Abstract
Background.
Independent predictors of preserved cognitive functioning and factors associated with maintaining high preserved cognitive function in women ≥80 years remain elusive.
Methods.
Two thousand two hundred twenty-eight women with a mean age of 85 years who participated in the Women’s Health Initiative Memory Study were classified as cognitively normal (n = 1,905, 85.5%), mild cognitive impairment (n = 88, 3.9%), dementia (n = 121, 5.4%) or other cognitive impairment (n = 114, n = 5.1%) by central adjudication. Global cognitive functioning was assessed using telephone interview for cognitive status-modified in those women who did not meet cognitive impairment criteria. Differences between women grouped by cognitive status with respect to each potential risk factor were assessed using chi-squared tests and t-tests. Backward stepwise logistic regression was used to select factors that were independently associated with cognitive status.
Results.
Factors associated with preserved cognitive functioning were younger age, higher education, and family incomes, being non-Hispanic white, better emotional wellbeing, fewer depressive symptoms, more insomnia complaints, being free of diabetes, and not carrying the apolipoprotein E-epsilon 4 allele. Cognitively normal women who demonstrated sustained high preserved cognition were younger, more educated, and endorsed better self-reported general health, emotional wellbeing, and higher physical functioning.
Conclusions.
Addressing sociodemographic disparities such as income inequality, and targeting interventions to improve depressive symptoms and vascular risk factors, including diabetes, may play an important role in preserving cognition among women who survive to 80 years of age. Person-centered approaches that combine interventions to improve physical, cognitive, and psychosocial functioning may promote maintenance of high preserved cognitive health in the oldest-old.
Key Words: Cognition, Cognitive aging, Successful aging
With the unprecedented increases in life expectancy, individuals who are octogenarians or older now comprise the fastest growing segment of the U.S. population. The numbers of oldest-old persons with mild cognitive impairment (MCI) and Alzheimer’s disease (AD) are expected to grow exponentially in the coming years. Some investigations have reported that the risk of MCI and dementia is more than 40% in those over the age of 80 (1,2). The lifetime risk of cognitive impairment is greater in women since they live longer and outnumber men at older ages. Cognitive aging research typically focuses on factors that differentiate those with MCI and AD from those who have normal cognition. However, a significant proportion of women who are 80 years or older maintain preserved cognitive function. Despite this, the factors associated with preserved or optimal cognitive aging in women aged 80 years or older are poorly understood.
The maintenance of optimal cognitive function in later years is critical to preserve independence, decrease disability, improve quality of life, and to achieve remarkable longevity (3,4). Recent studies have identified several modifiable and nonmodifiable factors that are associated with higher cognitive function in later years (5–7). These include demographic (younger age, higher levels of education and socioeconomic status, residing independently, being married), environmental (increased social activities, good social support), lifestyle (more exercise, less smoking, moderate alcohol consumption), vascular (less vascular risk factors and disease), and emotional (lower depressive symptom severity) factors, as well as higher physical functioning. However, the majority of these investigations was conducted in young-old and mixed gender samples, and/or defined preserved cognitive function based solely on cross-sectional evaluations (4,5).
Emerging findings from a handful of prospective studies have reported distinct categories of cognitive trajectories over time in the elderly (3,8,9). In the only study to our knowledge comprised of older women, cognitive maintainers were less likely to have comorbid medical conditions, difficulties with day-to-day functioning, and poor social networks, and were more likely to practice healthy lifestyle behaviors, relative to minor cognitive decliners (8). These findings are beginning to reveal characteristics of individuals who maintain higher cognitive functioning over time. However, these studies have largely depended on single measures of global cognitive function to categorize different cognitive groups; as such, they may have inadvertently misclassified participants with MCI and early dementia and included them in the analysis. The predictors of consistently high preserved cognitive function in those women who do not meet diagnostic criteria for MCI and probable dementia and have survived into their ninth decade therefore remain elusive.
The primary objectives of this study were to determine the independent predictors of preserved cognitive function in women aged 80 years or older relative to those with cognitive impairment, and to further characterize factors that are associated with sustaining high preserved cognitive function over time in those who are free of objectively defined cognitive impairment. We utilized the sub-cohort of women in the Women’s Health Initiative (WHI) who participated in the WHI Memory Study (WHIMS), were 80 years or older, had longitudinally been followed for a mean (interquartile range) of 15.1 (14.5–16.0) years, underwent annual cognitive assessments and were assigned presence or absence of a cognitive disorder diagnosis based on a central adjudication process.
Methods
The Women’s Health Initiative Hormone Therapy (WHI-HT) trials consisted of two parallel placebo-controlled trials of conjugated equine estrogen (CEE)-based HT regimens. Enrollees were 50–79 years of age and postmenopausal. Active therapies consisted of 0.625mg/day CEE in women post-hysterectomy and 0.625g/day CEE combined with 2.5mg/day medroxy progesterone acetate (MPA) in women with a uterus. The trial among women without prior hysterectomy was terminated in July 2002; the trial among women with prior hysterectomy was terminated in February 2004. In both instances, study therapy was stopped at these times and women were unmasked, but women continued to be followed. The WHIMS ancillary study enrolled 7,479 of these women who were 65 years of age and older (10). WHIMS participants were administered an annual cognitive screener. Scores below pre-set cut-points triggered comprehensive clinical and neurocognitive assessments and central adjudication to classify women to no cognitive impairment, mild cognitive impairment, or probable dementia. In 2008, WHI clinic visits ceased and WHIMS transitioned to an annual telephone-administered cognitive assessment battery (described later), for the WHIMS Epidemiology of Cognitive Health Outcomes (ECHO), which remains ongoing.
Cohort Selection
Women (n = 2,228) who met the following conditions were selected as the sub-cohort for this study: (a) previously enrolled in the WHIMS ancillary study; and an active participant in WHIMS ECHO and (b) at least 80 years of age and alive as of September 17, 2012.
