Abstract
Background:
Intensive Care Unit (ICU) acquired weakness is a common complication in critically ill patients affecting their prognosis. The handheld dynamometry is an objective method in detecting minimum muscle strength change, which has an impact on the physical function of ICU survivors. The minimal change in the force can be measured in units of weight such as pounds or kilograms.
Aim of the Study:
To detect the changes in peripheral muscle strength with handheld dynamometer in the early stage of ICU stay and to observe the progression of muscle weakness.
Methodology:
Three upper and three lower limb muscles force measured with handheld dynamometer during ICU stay. Data were analyzed using repeated measures ANOVA to detect changes in force generated by muscle on alternate days of ICU stay.
Results:
There was a reduction in peripheral muscle strength from day 3 to day 5 as well from day 5 to day 7 of ICU stay (P < 0.01). The average reduction in peripheral muscle strength was 11.8% during ICU stay.
Conclusion:
This study showed a progressive reduction in peripheral muscle strength as measured by handheld dynamometer during early period of ICU stay.
Keywords: Critical care units, critical illness polyneuromyopathy, immobilization, objective assessment, physiotherapy
Introduction
The Intensive Care Unit (ICU) is highly specified and sophisticated area of a hospital which is specifically designed, staffed, located, furnished and equipped, dedicated to the management of critically ill.[1] Prolonged stay in ICU may precipitate complications such as ventilator-associated pneumonia, deep vein thrombosis, ICU-acquired weakness (ICUAW), blood stream infections, and abnormal gastrointestinal bleeding.[2] Prolonged bed rest involving skeletal muscle dysfunction may hamper the rehabilitation of ICU survivors.[3] The significant decrease in muscle strength during ICU stay is a prognostic indicator of critical illness in ICU survivors.[4]
ICUAW is clinically detected weakness in critically ill patients in whom there is no plausible etiology other than critical illness.[5] It is multifactorial and predominantly due to muscle inactivity, sepsis, corticosteroid, neuromuscular blockers, and hyperglycemia that results in a reduction of force generating capacity of muscles.[5,6] The physical inactivity results in significant loss of muscle mass and strength up to 1% to 5% per day in critically ill patients.[3,7]
The incidence of weakness in mechanically ventilated critically ill patients has been noted to be 58% on electrophysiological evaluation and 25–33% on clinical evaluation.[8] As electrophysiological testing is not always practical or feasible in ICUs, its clinical applicability is limited for measuring ICUAW.[9,10] This highlights the need for objective and reliable methods to measure the presence of muscle weakness and to determine the effects of interventions on its progression.[11]
Measuring the peripheral muscle strength manually requires patient's cooperation. The Richmond Agitation and Sedation Scale (RASS, r = 0.956) is a valid and reliable tool for categorizing sedation and agitation levels in patients in ICU.[12] The different methods to assess ICUAW are an electrophysiological examination, manual muscle testing (MMT) (medical research council [MRC] score), and dynamometry. The electrophysiological examination can be performed at an early stage, but it is time-consuming, technically challenging, expensive, and not readily available in all ICUs.[5,13] The MRC scale is an ordinal measure limited by its sensitivity at the higher grades 4 and 5 that have been noticed in patients recovering from critical illness. The dynamometer can detect this change in a more objective manner as even minimum muscle strength loss can have an impact on the physical function of ICU survivors.[11,14,15,16] These devices usually record the force produced by loading through tension or compression and often used for quantification of muscle strength.[11,17] The minimal change in the peak torque value can be measured in units of weight such as pounds or kilograms.[18,19,20]
It is truly challenging to detect physical functional abnormalities early in the course of critical illness.[21] Hence, dynamometry has been accepted as reliable skeletal muscle strength measuring apparatus in ICU population,[11] however, its ability to detect the progression of ICUAW has not been tested.
There is a need for simple clinical assessment method to measure ICUAW in critically ill patients at the earliest. There is a paucity of literature about the hand-held dynamometer to detect the change in force generated by muscles in ICU patients. The aim was to observe the changes in dynamometry values for biceps, triceps, deltoid in upper extremity and quadriceps, dorsiflexors, plantar flexors in lower extremity during ICU stay.
