Abstract
Background
Pro-nerve growth factor must be cleaved to generate mature NGF, which was suggested to be a factor involved in ovarian physiology and pathology. Extracellular proNGF can induce cell death in many tissues. Whether extracellular proNGF exists in the ovary and may play a role in the death of follicular cells or atresia was unknown.
Material and Methods
Immunohistochemistry of human and Rhesus monkey ovarian sections was performed. IVF-derived follicular fluid and human granulosa cells were studied by RT-PCR, qPCR, Western blotting, ATP- and caspase-assays.
Results and Conclusions
Immunohistochemistry of ovarian sections identified proNGF in granulosa cells and Western blotting of human isolated granulosa cells confirmed the presence of proNGF. Ovarian granulosa cells thus produce proNGF. Recombinant human proNGF even at high concentrations did not affect the levels of ATP or the activity of caspase 3/7, indicating that in granulosa cells proNGF does not induce death. In contrast, mature NGF, which was detected previously in follicular fluid, may be a trophic molecule for granulosa cells with unexpected functions. We found that in contrast to proNGF, NGF increased the levels of the transcription factor early growth response 1 and of the enzyme choline acetyl-transferase. A mechanism for the generation of mature NGF from proNGF in the follicular fluid may be extracellular enzymatic cleavage. The enzyme MMP7 is known to cleave proNGF and was identified in follicular fluid and as a product of granulosa cells. Thus the generation of NGF in the ovarian follicle may depend on MMP7.
Keywords: granulosa cells, human ovary, matrix metalloprotease, neurotrophin
Introduction
The prototype neurotrophin nerve growth factor (NGF) regulates survival of nerve cells upon activation of the TrkA receptor [1]. The precursor of NGF, the larger molecule proNGF, appears to act in an opposite manner, inducing apoptosis upon binding to the p75 neurotrophin receptor (p75NTR) – sortilin complex. Clear evidence for p75NTR -mediated cell death was shown in different cellular systems, including glial cells and neurons [2] and more recently in the heart [3]. Increased levels of proNGF and p75NTR are typical for the brains of Alzheimer's patients [4] and it was suggested that the levels of proNGF may, in part, be responsible for apoptosis via p75NTR. Cells can secrete mature NGF and/or the precursor proNGF. In the latter case the levels of either factor in the extracellular space depend on extracellular processing of proNGF. Plasmin [5, 6], mast cell tryptase [7] and matrix metalloproteinase 7 (MMP7) [5, 8, 9] are well known extracellular enzymes, which are able to process proNGF.
In the female gonad cell proliferation, cell differentiation and cell death occur constantly and must be regulated by systemic and local factors. NGF has also been recognized as such an ovarian factor [10]. It was identified in the ovary of several species, including the human [11], in which also the pertinent receptors p75NTR and TrkA were found [12, 13]. The ovarian expression of sortilin, a rather ubiquitous protein, is not studied to our knowledge. Yet, the roles of NGF in human ovarian physiology, including the fate of ovarian follicles or possibly in ovarian pathologies are not fully known. Studies in human granulosa cells (GCs) provided some insights. For example, in human GCs, NGF increased the levels of FSHR mRNA [14]. NGF pretreatment of human GCs also resulted in higher secretion of oestradiol after exposure to follicle stimulating hormone (FSH) [15]. NGF also enhanced synthesis and secretion of the vascular endothelial growth factor (VEGF) from human GCs in a receptor-dependent manner [16] and has furthermore been implicated in ovarian cancer.
Whether proNGF is present in the (human) ovary, specifically in the extracellular space and in the follicular fluid (FF), was not known, despite of proteomic studies (see for example [17, 18]). ELISA studies indicated that immunoreactive NGF is produced by isolated human GCs and it was likewise found in human FF, derived from ovulatory follicles [19-22]. Its levels [20] in FF of PCOS (polycystic ovarian syndrome) – patients were reported to be lower than in women undergoing IVF (in vitro fertilization) for other reasons, or were elevated [23]. Differences in sampling and in selection criteria of PCOS-patients, as well as in IVF-protocols, and ELISA techniques might account for the discrepancies. Yet it is possible that several enzymes present in FF, which can process proNGF and/or NGF, could be at the heart of this difference. For example, MMP9 is present in FF and degrades NGF. Furthermore, higher MMP9 concentrations are reported in PCOS patients [24]. In addition, plasmin, able to cleave proNGF, is produced by GCs [25], while MMP7 or tryptase, which also cleave proNGF, are not well studied for their presence in FF.
