Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Risk of grade III-IV acute and chronic GVHD is significantly lower with haploidentical compared with URD transplantation.
Relapse risk, NRM, PFS, and OS was similar in haploidentical transplants compared with unrelated donor transplants.
Abstract
We evaluated 917 adult lymphoma patients who received haploidentical (n = 185) or HLA-matched unrelated donor (URD) transplantation either with (n = 241) or without antithymocyte globulin (ATG; n = 491) following reduced-intensity conditioning regimens. Haploidentical recipients received posttransplant cyclophosphamide-based graft-versus-host disease (GVHD) prophylaxis, whereas URD recipients received calcineurin inhibitor-based prophylaxis. Median follow-up of survivors was 3 years. The 100-day cumulative incidence of grade III-IV acute GVHD on univariate analysis was 8%, 12%, and 17% in the haploidentical, URD without ATG, and URD with ATG groups, respectively (P = .44). Corresponding 1-year rates of chronic GVHD on univariate analysis were 13%, 51%, and 33%, respectively (P < .001). On multivariate analysis, grade III-IV acute GVHD was higher in URD without ATG (P = .001), as well as URD with ATG (P = .01), relative to haploidentical transplants. Similarly, relative to haploidentical transplants, risk of chronic GVHD was higher in URD without ATG and URD with ATG (P < .0001). Cumulative incidence of relapse/progression at 3 years was 36%, 28%, and 36% in the haploidentical, URD without ATG, and URD with ATG groups, respectively (P = .07). Corresponding 3-year overall survival (OS) was 60%, 62%, and 50% in the 3 groups, respectively, with multivariate analysis showing no survival difference between URD without ATG (P = .21) or URD with ATG (P = .16), relative to haploidentical transplants. Multivariate analysis showed no difference between the 3 groups in terms of nonrelapse mortality (NRM), relapse/progression, and progression-free survival (PFS). These data suggest that reduced-intensity conditioning haploidentical transplantation with posttransplant cyclophosphamide does not compromise early survival outcomes compared with matched URD transplantation, and is associated with significantly reduced risk of chronic GVHD.
Introduction
Despite a generational shift toward targeted therapies, allogeneic hematopoietic cell transplantation (allo-HCT) retains a critical role in the management, and eventual cure, of relapsed and refractory Hodgkin and non-Hodgkin lymphomas (NHL). Unfortunately, a major limitation in widespread application of HCT is donor availability. In the absence of an HLA-identical sibling, an unrelated donor (URD) who is HLA-matched to the transplant recipient at the allele level at HLA-A, -B, -C, and -DRB1 is currently considered the preferred alternative donor.1 The likelihood of finding an HLA-matched URD varies among racial and ethnic groups, with the highest probability of success among whites of Western European descent (75%) and the lowest probability among blacks of South or Central America, at 16%.2 Other alternative donors including URD umbilical cord blood or haploidentical related donors are often considered when an HLA-matched URD is not available.3 Historically, in the haploidentical setting, the extensive in vivo or ex vivo T-cell depletion used to mitigate the risk of graft rejection and severe graft-versus-host disease (GVHD) resulted in a higher risk of nonrelapse mortality (NRM), disease relapse, and delayed immunereconstitution.4-8
More recently, several Asian centers have reported favorable outcomes of haploidentical transplantation, utilizing T-cell–replete grafts with intensive immunosuppression using antithymocyte globulin (ATG).9,10 A different strategy of T-cell–replete haploidentical transplantation being increasingly used involves administration of posttransplantation cyclophosphamide, which mitigates the risk of GVHD by targeting alloreactive T cells rapidly proliferating early after an HLA-mismatched transplant, relatively sparing regulatory T cells and leaving unaffected the nondividing hematopoietic stem and progenitor cells.11-15 Several reports, comprising mostly patients with myeloid malignancies and a recent registry study limited to patients with acute myeloid leukemia (AML), suggest similar outcomes after haploidentical transplantation using posttransplant cyclophosphamide, when compared with HLA-matched URD transplants.16-19 In lymphoid malignancies, small single institution reports have shown promising outcomes following haploidentical allo-HCT with posttransplantation cyclophosphamide.20-23 In lymphomas, outcomes of T-cell–replete haploidentical transplantation have not been compared against adult URD allo-HCT. The current analysis compares outcomes after haploidentical donor transplantation using posttransplant cyclophosphamide to 8 of 8 allele-level HLA-matched URD transplantation in adults with Hodgkin and NHL.
Materials and methods
Data sources
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a working group of >500 transplantation centers worldwide that contributes detailed data on HCT to a statistical center at the Medical College of Wisconsin (MCW). Participating centers are required to report all transplantations consecutively and compliance is monitored by onsite audits. Computerized checks for discrepancies, physicians’ review of submitted data, and onsite audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. The institutional review boards of the MCW and National Marrow Donor Program approved this study.
The CIBMTR collects data at 2 levels: transplant essential data (TED) and comprehensive report form (CRF) data. TED data include disease type, age, sex, pre-HCT disease stage and chemotherapy responsiveness, date of diagnosis, graft type, conditioning regimen, posttransplant disease progression and survival, development of a new malignancy, and cause of death. All CIBMTR centers contribute TED data. More detailed disease and pre- and posttransplant clinical information are collected on a subset of registered patients selected for CRF data by a weighted randomization scheme. TED- and CRF-level data are collected pretransplant, 100 days, and 6 months post-HCT and annually thereafter or until death. Data for the current analysis were retrieved from CIBMTR (TED and CRF) report forms.
Patients
Included in this analysis are adult (≥18 years) patients with Hodgkin or NHL, undergoing their first reduced-intensity (RIC) or nonmyeloablative (NMA) conditioning haploidentical or matched URD allo-HCT between 2008 and 2013. Recipients of haploidentical transplantation (mismatched by at least 2 or more HLA loci to donors) received unmanipulated, predominantly bone marrow (BM) grafts with GVHD prophylaxis consisting of posttransplant cyclophosphamide, followed typically by a calcineurin inhibitor (CNI) and mycophenolate mofetil (MMF) combination. Recipients of URD transplantation (matched at the allele level at HLA-A, -B, -C, and -DRB1) received predominantly peripheral blood grafts and CNI-based GVHD prophylaxis, either with or without ATG. Patients receiving ex vivo graft manipulation (T-cell–depleted or CD34-selected grafts) or those undergoing a planned tandem auto-allo-HCT were not included.
