Abstract
Purpose:
The choroid and retina receive most of the blood that enter to the eye, and this uptake may be affected by essential thrombocythemia (ET) in which thrombosis and hemorrhage is common. This study compares choroidal thickness, retinal vascular caliber, and ocular pulse amplitude (OPA) measurements between patients with ET and healthy adults.
Materials and Methods:
Thirty-seven patients with ET and 37 age-sex-matched healthy adults were recruited in this cross-sectional and comparative study. Spectral-domain optical coherence tomography was used to measure the subfoveal choroidal thickness (SFCT) and retinal vascular caliber measurements. The Pascal dynamic contour tonometer was used for OPA and intraocular pressure (IOP) measurements. The independent samples t-test was used for comparison of measurements between the groups. Pearson's correlation coefficient analysis was used to detect correlations between the variables. A P < 0.05 was statistically significant.
Results:
SFCT, OPA, and IOP measurements were not statistically significant differences between the study group and the control group (P > 0.05, all comparisons). Blood platelet counts were not associated with choroidal thickness, OPA, and IOP (P > 0.05). Retinal arteriolar and venular calibers were statistically, significantly thicker in healthy controls when compared to the study group (P < 0.05).
Conclusions:
Our results indicate that choroidal thickness and pulsatile blood flow are not significantly affected in ET and under high blood platelet counts. Retinal arteriolar and venular calibers are thinner in ET when compared to age-sex matched healthy controls.
Keywords: Blood Platelet Count, Choroid, Intraocular Pressure, Retinal Vasculature, Thrombocytosis
INTRODUCTION
Essential thrombocythemia (ET) is a rare acquired myeloproliferative disorder characterized by overproduction of platelets with a tendency for thrombosis and hemorrhage.1 It is usually diagnosed in the sixth decade of life, and more commonly affects females.2 ET is often asymptomatic, but vascular occlusive events include thromboses of large arteries and veins, and microvessels that generate neurological, cardiac, or peripheral arterial disabilities.2,3 The incidence of hemorrhagic complications is relatively rare.1
As a systemic and hematological disease, ET has the potential to influence many tissues and organs, including the eye. Ocular thromboembolic complications could deteriorate vision, especially when retinal vessels are involved. Central and branch retinal vein occlusions, central retinal artery occlusion, iritis, and partial third cranial nerve palsy are the reported ocular complications of ET.4,5,6,7,8 Also neurological events related to vision such as amaurosis fugax could occur due to ET.3
The majority of the blood coming to the eye is within the chorioretinal circulation.9 The choroid supplies blood to the outer layers of the retina and has the highest blood flow of any tissue. Hence, the choroid should be vulnerable to hematological disorders such as ET. Additionally, retinal vessels are prone to injury due to systemic hematological disorders. We hypothesized that ET may affect the choroid and retinal vessels due to ischemia, microembolism, thrombosis, or hemorrhage. Our aim was to demonstrate choroidal anatomy by measuring the subfoveal choroidal thickness (SFCT), choroidal pulsatile blood flow by measuring ocular pulse amplitude (OPA), and retinal vasculature by measuring retinal vessel caliber. To the best of our knowledge, this is the first study in the literature related to SFCT, OPA, and retinal vessel caliber measurements in patients with ET.
MATERIALS AND METHODS
In this prospective, cross-sectional, comparative study, 74 participants (37 patients in ET group and 37 age-sex-matched healthy adults in the control group) were recruited. This study was conducted in accordance with the ethical standards of the Declaration of Helsinki and was approved by the Institutional Ethical Committee.
Study population
The study group consisted of ET patients who were treated at the division of hematology. ET was diagnosed according to the criteria proposed by WHO in 2008.10 All of the patients in the study group were treated with acetylsalicylic acid. Hydroxyurea or anagrelide was also prescribed to the patients who had additional risk factors such as old age, a history of thrombosis, and platelet count over 1000 × 109/L. Exclusion criteria were any ocular surgery other than uneventful phacoemulsification and any ocular disease other than age-related cataract or mild refractive errors (<2.0 D spherical equivalent). Patients with systemic diseases such as diabetes mellitus and systemic hypertension that might affect ocular structures were also excluded.
