genotypes were compared with the reference sequence (AY648562) registered in GenBank. Three identical mutation sites were identified in H-FABP exon 2 in the two birds: G939A, G982A and C1014T.
Fig. 2.
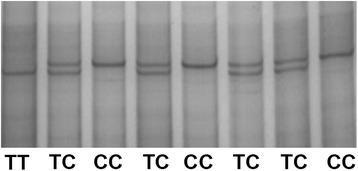
H-FABP genotypes of TYC and HTBC. Blood samples were collected from the wing vein of TYC at 70 d and HTBC at 120 d, anticoagulated via acid citrate dextrose (ACD), and DNA was extracted. Polymerase chain reaction-single strand conformation polymorphism (PCR-SSCP) was performed to analyze the polymorphism of the second exon of the H-FABP gene. The bands were named as TT, TC and CC
Table 1.
Genotypic and gene frequency of H-FABP in TYC and HTBC
| Breed | Genotypic Frequency, % | Gene Frequency, % | |||
|---|---|---|---|---|---|
| TT | TC | CC | T | C | |
| TYC | 0.467 (28/60) | 0.400 (24/60) | 0.133 (8/60) | 0.667 (80/120) | 0.333 (40/120) |
| HTBC | 0.417 (25/60) | 0.383 (23/60) | 0.200 (12/60) | 0.608 (73/120) | 0.391 (47/120) |
H-FABP heart-type fatty acid binding protein, TYC Three-yellow chicken, HTBC Hetian-black chicken
TT, TC and CC are genotype frequencies; T and C are alleles. These genotypes were analyzed using the POPGENE software package (v.1.31) in 60 chickens with a 1:1 sex ratio in each group
The genetic polymorphism parameters are presented in Table 2. Both values of expected heterozygosity (He) were higher than those of the observed heterozygosity (Ho), and both of the Polymorphic information contents (PICs) of the two breeds were in the range 0.25 < PIC < 0.5.
Table 2.
Hereditary character of H-FABP in TYC and HTBC
| Breed | Ho | He | Ne | PIC | χ2 | P |
|---|---|---|---|---|---|---|
| TYC | 0.400 | 0.448 | 1.800 | 0.346 | 0.708 | 0.40 |
| HTBC | 0.383 | 0.481 | 1.910 | 0.364 | 2.500 | 0.11 |
H-FABP heart-type fatty acid binding protein, TYC Three-yellow chicken, HTBC Hetian-black chicken
Ho observed heterozygosity, He expected heterozygosity, Ne effective number of alleles, PIC polymorphic information content
Ho, He, Ne and χ2 were analyzed using the POPGENE software package (v. 1.31), and the PIC was analyzed using the PowerMaker software package (v. 3.25)
The χ2 values were 0.708 (P = 0.400) in TYC and 2.500 (P = 0.114) in HTBC. The populations of both chicken breeds exhibited Hardy-Weinberg equilibrium (HWE).
Association between the H-FABP gene polymorphism and IMF content
The results of the association analysis between genotypic frequency and IMF content are displayed in Table 3. HTBC specimens with the TT genotype exhibited increased IMF content in the chest muscles compared with the TC genotype (P = 0.035) based on the least-square mean.
Table 3.