Definitions
The intensity of conditioning regimens was defined using consensus criteria.24 Complete remission (CR) to last line of therapy before allo-HCT on CIBMTR forms is defined as complete resolution of all known areas of disease on radiographic assessments, whereas partial remission (PR) is defined as ≥50% reduction in the greatest diameter of all sites of known disease and no new sites of disease. Resistant disease is defined as <50% reduction in the diameter of all disease sites, or development of new disease sites. Disease risk index (DRI) was defined as reported previously.25
Study end points
The primary end point was overall survival (OS); death from any cause was considered an event and surviving patients were censored at last contact. NRM was defined as death without evidence of lymphoma progression/relapse; relapse was considered a competing risk. Progression/relapse was defined as progressive lymphoma after HCT or lymphoma recurrence after a CR; NRM was considered a competing risk. For progression-free survival (PFS), a patient was considered a treatment failure at the time of progression/relapse or death from any cause. Patients alive without evidence of disease relapse or progression were censored at last follow-up. Acute GVHD26 and chronic GVHD27 were graded using standard criteria. Neutrophil recovery was defined as the first of 3 successive days with absolute neutrophil count (ANC) ≥500/µL after posttransplantation nadir. Platelet recovery was defined as achieving platelet counts ≥20 000/μL for at least 7 days, unsupported by transfusion. For neutrophil and platelet recovery, death without the event was considered a competing risk.
Statistical analysis
The haploidentical allo-HCT cohort was compared against (1) URD transplants using ATG (URD with ATG group) and (2) URD not using ATG (URD without ATG group). Probabilities of PFS and OS were calculated as described previously.28 Cumulative incidence of NRM, lymphoma progression/relapse, and hematopoietic recovery were calculated to accommodate for competing risks.29 Associations among patient-, disease-, and transplantation-related variables and outcomes of interest were evaluated using Cox proportional hazards regression. Backward elimination was used to identify covariates that influenced outcomes. Covariates with a P < .05 were considered statistically significant. The proportional hazards assumption for Cox regression was tested by adding a time-dependent covariate for each risk factor and each outcome. Covariates violating the proportional hazards assumption were added as time-dependent covariates in the Cox regression model. Interactions between the main effect and significant covariates were examined. Center effect was examined using the random effect score test30 for OS, PFS, relapse, and NRM. Results are expressed as relative risk (RR). The variables considered in multivariate analysis are shown in supplemental Table 1 (available on the Blood Web site). All statistical analyses were performed using SAS version 9.3 (SAS Institute).
Results
Baseline characteristics
Patients were divided into 3 groups: haploidentical (n = 185), URD without ATG (n = 491), and URD with ATG (n = 241). The baseline patient-, disease-, and transplantation-related characteristics are shown in Table 1 and supplemental Table 2. There was no significant difference across the 3 groups in terms of patient age, sex, stage at diagnosis, interval between diagnosis and allo-HCT, lines of prior therapy and BM or extranodal involvement at HCT. A greater proportion of African Americans were in the haploidentical group. Although more patients in the haploidentical group had a Karnofsky performance score (KPS) of ≥90 and an HCT–comorbidity index of <3 at transplantation, significantly more haploidentical cohort patients had intermediate or high DRI at HCT (P < .001). Diffuse large B-cell lymphoma (DLBCL) was the most common histology in the haploidentical and URD with ATG cohorts, whereas follicular lymphoma (FL) was the most common lymphoma in the URD without ATG group. Significantly more patients in the haploidentical (93%) and URD without ATG (88%) cohorts had chemosensitive disease relative to the URD with ATG group (81%; P = .02). Patients undergoing an URD allo-HCT were more likely to have received a prior autograft (P = .006). All patients in the haploidentical group received RIC with fludarabine, cyclophosphamide, and 2 Gy total body irradiation (TBI), whereas those in URD groups received conditioning with fludarabine plus either an alkylator (busulfan, cyclophosphamide, or melphalan) and/or 2 Gy TBI. The graft source was unmanipulated BM in 93% (n = 172) of haploidentical transplants and peripheral blood (PB) in 94% (n = 460) of URD without ATG and 91% (n = 279) of URD with ATG cohorts. All patients in the haploidentical group received posttransplant cyclophosphamide as GVHD prophylaxis, which in 95% (n = 175) of patients was combined with tacrolimus and MMF. The GVHD prophylaxis for patients in the URD cohort was CNI-based.
Table 1.
Baseline characteristics of lymphoma patients reported to the CIBMTR from 2008 to 2013
| Variable | Haploidentical | URD without ATG | URD with ATG | P |
|---|---|---|---|---|
| No. of patients | 185 | 491 | 241 | |
| No. of centers | 23 | 70 | 48 | |
| Median age at HCT (range), y | 55 (18-75) | 55 (19-74) | 55 (20-73) | .13 |
| Male sex | 118 (64) | 301 (61) | 163 (68) | .25 |
| Race | <.001 | |||
| Caucasian/white | 149 (81) | 469 (96) | 227 (94) | |
| Black | 28 (15) | 4 (<1) | 4 (2) | |
| Others* | 6 (3) | 8 (2) | 2 (<1) | |
| Missing | 2 (1) | 10 (2) | 8 (3) | |
| KPS ≥90 | 145 (78) | 311 (63) | 153 (63) | <.001 |
| HCT–comorbidity index | .001 | |||
| 0 | 79 (43) | 184 (37) | 65 (27) | |
| 1-2 | 51 (28) | 114 (23) | 77 (32) | |
| 3 or more | 55 (30) | 175 (36) | 88 (37) | |
| Missing | 0 | 18 (4) | 11 (5) | |
| Histology | <.001 | |||
| FL | 28 (15) | 130 (26) | 44 (18) | |
| DLBCL | 66 (36) | 94 (19) | 61 (25) | |
| Mantle cell lymphoma | 21 (11) | 83 (17) | 36 (15) | |
| Mature T- and NK-cell lymphomas† | 24 (13) | 79 (16) | 52 (22) | |
| Hodgkin lymphoma | 46 (25) | 105 (21) | 48 (20) | |
| Median interval from diagnosis to HCT (range), mo | 31 (<1-255) | 34 (<1-342) | 32 (4-460) | .19 |
| Remission status at HCT‡ | .02 | |||
| CR | 72 (39) | 215 (44) | 100 (41) | |
| PR | 99 (54) | 215 (44) | 96 (40) | |
| Chemorefractory | 10 (5) | 55 (11) | 40 (17) | |
| Untreated | 2 (1) | 3 (<1) | 3 (1) | |
| Unknown | 2 (1) | 3 (<1) | 2 (< 1) | |
| Disease risk index at HCT | <.001 | |||
| Low | 45 (24) | 199 (41) | 75 (31) | |
| Intermediate | 126 (68) | 263 (49) | 129 (54) | |
| High | 13 (7) | 48 (10) | 37 (15) | |
| Missing | 1 (<1) | 1 (<1) | 0 | |
| History of prior autologous HCT | 72 (39) | 254 (52) | 127 (53) | .006 |
| Conditioning regimen | <.001 | |||
| Flu/Bu ± rituximab | 0 | 130 | 136 | |
| Flu/Cy ± rituximab | 0 | 91 | 19 | |
| Flu/Cy/2 Gy TBI | 185 | 0 | 0 | |
| Flu/Mel ± rituximab | 0 | 153 | 83 | |
| Flu/2Gy TBI ± rituximab | 0 | 117 | 3 | |
| TBI in conditioning | 185 | 117 (24) | 3 (<1) | <.001 |
| Graft type | <.001 | |||
| BM | 172 (93) | 31 (6) | 26 (9) | |
| PB | 13 (7) | 460 (94) | 279 (91) | |
| Male donor to male recipient | 66 (36) | 227 (46) | 124 (51) | <.001 |
| Donor/recipient CMV status | .02 | |||
| ± | 40 (22) | 147 (30) | 70 (29) | |
| Other | 142 (76) | 340 (69) | 166 (69) | |
| Missing | 3 (2) | 4 (<1) | 5 (2) | |
| GVHD prophylaxis | <.001 | |||
| Posttransplant Cy§ | 185 (100) | 0 | 0 | |
| CNI + MMF ± others|| | 0 | 161 (33) | 86 (36) | |
| CNI + MTX ± others (except MMF)¶ | 0 | 253 (52) | 143 (59) | |
| CNI ± others (except MMF/MTX)# | 0 | 77 (16) | 12 (5) | |
| Median follow-up of survivors (range), mo | 36 (5-73) | 35 (4-74) | 35 (<1-75) |
Bu, busulfan; CMV, cytomegalovirus; Cy, cyclophosphamide; Flu, fludarabine; Mel, melphalan; MTX, methotrexate.