Cognitive Function
Trained and certified staff administered the telephone interview for cognitive status-modified (TICS-m), a 14-question test, with scores ranging from 0 to 50. Higher scores reflect better global cognitive function, and this instrument has been validated (11).
Cognitive Impairment
For women whose TICS-m scores were less than 31, a reliable informant who was previously identified by the participant was administered the dementia questionnaire (DQ) via telephone; a standardized, structured interview that assesses the history of cognitive and behavioral changes, functional impairments, and health events that can affect cognitive functioning (eg, stroke) (12). Data from all prior and recent WHIMS assessments and relevant supplemental information from the WHI were then submitted for central adjudication.
Covariates and Potential Confounders
Sociodemographic variables were collected at WHI baseline. Other factors were obtained from the most recent assessment prior to the cognitive assessment. Lifestyle factors included smoking status, physical activity, alcohol use, social support, and stressful life events; emotional and psychological factors included self-rated happiness, optimism, Geriatric Depression Scale-Short Form, SF-36 Pain subscale, WHI Insomnia Rating Scale, and self-rated health status; quality of life variables included self-reported physical functioning and emotional well-being from the SF-36; vascular and other physical health factors including body mass index (BMI), history of diabetes, and history of cardiovascular disease defined as myocardial infarction, angina, percutaneous transluminal coronary angioplasty, stroke, or coronary artery bypass surgery grafting.
Prior hormone use was assessed at baseline and the use of antidepressants, statins, and antihypertensive medications, at the last medication inventory before cognitive assessment. Hormone therapy assignment at randomization (HT, Placebo) was also included as a covariate. Apolipoprotein E (APOE) genotypes were assigned based on rs429358 and rs7412 genotype results from imputation and harmonization of genetic data across WHI genome-wide association studies within the WHI Clinical Trials and Observational studies. The imputation was conducted using the 1000 Genomes Project reference panel and the MaCH algorithm as implemented in Minimac [R2 = .98 for each SNP in the study population (13)].
Statistical Methods
Differences between women grouped by cognitive status with respect to each potential risk factor were assessed using chi-squared tests and t-tests. Backward stepwise logistic regression was used to select subsets that were independently associated with cognitive status. Factors with missing data were dichotomized using a median split and missingness was used to define a third level. For inclusion by the selection algorithm, factors were required to have associations with cognitive status at a p < .05 level. In supporting analyses, missing measures and responses were imputed (SAS Proc MI) to create five databases (14); backward stepwise logistic regression was applied to each to identify important predictors (missing APOE allelic status was not imputed, instead being treated as a separate category).
Results
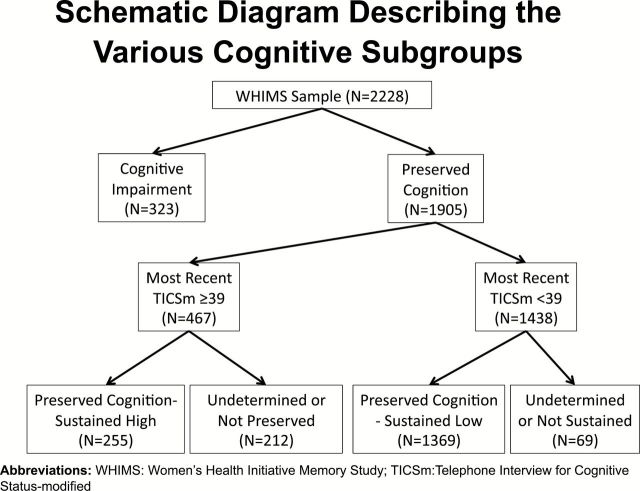
Figure 1 depicts the various cognitive subgroups used in different sets of analyses in this study. Among the 2,228 women included in our analyses, 121 (5.4%) women had met study criteria for probable dementia and an additional 88 (3.9%) had met criteria for MCI, but had not been observed to progress to dementia. An additional 114 (5.1%) women met criteria for cognitive impairment, but information was insufficient to differentiate probable dementia versus MCI.
Figure 1.
Schematic diagram describing the various cognitive subgroups.
Preserved Cognitive Function Versus Cognitive Impairment
Compared to the women with cognitive impairment, the remaining 1905 (85.5%) women (Table 1) tended to be younger, better educated, wealthier, and married; they were less likely to be from a racial/ethnic minority group. These women with preserved cognitive health were also more likely to report moderate alcohol intake and better general health and less likely to report diabetes. They had higher levels of social support, emotional wellbeing, optimism, and happiness, but also had higher mean insomnia rating scores. Women without cognitive impairment had fewer depressive symptoms and were less likely to carry the APOE-ε4 allele.
Table 1.
Characteristics of Women ≥80 Years of Age Grouped by Cognitive Impairment Status: Mean (SD) or Frequency (percent).