Methodology
This is an observational cohort study done after approval from Institutional Ethical Committee. Critically ill patients, who were admitted at least for 3 days in ICU, were screened. After taking informed consent, patients whose RASS score was −1 to +1 and Attention Screening Examination (ASE) score was ≥8, were included in this study. Biceps, triceps, deltoid in upper extremity and quadriceps, dorsiflexors, and plantar flexors in lower extremity were assessed bilaterally. The handheld dynamometer was used to measure the force generated by each muscle in critically ill patients on day 3. The procedure was repeated on 5th and 7th day of ICU stay by the same examiner. On each test day, the alertness and attention of critically ill subjects were assessed with the RASS and ASE score.
Subjects
The critically ill patients were screened in ICUs of tertiary care hospital, Karnataka from February 2013 to February 2014. Patients were excluded if they had an absolute contraindication that prevented peripheral muscle strength testing, an acute or preexisting neurologic condition, cardiovascular condition, organophosphate poisoning, a fracture in the assessment limb, a cognitive/intellectual/psychiatric impairment that impaired capacity to follow verbal instructions and those who started walking before 7th day from ICU admission. The patients on a mechanical ventilator and supplemental oxygen therapy were included in the study. The sample size was calculated using formula  anticipating a standard deviation (σ) = 5.13 (based on a pilot study of six critically ill patients), at 5% level of significance, clinically significance difference (d) =2, and 80% power, which was approximately 70.
anticipating a standard deviation (σ) = 5.13 (based on a pilot study of six critically ill patients), at 5% level of significance, clinically significance difference (d) =2, and 80% power, which was approximately 70.
Procedure for measurement of muscle strength using handheld dynamometer
The handheld dynamometer was used to measure the force generated by each muscle. One wooden board was placed under the limb to be tested to avoid the effect of water bed in ICU. The patients were instructed to perform maximum isometric contraction during dynamometry measures. The dynamometer was placed perpendicular to limb to be tested. The selected muscles were tested in the supine position as it was the most convenient for critically ill patients. Resistance was applied by the examiner to avoid movement of the limb that was tested. The rest period of one minute was given between two consecutive trials. The patients had to perform three efforts for each muscle and the best reading among three trials was recorded. The upper extremity muscles were assessed first and then the lower extremity muscles. Similar procedures were followed on 5th and 7th day of ICU stay. Standard physiotherapy management was delivered to the critical care patients as per the ICU protocol of the hospital.
Statistical analysis
SPSS version 15 (SPSS Inc. Released 2006. SPSS for Windows, Version 15.0. Chicago, SPSS Inc.) was used for data analysis. Repeated measures ANOVA were used to analyze the changes in force generated by the muscle on alternate days in ICU. The level of significance was set at < 0.05.
Results
A total of 184 patients were screened during the study period. Fifty-one subjects did not meet RASS and ASE score, to participate in the study. Seventy-eight subjects were recruited for the study. The assessment of 14 subjects was incomplete as they were shifted from ICU within 7 days of admission (n = 12), and death (n = 2) during the study period. Sixty-four subjects completed the study [Figure 1].
Figure 1.
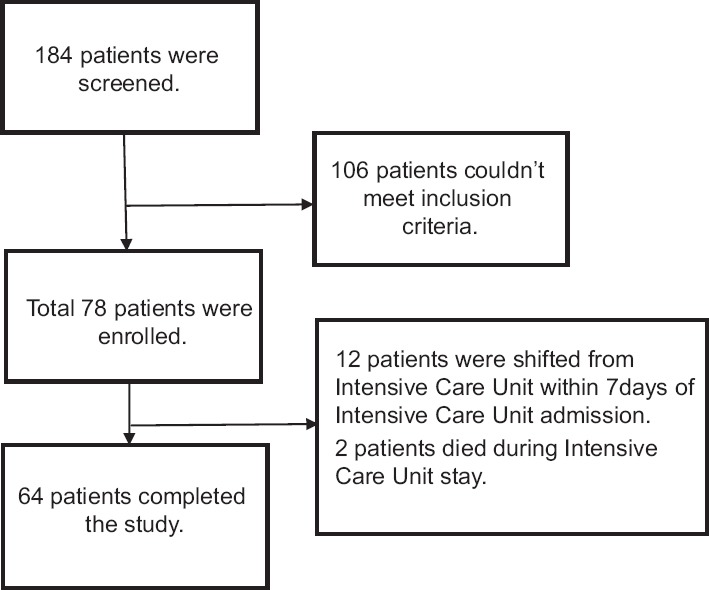
Flow of recruitment of subjects
Among the study population (n = 64) 33% subjects were on oxygen therapy. Fourteen subjects were on ventilator support with FiO2 ranges from 0.4 to 0.5. Six subjects had an acute exacerbation of COPD, maintaining oxygen saturation >90% during assessment [Table 1].