Haploidentical: Asian (n = 5), Native American (n = 1); URD: Asian (n = 13), Pacific Islander (n = 2).
Details of mature T-cell and NK-cell neoplasms included in the analysis. For the haploidentical group: PTCL-NOS (peripheral T-cell lymphoma–not otherwise specified) = 7, angioimmunoblastic T-cell lymphoma = 3, extranodal NK/T-cell lymphoma = 5, anaplastic large-cell lymphoma = 3, others = 6. For URD without ATG group: PTCL-NOS = 22, angioimmunoblastic T-cell lymphoma = 11, extranodal NK/T-cell lymphoma = 17, anaplastic large-cell lymphoma = 15, others = 14. For URD with ATG group: PTCL-NOS = 19, angioimmunoblastic T-cell lymphoma = 12, extranodal NK/T-cell lymphoma = 1, anaplastic large-cell lymphoma = 10, others = 10.
The haploidentical HCT, URD without ATG, and URD with ATG groups included 25, 67, and 27 patients, respectively, in CR1 or PR1 at the time of transplantation (P = .66).
Tacrolimus/MMF/Cy (n = 175), cyclosporine/MMF/Cy (n = 1), Cy alone (n = 3), tacrolimus/Cy (n = 4), tacrolimus/MTX/Cy (n = 1), MMF/Cy (n = 1).
CNI/MMF alone (n = 196), CNI/MMF/sirolimus (n = 13), CNI/MMF/MTX (n = 37), CNI/MMF/corticosteroids (n = 1).
CNI/MTX/corticosteroids (n = 3), CNI/MTX alone (n = 315), CNI/MTX/sirolimus (n = 78).
CNI/corticosteroids (n = 1), CNI alone (n = 12), CNI/sirolimus (n = 76).
Hematopoietic recovery
The cumulative incidence of neutrophil recovery at day 28 was 94% (95% confidence interval [CI], 90-97) in the haploidentical group compared with 97% (95% CI, 95-98) and 97% (95% CI, 94-99) in the URD without ATG and with ATG groups, respectively (P = .32). The day-28 cumulative incidence of platelet recovery in similar order was 63% vs 89% vs 84% (P < .001; Table 2).
Table 2.
Univariate analysis
| Outcomes | Haploidentical (1) | URD without ATG (2) | URD with ATG (3) | Pairwise P | Overall P | ||||
|---|---|---|---|---|---|---|---|---|---|
| N Eval | Prob % (95% CI) | N Eval | Prob % (95% CI) | N Eval | Prob % (95% CI) | 1 vs 2 | 1 vs 3 | ||
| ANC recovery >500/μL | 184 | 482 | 236 | ||||||
| 28 d | 94 (90-97) | 97 (95-98) | 97 (94-99) | .17 | .15 | .32 | |||
| 100 d | 98 (95-99) | 98 (97-99) | 97 (95-99) | .98 | .51 | .74 | |||
| Platelet recovery ≥20/μL | 184 | 481 | 234 | ||||||
| 28 d | 63 (56-70) | 89 (86-92) | 84 (79-89) | <.001 | <.001 | <.001 | |||
| 100 d | 93 (89-97) | 96 (94-97) | 91 (88-95) | .25 | .43 | .08 | |||
| Acute GVHD (II-IV)* | 49 | 93 | 41 | ||||||
| 100 d | 27 (15-40) | 40 (30-50) | 49 (34-64) | .10 | .03 | .07 | |||
| Acute GVHD (III-IV)* | 49 | 93 | 41 | ||||||
| 100 d | 8 (2-17) | 12 (6-19) | 17 (7-30) | .48 | .21 | .44 | |||
| Acute GVHD (II-IV) | 180 | 482 | 235 | ||||||
| 180 d | 52 (44-60) | 60 (55-65) | 56 (49-63) | .13 | .47 | .29 | |||
| Chronic GVHD | 183 | 475 | 230 | ||||||
| 1 y | 13 (8-18) | 51 (46-55) | 33 (27-39) | <.001 | <.001 | <.001 | |||
| 2 y | 15 (10-21) | 62 (57-67) | 37 (31-44) | <.001 | <.001 | <.001 | |||
| NRM | 184 | 488 | 240 | ||||||
| 1 y | 11 (7-17) | 13 (10-16) | 20 (15-25) | .64 | .02 | .03 | |||
| 2 y | 16 (11-22) | 18 (15-22) | 24 (19-30) | .56 | .04 | .10 | |||
| 3 y | 17 (12-23) | 22 (18-27) | 26 (21-33) | .14 | .02 | .08 | |||
| Relapse/progression | 184 | 488 | 240 | ||||||
| 1 y | 30 (24-37) | 22 (18-26) | 27 (21-33) | .03 | .45 | .07 | |||
| 2 y | 34 (27-41) | 27 (23-31) | 32 (26-38) | .09 | .67 | .15 | |||
| 3 y | 36 (29-43) | 28 (24-33) | 36 (29-43) | .08 | .99 | .07 | |||
| PFS | 184 | 488 | 240 | ||||||
| 1 y | 58 (51-65) | 65 (61-70) | 53 (47-60) | .10 | .31 | .007 | |||
| 2 y | 50 (42-57) | 55 (50-60) | 44 (37-50) | .25 | .23 | .02 | |||
| 3 y | 47 (40-55) | 49 (44-54) | 38 (31-45) | .63 | .07 | .02 | |||
| OS | 185 | 491 | 241 | ||||||
| 1 y | 76 (69-81) | 78 (74-81) | 65 (58-71) | .57 | .01 | .002 | |||
| 2 y | 63 (56-71) | 69 (65-73) | 55 (49-62) | .18 | .11 | .003 | |||
| 3 y | 60 (52-67) | 62 (57-67) | 50 (42-57) | .65 | .05 | .02 | |||
Probabilities of neutrophil and platelet recovery, platelet recovery, acute GVHD, chronic GVHD, treatment-related mortality, and progression/relapse were calculated using the cumulative incidence estimate. PFS and OS were calculated using the Kaplan-Meier product limit estimate.