| Characteristic | Preserved Cognitive Function, N = 1,905 | Cognitive Impairment*, N = 323 | p Value** |
|---|---|---|---|
| Current age | 84.6 (3.3) | 85.4 (3.6) | <0.001 |
| Education (Miss = 4) | |||
| High school or less | 453 (23.8) | 114 (35.4) | <0.001 |
| Post high school | 936 (49.2) | 138 (42.9) | |
| Post college graduation | 513 (27.0) | 70 (21.7) | |
| Ethnicity | |||
| African-American | 94 (4.9) | 35 (10.8) | <0.001 |
| Hispanic | 26 (1.4) | 7 (2.2) | |
| Other, multiple | 57 (3.0) | 12 (3.7) | |
| Non-Hispanic white | 1,728 (90.7) | 269 (83.3) | |
| Marriage status (Miss = 3) | |||
| Never/divorced | 275 (14.5) | 45 (13.9) | 0.002 |
| Widowed | 518 (27.2) | 119 (36.8) | |
| Married/relationship | 1,109 (58.3) | 159 (49.2) | |
| Family income (Miss = 115) | |||
| <$35K | 881 (48.7) | 194 (64.0) | <0.001 |
| >$35K | 929 (51.3) | 109 (36.0) | |
| Smoking (Miss = 20) | |||
| Never | 1,041 (55.2) | 193 (60.1) | 0.22 |
| Former | 760 (40.3) | 117 (36.4) | |
| Current | 86 (4.6) | 11 (3.4) | |
| Alcohol intake (Miss = 3) | |||
| None | 829 (43.6) | 187 (57.9) | <0.001 |
| <1/day | 887 (46.6) | 111 (34.4) | |
| 1+/day | 186 (9.8) | 25 (7.7) | |
| Self-reported health (Miss = 50) | |||
| Excellent | 174 (9.3) | 18 (5.7) | 0.002 |
| Very good | 745 (40.0) | 109 (34.7) | |
| Good | 769 (41.3) | 140 (44.6) | |
| Fair/poor | 176 (9.4) | 47 (15.0) | |
| BMI (Miss = 25) | |||
| <25kg/m2 | 523 (27.8) | 106 (33.2) | 0.14 |
| 25–29kg/m2 | 698 (37.0) | 109 (34.2) | |
| 30kg/m2 | 663 (35.2) | 104 (32.6) | |
| Recreational physical activity, METS h/wk (Miss = 1) | 12.0 (13.2) | 10.7 (13.7) | 0.10 |
| Moderate/strenuous activity, min/wk (Miss = 28) | 60.5 (99.5) | 60.8 (114.7) | 0.97 |
| Walking, METS h/wk (Miss = 28) | 2.94 (4.80) | 3.03 (5.08) | 0.75 |
| WHI-HT assignment | |||
| Placebo | 972 (51.0) | 167 (51.7) | 0.82 |
| HT | 933 (49.0) | 156 (48.3) | |
| Prior HT use | |||
| No | 1,327 (69.7) | 224 (69.4) | 0.91 |
| Yes | 578 (30.3) | 99 (30.6) | |
| Antidepressant use | |||
| No | 1,749 (91.8) | 297 (92.0) | 0.93 |
| Yes | 156 (8.2) | 26 (8.0) | |
| Antihypertensive use | |||
| No | 641 (33.6) | 120 (37.2) | 0.22 |
| Yes | 1,264 (66.4) | 203 (62.8) | |
| Lipid-lowering drug use | |||
| No | 1,102 (57.8) | 205 (63.5) | 0.06 |
| Yes | 803 (42.2) | 118 (36.5) | |
| Diabetes | |||
| No | 1,613 (84.7) | 254 (78.6) | 0.006 |
| Yes | 292 (15.3) | 69 (21.4) | |
| Cardiovascular disease | |||
| No | 1,472 (77.3) | 239 (74.0) | 0.20 |
| Yes | 433 (22.7) | 84 (26.0) | |
| SF-36 pain (Miss = 50) | 65.2 (25.8) | 64.5 (27.1) | 0.68 |
| Social support (Miss = 167) | 36.8 (7.6) | 35.4 (8.3) | 0.004 |
| Optimism (Miss = 78) | 23.7 (3.1) | 23.0 (3.0) | <0.001 |
| Emotional wellbeing (Miss = 80) | 82.0 (12.6) | 78.7 (14.5) | <0.001 |
| Happiness (Miss = 38) | |||
| All or most of the time | 1,351 (72.1) | 205 (64.9) | 0.009 |
| Less than most of the time | 523 (27.9) | 111 (35.1) | |
| Stressful life event score (Miss = 257) | 2.59 (2.70) | 2.65 (2.64) | 0.72 |
| Physical function score (Miss = 134) | 62.1 (25.4) | 59.9 (26.3) | 0.17 |
| GDS score (Miss = 20) | 1.80 (2.18) | 2.80 (2.91) | <0.001 |
| WHI Insomnia Rating Scale (Miss = 21) | 5.84 (4.10) | 5.04 (4.48) | 0.002 |
| APOE (imputed; Miss = 647) | |||
| ε4 carrier | 277 (20.2%) | 73 (34.4%) | <0.001 |
| Not ε4 carrier | 1,092 (79.8%) | 139 (65.6%) | |
Notes: Miss = missing; BMI = body mass index; METS = metabolic equivalent of tasks; WHI = women’s health initiative; HT = hormone therapy; GDS = Geriatric Depression Scale; APOE = apolipoprotein E; ε: Epsilon.
*Mild cognitive impairment, N = 88; Probable dementia, N = 121; Unable to classify—cognitive impairment, N = 114.
**Chi-square test or t-test.
Data were missing with some frequency for some measures (Table 1). Our principal approach was to categorize measures with missing data and to include missingness as a separate level. The following continuous variables were grouped into three levels (≤cohort median, >cohort median, missing): recreational activity (median = 7.5 METS), SF-36 pain (75), social support score (38), optimism score (24), emotional wellbeing score (84), stressful life events (2), physical functioning (65), GDS-Score (1), and insomnia rating score (5).
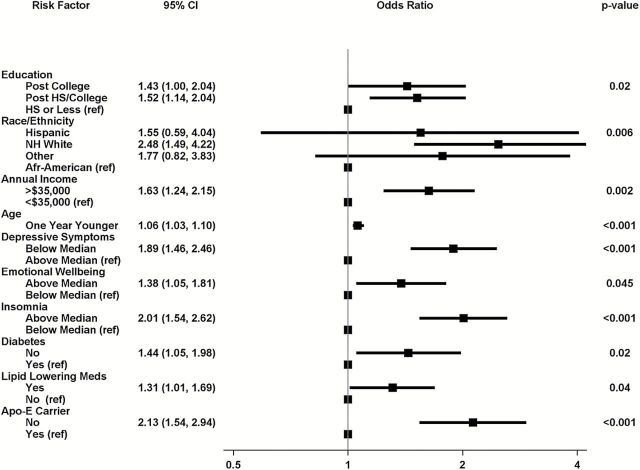
Figure 2 summarizes the results of applying backward stepwise logistic regression to identify a subset of variables in Table 1 that were independently associated with the odds of preserved cognitive function. Non-missing categories for the following characteristics were selected. Women whose depressive symptom scores were below the median had 1.89 [95% confidence interval: 1.46–2.46] greater odds of having preserved cognitive function. Women with post-college (1.43 [1.00–2.04]) and college (1.52 [1.14–2.04]) education had greater odds of having preserved cognitive function than those with high school or less education. Similarly, non-Hispanic white relative to African American women had 2.48 [1.49–4.22] higher odds of having preserved cognitive health, as did women with higher family incomes (odds ratio, OR = 1.63 [1.24–2.15]), women who were prescribed lipid lowering medications (OR = 1.31 [1.01–1.69], women who were younger (OR = 1.06/y [1.03–1.10]), women who had higher emotional wellbeing scores (OR = 1.38 [1.05–1.81]), women reporting more insomnia complaints (OR = 2.01 [1.54–2.62]), free of diabetes (OR = 1.44 [1.05–1.98]), and those not carrying the APOE-ε4 allele (OR = 2.13 [1.54–2.94].