Examination techniques
One eye of each subject was randomly selected. All subjects underwent an ophthalmic examination including measurement of visual acuity, biomicroscopy, air-puff tonometry, indirect retinoscopy, enhanced depth optical coherence tomography (OCT), and OPA measurements. The SFCT measurements were performed with a spectral-domain OCT (Spectralis; Heidelberg Engineering, Inc., Heidelberg, Germany). The SFCT was manually measured from the outer hyperreflective line corresponding to the retina pigment epithelium to the inner surface of the sclera. The retinal arteriolar caliber (RAC) and retinal venular caliber (RVC) measurements were also performed with the OCT. The caliber of temporal retinal arterioles and venules was measured at a distance of one disc diameter from the margin of the optic disc by using caliber tools included in the (Spectralis software version 5.8; Heidelberg Engineering Inc., Heidelberg, Germany) [Figure 1]. The mean thickness values of superior and inferior temporal retinal arterioles and venules were calculated for each eye.
Figure 1.
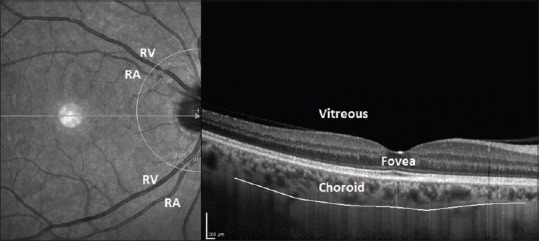
Macular enhanced depth optical coherence tomography screen of one of the patients in which the subfoveal choroidal thickness, retinal arteriole, and retinal venule caliber measurements were performed
The Pascal dynamic contour tonometer (Pascal DCT, Swiss Microtechnology AG, Port, Switzerland) was used for OPA and intraocular pressure (IOP) measurements. This noninvasive and contact device is attached to a slit-lamp biomicroscope, and it has an IOP sensor on its tip. The OPA is the difference between systolic and diastolic values of the pulsatile IOP. Blood platelet counts were also recorded as platelets per μL for analysis.
Data analysis
For statistical analysis, SPSS 17.0 Software for Windows (IBM Corp., New York, NY, USA) was used to analyze outcomes. A P < 0.05 was considered statistically significant. The independent samples t-test was used for comparison of SFCT, OPA, IOP, RAC, and RVC measurements between the study group and control group. Pearson's correlation coefficient analysis was used to detect correlations between OPA and SFCT, platelet count and OPA, platelet count and SFCT, platelet count and IOP.
RESULTS
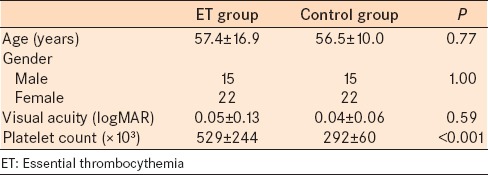
Thirty-seven eyes of 37 ET patients (study group) and 37 eyes of 37 healthy volunteers (control group) were examined and compared. Table 1 presents the demographic and clinical characteristics of the subjects. The participants were age and gender matched between the groups. The mean refractive error was 0.14 ± 0.46 D in the control group, and 0.25 ± 0.87 D in the study group (P = 0.53).
Table 1.
Demographic and clinical characteristics of subjects with ET and healthy control subjects
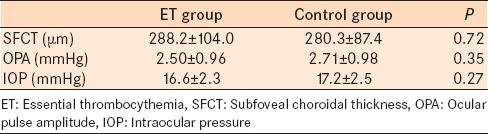
The mean SFCT, OPA, and IOP values of ET patients and controls are presented in Table 2. There were no statistically significant differences in the parameters between the groups. There was no correlation between OPA and SFCT in both groups. The correlations between OPA and SFCT in the study group and control group were r = −0.06 (P = 0.73) and r = 0.19 (P = 0.26), respectively.
Table 2.