Eval, evaluable; N, number; NA, not applicable; NE, not evaluable; Prob, probability.
Calculated from CRF-level data only.
Acute and chronic GVHD
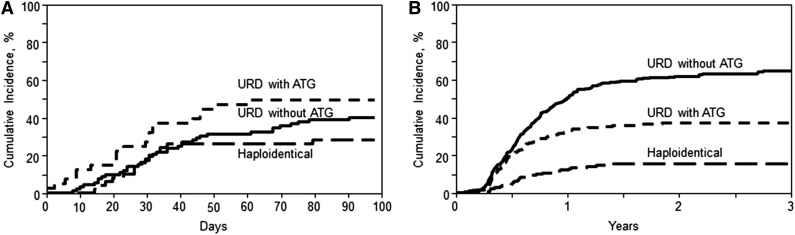
On univariate analysis, the cumulative incidence of grade II-IV acute GVHD at day 100 (Table 2; Figure 1A) in the haploidentical cohort was 27% (95% CI, 15-40), compared with 40% (95% CI, 30-50) and 49% (95% CI, 34-64) in the URD without ATG and URD with ATG groups, respectively (P = .07). The corresponding rates of severe (grades III-IV) acute GVHD were 8% (95% CI, 2-17), 12% (95% CI, 6-19), and 17% (95% CI, 7-30) in haploidentical, URD without ATG, and URD with ATG groups, respectively (P = .44). On multivariate analysis (Table 3), there was no significant difference in the risk of grade II-IV acute GVHD, in URD without ATG (RR = 1.22; 95% CI, 0.86-1.74; P = .27) and URD with ATG (RR = 1.26; 95% CI, 0.84-1.89; P = .25) groups, relative to the haploidentical group. However, the risk of grade III-IV acute GVHD was significantly higher following URD without ATG (RR = 2.87; 95% CI, 1.52-5.4; P = .001) as well as URD with ATG (RR = 2.45; 95% CI, 1.23-4.87; P = .01), relative to haploidentical HCT.
Figure 1.
Cumulative incidence of GVHD. (A) Acute GVHD II-IV. Cumulative incidence of grade II-IV acute GVHD in recipients of haploidentical donor, unrelated donor with ATG, and unrelated donor without ATG allo-HCT (overall, P = .07). (B) Chronic GVHD. Cumulative incidence of chronic GVHD in recipients of haploidentical donor, unrelated donor with ATG, and unrelated donor without ATG allo-HCT (overall, P < .001 at 1 year and 2 years).
Table 3.
Multivariate analysis results
| No. | No. of events | RR | 95% CI lower limit | 95% CI upper limit | P | Overall P | |
|---|---|---|---|---|---|---|---|
| Acute GVHD (grades 2-4)* | |||||||
| Haploidentical | 180 | 68 | 1 | .47 | |||
| URD without ATG | 482 | 206 | 1.22 | 0.86 | 1.74 | .27 | |
| URD with ATG | 235 | 104 | 1.26 | 0.84 | 1.89 | .25 | |
| Acute GVHD (grades 3-4)* | |||||||
| Haploidentical | 180 | 12 | 1 | .005 | |||
| URD without ATG | 482 | 82 | 2.87 | 1.52 | 5.40 | .001 | |
| URD with ATG | 235 | 35 | 2.45 | 1.23 | 4.87 | .01 | |
| Contrast | |||||||
| URD without ATG vs URD with ATG | 1.17 | 0.76 | 1.80 | .47 | |||
| Chronic GVHD | |||||||
| Haploidentical | 183 | 28 | 1 | <.0001 | |||
| URD without ATG | 475 | 289 | 5.85 | 3.96 | 8.64 | <.0001 | |
| URD with ATG | 230 | 82 | 3.64 | 2.37 | 5.60 | <.0001 | |
| Contrast | |||||||
| URD without ATG vs URD with ATG | 1.61 | 1.25 | 2.05 | .0002 | |||
| NRM | |||||||
| Haploidentical | 184 | 32 | 1 | .04 | |||
| URD without ATG | 488 | 98 | 1.03 | 0.68 | 1.56 | .88 | |
| URD with ATG | 240 | 60 | 1.54 | 0.98 | 2.4 | .06 | |
| Progression/relapse | |||||||
| Haploidentical | 184 | 65 | 1 | .27 | |||
| URD without ATG | 488 | 137 | 0.8 | 0.59 | 1.08 | .15 | |
| URD with ATG | 238 | 80 | 0.95 | 0.67 | 1.33 | .75 | |
| Contrast | |||||||
| URD without ATG vs URD with ATG | 0.85 | 0.64 | 1.12 | .24 | |||
| PFS | |||||||
| Haploidentical | 184 | 97 | 1 | .08 | |||
| URD without ATG | 488 | 235 | 0.90 | 0.71 | 1.16 | .42 | |
| URD with ATG | 240 | 140 | 1.16 | 0.88 | 1.51 | .29 | |
| Contrast | |||||||
| URD without ATG vs URD w/ATG | 0.78 | 0.63 | 0.97 | .02 | |||
| OS | |||||||
| Haploidentical | 185 | 76 | 1 | .004 | |||
| URD without ATG | 491 | 171 | 0.83 | 0.62 | 1.11 | .21 | |
| URD with ATG | 241 | 113 | 1.25 | 0.92 | 1.69 | .16 | |
| Contrast | |||||||
| URD without ATG vs URD with ATG | 0.67 | 0.52 | 0.85 | .001 |
Acute GVHD models used logistic regression.
On univariate analysis, the cumulative incidence of chronic GVHD at 1 year (Table 2; Figure 1B) after haploidentical transplants was 13% (95% CI, 8-18) compared with 51% (95% CI, 46-55) in URD without ATG and 33% (95% CI, 27-39) in URD with ATG cohorts (P < .001). Multivariate analysis (Table 3) showed a significantly higher risk of chronic GVHD in the recipients of URD HCT without ATG (RR = 5.85; 95% CI, 3.96-8.64; P < .0001) and URD HCT with ATG (RR = 3.64; 95% CI, 2.37-5.60; P < .0001). Among the URD allografts, not receiving ATG was associated with higher chronic GVHD risk (RR = 1.61; 95% CI, 1.25-2.05; P = .0002).
