Abstract
INTRODUCTION
Although pneumonia is a common reason for pediatric hospitalization among children with complex chronic conditions (CCC), treatment and outcomes have not been well-described. We characterized the presentation, management and outcomes of pneumonia in children with and without CCC and described how antibiotic management and outcomes vary among subgroups of children with CCC.
METHODS
We conducted a cohort study of children <18 years with pneumonia across a large sample of US hospitals. Children were grouped according to CCC subgroups. Differences in disease management and outcomes were assessed using multivariable regression.
RESULTS
Of the 31,684 children in our cohort, 11.9% had CCC. Children with CCC were more likely to receive intensive investigations and therapies, were less likely to receive aminopenicillins or third generation cephalosporins and were more likely to receive antibiotics against methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa and anaerobes. Compared with children without these conditions, children with CCC had significantly increased length of stay [relative risk 1.43, 95% confidence interval (CI) 1.39–1.48] and hospital costs (relative risk 1.38, 95% CI 1.33–1.43), with increased odds of antibiotic escalation (odds ratio 1.51, 95% CI 1.35–1.70), pneumonia complications (odds ratio 1.47, 95% CI 1.24–1.75) and readmission (odds ratio 4.0, 95% CI 3.2–5.0).
DISCUSSION
Children with CCC comprise a significant proportion of children hospitalized for pneumonia and are at substantially increased risk of adverse outcomes. They have high rates of treatment with broad spectrum antibiotics, both at the time of hospitalization and subsequently. Research is needed to inform decision-making and guideline development, with goals of reducing adverse outcomes and unnecessary variation in management among children with CCC.
Keywords: pneumonia, medical complexity, complex chronic conditions, child, adolescent, antibiotics
Introduction
Children with complex chronic medical conditions (CCC) have frequent encounters with healthcare providers in both inpatient and outpatient settings, accounting for approximately one-quarter of pediatric hospital days and more than one-third of hospital charges. These numbers represent an increasing proportion of pediatric hospitalizations and healthcare costs and highlight the importance of optimizing systems of care for children with these conditions.1 While recent surgical and medical advances have reduced pediatric mortality and contributed to increased survival of children with many CCC, this population remains at high risk for frequent and recurrent hospitalizations, with pneumonia being among the most common reasons for admission.2–6
The management of pneumonia among previously well children has been the focus of several recent studies exploring the comparative effectiveness of antibiotics and adjunctive therapies for disease management.7–12 However, considerably less is known about pneumonia treatment and outcomes among children with CCC despite their high burden of respiratory disease. Pneumonia etiology and subsequent management among children with CCC may be influenced by technology dependence, functional limitations, and frequent hospitalizations that often occur in this population. Despite this, patterns of antibiotic management are poorly understood.
The objectives of this study were to characterize the presentation, management and outcomes of pneumonia among children with CCC and to compare these to children without CCC in a large sample of US hospitals. Given the heterogeneous nature of pediatric complex conditions, we also examined antibiotic management and outcomes among subgroups of children with CCC.
Methods
Study Design & Eligibility Criteria
We conducted a retrospective cohort study of children less than 18 years of age admitted to hospitals that contribute data to the Perspective Data Warehouse (PDW) (Premier Healthcare Informatics, Charlotte, NC), a highly detailed administrative database that measures healthcare utilization, between July 1, 2007 and June 30, 2010. Geographically and structurally diverse hospitals across the United States contribute data to PDW, representing approximately one-sixth of hospitalizations nationally.13 This database has been shown to be sufficiently similar to the Agency for Healthcare Research and Quality Kids’ Inpatient Database, a nationally representative probablility-based sample of pediatric hospitalizations, and has been used in several previous studies of pediatric populations.13–16 It contains fully de-identified information including patient demographic characteristics, length of stay, all International Classification of Disease, 9th Edition, Clinical Modification (ICD-9-CM) discharge diagnoses and procedures, as well as a date-specific record of all services and items charged to the patient or insurer, including diagnostic tests, medications and their associated costs. Approximately 75% of hospitals that participate in PDW submit information on actual hospital costs, taken from internal cost accounting systems, whereas the remaining hospitals provide cost estimates based on Medicare cost-to-charge ratios.