Figure 2.
Factors selected by backward stepwise logistic regression as being independently predictive of preserved cognitive function.
Across five databases created by multiple imputation, fewer symptoms of depression, greater education, non-Hispanic white race/ethnicity, higher family income, younger age, better emotional well-being, more symptoms of insomnia, being free of diabetes, greater recreational physical activity, greater BMI, and not carrying the APOE-ε4 allele were selected from each as being independently associated with preserved cognitive function. Across the five imputed databases, the average odds ratio of recreational physical activity above the median compared with below the median was OR = 1.32 [1.02–1.69]. The average odds ratio for BMI 25–29kg/m2 and BMI ≥30kg/m2 compared with BMI <25kg/m2 and were OR = 1.30 [0.96–1.76] and OR = 1.56 [1.14–2.16], respectively. Lipid-lowering medications were only selected in one of the imputed databases, suggesting that its selection from the incomplete raw database may be associated with patterns of missing data.
Preserved Cognitive Function-Sustained High Versus Preserved Cognitive Function-Sustained Low
At their most recent assessment, 467 (21%) women had TICS-m scores within the clinically normal range of ≥39. Of these, 24 (5.1%) had no prior TICS-m measures and 32 (6.8%), 57 (12.2%), 83 (17.8%), 270 (57.8%), and 1 (0.2%) had 1, 2, 3, 4, and 5 prior TICS-m measures, respectively. We selected the subset of these women who had average TICS-m scores ≥39 across follow-up: N = 255 (including the 24 women with no prior assessment). These 255 women (8.9% of the full cohort) comprised our cohort of consistently high preserved cognitive functioning women and were compared with the 1,369 women with TICS-m <39 at the current visit, whose average TICS-m scores across follow-up was <39, and who had no adjudicated classification of dementia, MCI, or indeterminate impairment. Women with preserved cognition-sustained high (vs those with preserved cognition-sustained low) tended to be younger, more educated, wealthier, more physically active with walking and moderate/strenuous leisure time physical activity, and reported greater alcohol intake. They also were less likely to have a history of CVD. Women with preserved cognition-sustained high were less likely to take antihypertensive and lipid-lowering medications, and to have better psychosocial profiles (reflected in more favorable scores for pain, optimism, emotional wellbeing, happiness, physical function, and depressive symptoms; Table 2).
Table 2.
Characteristics of Women ≥80 Years of Age With Preserved Cognitive Functioning Separated Based on Sustained High Preserved Cognitive Function Relative to Comparison Group: Mean (SD) or Percent
| Characteristics | Preserved Cognitive Function-Sustained High (TICS-m ≥ 39), N = 255 | Preserved Cognitive Function-Sustained Low (TICS-m < 39), N = 1,369 | p Value* |
|---|---|---|---|
| Current age | 83.7 (3.0) | 84.9 (3.4) | <0.001 |
| Education (Miss = 3) | |||
| High school or less | 39 (15.4) | 352 (25.8) | <0.001 |
| Post high school | 117 (46.1) | 688 (50.3) | |
| Post college graduation | 98 (38.6) | 327 (23.9) | |
| Ethnicity | |||
| African-American | 9 (3.5) | 75 (5.5) | 0.35 |
| Hispanic | 4 (1.6) | 18 (1.3) | |
| Other | 5 (2.0) | 46 (3.4) | |
| Non-Hispanic white | 237 (92.9) | 1,230 (89.8) | |
| Marriage status (Miss = 2) | |||
| Never/divorced | 26 (10.2) | 199 (14.6) | 0.19 |
| Widowed | 73 (28.7) | 375 (27.4) | |
| Married/relationship | 155 (61.0) | 794 (58.0) | |
| Family income (Miss = 74) | |||
| <$35K | 99 (41.4) | 679 (51.8) | 0.003 |
| ≥$35K | 140 (58.6) | 632 (48.2) | |
| Smoking (Miss = 16) | |||
| Never | 136 (53.3) | 748 (55.3) | 0.31 |
| Former | 103 (40.4) | 549 (40.6) | |
| Current | 16 (6.3) | 56 (4.1) | |
| Alcohol intake (Miss = 2) | |||
| None | 95 (37.2) | 626 (45.8) | 0.03 |
| <1/day | 130 (51.0) | 622 (45.5) | |
| 1+/day | 30 (11.8) | 119 (8.7) | |
| Self-reported health (Miss = 37) | |||
| Excellent | 44 (17.5) | 105 (7.9) | <0.001 |
| Very good | 109 (43.2) | 518 (38.8) | |
| Good | 82 (32.5) | 578 (43.3) | |
| Fair/poor | 17 (6.8) | 134 (10.0) | |