NRM and relapse
Among recipients of haploidentical allografts, the 1-year NRM was 11% (95% CI, 7-17) compared with 13% (95% CI, 10-16) and 20% (95% CI, 15-25) in URD without ATG and URD with ATG groups, respectively (P = .03; Table 2). On multivariate analysis, compared with the haploidentical group, there was no significant difference in NRM with URD without ATG (RR = 1.03; 95% CI, 0.68-1.56; P = .88), or URD allo-HCT with ATG (RR = 1.54; 95% CI, 0.98-2.4; P = .06). Independent of the transplant type, recipient age >40 years, KPS <90 (RR = 1.48; 95% CI, 1.09-1.99; P = .01), and an HCT–comorbidity index ≥3 (RR = 1.4; 95% CI, 1.0-1.97; P = .048) were associated with higher risk of NRM (for details, see supplemental Table 4).
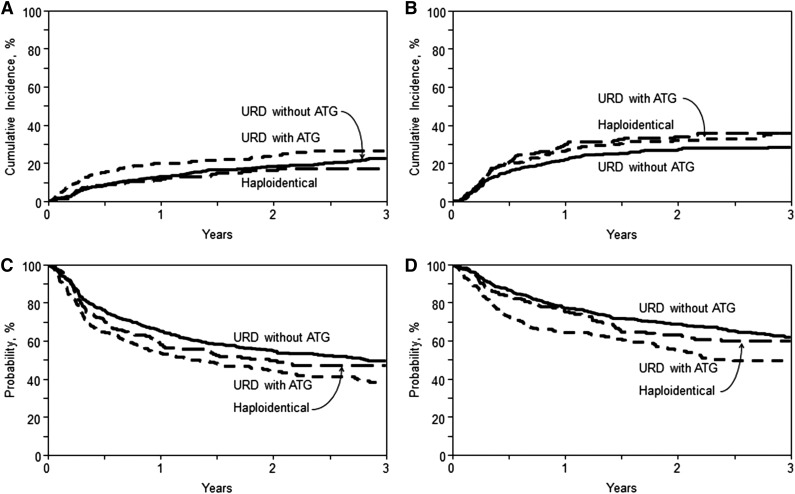
The cumulative incidence of relapse/progression at 3 years was 36% (95% CI, 29-43), 28% (95% CI, 24-33), and 36% (95% CI, 29-43) in the haploidentical, URD without ATG, and URD with ATG groups, respectively (P = .07; Table 2). On multivariate analysis, relative to the haploidentical group, there was no significant difference in the risk of relapse/progression following URD allo-HCT without ATG (RR = 0.8; 95% CI, 0.59-1.08; P = .15) or URD allo-HCT with ATG (RR = 0.95; 95% CI, 0.67-1.33; P = .75) (Table 3). Independent of the transplant type, lymphoma histologies other than FL and Hodgkin lymphoma were associated with increased relapse/progression risk within the first 7 months post-HCT. Beyond 7 months post-HCT, significantly increased relapse/progression risk was seen only in T-cell NHL (RR = 2.2; 95% CI, 1.05-4.63; P = .04) and Hodgkin lymphoma (RR = 4.95; 95% CI, 2.7-9.05; P < .0001) (supplemental Table 4). Other independent predictors of higher relapse/progression risk included not being in CR at allo-HCT (RR = 1.45 for PR; RR = 2.37 for chemoresistance), extranodal involvement (RR = 2.01), intermediate DRI (RR = 2.17), and high DRI (RR = 3.63), whereas sex-mismatched allografts reduced the risk of relapse/progression (RR = 0.68) (for details, see supplemental Table 4).
Progression-free survival
With a median follow-up of ∼3 years for surviving patients, the 3-year PFS was 47% (95% CI, 40-55), 49% (95% CI, 44-54), and 38% (95% CI, 31-45) in the haploidentical, URD without ATG, and URD with ATG groups, respectively (P = .02) (Table 2; Figure 2C). On multivariate analysis (Table 3), relative to the haploidentical group, there was no difference in the risk of therapy failure (inverse of PFS) with URD allo-HCT without ATG (RR = 0.90; 95% CI, 0.71-1.16; P = .42) or URD allo-HCT with ATG (RR = 1.16; 95% CI, 0.88-1.51; P = .29). Among those who received URD allografts, the absence of ATG was associated with a reduced risk of therapy failure (RR = 0.78; 95% CI, 0.63-0.97; P = .02). Independent of the transplant type, all disease histologies other than FL and Hodgkin lymphoma were associated with a higher risk of therapy failure within the first 7 months of allo-HCT (supplemental Table 4). However, beyond 7 months, higher therapy-failure risk was noted only in mantle cell lymphoma (RR = 1.64; 95% CI, 1.03-2.60; P = .04) and Hodgkin lymphoma (RR = 1.66; 95% CI, 1.07-2.58; P = .02). Other independent predictors of higher therapy-failure risk included history of prior autologous HCT (RR = 1.54), not being in CR at allo-HCT (RR = 1.35 for PR; RR = 1.99 for chemoresistance), intermediate DRI (RR = 1.69), and high DRI (RR = 2.54) (for details, see supplemental Table 4).
Figure 2.
Cumulative incidence and Kaplan-Meier estimates. (A) NRM. Cumulative incidence of NRM in recipients of haploidentical donor, unrelated donor with ATG, and unrelated donor without ATG allo-HCT (overall, P = .08 at 3 years). (B) Relapse/progression. Cumulative incidence of lymphoma relapse/progression in recipients of haploidentical donor, unrelated donor with ATG, and unrelated donor without ATG allo-HCT (overall, P = .07 at 3 years). (C) PFS. Kaplan-Meir estimate of PFS in recipients of haploidentical donor, unrelated donor with ATG, and unrelated donor without ATG allo-HCT (overall, P = .02 at 3 years). (D) OS. Kaplan-Meier estimate of OS in recipients of haploidentical donor, unrelated donor with ATG, and unrelated donor without ATG allo-HCT (overall, P = .02 at 3 years).
Overall survival
The 3-year OS in the haploidentical, URD without ATG, and URD with ATG groups was 60% (95% CI, 52-67), 62% (95% CI, 57-67), and 50% (95% CI, 42-57), respectively (P = .02) (Table 2; Figure 2D). On multivariate analysis (Table 3), no difference in mortality risk (inverse of OS) was seen in URD without ATG (RR = 0.83; 95% CI, 0.62-1.11; P = .21) or URD with ATG (RR = 1.25; 95% CI, 0.92-1.69; P = .16) groups relative to haploidentical transplants. Within the URD group, not receiving ATG was associated with reduced risk of mortality (RR = 0.67; 95% CI, 0.52-0.85; P = .001). Independent of the transplant type, variables associated with higher mortality included recipient age >60 years (RR = 1.91), KPS <90 (RR = 1.47), lymphoma histology other than FL, absence of CR prior to transplantation (RR = 1.27 for PR; RR = 1.62 for chemoresistance), intermediate DRI (RR = 1.66) and high DRI (RR = 2.07) (for details, see supplemental Table 4).