We included children with a principal ICD-9-CM diagnosis of pneumonia using a published algorithm.17 To ensure that patients were being treated for pneumonia that was present at the time of admission, we restricted the analysis to those in whom antibiotics were begun on the first day of hospitalization. Infants born in hospital and resident since birth were excluded. Patients transferred to or from outside facilities or who left hospital against medical advice were excluded as we were unable to accurately assess length of hospital stay (LOS) or full course of hospital treatments. Subjects meeting eligibility criteria were grouped according to the presence or absence of CCC, defined as, “medical conditions that can be reasonably expected to last at least 12 months (unless death intervenes) and to involve either several different organ systems or one system severely enough to require specialty pediatric care and probably some period of hospitalization in a tertiary care center” and identified using a previously established algorithm.18,19 This algorithm categorizes children with CCC into the following subgroups: (i) neuromuscular disorders, (ii) cardiovascular malformations, (iii) chronic respiratory conditions, (iv) renal conditions, (v) gastrointestinal conditions, (vi) hematologic or immunologic conditions, (vii) metabolic conditions, (viii) malignant neoplasms, and (ix) other congenital or genetic disorders.
Patient and hospital characteristics
Children with CCC were categorized into subgroups representing the three most common single CCC categories (neuromuscular disorders, cardiovascular malformations, other congenital or genetic defects), other single CCC, and those with two or more CCC. Subjects were then characterized on the basis of age, gender, race/ethnicity (as recorded by the staff of participating hospitals using hospital-defined options), and insurance status. We assessed the presence of co-morbid conditions that commonly present concurrently with pneumonia including asthma, influenza, and disorders of fluids and electrolytes. Characteristics of admitting hospitals included geographic region, bed size, urban/rural location, children’s hospital versus general community hospital, and teaching status. Children’s hospitals included both freestanding children’s hospitals and children’s hospitals within larger adult centers, defined as institutions that had at least ten pediatric subspecialties recorded in the dataset.
Pneumonia management and outcome variables
We examined detailed billing codes to identify the use of investigations and therapies for patients with pneumonia, including blood tests, chest imaging, antibiotics, provision of inotropes, beta-agonists, oral or intravenous steroids, blood transfusions, mechanical ventilation, and non-invasive ventilation using billing codes and ICD-9-CM procedure codes. Intensive chest imaging was defined as chest ultrasound or chest computed tomography. Initial intensive care was defined as intensive care unit (ICU) charges on the first day of hospitalization, while ICU transfer was defined as ICU charges on or after the second day of hospitalization. Antibiotics were categorized as: (i) typical Streptococcus pneumonaie coverage (parenteral aminopenicillins or third generation cephalosporins); (ii) coverage for atypical organisms (oral or intravenous macrolides);20 (iii) coverage for methicillin-resistant Staphylococcus aureus (MRSA) (intravenous clindamycin, vancomycin, oral or intravenous linezolid);20–22 (iv) coverage for Pseudomonas aeruginosa (anti-pseudomonal cephalosporins, anti-pseudomonal carbepenems, anti-pseudomonal beta-lactam/lactamase inhibitors, or antipseudomonal quinolones);21,22 or (v) coverage for anaerobic organisms (clindamycin, metronidazole, ampicillin/sulbactam, or piperacillin/tazobactam).22 Antibiotic coverage was categorized as early initiation (defined as initiation in the emergency department or on the first day of hospitalization) or later initiation (defined as initiation on or after the second day of hospitalization). Antibiotic escalation was defined as the addition of any one of the antibiotics listed above on or after the second day of hospitalization, excluding parenteral ampicillin or a switch from vancomycin to clindamycin.
Outcome variables included: (i) antibiotic escalation, (ii) pneumonia complications, including pulmonary, metastatic and systemic complications using a previously established algorithm (See Table, Supplemental Digital Content 1, illustrating ICD-9-CM codes);20,23 (iii) LOS, in days; (iv) total hospital costs; and (v) and all-cause readmissions within 30 days.