Relationship between H-FABP polymorphism and IMF content in TYC and HTBC
| Tissue | Breed | TT | TC | CC |
|---|---|---|---|---|
| Chest muscle | TYC | 5.029 ± 0.307 | 3.994 ± 0.332 | 4.314 ± 0.575 |
| HTBC | 7.535 ± 0.568a | 5.457 ± 0.581b | 6.133 ± 0.804ab | |
| Leg muscle | TYC | 7.301 ± 0.673 | 5.628 ± 0.727 | 6.506 ± 1.259 |
| HTBC | 10.643 ± 1.031 | 8.742 ± 1.053 | 9.320 ± 1.458 |
H-FABP heart-type fatty acid binding protein, TYC Three-yellow chicken, HTBC Hetian-black chicken
The correlation between H-FABP gene polymorphism and IMF content was performed using the SAS 9.0 software package’s PROC GLM procedures
a,bMeans within a row with no common superscript are different (P < 0.05)
Correlation between H-FABP gene mRNA expression and IMF content
The relative H-FABP gene expression in different tissues in these two chicken breeds is presented in Fig. 3. In the cardiac and leg muscles, the expression of H-FABP mRNA in HTBC was reduced compared with that in TYC (P < 0.001 and P = 0.02, respectively). No difference was noted in chest muscle H-FABP mRNA expression in the two chicken breeds.
Fig. 3.
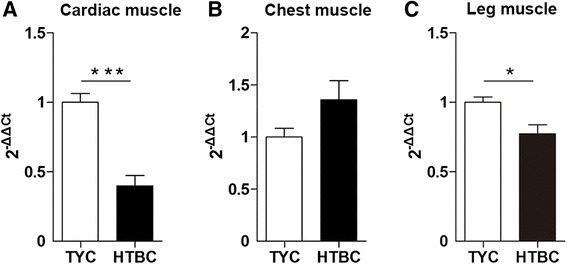
Relative expression of H-FABP mRNA in cardiac, chest and leg muscles of TYC and HTBC. Cardiac, chest and leg muscles were collected from TYC at 70 d and HTBC at 120 d. The relative expression of mRNA for the gene encoding H-FABP in cardiac (a), chest (b) and leg (c) muscles was analyzed using quantitative real-time PCR. The data are presented as the mean ± SEM for each tissue (n = 60 per group). * P < 0.05; *** P < 0.001. TYC, Three-yellow chicken; HTBC, Hetian-black chicken
The association coefficients of the H-FABP gene mRNA with the IMF contents in the cardiac, chest and leg muscles were -0.588 (P = 0.045), -0.649 (P = 0.012) and -0.441 (P > 0.05) in TYC and -0.667 (P = 0.018), -0.646 (P = 0.023) and -0.608 (P = 0.030) in HBTC, respectively. Negative correlations were observed in these tissues with the exception of the leg muscle in TYC (Table 4).
Table 4.
Correlation between H-FABP mRNA expression and IMF content in TYC and HTBC
| Tissue | TYC | HTBC |
|---|---|---|
| Cardiac muscle | −0.588* | −0.667* |
| Chest muscle | −0.649* | −0.646* |
| Leg muscle | −0.441 | −0.608* |
H-FABP heart-type fatty acid binding protein, TYC Three-yellow chicken, HTBC Hetian-black chicken
The correlation analysis between mRNA expression (2−ΔCt) and IMF content was assessed by Pearson’s correlation coefficient at slaughter time using the SPSS 17.0 software package, * P < 0.05
Interestingly, the trend of H-FABP protein expression in the different muscles mirrored the changes in H-FABP mRNA expression, but no statistical significance was observed at the protein level (Fig. 4).
Fig. 4.