| BMI (Miss = 17) | |||
| <25kg/m2 | 77 (30.4) | 378 (27.9) | 0.72 |
| 25–29kg/m2 | 90 (35.6) | 502 (37.1) | |
| ≥30kg/m2 | 86 (34.0) | 474 (35.0) | |
| Recreational physical activity, METS/wk (Miss = 1) | 12.9 (12.6) | 11.8 (13.4) | 0.23 |
| Moderate/strenuous activity, min/wk (Miss = 22) | 73.6 (114.6) | 58.9 (98.4) | 0.03 |
| Walking, METS, h/wk (Miss = 22) | 4.03 (5.97) | 2.83 (4.63) | <0.001 |
| WHI-HT assignment | |||
| Placebo | 135 (52.9) | 681 (49.7) | 0.35 |
| HT | 120 (47.1) | 688 (50.3) | |
| Prior HT use | |||
| No | 190 (74.5) | 936 (68.4) | 0.05 |
| Yes | 65 (25.5) | 433 (31.6) | |
| Antidepressant use | |||
| No | 237 (92.9) | 1,252 (91.4) | 0.43 |
| Yes | 18 (7.1) | 117 (8.6) | |
| Antihypertensive use | |||
| No | 108 (42.4) | 446 (32.6) | 0.002 |
| Yes | 147 (57.6) | 923 (67.4) | |
| Lipid-lowering drug use | |||
| No | 165 (64.7) | 790 (57.7) | 0.04 |
| Yes | 90 (35.3) | 579 (42.3) | |
| Diabetes | |||
| No | 36 (14.1) | 225 (16.4) | 0.35 |
| Yes | 219 (85.9) | 1,144 (83.6) | |
| Cardiovascular disease | |||
| No | 216 (84.7) | 1,041 (76.0) | 0.002 |
| Yes | 39 (15.3) | 328 (24.0) | |
| SF-36 pain (Miss = 38) | 68.7 (25.7) | 64.5 (25.8) | 0.02 |
| Social support (Miss = 108) | 36.9 (8.1) | 36.7 (7.7) | 0.63 |
| Optimism (Miss = 57) | 24.3 (3.3) | 23.6 (3.0) | <0.001 |
| Emotional wellbeing (Miss = 60) | 84.1 (12.1) | 81.4 (12.8) | 0.003 |
| Happiness (Miss = 28) | |||
| All or most of the time | 197 (77.6) | 948 (70.6) | 0.02 |
| Less than most of the time | 57 (22.4) | 394 (29.4) | |
| Stressful life event score (Miss = 193) | 2.38 (2.54) | 2.64 (2.72) | 0.18 |
| Physical function score (Miss = 90) | 69.6 (23.7) | 60.6 (25.7) | <0.001 |
| GDS score (Miss = 9) | 1.23 (1.66) | 1.94 (2.30) | <0.001 |
| Insomnia Rating Scale (Miss = 10) | 5.85 (4.03) | 5.83 (4.08) | 0.96 |
| Apo-E (imputed) (Miss = 469) | |||
| ε4 carrier | 33 (17.6) | 213 (22.0) | 0.17 |
| Not ε4 carrier | 155 (82.4) | 754 (78.0) | |
Notes: Miss = missing; BMI = body mass index; METS = metabolic equivalent of tasks; WHI = women’s health initiative; HT = hormone therapy; GDS = Geriatric Depression Scale; APOE = apolipoprotein E; ε = epsilon.
*Chi-square test or t-test.
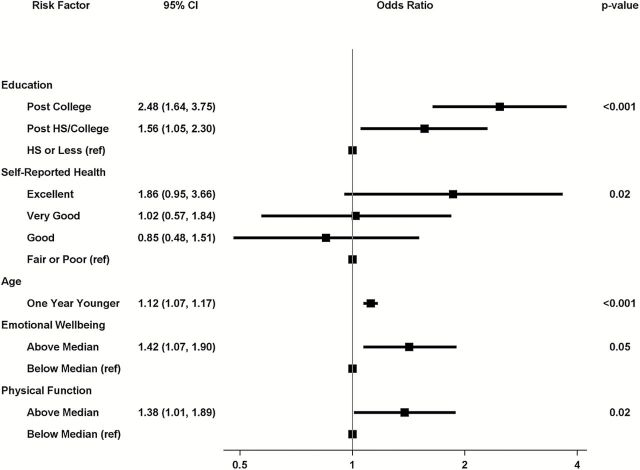
Application of backward stepwise logistic regression to the raw data yielded the following independent predictors for sustained high preserved cognitive function (Figure 3). Compared with women with a high school education or less, those with post-college (OR = 2.48, [1.64–3.75]) or a college education (OR = 1.56, [1.05–2.30]) had greater odds for sustained high preserved cognition. Compared to women reporting with fair/poor health, those with excellent self-reported health (OR = 1.86 [0.95–3.66]) were more likely to have sustained high preserved cognition. In addition, greater odds for sustained high preserved cognitive function were seen in those who had higher emotional wellbeing (OR = 1.42 [1.07–1.90]) and physical function (OR = 1.38 [1.01–1.89]) scores. Younger age was also associated with better odds for sustained high preserved cognitive functioning: OR = 1.12/y [1.07–1.17]. We repeated these analyses, eliminating the 24 women whose preserved cognitive functioning status was based on a single TICS-m score. Education, self-reported health, age, and emotional well-being were selected by the backward stepwise logistic regression, with comparable odds ratios and p-values. However, physical function was not selected. Instead, endorsing no prior history of cardiovascular disease was associated with better odds for sustained high preserved cognitive functioning, relative to history (OR = 1.55 [1.04–2.30], p = .03).
Figure 3.
Factors selected by backward stepwise logistic regression as being independently predictive of sustained high preserved cognitive function.
Across five databases created with multiple imputation, greater education, better general health, younger age, and better emotional well-being were selected every time as being predictive of consistently high preserved cognition. Higher physical functioning was selected three times. No prior history of CVD was selected twice. No other factors were selected in any of the five imputed databases.
