Abstract
Aim:
The purpose of this study was to evaluate the antibacterial efficacies of 2% chlorhexidine (CHX), N-acetyl cysteine (NAC) and assess their synergistic or antagonist action as intracanal medicament.
Materials and Methods:
Agar diffusion test was performed with 2% CHX, NAC, and their combination against E. faecalis planktonic cells. The diameters of the zones of bacterial inhibition were measured and recorded for each solution. The assay was further extended to 2 weeks old E. faecalis dentinal biofilm. Sixteen freshly extracted teeth were vertically sectioned into two halves resulting in a total of 32 samples. The samples were inoculated with bacterial suspension and incubated at 37°C for 2 weeks for biofilm formation. The samples were then divided into four experimental groups with 8 samples in each group. The samples were gently washed in saline and placed in culture wells containing the test solutions, i.e., 2% CHX, NAC, a combination of 2% CHX and NAC in 1:1 ratio, and a control group containing saline. The biofilm formed on the root canal surface were removed with a sterile scalpel and inoculated on blood agar plates to check for the formation of E. faecalis colonies.
Statistical Analysis:
For agar diffusion test, data were analyzed statistically using one-way analysis of variance and then by post-hoc Scheffe's test to compare the antimicrobial efficacy between the groups. Statistical analysis was not done for the cultures obtained from the biofilm as there was no growth in all the three test groups except the control group, i.e., saline.
Results:
In agar diffusion test, among the three groups tested, 2% CHX and NAC showed almost equal zones of inhibition whereas maximum inhibition was shown by a combination of NAC and 2% CHX suggesting a synergistic action. The results obtained were highly significant (P < 0.001) for the combination of medicament when compared to individual test group. In culture analysis, which was done for the biofilm, no growth was observed in all the three test groups. The results obtained were biologically significant but statistically insignificant.
Conclusion:
NAC has almost equal antimicrobial property as 2% CHX whereas their combination showed a synergistic action.
Keywords: Agar diffusion test, 2% chlorhexidine, E. faecalis dentinal biofilm, N-acetyl cysteine
INTRODUCTION
Even though E. faecalis makes up a small proportion of the flora in untreated canals, it is a persistent organism in root-filled teeth in which root canal therapy had failed. It can survive as single organism as well as a major component of the flora.[1] It becomes more resistant in the root canal system, whereas it can be easily destroyed in an open environment.[2] As E. faecalis can secrete proteases such as serine protease, gelatinase, and collagen-binding protein, it can easily bind to dentin firmly,[3] and thus it can live in dentinal tubules and endure prolonged times of starvation,[4] and E. faecalis biofilms are several folds more resistant to phagocytosis, antibodies, or antimicrobials than planktonic bacteria.[5]
Intracanal medicaments are an integral part and important adjunct to root canal therapy.
Chlorhexidine (CHX) has a wide range of antimicrobial activity against Gram-positive and Gram-negative bacteria, yeasts, dermatophytes, and some lipophilic viruses.[6] As an intracanal disinfectant, different forms of CHX (solution, gel, or controlled release devices) have been studied.[7,8,9]
N-acetyl cysteine (NAC) is a derivative of the amino acid L-cysteine.[10] It is a thiol-containing antioxidant and a mucolytic agent. It is a nonantibiotic drug with antibacterial properties which decreases biofilm formation by a variety of bacteria.[11] Disulfide bonds in mucus can be disrupted by this solution, and it thus reduces the viscosity of secretions.[12]
NAC exerts anti-inflammatory activity[13] as it has the ability to inhibit the expression and release of a variety of proinflammatory cytokines.[14] NAC solution is found to be an effective chemoprotectant and is used to protect the pulp and surrounding periradicular tissues.[15] NAC inhibits growth and eradicates biofilm of E. faecalis.[16] It effectively reduces extracellular polysaccharide production, disrupts mature biofilms, and therefore decreases the adhesion potential of the bacteria on surfaces.[17]
The present study was undertaken to evaluate the antibacterial efficacies of 2% CHX, NAC and assess their synergistic or antagonist action as intracanal medicament.
MATERIALS AND METHODS
Bacterial strain
The bacterial strains used in this study were E. faecalis (ATCC 29212) grown anaerobically on brain heart infusion (BHI) agar at 37°C for 24 h.