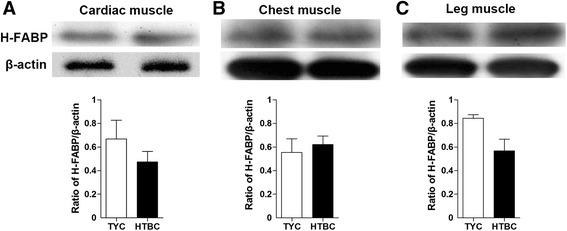
H-FABP protein expression in cardiac, chest and leg muscle in TYC and HTBC. Cardiac, chest and leg muscles were collected from TYC at 70 d and HTBC at 120 d. H-FABP protein expression in cardiac (a), chest (b) and leg (c) muscles was detected by western blot. Representative panels of H-FABP protein are shown. Expression of β-actin was measured as an internal control. The intensities of H-FABP and β-actin bands were determined using the Quantity One software package. The results are presented as the ratio of the H-FABP band intensity to the β-actin band intensity. The data are presented as the mean ± SEM for each tissue (n = 4 per group). H-FABP, heart-type fatty acid binding protein; TYC, Three-yellow chicken; HTBC, Hetian-black chicken
Discussion
Considerable efforts have been made to improve the speed of growth, daily weight gain and feed efficiency in chicken breeding over a long period of time. However, increased productivity led to dramatically decreased meat quality [2, 24]. As people’s quality of life has improved, higher requirements have been placed on the meat quality of chickens. Improving meat quality has become an important aim of breeding to meet people’s increased living standard. With their excellent meat and unique flavor, HTBCs have received considerable attention, but the native Chinese breed is going to become extinct. Therefore, the mechanism underlying the wonderful HTBC meat quality should be investigated immediately.
The growth and development process of fatty tissue is very complex because the procedure is associated with a variety of genes and pathways [31]. The IMF content, which refers to the deposition of fat within the muscles, affects the toughness of pork by changing the structure of the connective tissue [8] and affects the flavor and juiciness of chicken meat [32]. In the three tissues mentioned in the present experiment, the IMF contents in HTBC were increased compared with those in TYC, which could be the reason that slower-growing chickens have better flavor and meat quality than faster-growing chickens. The fact that HTBC meat is more popular than TYC meat in markets is consistent with this result. These results are consistent with the findings of Tu et al., who reported a similar phenomenon in Rugao and Luyuan chickens [10].
Fatty acid binding proteins expressed in mammalian tissues or cells serve as intracellular transporters to satisfy special cellular needs [13, 18]. Members of the FABP families are thought to be closely related to IMF deposition. Of these, H-FABP is detected in many species, ranging from arthropods to mammals. In addition, H-FABP plays a critical role in determining the IMF content [16]. In chickens, the H-FABP gene located on chicken chromosome 23 is composed of 3 introns and 4 exons that code for 132 amino acids. This gene is expressed in various types of tissues, such as liver, muscle and heart [14]. H-FABP is essential for the binding of long-chain fatty acids and the transportation of fatty acids from the cell membrane to the sites of fatty acid oxidation and triglyceride and phospholipid synthesis [33–35].
The results of the χ2 test indicated that the two populations in this study were in HWE, which could be a consequence of long-time natural and artificial selection [36]. Both He values were higher than the Ho values, indicating more homozygous samples than heterozygote samples. The PICs of both breeds were in the range of 0.25 < PIC < 0.5, thereby indicating that moderate polymorphisms were detected at this locus.
The autogenous variation of H-FABP has an important influence on IMF deposition and other biological traits of chickens [24]. The assay of PCR-SSCP had been used to analyze polymorphisms for cows [37], pigs [38] and chickens [39]. Ye et al. [40] assessed a SNP (C2054T) in the second intron of the Beijing-Oil chicken H-FABP gene that remarkably correlated with the IMF contents in the chest and leg muscles. Eight SNPs (G332A, G534A, C835T, -1131A, C1294A, C2329T, C2372T, and C2636T) in the H-FABP gene of Caoke chickens were detected and correlated with carcass traits. Their results indicated that the genotypes of one primer pair exhibited a significant difference in the half-eviscerated weight, body weight, chest weight, thigh weight and carcass weight; thus, H-FABP could have a strong impact on carcass traits or could be connected with genes that affect slaughter performance in chickens. Four SNPs (C260T, A675G, C783T, and A2778G) in the H-FABP gene in Fengkai Xinghua, Huiyang Huxu, Qingyuan Ma and Guangxi Xiayan chickens affect the IMF content [24]. In contrast with previous studies, three variations of the two breeds examined in this study were detected as follows: G939A, G982A and C1014T. One possible reason for the discrepancy in these results is that the chicken breeds used in our respective experiments have a different genetic background [41]. The IMF content in the chest muscle of HTBC with the TT genotype was increased compared with that of the TC genotype. Thus, the G982A mutable site could be considered as a gene marker for selecting HTBC with increased IMF content in the chest muscle.