Table 1.
Baseline characteristics of critically ill patients (n=64)

There was a reduction in force generated by each muscle from day 3 to day 5 as well from day 5 to day 7 of ICU stay [Table 2]. This reduction in value of force was significant with P < 0.05 in both upper limb and lower limb muscles.
Table 2.
Comparison of means of dynamometry values (in pounds) through day 3 to day 7

The average reduction in peripheral muscle strength observed in the study was 11.8% during ICU stay. The percentage reduction was 13% in deltoid and quadriceps muscle groups. The minimum reduction in force was 10% in dorsiflexors and plantarflexors during 7 days of ICU stay [Figure 2].
Figure 2.

Percentage (%) reduction in muscle strength with dynamometry values from day 3 to day 7
Discussion
We aimed to observe the changes in dynamometry values for biceps, triceps, deltoid in upper extremity and quadriceps, dorsiflexors, and plantar flexors in lower extremity during the subjects′ ICU stay. In our study, we observed a reduction in muscle force measured by handheld dynamometer in the above muscle groups through day 3 to day 7 of ICU stay. The hand-held dynamometer was found to be sensitive in detecting the muscle strength reduction in critically ill. Findings of our study were consistent with the earlier study by Garnacho-Montero et al.,[22] who similarly found that ICUAW is associated with reduced muscle strength, using electrophysiological examination.
In our study, the overall reduction in force detected with dynamometer was 10–13% for the analyzed peripheral muscles. As reported by Hermans et al.[6] weakness in the proximal muscles were more than distal muscle groups, in critically ill. We also observed a greater reduction in quadriceps muscle strength (13%) as compared to plantarflexors and dorsiflexors (10%). ICUAW reflected by reduced muscle force has been attributed to several underlying pathologies.[8,23] It has been suggested in previous studies that the quadriceps muscle has considerable protein reservoir that may be a target of increased proteolysis and preferential type II fiber atrophy.[24] Studies have shown that muscles with type I fibers have a high degree of immobilization-induced atrophy, such as adductor pollicis and diaphragm muscles.[25] It is known that type II fibers are responsible for force production as they are rich in glycogen. Quadriceps muscle contains approximately 48% type II fibers. This could be a probable reason for greater reduction in force in the quadriceps muscle. In the upright position, middle fibers of deltoid muscle continuously work to hold humerus in a neutral position. While in critically ill subjects, because of recumbent supine position, antigravity action of deltoid muscle may get hampered. This might have caused disuse muscle atrophy and impart marginal reduction (13%) in the strength of deltoid muscle during ICU stay.
Studies have shown that critically ill subjects with impaired peripheral circulation, show low skin and muscle temperature, possibly due to cytokine effect.[26] Moreover, drugs that cause vasoconstriction may also decrease blood flow to muscle, skin resulting in reduced temperature.[27] Studies on adductor pollicis muscle have shown that reduction in temperature affects the maximal force and isometric force in critically ill subjects.[25] Elevated reactive oxygen species or nitric oxide levels can result in contractile dysfunction causing muscle weakness and fatigue.[28]
The reduction in muscle strength in ICU subjects might have been due to systemic inflammation in the body, poor glycemic control, and muscle inactivity itself. Sedatives and corticosteroids are well-known drugs for enhancing muscle weakness in critically ill. It has been shown in few studies that inflammatory markers like plasma Interleukin-6 is associated with myosin loss in critically ill subjects.[29] Most of the previous studies have reported a predominance of myopathic changes in the form of muscle necrosis or myosin loss and muscle atrophy.[8] Hence, sepsis is considered as a major risk factor for ICUAW[27] although in our study only 11% subjects had sepsis. It is still unclear whether the reduction in muscle strength is related to ICUAW or due to the disease progression itself.
Though most of the earlier studies reported muscle strength reduction using MMT and MRC score, we quantified the loss of muscle strength using a dynamometer, which is a more objective method as compared to MMT. Thus, to mobilize the critically ill subjects, dynamometer can be proposed as an important alternative to MMT in routine physiotherapy assessment.
