Abstract
Herbal mouthwashes have been considered to be a more advantageous option to their chemical counterparts, for a long-time. The use of pomegranate fruit dates from ancient times and reports of its therapeutic abilities have echoed throughout the ages. To evaluate the effect on the salivary pH and the Streptococcus mutans count in healthy subjects before and after pomegranate mouthrinse. Fifty healthy patients were randomly divided into two groups of 25 subjects each. Group A was treated with 0.2% chlorhexidine mouthrinse; while Group B was treated with pomegranate peel extract (PPE) mouthrinse and the saliva samples were collected at three different intervals: Prerinse, after 10 min, and 60 min. The salivary pH was measured using a digital pH meter and the S. mutans count was determined by the commercial system Dentocult SM. The statistical analyses used in this study are Mann–Whitney U-test and t-test. PPE mouthrinse had an inhibitory effect on S. mutans count in adults. There was also an increase in the salivary pH after 10 min of the mouthrinse. PPE mouthrinse may be considered as a potential anticariogenic mouthrinse.
Key words: Chlorhexidine alternative, herbal mouthrinse, pomegranate extract
INTRODUCTION
Chlorhexidine, considered standard for an anti-plaque agent is one of the most competent antimicrobial agent but can cause tooth staining, horrid tang, and augmented calculus development.[1] This advocates the need for supplementary study and presents with new antibacterial agents that are strongly against Streptococcus mutans, with marginal consequences on the oral tissues, especially in children. Among the herbal alternatives, pomegranate finds a special mention.
Pomegranate, (botanical name: Punica granatum) is one of the oldest known edible fruits. The pomegranate is an ancient fruit that has not changed much throughout the history of man. It was found in the Indus Valley, so early that there is a word in Sanskrit for pomegranate. The pomegranate is also significant in Jewish, Christian and Muslim traditions.[2] The pomegranate is a native of Iran and Afghanistan, known in ancient Egypt.[3] It has been widely used in traditional medicine worldwide for the treatment of different types of diseases.[4] Furthermore, several antioxidant activities, including radical scavenging ability, ferrous ion chelating, and ferric ion reducing antioxidant power, were identified on P. granatum.
In various forms of traditional Asian medicine, pomegranate fruits were advocated as a health tonic and as a cure for plentiful conditions including diarrhea, dysentery, and diabetes.[5] Numerous studies have been done on the antimicrobial properties of pomegranate peel and its curative effects.[1]
Pomegranate is now finding vital uses in the arena of dental well-being.[5] Clinical reports ensure that this current antioxidant attacks the sources of tooth decay at the biochemical level, with significant vigor.[6]
The aim of this study was:
To estimate the influence of pomegranate extract mouthrinse on salivary pH and the S. mutans count
To compare the antibacterial and anticaries effect of a pomegranate extract mouthrinse with chlorhexidine.
SUBJECTS AND METHODS
Preparation of pomegranate extract
Pomegranate documented as P. granatum (Soans fruit technologies) was taken and used for the extract formulation.
Three types of pomegranate extract were primed. Pomegranate peel extract (PPE), pomegranate aril extract, and pomegranate juice. The most effective following the antibacterial efficacy test was used to prepare the mouthrinse used for the study.
PPE - Pomegranate peels were desiccated in sunlight and overnight in hot air oven at 60°C for 7 days. The dried peels were powdered. The acquired powder was used to formulate an aqueous extract in soxhlet extractor. 20 g of PPE was achieved at the end of 5 days.
Pomegranate aril extract - A parallel procedure was applied for pomegranate aril extract but required mandatory 15 days of drying in hot air oven at 60°C.
Pomegranate juice - Freshly prepared pomegranate juice was acquired using the sterilized grinder of the research laboratory. 400 ml of pomegranate juice was heated for 1 h to get a heavy concentrate.
Bacterial strain
The bacterial strain expended in this study was S. mutans Microbial Type Culture Collection (MTCC) 890, acquired from the MTCC and Gene Bank Institute of Microbial Technology, Chandigarh.