H-FABP participates in the transport procedure of fatty acids to the mitochondria during β-oxidation and exists in organs involved in high acid oxidation activity, such as skeletal muscle and cardiac muscle [27]. Our experimental results confirmed that H-FABP is expressed in various types of tissues, such as cardiac, breast and thigh muscles [10, 41–44]. Our findings indicate that the H-FABP mRNA level in the cardiac muscle of TYCs was increased compared with that in HTBCs. This result is consistent with the results of Wang et al., who reported that the H-FABP mRNA expression level in the cardiac muscle of Bai’er layers was increased compared with that in a fat line broiler at the age of 42 d [24].
Our findings demonstrated negative correlations between the H-FABP mRNA expression and IMF content in the three tissues of the two chicken breeds with the exception of the leg muscle of TYC. These findings are consistent with those of Tu et al., who indicated that the H-FABPmRNA expression level has a significant negative effect on the IMF of the cardiac, breast and leg muscles in Rugao and Luyuan chickens [10]. The results are also consistent with the results of Li et al., who reported that high H-FABP mRNA expression was correlated with low leg IMF content at 70 d in Baijing You chickens [25]. Furthermore, relatively increased H-FABP mRNA expression improved fatty metabolic activity and decomposed fat to produce more energy to satisfy the needs for growth and diversified physiological demands.
The H-FABP mRNA and protein expression trends were consistent in the current study, but no significant difference was observed at the protein level. H-FABP gene transcription and translation can directly or indirectly affect the synthesis and regulation of proteins in fatty acid metabolism [45]. Tissue-specific expression of FABP genes is considered to be primarily regulated at the transcriptional level [45]. Glatz et al. reported that H-FABP expression was mainly regulated via the process of transcription [46], and this viewpoint was confirmed in pigs [7]. Tyra et al. also drew a similar conclusion that the higher expression amount of mRNA was not consistent with higher H-FABP protein levels in pigs [22]. These results indicated that the distribution of fat among different fat deposits might be controlled by different mechanisms and possibly by different genes [47]. This finding indicates a low correlation between H-FABP mRNA and protein levels which is in agreement with the relationship between H-FABP mRNA level and protein expression level in pigs. [48].
Increasing the IMF content is economically desirable in chicken breeding [40]. Regarding the Chinese indigenous breed HTBC, the most important task would be to protect those genetic resources that have high IMF contents to ensure that precious experimental materials could be used for further study of H-FABP or other genes related to IMF content.
Conclusion
In conclusion, our results suggest that IMF content in the same tissues of HTBC is increased compared with TYC. The G982A mutational site could serve as a genetic marker for increased IMF content in selecting for the chest muscle of HTBC. H-FABP gene transcription had a negative impact on IMF content in the two chicken breeds.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Project No. 31160521), Tarim University Principal Fund (Project No. TDZKGG201504) and the project of Key Laboratory of Tarim Animal Husbandry Science and Technology, Xinjiang Production & Construction Group (Project No. HS201302).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JFW provided the experimental instruments and revised the manuscript. YW designed the study and drafted the initial manuscript. XHH and HEW performed the analysis and interpreted the data. YW, TK, CH, YC and JMX performed the experiments. All authors read and approved the final manuscript.
Contributor Information
Yong Wang, Email: wangyongdky@126.com.
Xiaohong Hui, Email: hxhdky@126.com.
Huie Wang, Email: whedky@126.com.
Tursunjan Kurban, Email: texdky@126.com.
Chao Hang, Email: hangchaodky@126.com.
Ying Chen, Email: chenyingdky@126.com.
Jinming Xing, Email: xjmdky@126.com.