Statistical analysis
We calculated patient-level summary statistics using frequencies and percents for categorical variables and medians and interquartile ranges for continuous variables. We assessed differences between children with and without CCC using chi-square tests for categorical variables and Wilcoxon rank-sum tests for continuous variables, applying a Bonferroni adjustment for multiple comparisons. Generalized estimating equation models with a logit link were used to assess odds of antibiotic escalation, pneumonia complications and readmission, while Poisson regression was used to assess differences in LOS. We used linear regression of log-transformed total hospital cost to assess differences between the groups and adjusted all models for within-hospital correlation. Costs were trimmed at 3 standard deviations above the mean. Covariates in the adjusted models included patient age, gender and payer, hospital region, location (urban/rural), teaching status, and hospital type (children’s hospital or general community hospital). All analyses were carried out using SAS 9.3 (Cary, NC: SAS Institute Inc). Because the data do not contain identifiable information, the Institutional Review Board at Baystate Medical Center determined that this study did not constitute human subjects research.
Results
A total of 31684 children meeting eligibility criteria were admitted to 284 hospitals contributing data to the PDW during the study period. Of these, 11.9 (n=3771) had CCC, including 22.8% (n= 861) with neuromuscular disorders, 20.8% (n=786) with cardiovascular malformations, 15.1% (n= 570) with chronic respiratory conditions, 2.5% (n= 93) with renal conditions, 1.7% (n= 63) with gastrointestinal conditions, 19.5% (n= 736) with hematologic or immunologic conditions, 3.6% (n= 134) with metabolic conditions, 5.9% (n=224) with malignant neoplasms, and 27.7% (n= 1043) with other congenital or genetic defects. A total of 16.9% (n=639) had two or more CCCs.
Children with CCC were more likely to be admitted to large, urban teaching hospitals than children without CCC. A total of 60.4% (n=2279) children with CCC were admitted to hospitals with more than 400 beds compared to 41.0% (n=11438) of children without CCC (p<0.001). More than half of children with CCC were admitted to teaching centers (55.6%, n=2098) compared with approximately one third of children without CCC (36.1%, n=10075, p<0.001), and 44.5% (n=1678) of children with CCC were admitted to children’s hospitals compared with 23.7% (n=6607) of children without CCC (p<0.001). A total of 14.2% of children with CCC (n=537) received their initial hospital care in the ICU compared to 4.5% (n=1254) of children without CCC (p<0.001).
Children with CCC were, on average, older and more likely to have public health insurance than children without CCC (See Table, Supplemental Digital Content 2, illustrating patient characteristics and initial pneumonia management). Asthma was a frequent comorbid condition among children with and without CCC, occurring in approximately one third of children. Children with CCC were significantly more likely to receive diagnostic testing and were three to nine times more likely to receive intensive therapies including inotropes, mechanical and non-invasive ventilation, and blood transfusions than children without these conditions.
Antibiotic usage also differed significantly between children with and without CCC (Figure, Supplemental Digital Content 3, illustrating antibiotic management). Children in all CCC subgroups, with the exception of children with cardiovascular malformations, were significantly less likely to receive typical S. pneumonaie coverage on the first day of hospitalization. However, again with the exception of children with cardiovascular malformations, they were two to five times more likely to receive initial antibiotic coverage against MRSA, anaerobic organisms and Pseudomonas. Children with CCC were also significantly more likely to have antibiotic coverage expanded on or after the second day of hospitalization. Children with neuromuscular disorders, other single CCC, and two or more CCC were most likely to have antibiotic coverage broadened, having a two- to four-fold increased likelihood of added coverage against MRSA and a three- to eight-fold increased likelihood of added coverage against Pseudomonas on or after the second hospital day.
In our unadjusted analyses, shown in Table 1, children with CCC were at significantly increased risk of adverse outcomes. Pneumonia complications occurred in more than 10% of children with CCC, a rate twice that seen among children without CCC. Length of hospital stay was considerably longer among children with CCC, with mean hospital costs that were twice those observed among children without CCC. Children with CCC had a four-fold increased likelihood of readmission within 30 days of discharge, with more than half of these readmissions being pneumonia-related.