Antibacterial activity-minimum inhibitory concentration
The antibacterial endeavor of each extract was tested alongside a strain of S. Mutans (MTCC 890). The lowest concentration of the extract that formed no evident bacterial development (turbidity) was documented as the minimum inhibitory concentration (MIC) (CLSI, 2006).[7]
The antibacterial assay was conducted by agar well diffusion method [8] with tripton soya agar as the media. The agar plates were spread with cultures of S. mutans incubated at 37°C for 24 h inoculated in a tripton soya broth. Wells (8 mm diameter) were punched in the agar and filled with 0.1 ml of extract and incubated for 24 h. Concentrations of 600, 300, 150, and 75 mg/ml were tested similarly. Antibacterial activities were evaluated by measuring the diameter of inhibition zone.
PPE showed well-defined inhibition zones at 600 and 300 mg/ml concentrations. Pomegranate aril extract showed inhibition zones at 600 mg/ml, but the diameter was less than PPE. However, pomegranate juice failed to show inhibition zones.
Preparation of mouthrinse
The PPE was selected to prepare the mouthrinse as it demonstrated higher antibacterial efficacy based on MIC.
The 300 mg/ml concentration of PPE was used to prepare the mouthrinse. About 18 g of PPE was dissolved in 60 ml of distilled water. Although no sweeteners were added, the taste was found to be acceptable.
Selection of study sample
Fifty patients aged 15–25 years who attended the Department of Restorative Sciences, Al Farabi College, who satisfied the eligibility criteria were selected for the study. The study protocol was reviewed and approved by the Institutional Ethical Committee.
The exclusion criteria were subjects with medical disorders such as diabetes mellitus, renal disease, gastrointestinal disorders, respiratory diseases, evidence of recent bronchitis, sinusitis, or tonsillitis, patients undergoing antibiotic or other antimicrobial therapy, smokers, those who, on prestudy clinical screening, presented a probing depth ≥4 mm, subjects with cavitated caries lesion or naso-pharyngeal alterations, mouth breathers, and patients with prostheses, orthodontic or dental appliances. In addition, the participants in the study were required to have a normal unstimulated (resting) whole saliva flow rate (0.5 ml/min). The nature of the study was explained and written informed consent obtained from all the subjects.
The selected patients were randomly divided into two groups of 25 subjects each. Group A was treated with 0.2% chlorhexidine mouthrinse; while Group B was treated with PPE mouthrinse.
Estimation of salivary pH
Before saliva collection, patients were kept seated for 5 min, relaxed and silent. Following an initial swallow, 5 ml of unstimulated saliva was collected in a sterilized airtight container by allowing the saliva to flow over a period of 5 min into the containers by tilting their heads forward (set 1).
The Group A subjects were given chlorhexidine mouthwash and asked to rinse for 2 min and expectorate. Subsequent to baseline estimation, each participant underwent two sets of saliva sample collection along with estimation of salivary pH in two phases: Ten minutes after the mouthrinse (set 2), and 60 min after the mouthrinse (set 3). None were allowed to eat or drink in between the phases. Similarly, Group B participants were asked to rinse with 5 ml of pomegranate extract for 2 min and subsequently, the saliva samples (set 1, 2, and 3) were collected. The pH of each collection was noted using a digital pH meter.
Estimation of SM count
S. mutans count was determined by the commercial system Dentocult SM (Vivadent, Vivacare, Schaan, Liechtenstein). All bacterial cultures were cultivated in the manufacturer's thermostat at a standard temperature of 36.5°C. Culture media were stored and prepared according to the manufacturer's instructions. These instructions were followed on the sampling procedure. The results obtained by culture in the thermostat were read by use of the model chart supplied by the manufacturer.
Statistical analysis
The data obtained were analyzed using Statistical Package for Social Sciences software version 15.0 (SPSS Inc., Chicago, IL, USA). Mann–Whitney U-test was used for significance in intra-group analysis where significant P = 5%, and t-test was used for inter-group comparison.