Jiufeng Wang, Email: Jiufeng_wang@hotmail.com.
References
- 1.Guo XL, Tang RY, Wang W, Liu DY, Wang KN. Effects of dietary protein/carbohydrate ratio on fat deposition and gene expression of peroxisome proliferator activated receptor γ and heart fatty acid-binding protein of finishing pigs. Livest Sci. 2011;140(1):111–116. doi: 10.1016/j.livsci.2011.02.016. [DOI] [Google Scholar]
- 2.Li WJ, Li HB, Chen JL, Zhao GP, Zheng MQ, Wen J. Gene expression of heart-and adipocyte-fatty acid-binding protein and correlation with intramuscular fat in Chinese chickens. Anim Biotechnol. 2008;19(3):190–194. doi: 10.1080/10495390802058319. [DOI] [PubMed] [Google Scholar]
- 3.Chen JL, Zhao GP, Zheng MQ, Wen J, Yang N. Estimation of genetic parameters for contents of intramuscular fat and inosine-5’-monophosphate and carcass traits in Chinese Beijing-You chickens. Poult Sci. 2008;87(6):1098–1104. doi: 10.3382/ps.2007-00504. [DOI] [PubMed] [Google Scholar]
- 4.Rizzi C, Marangon A, Chiericato G. Effect of genotype on slaughtering performance and meat physical and sensory characteristics of organic laying hens. Poult Sci. 2007;86(1):128–135. doi: 10.1093/ps/86.1.128. [DOI] [PubMed] [Google Scholar]
- 5.Zhao GP, Chen JL, Zheng MQ, Wen J, Zhang Y. Correlated responses to selection for increased intramuscular fat in a Chinese quality chicken line. Poult Sci. 2007;86(11):2309–2314. doi: 10.1093/ps/86.11.2309. [DOI] [PubMed] [Google Scholar]
- 6.Wood J, Nute G, Richardson R, Whittington F, Southwood O, Plastow G, et al. Effects of breed, diet and muscle on fat deposition and eating quality in pigs. Meat Sci. 2004;67(4):651–667. doi: 10.1016/j.meatsci.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Zhao SM, Ren LJ, Guo L, Cheng ML, Zhang X, Ge CR, et al. Muscle lipid metabolism gene expression in pigs with different H-FABP genotypes. Livest Sci. 2010;128(1–3):101–107. doi: 10.1016/j.livsci.2009.11.005. [DOI] [Google Scholar]
- 8.Schwab C, Baas T, Stalder K, Mabry J. Effect of long-term selection for increased leanness on meat and eating quality traits in Duroc swine. J Anim Sci. 2006;84(6):1577–1583. doi: 10.2527/2006.8461577x. [DOI] [PubMed] [Google Scholar]
- 9.Thompson J. The effects of marbling on flavour and juiciness scores of cooked beef, after adjusting to a constant tenderness. Anim Prod Sci. 2004;44(7):645–652. doi: 10.1071/EA02171. [DOI] [Google Scholar]
- 10.Tu YJ, Su YJ, Wang KH, Zhang XY, Gao YS. Gene expression of heart and adipocyte fatty acid-binding protein in chickens by FQ-RT-PCR. Asian-Australas J Anim Sci. 2010;23(8):987–992. doi: 10.5713/ajas.2010.90556. [DOI] [Google Scholar]
- 11.Suzuki K, Irie M, Kadowaki H, Shibata T, Kumagai M, Nishida A. Genetic parameter estimates of meat quality traits in Duroc pigs selected for average daily gain, longissimus muscle area, backfat thickness, and intramuscular fat content. J Anim Sci. 2005;83(9):2058–2065. doi: 10.2527/2005.8392058x. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Li H, Li N, Leng L, Wang Y, Tang Z. Identification of single nucleotide polymorphism of adipocyte fatty acid-binding protein gene and its association with fatness traits in the chicken. Poult Sci. 2006;85(3):429–434. doi: 10.1093/ps/85.3.429. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerman AW, Veerkamp JH. New insights into the structure and function of fatty acid-binding proteins. Cell Mol Life Sci. 2002;59(7):1096–1116. doi: 10.1007/s00018-002-8490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ockner RK, Manning JA, Poppenhausen RB, Ho WKL. A binding protein for fatty acids in cytosol of intestinal mucosa, liver, myocardium, and other tissues. Science. 1972;177(4043):56–58. doi: 10.1126/science.177.4043.56. [DOI] [PubMed] [Google Scholar]
- 15.Wang QG, Guan TZ, Li H, Bernlohr DA. A novel polymorphism in the chicken adipocyte fatty acid-binding protein gene (FABP4) that alters ligand-binding and correlates with fatness. Comp Biochem Physiol B: Biochem Mol Biol. 2009;154(3):298–302. doi: 10.1016/j.cbpb.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai JL, Xu HW, Zang RX, He HJ, Cai Y, Cao X, et al. Cloning of the heart fatty acid-binding protein (H-FABP) gene and its tissue-specific expression profile in the Lanzhou fat-tailed sheep, Ovis aries. Small Rumin Res. 2013;112(1–3):114–122. doi: 10.1016/j.smallrumres.2012.12.016. [DOI] [Google Scholar]
- 17.Beniyama Y, Matsuno K, Miyachi H. Structure-guided design, synthesis and in vitro evaluation of a series of pyrazole-based fatty acid binding protein (FABP) 3 ligands. Bioorg Med Chem Lett. 2013;23(6):1662–1666. doi: 10.1016/j.bmcl.2013.01.054. [DOI] [PubMed] [Google Scholar]
- 18.Storch J, Corsico B. The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu Rev Nutr. 2008;28:73–95. doi: 10.1146/annurev.nutr.27.061406.093710. [DOI] [PubMed] [Google Scholar]
- 19.Vogel Hertzel A, Bernlohr DA. The mammalian fatty acid-binding protein multigene family: molecular and genetic insights into function. Trends Endocrinol Metab. 2000;11(5):175–180. doi: 10.1016/S1043-2760(00)00257-5. [DOI] [PubMed] [Google Scholar]
- 20.Chmurzynska A. The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet. 2006;47(1):39–48. doi: 10.1007/BF03194597. [DOI] [PubMed] [Google Scholar]
- 21.Niewold TA, Meinen M, van der Meulen J. Plasma intestinal fatty acid binding protein (I-FABP) concentrations increase following intestinal ischemia in pigs. Res Vet Sci. 2004;77(1):89–91. doi: 10.1016/j.rvsc.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Tyra M, Ropka-Molik K, Eckert R, Piórkowska K, Oczkowicz M. H-FABP and LEPR gene expression profile in skeletal muscles and liver during ontogenesis in various breeds of pigs. Domest Anim Endocrinol. 2011;40(3):147–154. doi: 10.1016/j.domaniend.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Shi H, Wang QG, Wang YX, Leng L, Zhang QQ, Shang ZC, et al. Adipocyte fatty acid-binding protein: An important gene related to lipid metabolism in chicken adipocytes. Comp Biochem Physiol B: Biochem Mol Biol. 2010;157(4):357–363. doi: 10.1016/j.cbpb.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Shu DM, Li L, Qu H, Yang C, Zhu Q. Identification of single nucleotide polymorphism of H-FABP gene and its association with fatness traits in chickens. Asian-Australas J Anim Sci. 2007;20(12):1812. doi: 10.5713/ajas.2007.1812. [DOI] [Google Scholar]