Table 1.
Unadjusted outcomes among children with and without complex chronic conditions admitted with pneumonia.
| Outcome | Children without CCC (n=27913) |
Children with CCC ( n=3771) |
p-value |
|---|---|---|---|
| Antibiotic escalation, n(%) | 4094 (14.7%) | 863 (22.9%) | <0.001 |
| Pneumonia complications, n (%) | 1520 (5.4%) | 442 (11.7%) | <0.001 |
| Length of stay in days, mean, median [IQR] | 2.6, 2 [2–3] | 4.5, 3 [2–5] | <0.001 |
| Total hospital costs in USD, mean, median, [IQR] | 4379, 3336 [2250–5093] | 9168, 5141 [3009–9353] | <0.001 |
| All cause <30 day readmissions | 386 (1.4%) | 226 (6.0%) | <0.001 |
Accounting for within-hospital correlation as well as age, gender, payer, hospital region, teaching status, and hospital type, children with CCC had significantly greater odds of all outcomes examined (Figure 1), and these differences were consistent across most CCC subgroups. Children with CCC had a 51% increased odds of antibiotic escalation (adjusted OR 1.51, 95% CI 1.35–1.70), 47% increased odds of pneumonia complications (adjusted OR 1.47, 95% CI 1.24–1.75), 43% increased LOS (adjusted RR 1.43 95% CI 1.39–1.48), 38% increased cost (adjusted RR 1.38, 95% CI 1.33–1.43), and four times the odds or readmission within 30 days (adjusted OR 4.0, 95% CI 3.2–5.0).
Figure 1.
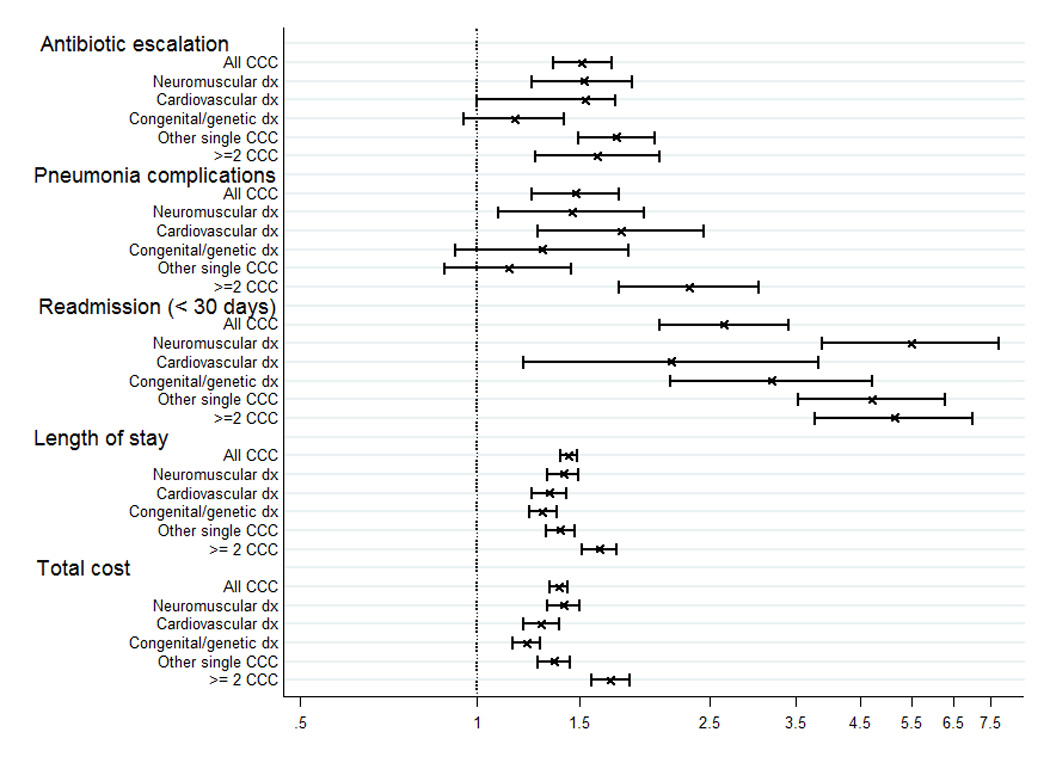
Odds of antibiotic escalation, pneumonia complications and readmission, and rate ratios of length of stay and total hospital costs among children with complex chronic conditions relative to those without complex chronic conditions, adjusted for patient and hospital covariaties and clustering within hospitals.