The limitation of this study is that we did not assess the fatigue level of subjects during dynamometry measurement that may have influenced the results. Since subjects with critical illness are not routinely subjected to electrophysiological examination on admission, any previously undiagnosed neuropathies were not segregated in our study. This could have been a possible confounding factor as we were trying to detect newly AW and its progression. Age-related neuropathic changes could have also influenced the study as 9% of the subjects were more than 65 years. Our study had 5 chronic obstructive pulmonary diseases which could have also influenced the study results. However, we chose not to exclude as acute exacerbation of COPD is one of the conditions which require ICU admission.
To avoid the impact on sedation level on muscle strength performance, and reduction in muscle strength, RASS score was recorded each time prior to assessment. Gross peripheral edema that can affect muscle performance was not existent in any of our study subjects. There were no adverse events recorded during assessment in critically ill subjects.
ICUAW is considered as a rapidly progressing complication among critically ill subjects. Bed-mobility exercises alone may not be sufficient to alleviate the progression of muscle weakness. In our study, all subjects who were only on bed mobility exercises showed reduced muscle strength. So for hemodynamically stable critically ill subjects, strengthening exercises may be encouraged.
The significant reduction in strength of antigravity muscles may have an impact on rehabilitation of ICU survivors. Early detection of a loss in muscle strength and adopting appropriate measures to prevent such muscle weakness may help in the early and successful rehabilitation of subjects who are critically ill.
Scope of future research
The validation of dynamometry needs to be established with a gold standard method such as electrophysiological examination.
Conclusion
This study showed a progressive reduction in peripheral muscle strength as measured by handheld dynamometer during the early period of ICU stay.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.ICU Planning and Designing in India-Guidelines 2010. Indian Society for Critical Care Medicine. 2010. [Last cited on 2015 Sep 16]. Available from: http://www.isccm.org/images/Section1.pdf .
- 2.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011;39:371–9. doi: 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- 3.Chambers MA, Moylan JS, Reid MB. Physical inactivity and muscle weakness in the critically ill. Crit Care Med. 2009;37(10 Suppl):S337–46. doi: 10.1097/CCM.0b013e3181b6e974. [DOI] [PubMed] [Google Scholar]
- 4.Puthucheary Z, Montgomery H, Moxham J, Harridge S, Hart N. Structure to function: Muscle failure in critically ill patients. J Physiol. 2010;588(Pt 23):4641–8. doi: 10.1113/jphysiol.2010.197632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schefold JC, Bierbrauer J, Weber-Carstens S. Intensive care unit-acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J Cachexia Sarcopenia Muscle. 2010;1:147–157. doi: 10.1007/s13539-010-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermans G, Clerckx B, Vanhullebusch T, Segers J, Vanpee G, Robbeets C, et al. Interobserver agreement of medical research council sum-score and handgrip strength in the intensive care unit. Muscle Nerve. 2012;45:18–25. doi: 10.1002/mus.22219. [DOI] [PubMed] [Google Scholar]
- 7.De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, et al. Paresis acquired in the intensive care unit: A prospective multicenter study. JAMA. 2002;288:2859–67. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 8.Deem S. Intensive-care-unit-acquired muscle weakness. Respir Care. 2006;51:1042–52. [PubMed] [Google Scholar]
- 9.Waak K, Zaremba S, Eikermann M. Muscle strength measurement in the intensive care unit: Not everything that can be counted counts. J Crit Care. 2013;28:96–8. doi: 10.1016/j.jcrc.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Bednarik J, Lukas Z, Vondracek P. Critical illness polyneuromyopathy: The electrophysiological components of a complex entity. Intensive Care Med. 2003;29:1505–14. doi: 10.1007/s00134-003-1858-0. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin CE, Paratz JD, Bersten AD. Muscle strength assessment in critically ill patients with handheld dynamometry: An investigation of reliability, minimal detectable change, and time to peak force generation. J Crit Care. 2013;28:77–86. doi: 10.1016/j.jcrc.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O′Neal PV, Keane KA, et al. The Richmond agitation-sedation scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 13.Deem S, Lee CM, Curtis JR. Acquired neuromuscular disorders in the intensive care unit. Am J Respir Crit Care Med. 2003;168:735–9. doi: 10.1164/rccm.200302-191UP. [DOI] [PubMed] [Google Scholar]