Discussion
The predictors of preserved cognitive health in women ≥80 years included fewer depressive symptoms, greater education, non-Hispanic race/ethnicity, higher family income, younger age, reporting better emotional wellbeing, reporting more symptoms of insomnia, lack of diabetes history, being on lipid lowering medications, increased recreational physical activity, and not carrying the APOE-ε4 allele. Most of our findings are consistent with factors that are associated with less age-related cognitive decline and lower incidence of MCI and AD in older adults (2,6,7).
Few studies have examined the factors associated with cognitive impairment in those who survive to 80 years of age. In the Women, Cognitive Impairment Study of Exceptional Aging (WISE), oldest-old women with cognitive impairment had lower education, history of stroke, and higher prevalence of depressive symptoms, relative to those with normal cognition (2). Elevated depressive symptoms were also associated with increased incidence of MCI and dementia among women living to their ninth decades (15). On the other hand, APOE status did not differentiate those with cognitive impairment from those with normal cognition in the WISE (2), which is in contrast to our results. The presence of APOE-ε4 allele is thought to lose its significance in predicting AD, the most prevalent type of dementia, with advancing age (16). However, recent studies have found that plaques and tangles are predictors of dementia independent of age (17). In the 90+ Study, APOE-ε4 carriers were more likely to have dementia and higher beta-amyloid neuropathology, whereas ε2 carriers had better cognition and lower total beta-amyloid burden (18). Taken together, our results add to the literature by suggesting that not possessing the APOE-ε4 allele continues to play an important role as a predictor of preserved cognitive function even in women 80 years or older.
Women without diabetes and those being prescribed lipid-lowering medications (a proxy for history and treatment of hyperlipidemia in our cohort) had increased odds of having preserved cognitive health. In supporting multiple imputation analyses, we also found evidence that increased physical activity and higher BMI (with control for diabetes status) were associated with preserved cognitive function (19,20). However, we found no associations for hypertension, smoking and history of cardiovascular disease. The relationships of vascular risk factors and cardiovascular disease with cognitive functioning may attenuate with age due to differential survival rates. Those with these conditions are more likely to have poorer health, which may result in premature mortality. Our results however point to an alternate process, which some have described as a failure-of-success effect (21). That is, as the health benefits from effective disease prevention, improved treatment interventions and healthier lifestyle practices advance life expectancy, the 80+ cohort will include survivors with relatively poorer health who may have died sooner if they had been born in a time period with less favorable conditions. Our findings therefore suggest that the absence (eg, diabetes in our cohort) or adequate treatment (ie, hyperlipidemia) of certain vascular factors may remain significant predictors of preserved cognitive function as women enter into the oldest-old cohort. Another possibility is that the association of lipid lowering drugs with better cognitive function may reflect better access to health care rather than adequate management of hyperlipidemia. Also, the association between higher BMI and preserved cognition may reflect weight loss with onset of MCI and AD, rather than a protective effect of higher BMI.
Our results that higher insomnia complaints are associated with preserved cognitive functioning are in contrast to some but not all studies. Insomnia has been associated with increased incidence of cognitive decline, MCI and AD (22,23). However, others have found no such relationships (24). Sleep deprivation increases soluble beta-amyloid concentration in the brain; this risk may be higher for those with chronic insomnia (25,26). Shorter sleep duration and poorer sleep quality have also been associated with greater beta-amyloid burden in community-dwelling older adults (27). However, others have reported that excessive daytime sleepiness and not nighttime insomnia predicts cognitive decline in those who develop dementia (24). Frequent napping occurs with preclinical cognitive decline. Changes in the regular patterns of sleep over time, and not increase or decrease in duration, may be associated with poorer cognitive function (28). Furthermore, sleep disordered breathing is associated with AD biomarkers and an increased risk of dementia in the elderly, suggesting a role of hypoxia and not sleep fragmentation as a link between sleep disturbance and incident cognitive decline (29). Our study only evaluated complaints of nocturnal insomnia, and did not assess for chronicity of insomnia complaints, daytime sleepiness, and different etiologies of sleep disturbances or for sleep medication use in our cohort. Moreover, while insomnia scores were significantly higher in the preserved cognitive function group than those with cognitive impairment, the severity of insomnia complaints in both groups was below the threshold that signified problematic insomnia (30). A more comprehensive understanding of the bidirectional associations of sleep-wake cycle characteristics with AD neuropathology and cognitive decline may have important therapeutic implications in different stages of preclinical and syndromal dementia states (31).
As expected, the predictors of sustained high preserved cognitive function included younger age and greater education (9). The effects of education further underscore the role of cognitive reserve capacity in maintaining cognitive function even into later years (32). Also, other factors that have been associated with maintenance of preserved cognitive function in prior studies included lack of vascular factors (hypertension, diabetes), healthier lifestyle choices (lack of smoking, moderate alcohol consumption, and physical exercise), better activities of daily living and social networks. However, we did not find environmental and medical/psychiatric factors to predict maintenance of high preserved cognition; instead, quality of life measures (better self-reported general health, and better emotional well-being and physical functioning) were more strongly associated with consistently high preserved cognitive performance in the oldest-old women. Discrepancies between our findings and prior studies are most likely due to the differences among studies in the age distributions, duration of follow-up, definition of cognitive groups, and assessment methods. The previous study also did not formally assess for MCI or probable dementia and therefore may have included those with cognitive impairment.
The major component that was consistently used in defining successful aging in earlier studies was physical functioning and disability, with self-reported general health and emotional variables included less often (33). However, a greater emphasis is placed on factors associated with health-related quality of life (including physical and emotional functioning) and less on disability, diseases and demographic characteristics when older adults are asked for their own definitions of successful aging (34,35). There is also an increased recognition that “successful aging” is a multidimensional phenotype that includes preserved cognitive functioning especially in those who have survived into later years of life despite having chronic diseases and disabilities. Maintainers of cognitive function also have lower mortality and incident disability than minor and major decliners among older adults, supporting the view that maintenance of preserved cognitive function is an important component in defining successful aging.