Footnote:
*CCC – complex chronic conditions
Discussion
In this study of children with pneumonia from 284 hospitals in the United States, we found that children with CCC presented to hospital with greater illness severity and were considerably more likely to receive intensive diagnostic tests and therapies than children without CCC. They were significantly more likely to receive broad spectrum antibiotics both at the time of admission and subsequently. Despite this, their outcomes were consistently worse, including significantly increased likelihood of pneumonia complications and readmission, and increased LOS and total hospital costs.
Several recent studies have highlighted the growing proportion of hospital admissions, readmissions and healthcare costs attributed to children with CCC.1–3,24,25 Using the same classification scheme applied in previous studies to identify children with CCC, our findings illustrate that poorer outcomes and increased resource utilization are not limited to specialized conditions or procedures but extend to this common pediatric infection. Although previous studies have shown that pneumonia is a common reason for hospitalization among children with cerebral palsy and other neurological impairments, this is, to our knowledge, the first study to examine how management patterns and outcomes differ among children with CCCs. 26,27
Children with CCCs were considerably more likely to receive broad spectrum coverage against anaerobes, MRSA and Pseudomonas, with children with neuromuscular disorders and multiple CCC most likely to receive broad spectrum coverage. These prescribing patterns may be influenced by perceived differences in disease etiology, perhaps influenced by aspiration risk, technology dependence, co-morbidities, past reasons for hospitalization, and other patient- and hospital-associated factors. In 30–40% of cases, children with CCCs had their antibiotic coverage broadened against these organisms on or after the second day of hospitalization. These changes in antibiotic coverage may be related to clinical deterioration, a lack of expected clinical improvement, or changes in providers’ perspectives regarding optimal antibiotic therapies. These patterns of antibiotic management, taken together with this cohort’s more severe disease presentation and poorer outcomes, highlight the need for research to characterize the role of multidrug resistant organisms in pneumonia pathogenesis among children with CCC. Research questions that have been addressed among previously well children, including the comparative effectiveness of antibiotics for pneumonia management, emerge as questions of significant relevance to children with CCCs.
Although there is no categorization of adult patients that is directly comparable to classification schemes applied to identify children with CCCs, the American Thoracic Society has developed risk criteria to identify and treat adult patients at increased risk of “healthcare-associated pneumonia” (HCAP), which has an increased likelihood of being caused by multidrug resistant organisms.21 HCAP is unique from hospital-acquired pneumonia in that patients need not develop signs and symptoms of pneumonia while in hospital, but present from community settings with characteristics that may increase their risk for colonization and infection with multi-drug resistant pathogens.
Analogous to HCAP among adults, children with CCC comprise a diverse population who have frequent contact with the healthcare system. As a result, they are expected to be at risk of infection with multi-drug resistant organisms and hence may benefit from tailored treatment recommendations that recognize how they differ from children without CCC. Similar to the recently published national guidelines for pneumonia management among previously well children, guidelines for children with CCC could aid decision-making regarding diagnostic testing and antibiotic coverage, with goals of reducing adverse outcomes and unnecessary variation in antibiotic management.20
Our results should be interpreted in light of a number of limitations. We used ICD-9-CM codes to identify patients with pneumonia, which may have resulted in misclassification. We attempted to minimize this by using a previously validated ICD-9-CM algorithm17 and by limiting our analysis to children who received an antibiotic on the first day of hospitalization. We used frequently cited antibiotic references to develop our classification scheme of antibiotic regimens. While this scheme does not reflect all possible antibiotic combinations and does not take into account local antibiotic sensitivity patterns, potential misclassification would have influenced all groups similarly. Because our analysis is limited to highly-detailed administrative data, we are unable to ascertain factors associated with providers’ management decisions, including antibiotic management as well as use of adjunctive therapies and tests. We are also unable to distinguish between pneumonia complications that were present at the time of admission from those that developed during the hospital stay. Lastly, readmissions to a hospital different from the original admission are not captured in PDW, which may have resulted in underestimates of readmission rates. In contrast, study strengths include our large sample size of children with CCC admitted to a large number of structurally diverse hospitals, allowing us to examine management and outcomes among CCC subgroups while controlling for patient and hospital factors and clustering within hospitals.