We used IVF-derived human FFs, human and Rhesus monkey ovarian sections, and human IVF-derived GCs to study whether in addition to NGF, proNGF could be a factor in the follicle and to explore its function.
Materials and Methods
Human GC preparation and culture
FF containing GCs was obtained from IVF-patients stimulated according to routine protocols. The ethics committee of the Ludwig-Maximilian-University (LMU) of Munich approved the use of follicular aspirates and GCs for scientific experiments. Written consent of the patients was obtained. The study was carried out according to the guidelines of the 1975 Declaration of Helsinki. All samples and clinical information were anonymized. Aspirates with cells from two to five patients were pooled for the experiments. Cells were separated by centrifugation at 560 × g for 3 min and subsequently washed in serum-free DMEM/Ham's F12 media (PAA, Cölbe, Germany). The supernatant, i.e. FF, was frozen at -20°C until use for Western blot and proNGF ELISA studies. Washed cells were re-suspended in culture media supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml) and 10% (v/v) fetal calf serum (FCS) (all from PAA) as previously described [26-30]. Experiments were performed on day 2 or 3 of cell culture, unless otherwise indicated. Stimulations of the cells were performed in culture media without serum and penicillin/streptomycin. The numbers of replicates are provided for each experiment.
Ovarian samples, immunohistochemistry and immunocytochemistry
Human ovarian sections (from women of the reproductive phase) were derived from the tissue collection of the Institute of Anatomy and Cell Biology (Munich). Monkey ovarian samples from Rhesus macaques (Macaca Mulatta, age 5-6 years) were the same as used in previous studies [29]. Collection of tissues was approved by the Oregon National Primate Research Center Institutional Animal Care and Use Committee. Sections (4 μm) were cut from all paraffin-embedded samples and were used for immunohistochemistry, as described [30]. In brief, sections were deparaffinized, subjected to antigen retrieval by heating in citrate buffer (1.8 mM citric acid, 8.2 mM sodium citrate, pH 6.0) at 90°C for 30 min and endogenous peroxidase activity was blocked. Sections were incubated over night with different antisera, followed by a secondary antibody (biotinylated anti rabbit, 1:500, Dianova, Hamburg, Germany) and avidin-biotin complex peroxidase (ABC, Vector Laboratories, Burlingame, CA, USA). The binding was visualized with 3,3′diaminobenzidin (DAB, Sigma-Aldrich, St. Luis, MO, USA). Incubation with normal non-immune serum instead of the specific antiserum or omission of the primary antiserum served as control as well as preincubation of the antiserum with a specific blocking peptide (Table 1 and 2). The antiserum to proNGF does not cross-react with mature NGF and specifically recognizes a region of the proform. The antiserum to NGF reacted with NGF and proNGF. The specificities of the antisera were tested in a previous study [7].
Table 1. Antibodies and antisera used for immunohistochemistry and Western blotting.
| Antibody | Dilution | Company | |
|---|---|---|---|
| IHC | WB | ||
|
| |||
| β-actin (mouse) | 1:5000 | Sigma-Aldrich, St. Luis, MO, USA | |
| Biotinylated goat anti rabbit | 1:500 | Dianova, Hamburg, Germany | |
| ChAT (rabbit) | 1:500 | Millipore, Billerica, MA, USA | |
| egr-1 (rabbit) | 1:500 | Santa Cruz Biotechnology, Santa Cruz, CA, USA | |
| MMP7 (rabbit) | 1:100 | 1:1000 | Acris, Herford, Germany |
| NGF-beta (rabbit) | 1:500 | 1:500 | Chemicon, Billerica, MA, USA |
| NGFR (p75NTR) (rabbit) | 1:1000 | Sigma-Aldrich, St. Luis, MO, USA | |
| Pox donkey anti mouse | 1:10000 | Dianova, Hamburg, Germany | |
| Pox goat anti rabbit | 1:10000 | Dianova, Hamburg, Germany | |
| proNGF (rabbit) | 1:100 | 1:500 | Alomone Labs, Jerusalem, Israel |
| sortilin (rabbit) | 1:500 | Alomone Labs, Jerusalem, Israel | |
Table 2. Blocking peptides used for immunohistochemistry and Western blot experiments.
| Antibody | Dilution | Company |
|---|---|---|
| egr-1 | 2 μg peptide per 1 μg AB | Santa Cruz Biotechnology, Santa Cruz, CA, USA |
| proNGF | 2 μg peptide per 1 μg AB | Alomone Labs, Jerusalem, Israel |
| sortilin | 2 μg peptide per 1 μg AB | Alomone Labs, Jerusalem, Israel |
Immunocytochemistry on cultured cells (day 1) was performed as described, using anti sortilin antiserum and non-immune serum [7, 31, 32].