PFS and OS posttransplantation stratified according to lymphoma subtypes is provided in supplemental Table 3. Among the URD with ATG group, the ATG dose administered had no effect on OS (supplemental Table 6).
Center effect
Haploidentical transplantations were performed at 23 transplant centers compared with 70 and 43 centers performing URD HCT without and with ATG, respectively. To ensure that outcomes reported in the current analysis were not driven by institutional expertise, the transplant center effect was examined. We found no center effect on the hazard of OS (P = .63) and PFS (P = .78), and the cause-specific hazard of relapse (P = .45) and NRM (P = .12), using the random-effect score test.30
Causes of death
At last follow-up, 41% (n = 76) of haploidentical recipients, 35% (n = 171) of URD without ATG, and 47% (n = 113) of URD with ATG recipients had died (supplemental Table 5). The most common cause of death in all 3 cohorts was recurrent/progressive lymphoma; 45% (n = 45), 34% (n = 58), and 41% (n = 46) in haploidentical, URD without ATG, and URD with ATG groups, respectively. Although GVHD was the cause of death in 6% (n = 10) and 12% (n = 13) of recipients of URD without ATG and URD with ATG respectively, only 1 death in the haploidentical group was attributed to GVHD. Infectious complications led to the death of 7% (n = 7) in the haploidentical group, 8% (n = 18) in URD without ATG, and 11% (n = 14) in the URD with ATG group.
Subset analysis
Because the predominant graft source differed between the haploidentical and URD cohorts, subset analysis of transplantation outcomes was performed in haploidentical transplants receiving BM grafts (n = 172) only and URD HCT with (n = 217) or without (n = 460) ATG receiving PB grafts (Table 4). The results were in line with the outcomes of the entire study population. URD allo-HCT without ATG was associated with higher risk of grade III-IV acute GVHD (RR = 2.41, 95% CI, 1.27-4.57, P = .007) and chronic GVHD (RR = 6.72, 95% CI, 4.39-10.31, P < .0001) relative to haploidentical allo-HCT. Similarly, the URD with ATG group was also associated with higher risk of grade III-IV acute GVHD (RR = 2.2; 95% CI, 1.09-4.43; P = .03) and chronic GVHD (RR = 4.44; 95% CI, 2.78-7.08; P < .0001) compared with haploidentical group. Within the URD group, not receiving ATG increased the risk of chronic GVHD (RR = 1.5; 95% CI, 1.17-1.95; P = .001). There were no significant differences in the risk of NRM, relapse/progression, therapy failure, and mortality between haploidentical BM grafts compared with URD PB grafts with or without ATG. Among URD transplant recipients, the absence of ATG in the conditioning regimen was associated with lower NRM risk (RR = 0.67; 95% CI, 0.47-0.95; P = .02) and reduced mortality risk (RR = 0.70; 95% CI, 0.54-0.91; P = .007).
Table 4.
Multivariate analysis restricted to haploidentical HCT using BM grafts vs unrelated donor transplants using PB grafts only
| No. | Relative risk | 95% CI lower limit | 95% CI upper limit | P | Overall P | |
|---|---|---|---|---|---|---|
| Acute GVHD (grades 2-4) | ||||||
| Haploidentical | 167 | 1 | .32 | |||
| URD without ATG | 451 | 1.30 | 0.90 | 1.89 | .16 | |
| URD with ATG | 213 | 1.34 | 0.88 | 2.06 | .17 | |
| Acute GVHD (grades 3-4) | ||||||
| Haploidentical | 167 | 1 | .005 | |||
| URD without ATG | 451 | 2.41 | 1.27 | 4.57 | .007 | |
| URD with ATG | 213 | 2.2 | 1.09 | 4.43 | .03 | |
| Chronic GVHD | ||||||
| Haploidentical | 170 | 1 | <.0001 | |||
| URD without ATG | 446 | 6.72 | 4.39 | 10.31 | <.0001 | |
| URD with ATG | 208 | 4.44 | 2.78 | 7.08 | <.0001 | |
| Contrast | ||||||
| URD without ATG vs URD with ATG | 1.51 | 1.17 | 1.95 | .001 | ||
| NRM | ||||||
| Haploidentical | 171 | 1 | .051 | |||
| URD without ATG | 457 | 1.04 | 0.67 | 1.62 | .84 | |
| URD with ATG | 216 | 1.56 | 0.97 | 2.5 | .06 | |
| Contrast | ||||||
| URD without ATG vs URD with ATG | 0.67 | 0.47 | 0.95 | .02 | ||
| Progression/relapse | ||||||
| Haploidentical | 171 | 1 | .30 | |||
| URD without ATG | 457 | 0.78 | 0.57 | 1.07 | .12 | |
| URD with ATG | 214 | 0.86 | 0.60 | 1.22 | .40 | |
| PFS | ||||||
| Main effect | ||||||
| Haploidentical | 171 | 1 | .16 | |||
| URD without ATG | 457 | 0.87 | 0.68 | 1.13 | .30 | |
| URD with ATG | 216 | 1.08 | 0.81 | 1.43 | .60 | |
| OS | ||||||
| Haploidentical | 172 | 1 | .02 | |||
| URD without ATG | 460 | 0.82 | 0.61 | 1.11 | .21 | |
| URD with ATG | 217 | 1.17 | 0.85 | 1.62 | .33 | |
| Contrast | ||||||
| URD without ATG vs URD with ATG | 0.70 | 0.54 | 0.91 | .007 |
Discussion
Studies comparing outcomes after haploidentical transplantation to that after HLA-matched URD allo-HCT in lymphoma patients have not been performed. Here, we performed a registry analysis of patients undergoing RIC/NMA conditioning followed by (1) haploidentical transplantation using posttransplant cyclophosphamide-based GVHD prophylaxis compared with (2) URD transplantation with or (3) without ATG, constituting the largest series to explore haploidentical transplantation in lymphomas. The current study provides several important observations. First, a similar 3-year survival was noted when pursuing haploidentical HCT compared with URD transplants regardless of ATG use. Second, haploidentical transplantation resulted in comparable rates of neutrophil recovery, relapse risk, and NRM compared with URD transplant. Third, haploidentical allo-HCT with posttransplant cyclophosphamide was associated with lower rates of acute grade III-IV as well as chronic GVHD. Finally, mortality secondary to GVHD following haploidentical transplantation was rare.