RESULTS
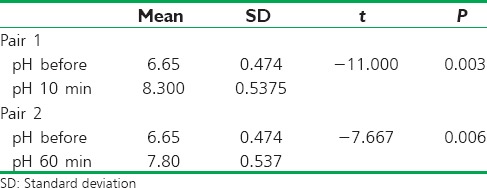
There was a significant increase in the pH of saliva sample in both Group A and Group B after the mouthrinses. However, the difference between the pH between set 2 (10 min after rinse) and set 3 (60 min after rinse) was not significant [Table 1].
Table 1.
Comparison of the salivary pH changes after 10 min and 60 min
In regard to the S. mutans count, PPE mouthrinse showed a significant decrease in the S. mutans count. However, the difference in the S. mutans count at set 2 (10 min after oral rinse) and set 3 (60 min after oral rinse) was not found to be significant [Graph 1].
Graph 1.
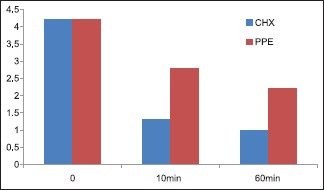
Comparison of the mean of log10 values of Streptococcus mutans count after chlorhexidine and pomegranate peel extract mouthrinse
Statistical analysis of the results showed a significant difference in the number of S. mutans after PPE mouthwash between sets 1 and 2 (mean ± standard deviation, 4.275 ± 0.8459 vs. 2.809 ± 2.0911; t = 2.8783 and P = 0.0031), and between sets 1 and 3 (4.275 ± 0.8459 vs. 2.4184 ± 1.9999; t = 4.8589 and P < 0.001).
These results indicate a reduction in the number of S. mutans, implying an inhibitory effect of PPE on bacterial growth both after the beginning of mouthrinse (set 2) and at the end of treatment that is, 1 h after mouthrinse (set 3).
DISCUSSION
Various studies have already shown that pomegranate's active components, including polyphenolic flavonoids like punicalagins and ellagic acid are believed to prevent gingivitis through a number of mechanisms including the reduction of oxidative stress in oral cavity, direct anti-oxidant activity anti-inflammatory effects, antibacterial activity, and direct removal of plaque from the teeth.[9,10,11]
When used regularly in combination with toothpaste that has been reinforced with bioactive botanical extracts, pomegranate containing mouthwash may fight dental plaque and tartar formation by inhibiting the activities of the microorganisms that cause plaque. In addition, pomegranate compounds possess anti-inflammatory properties that may help soothe irritated tissues.[12,13]
Pomegranate extract suppresses the ability of these microorganisms to adhere to the surface of the tooth.[14] Plaque may involve four or more different microorganisms combining forces to colonize the surface of the teeth. Remarkably, nature's own pomegranate fights the organisms' ability to adhere by interfering with the production of the very chemicals the bacteria use for adhesion.[15]
In an Ohio State study, those subjects who rinsed with pomegranate solution experienced a reduction in saliva total protein content which is normally higher among people with gingivitis and may correlate with plaque-forming bacterial content.[16]
Pomegranate rinsing also lowered saliva activities of alpha-glucosidase, an enzyme that breaks down sucrose, while it increased the activities of ceruloplasmin, an antioxidant enzyme.[17] Therefore, pomegranate may exhibit anticariogenic effect as well, which may be utilized to prevent dental caries in individuals.
The present study indicates that PPE mouthrinse significantly reduced the salivary count of S. mutans count in the subjects as compared to the standard chlorhexidine mouthwash. The herbal mouthrinse also increased the salivary pH significantly within a short-time interval of 10 min after the mouthrinse; thereby proving its potential as an anti-cariogenic agent.
CONCLUSION
This study implies that, PPE mouthrinse possesses remarkable antimicrobial activity against S. mutans present in the oral cavity as tested in vivo, and may be used as an adjunct to prevent dental caries and maintain good oral hygiene.