The SFCT, OPA, and IOP comparisons between subjects with ET and healthy control subjects
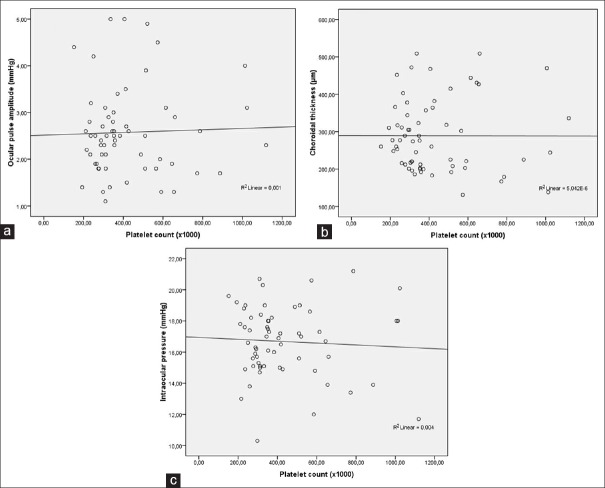
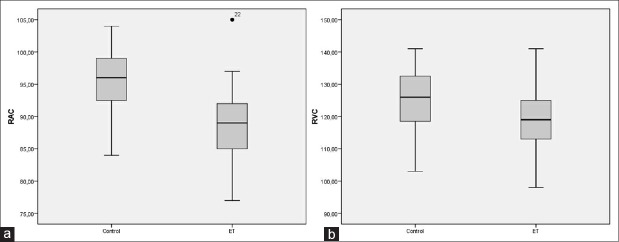
There was no correlation between OPA and blood platelet counts (r = 0.03, P = 0.80) [Figure 2a]. There was no correlation between SFCT and platelet counts (r = −0.002, P = 0.98) [Figure 2b]. There was no correlation between IOP and platelet counts (r = −0.06, P = 0.65) [Figure 2c]. Figure 3 demonstrates the box plot graphics of RAC (A) and RVC (B) in the study and control groups.
Figure 2.
Scatter plot graphics showing the correlation between ocular pulse amplitude and blood platelet counts (a), subfoveal choroidal thickness and blood platelet counts (b), and intraocular pressure and blood platelet counts (c)
Figure 3.
Box plot graphics showing the retinal arteriolar (a) and venular (b) caliber (μm) ranges of the essential thrombocythemia group and control group
The mean RAC value was 88.5 ± 5.7 µm in the study group, and 95.5 ± 4.8 µm in the control group (P < 0.001). The mean RVC value was 119.6 ± 11.4 µm in the study group, and 125.6 ± 8.6 µm in the control group (P = 0.02). There was a significant negative correlation between RAC and platelet counts (r = −0.35, P = 0.008). RVC was not associated to platelet counts (r = 0.10, P = 0.44).
DISCUSSION
Our results show that OPA and SFCT values do not differ in patients with ET and healthy control patients. Additionally, RAC is markedly thinner in ET. It would be expected that as a systemic blood disease, ET could cause some alterations in the choroid and retina which have significant blood flow. Platelets in ET may aggregate at unpredictable times, regardless of platelet count.2,11 This condition may create a risk for ocular vascular complications in patients under treatment.
In addition to thromboembolic and hemorrhagic complications, ET, as a myeloproliferative disorder, may rarely evolve into the acute myeloid leukemia.1,2 It has been reported that leukemias are associated with choroidal infiltration.12,13,14 Additionally, amaurosis fugax is has been reported in some ET patients that may be due episodic choroidal ischemic attacks.3 Hence, ET may affect choroidal thickness. However, we did not find a statistically important SFCT alteration in ET patients and this may be interpreted as ET is not associated with gross ischemic or hemorrhagic choroidal events. In other words, choroid may be protected from systemic hematological threats to some extent. Another possible explanation for this condition may be the rapid blood flow rates in the choroid.
OPA is a surrogate for pulsatile choroidal blood flow, is affected by various systemic vascular conditions such as aortic regurgitation, carotid artery stenosis, and water drinking;15,16,17 on the other hand OPA is not influenced by systemic blood pressure levels.18 In the present study, OPA was similar in ET patients and healthy controls. Additionally, we did not find an association between OPA and SFCT in the ET patients and the control group. De Moraes et al. reported a strong positive correlation between OPA and choroidal thickness during the water drinking test.19
Influence of hematological disorders on IOP has been investigated in several studies.20,21,22 Yoshizumi and Townsend-Pico reported a case of neovascular glaucoma secondary to central retinal vein occlusion in ET.5 However, the possible impact of ET on IOP, other than neovascularization, is not clear. Additionally, increased oxidative stress in patients with ET was revealed.23 It is already known that lower systemic antioxidant capacity is involved in the pathogenesis of glaucoma.24 In this study, we did not find IOP increase in ET.