At the outset, it is important to highlight that the haploidentical transplantation cohort in our study received uniform conditioning (fludarabine/cyclophosphamide/2 Gy TBI) and GVHD prophylaxis (posttransplant cyclophosphamide-based approach). To ensure that haploidentical transplantation in our study was compared against a relatively uniform URD cohort, conditioning regimens in URD allografts were limited to fludarabine plus either an alkylator and/or 2 Gy TBI and GVHD prophylaxis to CNI-based approaches. Preliminary multivariate models restricted to only the URD cohort confirmed no significant differences between individual conditioning regimens and GVHD prophylactic regimens in terms of NRM, relapse/progression, PFS, and OS (data not shown). This additional step ensured that survival outcomes in URD transplants were not impacted by the heterogeneity of conditioning and GVHD prophylaxis approaches. In addition, we elected to divide the URD allografts into a group with and one without ATG. The observed outcome differences between the 2 approaches on multivariate analysis (Tables 3 and 4) not only lend support to the approach of analyzing these 2 groups separately, but also provide an opportunity to compare haploidentical allografts against 2 commonly used URD allo-HCT approaches. Despite the above-mentioned methodology, as expected in a registry study, the baseline characteristics differed between the 3 groups. Recipients of haploidentical allografts had better performance status and lower HCT–comorbidity index. Despite these differences, significantly more haploidentical patients had intermediate or high DRI. Of note, DRI is a validated tool that was developed based on type and status of disease at the time of transplantation and stratifies patients into risk groups.25,31 These observations suggest that despite differences in KPS and HCT–comorbidity index, the haploidentical cohort in our study included significantly more patients with biologically higher-risk disease. Not surprisingly, in this study, both chemonsensitivity at HCT and DRI were independently associated with increased relapse risk and inferior PFS and OS.
The similar rates of relapse/progression seen with haploidentical and URD transplants in our study are noteworthy and address the concerns that posttransplant cyclophosphamide could negatively influence potential graft-versus-malignancy (GVM) effects leading to higher relapse risk. The histologic subtype of the lymphoma in this study also strongly predicted the risk of relapse, therapy failure, and mortality. Independent of transplant type, FL was associated with the most favorable relapse risk and survival, consistent with prior registry data.32 Outcomes in other subtypes appeared to vary by histology in a time-dependent manner. For instance, in DLBCL and mantle cell lymphoma, the risk of disease relapse/progression was only significant within the first 7 months postallograft and not afterward, potentially suggesting evolution of durable GVM effects beyond that timeframe. Conversely, for Hodgkin lymphoma, the relapse risk increased beyond 7 months postallograft. Whether application of novel post-HCT maintenance/consolidation approaches could mitigate the risk of late failures after allografting in Hodgkin lymphoma is not known. Results of ongoing studies using post-allo-HCT brentuximab vedotin maintenance will help clarify this question in future (NCT02098512). We also assessed the effect of transplant modality on survival outcomes stratified by disease histology and found no significant differences (supplemental Table 3).
Progressive lymphoma was the most common cause of death in all 3 groups. Mortality due to infectious complications was lower in haploidentical transplants (7%) and slightly higher in URD allo-HCT using ATG (11%). The nature of data available to our registry precludes a detailed analysis of immune reconstitution across various donor sources. Mortality due to GVHD was lowest in the haploidentical transplantation group (1%) likely related to the low rates of grade III-IV acute and chronic GVHD in this group. Twelve percent of deaths in recipients of URD with ATG were due to GVHD as opposed to 6% in the URD cohort not receiving ATG.
Cumulative incidence of grade III-IV acute and chronic GVHD was significantly lower after haploidentical transplantation compared with URD transplant irrespective of whether patients received ATG or not. Within the URD transplants, the use of ATG lowered the risk of chronic but not acute GVHD, consistent with prior data.33,34 Among URD transplants, ATG use was associated with inferior survival, warranting caution with the routine use of this strategy in RIC/NMA transplantation. The lower chronic GVHD rates with haploidentical transplant are in line with recent reports in AML patients19 and this may be explained by (1) the posttransplant cyclophosphamide-based GVHD prophylaxis used or (2) by the use of BM as the predominant graft source in haploidentical recipients.35,36 Whether the low rate of GVHD in the current analysis is predominantly due to the donor source or use of posttransplant cyclophosphamide or the combination of both cannot be dissected by this analysis. The recently activated BMT CTN 1301 (NCT02345850) and 1203 (NCT02208037) trials are evaluating the role of posttransplant cyclophosphamide as GVHD prophylaxis in allo-HCT recipients with BM and PB as graft sources, respectively. Upon completion, we may better understand the impact of the posttransplant cyclophosphamide for GVHD prophylaxis relative to the standard CNI-based prophylaxis.
This study has inherent limitations. The nature of the data captured by the registry does not allow us to evaluate why a specific donor or graft source was chosen for any given patient included in the analysis. Although some of the patients may not have had a suitable HLA-matched adult URD, others may have been offered haploidentical transplantation based on institutional preference. More frequent application of prior autografts in the URD cohort could be reflective of institutional practice differences; however, the similar time interval between diagnosis and allo-HCT and median lines of prior therapies across the 3 groups suggest that no 1 group was overrepresented by lymphomas earlier in the disease course. The sample size limits the power to detect small differences in survival in our population. The study included various histologies, and although outcomes reported here were adjusted for lymphoma subtypes, with the current analysis we cannot demonstrate potential benefit or lack thereof of 1 donor source over another for any given lymphoma subtype. In addition, with the available data in the registry, we cannot evaluate potential differences between the 3 cohorts in terms of histology-specific prognostic scores (eg, international prognostic index for DLBCL), health care cost-effectiveness, and resource utilization (eg, secondary to infectious complications). Notwithstanding these limitations, this analysis is the only comparative study evaluating outcomes of haploidentical allo-HCT with posttransplant cyclophosphamide vs URD allografts in lymphoma patients. It is noteworthy that 15% of patients in the haploidentical group were African Americans compared with <1% in the URD groups, emphasizing the limited availability of HLA-matched URD for non-Caucasians. In the absence of randomized data, our results suggest that RIC/NMA conditioning followed by haploidentical allo-HCT with posttransplant cyclophosphamide should be considered an acceptable option for lymphoma patients without HLA-identical siblings. Our encouraging data warrant confirmation in randomized studies. These observations, if confirmed prospectively, will herald a paradigm shift in alternative donor selection.
Acknowledgments
The authors thank Morgan Geronime for administrative support.
The CIBMTR was supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Institutes of Health National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases; a grant/cooperative agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/US Department of Health and Human Services [DHHS]); grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc; *Amgen, Inc; anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc; *Gamida Cell Teva Joint Venture Ltd; Genentech, Inc; *Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc Roswell Park Cancer Institute; HistoGenetics, Inc; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc; Millennium: The Takeda Oncology Co; *Milliman USA, Inc; *Miltenyi Biotec, Inc; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc; Osiris Therapeutics, Inc; Otsuka America Pharmaceutical, Inc; Perkin Elmer, Inc; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc; St. Baldrick’s Foundation; StemCyte, a Global Cord Blood Therapeutics Co; Stemsoft Software, Inc; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc; *THERAKOS, Inc; University of Minnesota; University of Utah; and *Wellpoint, Inc (*corporate members).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.S.K., A.M., M.A.K.-D. and M. Hamadani conceived and designed the study; A.D. and M. Hamadani collected and assembled data; K.W.A., A.D., and M. Hamadani analyzed data; A.S.K., A.M., M.A.K.-D., and M. Hamadani prepared the first draft of the manuscript; and all authors contributed to data interpretation, helped revise the manuscript, and gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration, or any other agency of the US government.