Determination of zones of inhibition through agar diffusion test
Initially, a qualitative assay was performed using agar well diffusion test. Eight Petri dishes containing BHI agar enriched with defibrinated sheep blood were seeded with E. faecalis isolates. In each Petri dish, three wells measuring 6 mm diameter were prepared, and the three test solutions were delivered in the prepared wells through a micropipette. Agar plates were incubated for 24 h at 37°C.
The experimental groups for agar diffusion test were as follows:
Group 1: 2% CHX
Group 2: NAC (200 mg/ml) (Sisco Research Laboratories, Mumbai, India)
Group 3: A combination of 2% CHX and NAC.
NAC solution at a concentration of 200 mg/mL was freshly prepared by dissolving 0.2 g in 1 mL of sterile distilled water according to Quah et al.[16] The diameters of the zones of bacterial inhibition were measured with a digital Vernier caliper (Precision Scientific Instruments Corporation, Delhi, India). Measurement accuracy: ±0.03 mm and recorded for each solution. The assay was further extended to 2 weeks in vitro biofilm formed on tooth samples.
Experimental groups for culture analysis on biofilm were as follows:
Group 1: Saline (control)
Group 2: 2% CHX
Group 3: NAC (200 mg/ml) (Sisco Research Laboratories, Mumbai, India)
Group 4: Combination of NAC and 2% CHX.
Biofilm formation on tooth substrate
The tooth samples for biofilm formation were prepared and sterilized.[18] Sixteen intact, unrestored noncarious, mature human single-rooted freshly extracted teeth for orthodontic or periodontal reason were selected. Teeth were cleaned and stored in normal saline solution at room temperature and used within 3 months after extraction. Teeth were decoronated using a diamond disc in a low speed straight handpiece. Then, working length for each root was recorded using a size 10 K-file. Apical enlargement of all samples was done up to size 40, and the root canals were prepared with step back technique using hand K-files. During the process of instrumentation, canals were irrigated with 2 ml of 3% NaOCl. All the teeth were then vertically sectioned into two halves to obtain 32 samples. The concave tooth surface was minimally grounded to achieve a flat surface to enable placement in the tissue culture wells, exposing the root canal surface to E. faecalis to form a biofilm. The sectioned samples were then divided into four experimental groups. Each group consisted of eight samples. The samples were placed in the tissue culture wells. Wells containing tooth samples were inoculated with 2 ml of bacterial suspension and incubated at 37°C for 2 weeks. The 2 weeks old biofilm was gently washed in saline and placed in culture wells containing the test solutions, i.e., 2% CHX, NAC, a combination of 2% CHX and NAC in 1:1 ratio, and a control group consisting saline. After 7 days, the tooth samples were washed with saline solution for 5 min.
Quantitative assay
The biofilm formed on the root canal surface were removed with sterile scalpel and inoculated into 1 mL of BHI broth and gently vortexed. Spread plate method of inoculation was performed with 10 μL of the BHI broth onto blood agar plates, incubated at 37°C for 24 h. Colony forming units per milliliter was calculated by viable plate count method.
Statistical analysis
SPSS Statistics Version 17.0. (Chicago: SPSS Inc.) was used for statistical analysis. One-way analysis of variance (ANOVA) was performed and followed by post-hoc Scheffe's test to compare the antimicrobial efficacy between the groups for agar diffusion test.
Statistical analysis was not done for the cultures obtained from the biofilm as there was no growth in all the three test groups except the control group, i.e., saline.
RESULTS
Among the three groups tested, 2% CHX and NAC showed almost equal zones of inhibition whereas maximum inhibition was shown by combination of NAC and 2% CHX suggesting a synergistic action.
The mean and standard deviations for zones of inhibition in millimeters were calculated for each group [Table 1] and analyzed using ANOVA test. The maximum zone of inhibition value was recorded for Group 3, where a combination of CHX and NAC was used.
Table 1.