Isolation of RNA and RT-PCR
Total RNA of cultured human GCs (days 1-5 after isolation) was isolated using RNeasy Mini Kit (Quiagen, Hilden, Germany). As previously described [33, 34] 400 ng of total RNA were used for reverse transcription using random hexamer primers and Superscript II (Life Technologies, Karlsruhe, Germany). RT-PCR was performed using different oligonucleotide primers. Controls with H2O and RNA instead of cDNA were performed. Amplified PCR products were separated and visualized by ethidium bromide stained agarose gels. The identities of all PCR products were verified by sequencing [30, 35]. For primer sequence information, please see Table 3.
Table 3. Oligonucleotide primers used in RT-PCR studies.
| Gene | Primer sequence (5′ – 3′) | GeneBank accession no. |
|---|---|---|
| β-actin fw | TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA | NM_001101 |
| β-actin rev | CTA GAA GCA TTG CGG TGG ACG ATG GAG GG | |
| MMP7 fw | GAGTGCCAGATGTTGCAGAA | NM_002423 |
| MMP7 rev | AAATGCAGGGGGATCTCTTT | |
| MMP9 fw | TTGACAGCGACAAGAAGTGG | NM_004994 |
| MMP9 rev | GCCATTCACGTCGTCCTTAT | |
| NGF fw | CAC ACT GAG GTG CAT AGC GTA | NM_002506 |
| NGF rev | CAG GGA CAT TGC TCT CTG AGT | |
| p75NTR fw | AGC CAA CCA GAC CGT GTG TG | NM_002507 |
| p75NTR rev | TTG CAG CTG TTC CAC CTC TT | |
| Sortilin fw | TCCTGGGTTGGAGATAGCAC | NM_002959 |
| Sortilin rev | TTCCTCCAGACACCTCTGCT | |
| TrkA fw | TCAACAAATGTGGACGGAGA | NM_001007792 |
| TrkA rev | GTGGTGAACACAGGCATCAC | |
| TrkA rev seminested | GAGCTGCCACCCAATGTC |
Western blot
Western blotting was performed as previously described [33]. In brief, human GCs were cultured on 60 mm dishes (Sarstedt, Nürnbrecht, Germany) in DMEM/Ham's F12 media supplemented with 10% (v/v) FCS and 1% (v/v) penicillin/streptomycin. To detect possible small differences in loading and to correct the results accordingly, β-actin served as internal standard. Some antibodies were also used for immunohistochemistry. For control purposes, some antibodies, including proNGF, were pre-incubated with a blocking peptide. This antibody was also used for immunohistochemistry. Western blot bands were visualized with chemiluminescence reagents (Pierce – Thermo Scientific, Waltham, USA). For the list of antibodies and blocking peptides used, please see Tables 1 and 2. Human GCs were incubated with recombinant proNGF (Cedarlane, Burlington, Canada) and recombinant NGF (Alomone Labs, Jerusalem, Israel) each with 50 ng/ml for 1 h or 24 h in DMEM/Ham's F12 media without FCS, to examine downstream effects on early growth response 1 (EGR1) and choline acetyl-transferase (CHAT) level exerted by the factors. Bands were analyzed with ImageJ Software (National Institute of Health, Bethesda, MD, USA; version 1.45s) and the results normalized to the untreated control.
Cell viability assay
Cell viability was estimated by measuring ATP content, which correlates with cell number and/or viability [34]. For the ATP assay, cells were cultured for 2 days, seeded on white walled 96-well plates (20000 cells per well) and then exposed to different concentrations of proNGF and NGF for 24 h. Staurosporine (10 μM; Sigma-Aldrich) was used as control. 100 μl of CellTiter-Glo® reagent (Promega, Mannheim, Germany) were added to each well containing 100 μl of untreated control cells or stimulated cells, avoiding direct light. The contents were mixed for 2 min at 500 rpm on a plate shaker and then incubated for 10 min at room temperature. The fluorescence of the samples was measured in a luminometer (Fluostar, BMG Labtech, Ortenberg, Germany).
Caspase assay
The activities of the effector caspases 3/7 were determined following exposure of human GCs to proNGF and NGF [34, 35]. Cells were cultured for 2 days, seeded on white walled 96-well plates (20000 cells per well) and then treated with different concentrations of proNGF and NGF for 24 h. Staurosporine (1 μM; Sigma-Aldrich) was used as positive control. 100 μl of Caspase-Glo® 3/7 reagent (Promega) were added to each well containing 100 μl of untreated control cells or stimulated cells, avoiding direct light. Contents of the plate were mixed on a plate shaker for 30 s at 500 rpm and then incubated for 1 h at room temperature. The luminescence of each sample was measured in a luminometer (Fluostar, BMG Labtech).