- 25.Li H, Wu G, Zhang J, Yang N. Identification of the heart-type fatty acid-binding protein as a major gene for chicken fatty acid metabolism by Bayesian network analysis. Poult Sci. 2010;89(9):1825–1833. doi: 10.3382/ps.2010-00699. [DOI] [PubMed] [Google Scholar]
- 26.Brandstetter AM, Sauerwein H, Veerkamp JH, Geay Y, Hocquette JF. Effects of muscle type, castration, age and growth rate on H-FABP expression in bovine skeletal muscle. Livest Prod Sci. 2002;75(2):199–208. doi: 10.1016/S0301-6226(01)00318-9. [DOI] [Google Scholar]
- 27.Zhang YJ, Liu YQ, Song J, Cheng SY, Dong LX. Effects of dietary energy level on the transcription of the H-FABP gene in different tissues of sheep. Small Rumin Res. 2013;115(1–3):29–33. doi: 10.1016/j.smallrumres.2013.09.007. [DOI] [Google Scholar]
- 28.National Research Council. Subcommittee on Poultry N . Nutrient requirements of poultry. Washington, D.C: National Academy Press; 1994. [Google Scholar]
- 29.Li XQ, Zhu YH, Zhang HF, Yue Y, Cai ZX, Lu QP, et al. Risks associated with high-dose Lactobacillus rhamnosus in an Escherichia coli model of piglet diarrhoea: intestinal microbiota and immune imbalances. PLoS One. 2012;7(7):e40666. doi: 10.1371/journal.pone.0040666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Q, Yao HD, Zhang ZW, Zhang B, Meng FY, Xu S-W, et al. Possible Correlation between Selenoprotein W and Myogenic Regulatory Factors in Chicken Embryonic Myoblasts. Biol Trace Elem Res. 2012;150(1-3):166–172. doi: 10.1007/s12011-012-9520-8. [DOI] [PubMed] [Google Scholar]
- 31.Shi H, Wang Q, Zhang Q, Leng L, Li H. Tissue expression characterization of chicken adipocyte fatty acid-binding protein and its expression difference between fat and lean birds in abdominal fat tissue. Poult Sci. 2010;89(2):197–202. doi: 10.3382/ps.2009-00397. [DOI] [PubMed] [Google Scholar]
- 32.Chizzolini R, Zanardi E, Dorigoni V, Ghidini S. Calorific value and cholesterol content of normal and low-fat meat and meat products. Trends Food Sci Technol. 1999;10(4):119–128. doi: 10.1016/S0924-2244(99)00034-5. [DOI] [Google Scholar]
- 33.Tyra M, Żak G. Analysis of relationships between fattening and slaughter performance of pigs and the level of intramuscular fat (IMF) in longissimus dorsi muscle. Ann Anim Sci. 2012;12(2):169–178. doi: 10.2478/v10220-012-0014-6. [DOI] [Google Scholar]
- 34.Binas B, Han XX, Erol E, Luiken JJFP, Glatz JFC, Dyck DJ, et al. A null mutation in H-FABP only partially inhibits skeletal muscle fatty acid metabolism. Am J Physiol Endocrinol Metab. 2003;285(3):E481–E489. doi: 10.1152/ajpendo.00060.2003. [DOI] [PubMed] [Google Scholar]
- 35.Wang S, Cai X, Xue K, Chen H. Polymorphisms of MRF4 and H-FABP genes association with growth traits in Qinchuan cattle and related hybrids. Mol Biol Rep. 2011;38(2):1013–1020. doi: 10.1007/s11033-010-0197-9. [DOI] [PubMed] [Google Scholar]