Mean values (mm) and SD of zones of inhibition of 2% CHX, NAC, and a combination of 2% CHX and NAC
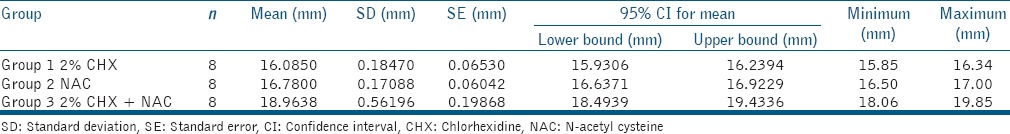
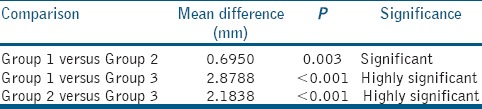
An intergroup comparison for the zones of inhibition was done using post-hoc Scheffe's test [Table 2], which revealed a statistically highly significant difference between Group 1 and 3 and Group 2 and 3 (P < 0.001), whereas a statistical significant difference was observed in Group 1 and 2 with a mean difference of 0.69 and P = 0.003, suggesting that the zone of inhibition for Group 2, i.e., NAC was slightly larger than Group 1, i.e., 2% CHX.
Table 2.
Intergroup comparison for the zone of inhibition and its statistical significance
In quantitative analysis, i.e., culture analysis obtained from the biofilm grown on dentin, no growth was observed in all the three test groups when compared to the control group, i.e., saline. The results obtained were biologically significant but statistically insignificant.
DISCUSSION
This study was conducted on E. faecalis because these microorganisms are commonly encountered in recalcitrant endodontic infections. The strains studied were E. faecalis ATCC 29212, a strain of reference used in antimicrobial studies.[19] Susceptibility of both planktonic and biofilm form was checked through agar well diffusion test and culture analysis, respectively.
Dentin was selected because it represents the primary substratum for bacterial adhesion and biofilm formation.[20]
Intracanal medicaments between visits have a definite and important role to play in endodontic therapy. They penetrate into areas not reached by instruments or irrigants and prevent regrowth of residual microorganism.
2% CHX has been proposed as an intracanal dressing because of its wide range of antimicrobial activity.[21]
NAC is a thiol-containing antioxidant that possess antibacterial property.[22,23] The active moiety is the thiol (-SH) site, which plays a role in free radical scavenging and destruction of intermolecular or intramolecular disulfide bonds in proteins.[24] The mechanism of antibacterial effects of NAC is due to the reaction of its –SH group with the disulfide bonds of bacterial proteins, leading to the irreversible damage of bacterial proteins that are essential for bacterial growth and metabolism.[24] The biofilm disruption action of NAC is likely due to its effect on exopolysaccharide (EPS) production, which is one of the major components in the biofilm. This can occur through several ways. First, the disulfide bonds of the bacterial enzyme involved in EPS production can be disrupted by the sulfhydryl group of NAC, or there can be an excretion through the thiol-disulfide exchange. Second, as NAC is antioxidant, it can exert an indirect effect on bacterial cell metabolism as well as on EPS production.[17,25]
Portenier et al.[26] observed that inhibition of the antibacterial activity of CHX occurs by dentin. However, Quah et al.[16] found antibacterial property of NAC is unaffected by the presence of dentin which can be considered as an advantage of NAC over CHX.
This study aimed to evaluate the antimicrobial activity of CHX and NAC alone and their combination against E. faecalis isolates and biofilm. The presence of EPS is one mechanism that may explain why bacteria in the form of biofilm are less sensitive to antimicrobial agents than planktonic forms.[27] EPS acts as a barrier limiting the penetration by the agent.[28] In this sense, the association of an antimicrobial substance – CHX – with an agent of proven mucolytic activity or a potential EPS disruptor such as NAC should enhance antimicrobial efficacy against biofilms and this enhanced antimicrobial activity is observed in agar diffusion test with an increase in zone of inhibition on combination of the two medicaments when compared to individual group.
However, although NAC shows good antimicrobial and biofilm eradication efficacies and has potential as a method of irrigating or treating infected root canals, the short- and long-term effects of NAC on dentin are currently unknown and remain to be tested. Furthermore, the toxicity of by-products formed on combining CHX and NAC has to be tested.
CONCLUSION
Within the limitations of this study, 2% CHX and NAC showed almost equal zones of inhibitions whereas their combination showed the maximum zone of inhibition suggesting a synergistic action.