Retinal vessel caliber measurements show anatomical microcirculation and may reflect the vascular effects of some systemic diseases.25,26,27 Narrower RAC is associated with older age, systemic arterial hypertension, and coronary heart disease.26,27 Wider RVC is associated with diabetes mellitus, lipid disorders, cigarette smoking, and coronary heart disease.26,27 In the present study, ET was associated with narrower RAC and RVC. Similar to our results, Liew et al. found that blood platelet count is associated with narrower RAC;28 but apart from our results they found that blood platelet count is associated with wider RVC. The difference in RVC measurements may be due the many pathological aspects of ET and just increased platelet count.
Our study has several limitations. One limitation was the inability to perform the measurements in active and untreated disease status because all of the patients were taking medications for ET. However, it was shown that the vascular complications of ET could occur in normal platelet counts, regardless of the disease activity.29 Another limitation is the lack of retina-choroidal Doppler ultrasound investigation that might support our findings.
CONCLUSION
ET does not cause anatomical or physiological alterations in the choroid. But RAC is negatively correlated with blood platelet counts and decreased in ET. Our results may indicate that choroid has strong autoregulatory mechanisms for countering blood platelet aggregation and micro thrombosis. Further studies including indocyanine green and fluorescein angiography examinations may provide additional insight on this topic.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Brière JB. Essential thrombocythemia. Orphanet J Rare Dis. 2007;2:3. doi: 10.1186/1750-1172-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchez S, Ewton A. Essential thrombocythemia: A review of diagnostic and pathologic features. Arch Pathol Lab Med. 2006;130:1144–50. doi: 10.5858/2006-130-1144-ET. [DOI] [PubMed] [Google Scholar]
- 3.Billot S, Kouroupi EG, Le Guilloux J, Cassinat B, Jardin C, Laperche T, et al. Neurological disorders in essential thrombocythemia. Haematologica. 2011;96:1866–9. doi: 10.3324/haematol.2011.050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asensio Sánchez VM, Manteca Jiménez G, Cano Navarro E. Essential thrombocythemia and retinal vein thrombosis. Arch Soc Esp Oftalmol. 2004;79:629–32. doi: 10.4321/s0365-66912004001200010. [DOI] [PubMed] [Google Scholar]
- 5.Yoshizumi MO, Townsend-Pico W. Essential thrombocythemia and central retinal vein occlusion with neovascular glaucoma. Am J Ophthalmol. 1996;121:728–30. doi: 10.1016/s0002-9394(14)70649-x. [DOI] [PubMed] [Google Scholar]
- 6.Strassman I, Silverstone BZ, Seelenfreund MH, Sheer A, Berson D. Essential thrombocythemia: A rare case of central retinal artery occlusion. Metab Pediatr Syst Ophthalmol. 1991;14:18–20. [PubMed] [Google Scholar]
- 7.Arıkan G, Saatci AO, Kahraman S, Pişkin Ö, Men S, Ündar B. Central retinal artery occlusion as the presenting sign of essential thrombocythemia. Turk J Hematol. 2011;28:146–8. doi: 10.5152/tjh.2011.33. [DOI] [PubMed] [Google Scholar]
- 8.Sørensen TL, Mortzos P. Iritis, ptosis, and sequential severe loss of vision in a patient with essential thrombocytosis. Eye (Lond) 2010;24:928. doi: 10.1038/eye.2009.213. [DOI] [PubMed] [Google Scholar]
- 9.Zion IB, Harris A, Siesky B, Shulman S, McCranor L, Garzozi HJ. Pulsatile ocular blood flow: Relationship with flow velocities in vessels supplying the retina and choroid. Br J Ophthalmol. 2007;91:882–4. doi: 10.1136/bjo.2006.108340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiele J, Kvasnicka HM. The 2008 WHO diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis. Curr Hematol Malig Rep. 2009;4:33–40. doi: 10.1007/s11899-009-0005-6. [DOI] [PubMed] [Google Scholar]
- 11.Michiels JJ, Thiele J. Clinical and pathological criteria for the diagnosis of essential thrombocythemia, polycythemia vera, and idiopathic myelofibrosis (agnogenic myeloid metaplasia) Int J Hematol. 2002;76:133–45. doi: 10.1007/BF02982575. [DOI] [PubMed] [Google Scholar]