In a previous study that included women aged 65 years and older from the WHI cohort, physical-social functioning and emotional functioning (that included self-reported general health and emotional wellbeing measures) constituted factors that strongly predicted outcomes related to positive aging phenotype (36). Several demographic, psychosocial, health- and lifestyle-related factors were found to influence distinct trajectories of change in the physical-social and emotional functioning dimensions over time, further suggesting the multidimensional nature of successful aging (37).
It is interesting that better emotional wellbeing and lower depressive symptom severity independently predicted better cognition, suggesting that wellbeing captures an important aspect of health that is independent of depression. This finding harkens back to the critical question of whether so-called “negative” psychological experience hinders healthy aging, “positive” psychological experience optimizes it, or both (38). In this study of maintenance of cognition into older age, it appears that both are important. It is also interesting that optimism was not associated with maintenance of cognition in this sample of WHIMS participants in adjusted models. One possible explanation is that “effect” sizes for health outcomes are moderate, requiring a larger sample size or a comparison of individuals at the extremes of the character trait (ie, high pessimism vs high optimism). This question requires further study.
The women included in this study participated in the WHIMS hormone therapy (HT) trial. Our findings suggest that HT exposure was not related to cognitive status in the current cohort. These results may therefore indicate a lack of long-term detrimental effects on cognition from hormone use in this oldest-old cohort. However, prior studies across broader WHIMS cohorts have found differences between the intervention groups to persist for some time even after the HT trial had ceased, both for cognitive function and for brain volumes (39–41).
Specific biomarkers, including genetic, inflammatory, and oxidative stress may influence successful cognitive aging (42). While we found that the absence of APOE-ε4 allele predicted preserved cognitive function, we did not find an association between APOE status and sustained high preserved cognitive performance in cognitively normal women. It is plausible that other genetic vulnerabilities and neurobiologic mechanisms and their interactions with medical, psychosocial and behavioral factors may play a role in sustaining high preserved cognitive function in the oldest-old women, and that by excluding women with MCI or dementia from these analyses we may have attenuated relationships.
Some limitations may affect the interpretation of our results. Our cohort comprised of only those women who participated in the WHIMS HT trial; therefore, generalizability of our findings needs to be demonstrated. We cannot make causal inferences. Although we included the most contemporaneous values for predictors, some are distributed across a broad timeframe that varied among women. We cannot rule out the possibility of residual confounding bias. In addition, some measures were occasionally missing; therefore, multiple imputations were used in supporting analyses. Losses to follow-up differentially culled women with chronic diseases, lower education, and lower cognitive function, which may have attenuated some relationships (43). We defined risk factors (eg, vascular, lifestyle factors) and treatment (eg, medication use) based on the most recent assessment or last medication inventory prior to the cognitive assessment. Inclusion of data regarding persistence of specific conditions or treatment in the analysis may have served as better predictors of cognitive function; however, because the prior observation periods among women varied, given the study design, it is difficult to address this issue in this study. Although we used a single validated measure of overall cognitive function to define consistently high performers, it is possible that factors that predict changes in specific cognitive domains over time are different.
In summary, addressing certain sociodemographic factors such as income inequality and targeting specific treatment interventions to improve depressive symptoms, and vascular risk factors may play an important role in preserving cognitive functioning among women who survive to 80 years of age. Person-centered approaches that combine interventions to improve physical and cognitive functioning and specific psychosocial treatments to address emotional wellbeing may promote maintenance of preserved cognitive aging in the oldest-old. Such treatments may be crucial to improve quality of life in those women who survive into their later years without pathological cognitive decline.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
The Women’s Health Initiative (WHI) program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100–2, 32105–6, 32108–9, 32111–13, 32115, 32118–32119, 32122, 42107–26, 42129–32, and 44221. S.M.R. is supported by the Intramural Research Program, National Institute on Aging, and National Institutes of Health. J.S.G. is supported by the Alzheimer’s Association New Investigator NIRG-11-204070 and Extendicare Foundation. The active study drug and placebo were supplied by Wyeth-Ayerst Research Laboratories, Philadelphia, Pennsylvania. The Women’s Health Initiative Memory Study was funded in part by Wyeth Pharmaceuticals as an ancillary study to the WHI. Wyeth Pharmaceuticals did not participate in the design and conduct of the studies, in the collection, analysis, and interpretation of the data, or in preparation, review or approval of this manuscript.
Supplementary Material
Acknowledgment
This work was accepted as an oral presentation at the Gerontological Society of America 2014 Annual Meeting in Washington, DC (November 5–9, 2014).