In culture analysis, equal antibacterial efficacy was observed in all the three groups.
NAC has almost equal antimicrobial property as 2% CHX which opens new avenues to be used as newer irrigant/medicament.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Evans M, Davies JK, Sundqvist G, Figdor D. Mechanisms involved in the resistance of Enterococcus faecalis to calcium hydroxide. Int Endod J. 2002;35:221–8. doi: 10.1046/j.1365-2591.2002.00504.x. [DOI] [PubMed] [Google Scholar]
- 2.Roach RP, Hatton JF, Gillespie MJ. Prevention of the ingress of a known virulent bacterium into the root canal system by intracanal medications. J Endod. 2001;27:657–60. doi: 10.1097/00004770-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Hubble TS, Hatton JF, Nallapareddy SR, Murray BE, Gillespie MJ. Influence of Enterococcus faecalis proteases and the collagen-binding protein, Ace, on adhesion to dentin. Oral Microbiol Immunol. 2003;18:121–6. doi: 10.1034/j.1399-302x.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- 4.Love RM. Enterococcus faecalis — A mechanism for its role in endodontic failure. Int Endod J. 2001;34:399–405. doi: 10.1046/j.1365-2591.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- 5.Distel JW, Hatton JF, Gillespie MJ. Biofilm formation in medicated root canals. J Endod. 2002;28:689–93. doi: 10.1097/00004770-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Russell AD, Hammond SA, Morgan JR. Bacterial resistance to antiseptics and disinfectants. J Hosp Infect. 1986;7:213–25. doi: 10.1016/0195-6701(86)90071-x. [DOI] [PubMed] [Google Scholar]
- 7.Neelakantan P, Sanjeev K, Subbarao CV. Duration-dependent susceptibility of endodontic pathogens to calcium hydroxide and chlorhexidene gel used as intracanal medicament: An in vitro evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:e138–41. doi: 10.1016/j.tripleo.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 8.Estrela C, Ribeiro RG, Estrela CR, Pécora JD, Sousa-Neto MD. Antimicrobial effect of 2% sodium hypochlorite and 2% chlorhexidine tested by different methods. Braz Dent J. 2003;14:58–62. doi: 10.1590/s0103-64402003000100011. [DOI] [PubMed] [Google Scholar]
- 9.Siqueira JF, Jr, de Uzeda M. Intracanal medicaments: Evaluation of the antibacterial effects of chlorhexidine, metronidazole, and calcium hydroxide associated with three vehicles. J Endod. 1997;23:167–9. doi: 10.1016/S0099-2399(97)80268-3. [DOI] [PubMed] [Google Scholar]
- 10.Ehsani M, Moghadamnia AA, Zahedpasha S, Maliji G, Haghanifar S, Mir SM, et al. The role of prophylactic ibuprofen and N-acetylcysteine on the level of cytokines in periapical exudates and the post-treatment pain. Daru. 2012;20:30. doi: 10.1186/2008-2231-20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwandt LQ, Van Weissenbruch R, Stokroos I, Van der Mei HC, Busscher HJ, Albers FW. Prevention of biofilm formation by dairy products and N-acetylcysteine on voice prostheses in an artificial throat. Acta Otolaryngol. 2004;124:726–31. doi: 10.1080/00016480410022516. [DOI] [PubMed] [Google Scholar]
- 12.Livingstone CR, Andrews MA, Jenkins SM, Marriott C. Model systems for the evaluation of mucolytic drugs: Acetylcysteine and S-carboxymethylcysteine. J Pharm Pharmacol. 1990;42:73–8. doi: 10.1111/j.2042-7158.1990.tb05357.x. [DOI] [PubMed] [Google Scholar]
- 13.Sadowska AM, Manuel-Y-Keenoy B, De Backer WA. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: Discordant in vitro and in vivo dose-effects: A review. Pulm Pharmacol Ther. 2007;20:9–22. doi: 10.1016/j.pupt.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Lappas M, Permezel M, Rice GE. N-Acetyl-cysteine inhibits phospholipid metabolism, proinflammatory cytokine release, protease activity, and nuclear factor-kappaB deoxyribonucleic acid-binding activity in human fetal membranes in vitro. J Clin Endocrinol Metab. 2003;88:1723–9. doi: 10.1210/jc.2002-021677. [DOI] [PubMed] [Google Scholar]