Further investigations may be performed to establish the antibacterial and anticariogenic activity of pomegranate extract and to attain the appropriate concentration to be used as a mouthwash regularly.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Eley BM. Antibacterial agents in the control of supragingival plaque – A review. Br Dent J. 1999;186:286–96. doi: 10.1038/sj.bdj.4800090. [DOI] [PubMed] [Google Scholar]
- 2.Seeram PN, Schulmann NR, Heber D. Pomegranates: Ancient Roots to Modern Medicine. Boca Raton, FL, USA: CRC Press; 2006. [Google Scholar]
- 3.Bakhru HK. Foods That Heal: The Natural Way to Good Health. Delhi: Orient Paperbacks; 2009. [Google Scholar]
- 4.Olapour S, Najafzadeh H. Evaluation analgesic, anti-inflammatory and antiepileptic effect of hydro alcoholic peel extract of Punica granatum (Pomegranate) Asian J Med Sci. 2010;2:266–70. [Google Scholar]
- 5.Kapoor LD. Handbook of Ayurvedic Medicinal Plants. Boca Raton, Florida: CRC Press; 1990. [Google Scholar]
- 6.Naovi SA, Khan MS, Vohora SB. Antibacterial, antifungal and anthelmintic investigations on Indian medicinal plants. Fitoterapia. 1991;62:221–8. [Google Scholar]
- 7.CLSI. Approved Standard M7-A7. 7th ed. Vol. 26. Wayne PA, USA: CLSI; 2006. Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; p. 2. [Google Scholar]
- 8.Perez C, Pauli M, Bazerque P. An antibacterial assay by agar well diffusion method. Acta Biol Med Exp. 1990;15:113–5. [Google Scholar]
- 9.Taguri T, Tanaka T, Kouno I. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol Pharm. 2004;27:1965–9. doi: 10.1248/bpb.27.1965. [DOI] [PubMed] [Google Scholar]
- 10.Menezes SM, Cordeiro LN, Viana GS. Punica granatum (pomegranate) extract is active against dental plaque. J Herb Pharmacother. 2006;6:79–92. [PubMed] [Google Scholar]
- 11.Sastravaha G, Yotnuengnit P, Booncong P, Sangtherapitikul P. Adjunctive periodontal treatment with Centella asiatica and Punica granatum extracts. A preliminary study. J Int Acad Periodontol. 2003;5:106–15. [PubMed] [Google Scholar]
- 12.Vasconcelos LC, Sampaio FC, Sampaio MC, Pereira Mdo S, Higino JS, Peixoto MH. Minimum inhibitory concentration of adherence of Punica granatum Linn (pomegranate) gel against S. mutans, S. mitis and C. albicans. Braz Dent J. 2006;17:223–7. doi: 10.1590/s0103-64402006000300009. [DOI] [PubMed] [Google Scholar]
- 13.Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109:177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Shukla M, Gupta K, Rasheed Z, Khan KA, Haqqi TM. Consumption of hydrolyzable tannins-rich pomegranate extract suppresses inflammation and joint damage in rheumatoid arthritis. Nutrition. 2008;24:733–43. doi: 10.1016/j.nut.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Wen S, Kota BP, Peng G, Li GQ, Yamahara J, et al. Punica granatum flower extract, a potent alpha-glucosidase inhibitor, improves postprandial hyperglycemia in Zucker diabetic fatty rats. J Ethnopharmacol. 2005;99:239–44. doi: 10.1016/j.jep.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 16.Rudney JD, Krig MA, Neuvar EK. Longitudinal study of relations between human salivary antimicrobial proteins and measures of dental plaque accumulation and composition. Arch Oral Biol. 1993;38:377–86. doi: 10.1016/0003-9969(93)90208-4. [DOI] [PubMed] [Google Scholar]
- 17.Beighton D, Radford JR, Naylor MN. Glycosidase activities in gingival crevicular fluid in subjects with adult periodontitis or gingivitis. Arch Oral Biol. 1992;37:343–8. doi: 10.1016/0003-9969(92)90016-2. [DOI] [PubMed] [Google Scholar]