- 14.Vanpee G, Segers J, Van Mechelen H, Wouters P, Van den Berghe G, Hermans G, et al. The interobserver agreement of handheld dynamometry for muscle strength assessment in critically ill patients. Crit Care Med. 2011;39:1929–34. doi: 10.1097/CCM.0b013e31821f050b. [DOI] [PubMed] [Google Scholar]
- 15.Hough CL, Lieu BK, Caldwell ES. Manual muscle strength testing of critically ill patients: Feasibility and interobserver agreement. Crit Care. 2011;15:R43. doi: 10.1186/cc10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan E, Ciesla ND, Truong AD, Bhoopathi V, Zeger SL, Needham DM. Inter-rater reliability of manual muscle strength testing in ICU survivors and simulated patients. Intensive Care Med. 2010;36:1038–43. doi: 10.1007/s00134-010-1796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O′Shea SD, Taylor NF, Paratz JD. Measuring muscle strength for people with chronic obstructive pulmonary disease: Retest reliability of hand-held dynamometry. Arch Phys Med Rehabil. 2007;88:32–6. doi: 10.1016/j.apmr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Fan E, Zanni JM, Dennison CR, Lepre SJ, Needham DM. Critical illness neuromyopathy and muscle weakness in patients in the intensive care unit. AACN Adv Crit Care. 2009;20:243–53. doi: 10.1097/NCI.0b013e3181ac2551. [DOI] [PubMed] [Google Scholar]
- 19.Judemann K, Lunz D, Zausig YA, Graf BM, Zink W. Intensive care unit-acquired weakness in the critically ill: Critical illness polyneuropathy and critical illness myopathy. Anaesthesist. 2011;60:887–901. doi: 10.1007/s00101-011-1951-7. [DOI] [PubMed] [Google Scholar]
- 20.Click Fenter P, Bellew JW, Pitts TA, Kay RE. Reliability of stabilised commercial dynamometers for measuring hip abduction strength: A pilot study. Br J Sports Med. 2003;37:331–4. doi: 10.1136/bjsm.37.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JJ, Waak K, Grosse-Sundrup M, Xue F, Lee J, Chipman D, et al. Global muscle strength but not grip strength predicts mortality and length of stay in a general population in a surgical intensive care unit. Phys Ther. 2012;92:1546–55. doi: 10.2522/ptj.20110403. [DOI] [PubMed] [Google Scholar]
- 22.Garnacho-Montero J, Madrazo-Osuna J, García-Garmendia JL, Ortiz-Leyba C, Jiménez-Jiménez FJ, Barrero-Almodóvar A, et al. Critical illness polyneuropathy: Risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med. 2001;27:1288–96. doi: 10.1007/s001340101009. [DOI] [PubMed] [Google Scholar]
- 23.Schweickert WD, Hall J. ICU-acquired weakness. Chest. 2007;131:1541–9. doi: 10.1378/chest.06-2065. [DOI] [PubMed] [Google Scholar]
- 24.Bierbrauer J, Koch S, Olbricht C, Hamati J, Lodka D, Schneider J, et al. Early type II fiber atrophy in intensive care unit patients with nonexcitable muscle membrane. Crit Care Med. 2012;40:647–50. doi: 10.1097/CCM.0b013e31823295e6. [DOI] [PubMed] [Google Scholar]
- 25.de Ruiter CJ, Jones DA, Sargeant AJ, de Haan A. Temperature effect on the rates of isometric force development and relaxation in the fresh and fatigued human adductor pollicis muscle. Exp Physiol. 1999;84:1137–50. doi: 10.1017/s0958067099018953. [DOI] [PubMed] [Google Scholar]
- 26.Wang GC, Chi WM, Perng WC, Huang KL. Body temperature control in sepsis-induced acute lung injury. Chin J Physiol. 2003;46:151–7. [PubMed] [Google Scholar]
- 27.Wiles CM, Edwards RH. The effect of temperature, ischaemia and contractile activity on the relaxation rate of human muscle. Clin Physiol. 1982;2:485–97. doi: 10.1111/j.1475-097x.1982.tb00055.x. [DOI] [PubMed] [Google Scholar]
- 28.Smith MA, Reid MB. Redox modulation of contractile function in respiratory and limb skeletal muscle. Respir Physiol Neurobiol. 2006;151:229–41. doi: 10.1016/j.resp.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 29.van Hees HW, Schellekens WJ, Linkels M, Leenders F, Zoll J, Donders R, et al. Plasma from septic shock patients induces loss of muscle protein. Crit Care. 2011;15:R233. doi: 10.1186/cc10475. [DOI] [PMC free article] [PubMed] [Google Scholar]