- 15.Minamikawa H, Yamada M, Deyama Y, Suzuki K, Kaga M, Yawaka Y, et al. Effect of N-acetylcysteine on rat dental pulp cells cultured on mineral trioxide aggregate. J Endod. 2011;37:637–41. doi: 10.1016/j.joen.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Quah SY, Wu S, Lui JN, Sum CP, Tan KS. N-acetylcysteine inhibits growth and eradicates biofilm of Enterococcus faecalis. J Endod. 2012;38:81–5. doi: 10.1016/j.joen.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Olofsson AC, Hermansson M, Elwing H. N-acetyl-L-cysteine affects growth, extracellular polysaccharide production, and bacterial biofilm formation on solid surfaces. Appl Environ Microbiol. 2003;69:4814–22. doi: 10.1128/AEM.69.8.4814-4822.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prabhakar J, Senthilkumar M, Priya MS, Mahalakshmi K, Sehgal PK, Sukumaran VG. Evaluation of antimicrobial efficacy of herbal alternatives (Triphala and green tea polyphenols), MTAD, and 5% sodium hypochlorite against Enterococcus faecalis biofilm formed on tooth substrate: An in vitro study. J Endod. 2010;36:83–6. doi: 10.1016/j.joen.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 19.Silveira LF, Baca P, Arias-Moliz MT, Rodríguez-Archilla A, Ferrer-Luque CM. Antimicrobial activity of alexidine alone and associated with N-acetylcysteine against Enterococcus faecalis biofilm. Int J Oral Sci. 2013;5:146–9. doi: 10.1038/ijos.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Portenier I, Waltimo TM, Haapasalo M. Enterococcus faecalis — The root canal survivor and ‘star’ in posttreatment disease. Endod Topics. 2003;6:135–59. [Google Scholar]
- 21.Mohammadi Z, Abbott PV. The properties and applications of chlorhexidine in endodontics. Int Endod J. 2009;42:288–302. doi: 10.1111/j.1365-2591.2008.01540.x. [DOI] [PubMed] [Google Scholar]
- 22.Marchese A, Bozzolasco M, Gualco L, Debbia EA, Schito GC, Schito AM. Effect of fosfomycin alone and in combination with N-acetylcysteine on E. coli biofilms. Int J Antimicrob Agents. 2003;22(Suppl 2):95–100. doi: 10.1016/s0924-8579(03)00232-2. [DOI] [PubMed] [Google Scholar]
- 23.del Prado G, Ruiz V, Naves P, Rodríguez-Cerrato V, Soriano F, del Carmen Ponte M. Biofilm formation by Streptococcus pneumoniae strains and effects of human serum albumin, ibuprofen, N-acetyl-l-cysteine, amoxicillin, erythromycin, and levofloxacin. Diagn Microbiol Infect Dis. 2010;67:311–8. doi: 10.1016/j.diagmicrobio.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Sevier CS, Kaiser CA. Formation and transfer of disulphide bonds in living cells. Nat Rev Mol Cell Biol. 2002;3:836–47. doi: 10.1038/nrm954. [DOI] [PubMed] [Google Scholar]
- 25.Pérez-Giraldo C, Rodríguez-Benito A, Morán FJ, Hurtado C, Blanco MT, Gómez-García AC. Influence of N-acetylcysteine on the formation of biofilm by Staphylococcus epidermidis. J Antimicrob Chemother. 1997;39:643–6. doi: 10.1093/jac/39.5.643. [DOI] [PubMed] [Google Scholar]
- 26.Portenier I, Haapasalo H, Rye A, Waltimo T, Ørstavik D, Haapasalo M. Inactivation of root canal medicaments by dentine, hydroxylapatite and bovine serum albumin. Int Endod J. 2001;34:184–8. doi: 10.1046/j.1365-2591.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Wen YM. The role of bacterial biofilm in persistent infections and control strategies. Int J Oral Sci. 2011;3:66–73. doi: 10.4248/IJOS11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–8. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]