Our study has important implications for the care of children with CCCs who are admitted to hospital with pneumonia and establishes several important areas for future studies. Children with CCC presented to hospital with greater disease severity, were more likely to receive broad spectrum antibiotics at the time of hospitalization, and had considerably increased likelihood of antibiotic escalation during their hospital stay. Research is needed to describe pneumonia microbiology, ascertain specific risk factors for adverse pneumonia outcomes among children with CCCs and to inform optimal management strategies to mitigate these. This research is an essential prerequisite to inform future treatment guidelines to optimize processes of care and outcomes among this population of children while minimizing potential adverse effects of unnecessary broad spectrum antibiotic coverage.
Supplementary Material
Acknowledgments
This study was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 RR025752. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Pekow had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Simon T, Berry J, Feudtner C, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):1–10. doi: 10.1542/peds.2009-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry JG, Hall DE, Kuo DZ, Hall M, Kueser J, Kaplan W. Hospital Utilization and Characteristics of Patients Experiencing Recurrent Readmissions Within Children’s Hospitals. JAMA. 2011;305(7):682–690. doi: 10.1001/jama.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry JG, Hall M, Hall DE, et al. Inpatient Growth and Resource Use in 28 Children’s Hospitals: A Longitudinal, Multi-institutional Study. JAMA pediatrics. 2013;167(2):170–177. doi: 10.1001/jamapediatrics.2013.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kun SS, Edwards JD, Ward SLD, Keens TG. Hospital readmissions for newly discharged pediatric home mechanical ventilation patients. Pediatric pulmonology. 2012;47(4):409–414. doi: 10.1002/ppul.21536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loddenkemper T, Syed TU, Ramgopal S, et al. Risk factors associated with death in in-hospital pediatric convulsive status epilepticus. PloS one. 2012;7(10):e47474. doi: 10.1371/journal.pone.0047474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plioplys AV, Kasnicka I. Nebulized tobramycin: prevention of pneumonias in patients with severe cerebral palsy. J Pediatr Rehabil Med. 2011;4(2):155–158. doi: 10.3233/PRM-2011-0168. [DOI] [PubMed] [Google Scholar]
- 7.Ambroggio L, Taylor Ja, Tabb LP, Newschaffer CJ, Evans Aa, Shah SS. Comparative effectiveness of empiric β-lactam monotherapy and β-lactam-macrolide combination therapy in children hospitalized with community-acquired pneumonia. The Journal of pediatrics. 2012;161(6):1097–1103. doi: 10.1016/j.jpeds.2012.06.067. [DOI] [PubMed] [Google Scholar]
- 8.Shah SS, Test M, Sheffler-Collins S, Weiss AK, Hall M. Macrolide therapy and outcomes in a multicenter cohort of children hospitalized with Mycoplasma pneumoniae pneumonia. Journal of hospital medicine. 2012;7(4):311–317. doi: 10.1002/jhm.1904. [DOI] [PubMed] [Google Scholar]
- 9.Pati S, Lorch SA, Lee GE, Sheffler-Collins S, Shah SS. Health insurance and length of stay for children hospitalized with community-acquired pneumonia. Journal of hospital medicine. 2012;7(4):304–310. doi: 10.1002/jhm.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss AK, Hall M, Lee GE, Kronman MP, Shah SS, Weiss AAK. Adjunct Corticosteroids in Children Hospitalized With Community-Acquired Pneumonia. Pediatrics. 