Correspondence: Mehdi Hamadani, Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, 9200 W Wisconsin Ave, Suite C5500, Milwaukee, WI 53226; e-mail: mhamadani@mcw.edu.
References
- 1.Appelbaum FR. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia when a matched related donor is not available. Hematology Am Soc Hematol Educ Program. 2008;2008(1):412–417. doi: 10.1182/asheducation-2008.1.412. [DOI] [PubMed] [Google Scholar]
- 2.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122(4):491–498. doi: 10.1182/blood-2013-02-453175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henslee-Downey PJ, Parrish RS, MacDonald JS, et al. Combined in vitro and in vivo T lymphocyte depletion for the control of graft-versus-host disease following haploidentical marrow transplant. Transplantation. 1996;61(5):738–745. doi: 10.1097/00007890-199603150-00012. [DOI] [PubMed] [Google Scholar]
- 5.Kanda Y, Oshima K, Asano-Mori Y, et al. In vivo alemtuzumab enables haploidentical human leukocyte antigen-mismatched hematopoietic stem-cell transplantation without ex vivo graft manipulation. Transplantation. 2005;79(10):1351–1357. doi: 10.1097/01.tp.0000158718.49286.14. [DOI] [PubMed] [Google Scholar]
- 6.Dodero A, Carniti C, Raganato A, et al. Haploidentical stem cell transplantation after a reduced-intensity conditioning regimen for the treatment of advanced hematologic malignancies: posttransplantation CD8-depleted donor lymphocyte infusions contribute to improve T-cell recovery. Blood. 2009;113(19):4771–4779. doi: 10.1182/blood-2008-10-183723. [DOI] [PubMed] [Google Scholar]
- 7.Aversa F, Tabilio A, Velardi A, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339(17):1186–1193. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 8.Aversa F, Tabilio A, Terenzi A, et al. Successful engraftment of T-cell-depleted haploidentical “three-loci” incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood. 1994;84(11):3948–3955. [PubMed] [Google Scholar]
- 9.Lu DP, Dong L, Wu T, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006;107(8):3065–3073. doi: 10.1182/blood-2005-05-2146. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Liu DH, Liu KY, et al. Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer. 2013;119(5):978–985. doi: 10.1002/cncr.27761. [DOI] [PubMed] [Google Scholar]
- 11.O’Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8(7):377–386. doi: 10.1053/bbmt.2002.v8.pm12171484. [DOI] [PubMed] [Google Scholar]
- 12.Brunstein CG, Fuchs EJ, Carter SL, et al. Blood and Marrow Transplant Clinical Trials Network. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciurea SO, Mulanovich V, Saliba RM, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(12):1835–1844. doi: 10.1016/j.bbmt.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98(12):3456–3464. doi: 10.1182/blood.v98.12.3456. [DOI] [PubMed] [Google Scholar]
- 15.Kanakry CG, Ganguly S, Zahurak M, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med. 2013;5(211):211ra157. doi: 10.1126/scitranslmed.3006960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon SR, Sizemore CA, Sanacore M, et al. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant. 2012;18(12):1859–1866. doi: 10.1016/j.bbmt.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Raiola AM, Dominietto A, di Grazia C, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014;20(10):1573–1579. doi: 10.1016/j.bbmt.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Bashey A, Zhang X, Sizemore CA, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31(10):1310–1316. doi: 10.1200/JCO.2012.44.3523. [DOI] [PubMed] [Google Scholar]
- 19.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033–1040. doi: 10.1182/blood-2015-04-639831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castagna L, Bramanti S, Furst S, et al. Nonmyeloablative conditioning, unmanipulated haploidentical SCT and post-infusion CY for advanced lymphomas. Bone Marrow Transplant. 2014;49(12):1475–1480. doi: 10.1038/bmt.2014.197. [DOI] [PubMed] [Google Scholar]
- 21.Kanakry JA, Kasamon YL, Gocke CD, et al. Outcomes of related donor HLA-identical or HLA-haploidentical allogeneic blood or marrow transplantation for peripheral T cell lymphoma. Biol Blood Marrow Transplant. 2013;19(4):602–606. doi: 10.1016/j.bbmt.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raiola A, Dominietto A, Varaldo R, et al. Unmanipulated haploidentical BMT following non-myeloablative conditioning and post-transplantation CY for advanced Hodgkin’s lymphoma. Bone Marrow Transplant. 2014;49(2):190–194. doi: 10.1038/bmt.2013.166. [DOI] [PubMed] [Google Scholar]
- 23.Kanakry JA, Gocke CD, Bolaños-Meade J, et al. Phase II study of nonmyeloablative allogeneic bone marrow transplantation for B cell lymphoma with post-transplantation rituximab and donor selection based first on non-HLA factors. Biol Blood Marrow Transplant. 2015;21(12):2115–2122. doi: 10.1016/j.bbmt.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 27.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Zhang MJ. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101(1):87–93. doi: 10.1016/j.cmpb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Commenges D, Andersen PK. Score test of homogeneity for survival data. Lifetime Data Anal. 1995;1(2):145–156-159. doi: 10.1007/BF00985764. [DOI] [PubMed] [Google Scholar]
- 31.McCurdy SR, Kanakry JA, Showel MM, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125(19):3024–3031. doi: 10.1182/blood-2015-01-623991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachanova V, Burns LJ, Ahn KW, et al. Center for International Blood and Marrow Transplant Research Lymphoma Working Committee. Impact of pretransplantation (18)F-fluorodeoxy glucose-positron emission tomography status on outcomes after allogeneic hematopoietic cell transplantation for non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2015;21(9):1605–1611. doi: 10.1016/j.bbmt.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavletic SZ, Fowler DH. Are we making progress in GVHD prophylaxis and treatment? Hematology Am Soc Hematol Educ Program. 2012;2012(1):251–264. doi: 10.1182/asheducation-2012.1.251. [DOI] [PubMed] [Google Scholar]
- 35.Eapen M, Logan BR, Confer DL, et al. Peripheral blood grafts from unrelated donors are associated with increased acute and chronic graft-versus-host disease without improved survival. Biol Blood Marrow Transplant. 2007;13(12):1461–1468. doi: 10.1016/j.bbmt.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anasetti C, Logan BR, Lee SJ, et al. Blood and Marrow Transplant Clinical Trials Network. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]