- 12.Madjlessi F, Dann K, Althaus C, Sundmacher R, Meckenstock G. Choroid infiltration in myelodysplastic syndrome. Klin Monbl Augenheilkd. 1998;213:51–4. doi: 10.1055/s-2008-1034944. [DOI] [PubMed] [Google Scholar]
- 13.Campbell RA, Bouldin TW, Peiffer RL. Large histiocytes in the choroid of leukemic patients. Arch Pathol Lab Med. 1990;114:210–1. [PubMed] [Google Scholar]
- 14.Rosenthal AR. Ocular manifestations of leukemia. A review. Ophthalmology. 1983;90:899–905. doi: 10.1016/s0161-6420(83)80013-x. [DOI] [PubMed] [Google Scholar]
- 15.McKee HD, Saldaña M, Ahad MA. Increased ocular pulse amplitude revealing aortic regurgitation. Am J Ophthalmol. 2004;138:503. doi: 10.1016/j.ajo.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Knecht PB, Menghini M, Bachmann LM, Baumgartner RW, Landau K. The ocular pulse amplitude as a noninvasive parameter for carotid artery stenosis screening: A test accuracy study. Ophthalmology. 2012;119:1244–9. doi: 10.1016/j.ophtha.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 17.Mansouri K, Medeiros FA, Marchase N, Tatham AJ, Auerbach D, Weinreb RN. Assessment of choroidal thickness and volume during the water drinking test by swept-source optical coherence tomography. Ophthalmology. 2013;120:2508–16. doi: 10.1016/j.ophtha.2013.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grieshaber MC, Katamay R, Gugleta K, Kochkorov A, Flammer J, Orgül S. Relationship between ocular pulse amplitude and systemic blood pressure measurements. Acta Ophthalmol. 2009;87:329–34. doi: 10.1111/j.1755-3768.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- 19.De Moraes CG, Reis AS, Cavalcante AF, Sano ME, Susanna R., Jr Choroidal expansion during the water drinking test. Graefes Arch Clin Exp Ophthalmol. 2009;247:385–9. doi: 10.1007/s00417-008-0969-2. [DOI] [PubMed] [Google Scholar]
- 20.Charles KS, Leelah N, Boodoo L, Murray DC. Ophthalmic manifestations of haematological disorders. West Indian Med J. 2013;62:99–103. [PubMed] [Google Scholar]
- 21.Lee MH, Park MY, Lee JW. Leukemic glaucoma in a patient with chronic myeloid leukemia treated by intracameral methotrexate. Jpn J Ophthalmol. 2010;54:362–4. doi: 10.1007/s10384-010-0827-8. [DOI] [PubMed] [Google Scholar]
- 22.Steinmann W, Stone R, Nichols C, Werner E, Schweitzer J, Keates E, et al. A case-control study of the association of sickle cell trait and chronic open-angle glaucoma. Am J Epidemiol. 1983;118:288–93. doi: 10.1093/oxfordjournals.aje.a113635. [DOI] [PubMed] [Google Scholar]
- 23.Durmus A, Mentese A, Yilmaz M, Sumer A, Akalin I, Topal C, et al. Increased oxidative stress in patients with essential thrombocythemia. Eur Rev Med Pharmacol Sci. 2013;17:2860–6. [PubMed] [Google Scholar]
- 24.Tanito M, Kaidzu S, Takai Y, Ohira A. Status of systemic oxidative stresses in patients with primary open-angle glaucoma and pseudoexfoliation syndrome. PLoS One. 2012;7:e49680. doi: 10.1371/journal.pone.0049680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong TY. Is retinal photography useful in the measurement of stroke risk? Lancet Neurol. 2004;3:179–83. doi: 10.1016/s1474-4422(04)00682-9. [DOI] [PubMed] [Google Scholar]
- 26.Ikram MK, Ong YT, Cheung CY, Wong TY. Retinal vascular caliber measurements: Clinical significance, current knowledge and future perspectives. Ophthalmologica. 2013;229:125–36. doi: 10.1159/000342158. [DOI] [PubMed] [Google Scholar]
- 27.Sun C, Wang JJ, Mackey DA, Wong TY. Retinal vascular caliber: Systemic, environmental, and genetic associations. Surv Ophthalmol. 2009;54:74–95. doi: 10.1016/j.survophthal.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Liew G, Wang JJ, Rochtchina E, Wong TY, Mitchell P. Complete blood count and retinal vessel calibers. PLoS One. 2014;9:e102230. doi: 10.1371/journal.pone.0102230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schafer AI. Thrombocytosis. N Engl J Med. 2004;350:1211–9. doi: 10.1056/NEJMra035363. [DOI] [PubMed] [Google Scholar]