Data analysis and statistics
Results obtained were analyzed and depicted using Excel and Prism (GraphPad). One-way ANOVA-analyses followed by a post hoc test (Newman-Keuls multiple comparison test) were performed. All data are expressed as mean ± SEM.
Results
We examined proNGF and NGF by immunohistochemistry in human ovarian large antral follicles (Fig. 1A, B). We observed that both GCs and theca cells (TCs) were stained. Staining of proNGF disappeared upon pre-adsorption of the antibody with proNGF. Further controls, including non-immune serum, also yielded negative results. To examine the specificities and cross-reactivities of the antibodies used for immmunohistochemistry, they were used for Western blotting. In GC-pellets proNGF was readily detected (Fig. 2, left panel shows examples from cells at day 2 of culture). It was also detected in human GCs on culture day 1, 3, 4 and 5 (data not shown). Specificity is illustrated by the absence of signals in Western blots when the proNGF antibody was pre-adsorbed (Fig. 2, middle panel). Western blotting using a NGF antibody, which as expected detects both NGF and the NGF precursor proNGF, revealed in all batches of GCs examined (n = 7) abundant proNGF, recognized by its size of approximately 37 kDa. Only in 3 out of the 7 samples this antibody showed concomitantly mature NGF of approximately 17 kDa, which was however less abundant than proNGF (Fig. 2, right panel). In the other 4 out of the 7 cases immunoreactive NGF was not observed (not shown). Thus, human GCs produce and contain mainly the pro-form of NGF, and its presence in FF suggests that mainly proNGF is secreted into the FF by human GCs.
Figure 1. The NGF precursor proNGF (A) and mature NGF (B) are present in the cells of the follicular wall in human ovaries.
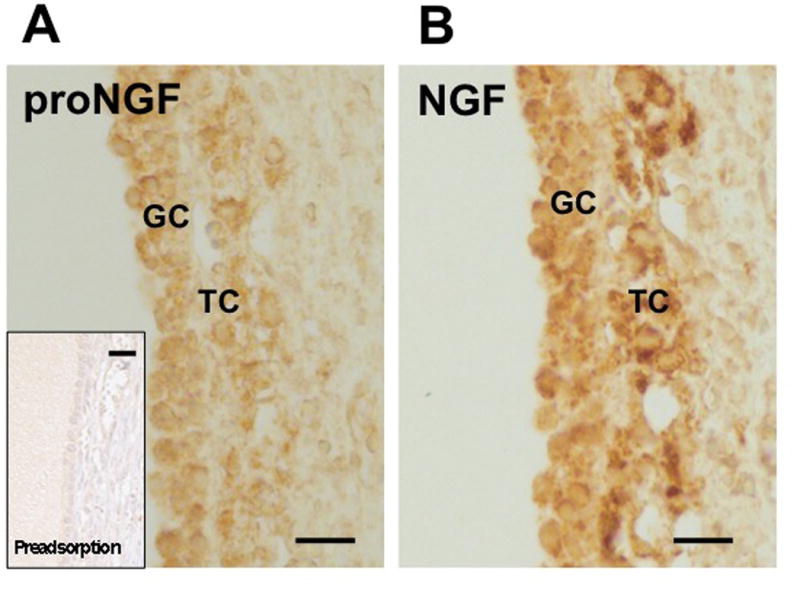
Immunohistochemical staining of sections of human ovaries reveals that proNGF is present in cells of a large antral follicle, namely in GCs and theca cells (TCs). Positive staining reaction is indicated by the brown color. Note that the antiserum is specific for the pro-region of the proNGF molecule and does not recognize mature NGF. Bar: 30 μm. Insert control: Result of an experiment in which the proNGF antiserum was pre-adsorbed.
Figure 2. ProNGF and NGF in human GCs.

Left panel: Example of a Western blot: ProNGF is detected in human GCs (cells cultured for 2 and 3 days are shown); middle panel: Control blot, in which pre-adsorbed anti-proNGF antiserum was used; right panel: Example of a Western blot, in which an antiserum to NGF was used. Note that this antiserum, as expected, recognizes both NGF and proNGF and that the GCs shown contain abundant proNGF, while NGF was much less abundant.