- 36.Gao Y, Zhang YH, Zhang S, Li F, Wang S, Dai L, et al. Association of A-FABP gene polymorphism in intron 1 with meat quality traits in Junmu No. 1 white swine. Gene. 2011;487(2):170–173. doi: 10.1016/j.gene.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Chen R, Yan Z, Ji D, Mao Y, Chen Y, Li Y, et al. Polymorphisms of the IL8 gene correlate with milking traits, SCS and mRNA level in Chinese Holstein. Mol Biol Rep. 2011;38:4083–4088. doi: 10.1007/s11033-010-0528-x. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Dai L, Ma T, Wang S, Guo J, Li F, et al. Association of T1740C polymorphism of L-FABP with meat quality traits in Junmu No. 1 white swine. Gene Mol Res. 2013;12:235. doi: 10.4238/2013.January.30.9. [DOI] [PubMed] [Google Scholar]
- 39.Zhao ZY, Zhang L, Du BW, Cao NX, Jiang XS, Tian WX, et al. Polymorphisms in chicken extracellular fatty acid binding protein gene. Mol Biol Rep. 2012;39(3):2677–82. doi: 10.1007/s11033-011-1021-x. [DOI] [PubMed] [Google Scholar]
- 40.Ye MH, Cao HH, Wen J. RFLPS at Heart and Adipocyte Fatty Acid Binding Protein Genes in Beijing Oil Chick and Ai-jiao Chick. Acta Veterinaria et Zootechnica Sinica. 2003;34(5):422–426. [Google Scholar]
- 41.Chao Z, Wang F, Deng CY, Wei LM, Sun RP, Liu HL, et al. Distribution and linkage disequilibrium analysis of polymorphisms of MC4R, LEP, H-FABP genes in the different populations of pigs, associated with economic traits in DIV2 line. Mol Biol Rep. 2012;39(5):6329–35. doi: 10.1007/s11033-012-1454-x. [DOI] [PubMed] [Google Scholar]
- 42.Gerbens F, Van Erp A, Harders F, Verburg F, Meuwissen T, Veerkamp J, et al. Effect of genetic variants of the heart fatty acid-binding protein gene on intramuscular fat and performance traits in pigs. J Anim Sci. 1999;77(4):846–852. doi: 10.2527/1999.774846x. [DOI] [PubMed] [Google Scholar]
- 43.Ye MH, Chen JL, Zhao GP, Zheng MQ, Wen J. Associations of A-FABP and H-FABP markers with the content of intramuscular fat in Beijing-You chicken. Anim Biotechnol. 2009;21(1):14–24. doi: 10.1080/10495390903328116. [DOI] [PubMed] [Google Scholar]
- 44.Li W, Zhao G, Chen J, Zheng M, Wen J. Influence of dietary vitamin E supplementation on meat quality traits and gene expression related to lipid metabolism in the Beijing-you chicken. Br Poult Sci. 2009;50(2):188–198. doi: 10.1080/00071660902755409. [DOI] [PubMed] [Google Scholar]
- 45.Glatz JF, van der Vusse GJ. Cellular fatty acid-binding proteins: their function and physiological significance. Prog Lipid Res. 1996;35(3):243–282. doi: 10.1016/S0163-7827(96)00006-9. [DOI] [PubMed] [Google Scholar]
- 46.Glatz J, Schaap F, Binas B, Bonen A, Van Der Vusse G, Luiken J. Cytoplasmic fatty acid binding protein facilitates fatty acid utilization by skeletal muscle. Acta Physiol Scand. 2003;178(4):367–371. doi: 10.1046/j.1365-201X.2003.01166.x. [DOI] [PubMed] [Google Scholar]
- 47.Doran O, Moule SK, Teye GA, Whittington FM, Hallett KG, Wood JD. A reduced protein diet induces stearoyl-CoA desaturase protein expression in pig muscle but not in subcutaneous adipose tissue: relationship with intramuscular lipid formation. Br J Nutr. 2006;95(03):609–617. doi: 10.1079/BJN20051526. [DOI] [PubMed] [Google Scholar]
- 48.Gerbens F, Verburg FJ, Van Moerkerk HT, Engel B, Buist W, Veerkamp JH, et al. Associations of heart and adipocyte fatty acid-binding protein gene expression with intramuscular fat content in pigs. J Anim Sci. 2001;79(2):347–354. doi: 10.2527/2001.792347x. [DOI] [PubMed] [Google Scholar]