References
- 1. Hebert LE, Scherr PA, Beckett LA, et al. Age-specific incidence of Alzheimer’s disease in a community population. JAMA. 1995;273:1354–1359. [PubMed] [Google Scholar]
- 2. Yaffe K, Middleton LE, Lui LY, et al. Mild cognitive impairment, dementia, and their subtypes in oldest old women. Arch Neurol. 2011;68:631–636. doi:10.1001/archneurol.2011.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yaffe K, Lindquist K, Vittinghoff E, et al. The effect of maintaining cognition on risk of disability and death. J Am Geriatr Soc. 2010; 58:889–894. doi:10.1111/j.1532-5415.2010.02818.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fiocco AJ, Yaffe K. Defining successful aging: the importance of including cognitive function over time. Arch Neurol. 2010;67:876–880. doi:10.1001/archneurol.2010.130 [DOI] [PubMed] [Google Scholar]
- 5. Albert MS, Jones K, Savage CR, et al. Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychol Aging. 1995;10:578–589. [DOI] [PubMed] [Google Scholar]
- 6. Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:13–22. [DOI] [PubMed] [Google Scholar]
- 7. Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–828. doi:10.1016/S1474-4422(11)70072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnes DE, Cauley JA, Lui LY, et al. Women who maintain optimal cognitive function into old age. J Am Geriatr Soc. 2007;55:259–264. [DOI] [PubMed] [Google Scholar]
- 9. Yaffe K, Fiocco AJ, Lindquist K, et al. Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology. 2009;72:2029–2035. doi:10.1212/WNL.0b013e3181a92c36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shumaker SA, Reboussin BA, Espeland MA, et al. The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials. 1998;19:604–621. [DOI] [PubMed] [Google Scholar]
- 11. Rapp SR, Legault C, Espeland MA, et al. Validation of a cognitive assessment battery administered over the telephone. J Am Geriatr Soc. 2012;60:1616–1623. doi:10.1111/j.1532-5415.2012.04111.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ellis RJ, Jan K, Kawas C, et al. Diagnostic validity of the dementia questionnaire for Alzheimer disease. Arch Neurol. 1998;55:360–365. [DOI] [PubMed] [Google Scholar]
- 13. Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet. 2012;44:955–959. doi:10.1038/ng.2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yuan Y. Multiple imputation using SAS software. J Statist Software. 2011;45:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeki Al Hazzouri A, Vittinghoff E, Byers A, et al. Long-term cumulative depressive symptom burden and risk of cognitive decline and dementia among very old women. J Gerontol A Biol Sci Med Sci. May 2014;69(5):595–601. doi:10.1093/gerona/glt139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Breitner JC, Wyse BW, Anthony JC, et al. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53:321–331. [DOI] [PubMed] [Google Scholar]
- 17. Dolan D, Troncoso J, Resnick SM, Crain BJ, Zonderman AB, O’Brien RJ. Age, Alzheimer’s disease and dementia in the Baltimore Longitudinal Study of Ageing. Brain. 2010;133(Pt 8):2225–2231. doi:10.1093/brain/awq141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berlau DJ, Corrada MM, Robinson JL, et al. Neocortical β-amyloid area is associated with dementia and APOE in the oldest-old. Alzheimers Dement. 2013;9:699–705. doi:10.1016/j.jalz.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–342. doi:10.1001/archneurol.2008.582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang S, Luo X, Barnes D, Sano M, Yaffe K. Physical activity and risk of cognitive impairment among oldest-old women. Am J Geriatr Psychiatry. November 2014;22(11)1149–1157. 10.1016/j.jagp.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Christensen K, Thinggaard M, Oksuzyan A, et al. Physical and cognitive functioning of people older than 90 years: a comparison of two Danish cohorts born 10 years apart. Lancet. 2013;382:1507–1513. doi:10.1016/S0140-6736(13)60777-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Osorio RS, Pirraglia E, Agüera-Ortiz LF, et al. Greater risk of Alzheimer’s disease in older adults with insomnia. J Am Geriatr Soc. 2011;59:559–562. doi:10.1111/j.1532-5415.2010.03288.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006;20:41–48. [DOI] [PubMed] [Google Scholar]
- 24. Jaussent I, Bouyer J, Ancelin ML, et al. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep. 2012;35:1201–1207. doi:10.5665/sleep.2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Naidoo N, Ferber M, Master M, Zhu Y, Pack AI. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci. 2008;28:6539–6548. doi:10.1523/JNEUROSCI.5685-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi:10.1126/science.1180962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spira AP, Gamaldo AA, An Y, et al. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurology. 2013; 70: 1537–1543. doi:10.1001/jamaneurol.2013.4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimäki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011;34:565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi:10.1001/jama.2011.1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levine DW, Kripke DF, Kaplan RM, et al. Reliability and validity of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15:137–148. [DOI] [PubMed] [Google Scholar]
- 31. Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol. 2014;10:115–119. doi:10.1038/nrneurol.2013.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–1012. doi:10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Depp CA, Jeste DV. Definitions and predictors of successful aging: a comprehensive review of larger quantitative studies. Am J Geriatr Psychiatry. 2006;14:6–20. [DOI] [PubMed] [Google Scholar]
- 34. Bowling A, Dieppe P. What is successful ageing and who should define it? BMJ. 2005;331:1548–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Montross LP, Depp C, Daly J, et al. Correlates of self-rated successful aging among community-dwelling older adults. Am J Geriatr Psychiatry. 2006;14:43–51. [DOI] [PubMed] [Google Scholar]
- 36. Woods NF, Cochrane BB, LaCroix AZ, et al. Toward a positive aging phenotype for older women: observations from the women’s health initiative. J Gerontol A Biol Sci Med Sci. 2012;67:1191–1196. doi:10.1093/gerona/gls117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zaslavsky O, Cochrane BB, Woods NF, et al. Trajectories of positive aging: observations from the women’s health initiative study. Int Psychogeriatr. 2014;26:1–12. doi:10.1017/S1041610214000593 [DOI] [PubMed] [Google Scholar]
- 38. Tindle H, Davis E, Kuller L. Attitudes and cardiovascular disease. Maturitas. 2010;67:108–113. doi:10.1016/j.maturitas.2010.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Espeland MA, Brunner RL, Hogan PE, et al. Long-term effects of conjugated equine estrogen therapies on domain-specific cognitive function: results from the Women’s Health Initiative study of cognitive aging extension. J Am Geriatr Soc. 2010;58:1263–1271. doi:10.1111/j.1532-5415.2010.02953.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Espeland MA, Tindle HA, Bushnell CA, et al. Brain volumes, cognitive impairment, and conjugated equine estrogens. J Gerontol A Biol Sci Med Sci. 2009;64:1243–1250. doi:10.1093/gerona/glp128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Resnick SM, Espeland MA, Jaramillo SA, et al. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology. 2009;72:135–142. doi:10.1212/01.wnl.0000339037.76336.cf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Glatt SJ, Chayavichitsilp P, Depp C, Schork NJ, Jeste DV. Successful aging: from phenotype to genotype. Biol Psychiatry. 2007;62:282–293. [DOI] [PubMed] [Google Scholar]
- 43. Espeland MA, Pettinger M, Falkner KL, et al. Demographic and health factors associated with enrollment in posttrial studies: the Women’s Health Initiative Hormone Therapy Trials. Clin Trials. 2013;10:463–472. doi:10.1177/1740774513477931 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.