2011;127(2):e255–e263. doi: 10.1542/peds.2010-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.JK L, Shieh M, Lagu T, Pekow PS, Lindenauer PK. Comparative effectiveness of ceftriaxone alone relative to ceftriaxone in combination with a macrolide for the management of pediatric community acquired pneumonia. Pediatr Infect Dis Journal. doi: 10.1097/INF.0000000000000119. Epub ahead of print Oct 28, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams DJ, Hall M, Shah SS, et al. Narrow vs Broad-spectrum Antimicrobial Therapy for Children Hospitalized With Pneumonia. Pediatrics. 2013;132(5):e1141–e1148. doi: 10.1542/peds.2013-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lasky T, Ernst FR, Greenspan J, Wang S, Gonzalez L. Estimating pediatric inpatient medication use in the United States. Pharmacoepidemiology and drug safety. 2011;20:76–82. doi: 10.1002/pds.2063. [DOI] [PubMed] [Google Scholar]
- 14.Feudtner C, Dai D, Hexem KR, Luan X, Metjian TA. Prevalence of polypharmacy exposure among hospitalized children in the United States. Archives of pediatrics & adolescent medicine. 2012;166(1):9–16. doi: 10.1001/archpediatrics.2011.161. [DOI] [PubMed] [Google Scholar]
- 15.Jan S, Slap G, Smith-Whitley K, Dai D, Keren R, Rubin DM. Association of hospital and provider types on sickle cell disease outcomes. Pediatrics. 2013;132(5):854–861. doi: 10.1542/peds.2013-0089. [DOI] [PubMed] [Google Scholar]
- 16.Lasky T, Greenspan J, Ernst F, Gonzalez L. Pediatric vancomycin use in 421 hospitals in the United States, 2008. PloS one. 2012;7(8):e43258. doi: 10.1371/journal.pone.0043258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams DJ, Shah SS, Myers A, et al. Identifying Pediatric Community-Acquired Pneumonia Hospitalizations: Accuracy of Administrative Billing Codes. JAMA pediatrics. 2013;37232:1–8. doi: 10.1001/jamapediatrics.2013.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feudtner C, Christakis DA, Connell FA. Pediatric Deaths Attributable to Complex Chronic Conditions: A population based study of Washington State: 1980–1997. Pediatrics. 2000;106(1):205–209. [PubMed] [Google Scholar]
- 19.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths Attributed to Pediatric Complex Chronic Conditions: National Trends and Implications for Supportive Care Services. Pediatrics. 2001;107(6):e99. doi: 10.1542/peds.107.6.e99. [DOI] [PubMed] [Google Scholar]
- 20.Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clinical infectious diseases. 2011;53(7):e25–e76. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. American journal of respiratory and critical care medicine. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert DN, Moellering RC Jr, Eliopoulos GM, Chambers HF, Saag MS, editors. The Sanford Guide to Antimicrobial Therapy. 39th. Sperryville, VA: Antimicrobial Therapy; 2009. [Google Scholar]
- 23.Lee GE, Lorch SA, Sheffler-Collins S, Kronman MP, Shah SS. National hospitalization trends for pediatric pneumonia and associated complications. Pediatrics. 2010;126(2):204–213. doi: 10.1542/peds.2009-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berry JG, Graham Da, Graham RJ, et al. Predictors of clinical outcomes and hospital resource use of children after tracheotomy. Pediatrics. 2009;124(2):563–572. doi: 10.1542/peds.2008-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130(6):e1463–e1470. doi: 10.1542/peds.2012-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veugelers R, Calis EaC, Penning C, et al. A population-based nested case control study on recurrent pneumonias in children with severe generalized cerebral palsy: ethical considerations of the design and representativeness of the study sample. BMC pediatrics. 2005;5:25. doi: 10.1186/1471-2431-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berry JG, Poduri A, Bonkowsky JL, et al. Trends in resource utilization by children with neurological impairment in the United States inpatient health care system: a repeat cross-sectional study. PLoS medicine. 2012;9(1):e1001158. doi: 10.1371/journal.pmed.1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