To explore actions of proNGF and NGF, we used human GCs from culture days 2-3 for functional studies, since qPCR analysis showed low proNGF/NGF mRNA levels at that time of culture (results not shown). Thus actions of exogenous proNGF/ NGF should not be confounded by endogenous production. We assessed, whether proNGF and/or NGF can influence cell vitality of human GCs. Different amounts of proNGF and NGF (5 ng/ml – 200 ng/ml) were added for 24 h but neither proNGF, nor NGF changed ATP levels (Fig. 3A). Furthermore, caspase assays did not indicate induction of apoptosis in the proNGF- or NGF-treated cells (Fig. 3B). Thus, in cultured human GCs proNGF does not cause cell death and NGF does not promote cell proliferation of cultured GCs.
Figure 3. Stimulation of GCs with proNGF and NGF has no effects on the viability and the apoptosis of human GCs (A and B) and presence of the receptors for NGF and proNGF in human GCs (C – E).
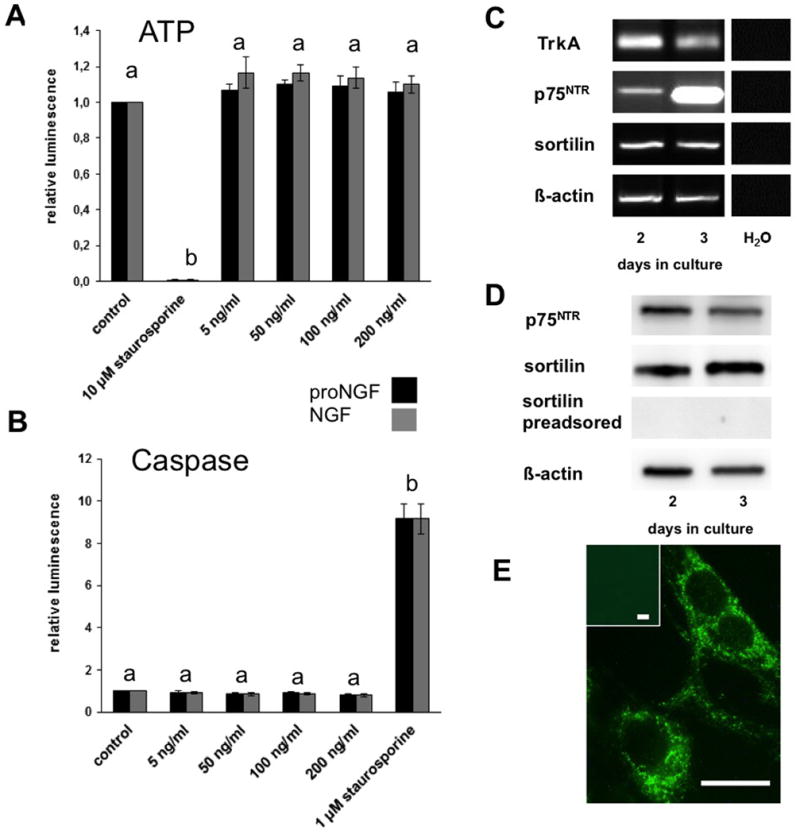
A: Results of ATP assays with human GCs. The stimulation was performed on day 2-3 for 24 h with different concentrations of proNGF and NGF. Staurosporin (10 μM) was used as positive control. Both recombinant proteins did not significantly affect viability of GCs. Each column represents the relative luminescence of four independent experiments (mean ± SEM) per group. Different letters indicate statistically significant differences between the staurosporine-group and the other groups (p< 0.05).
B: Results of caspase assays with human GCs. Stimulation was performed on day 2-3 for 24 h with different concentrations of proNGF and NGF and staurosporine (1 μM) as positive control. Neither proNGF nor NGF promoted apoptotic cell death in the human GCs. Each column represents the relative luminescence of three independent experiments (mean ±SEM). Different letters indicate statistically significant differences between the staurosporine-group and the other groups (p< 0.05).
C: Result of RT-PCR experiment detecting the receptors for NGF (TrkA and p75NTR) and for proNGF (p75NTR and sortilin) in human GCs on different days of culture; β-actin was used as internal standard. Controls were performed without cDNA.
D: Western blot of the proNGF receptors in human GCs on different days of culture; preadsorption with sortilin was used as control and β-actin as internal standard.
E: Intracellular localization of sortilin in GCs (day 1). Insert as control, in which non-immune serum was used. Bars: 10 μm
Actions of NGF/proNGF depend on specific receptors. We detected by RT-PCR and Western blotting that human GCs express TrkA/p75NTR and p75NTR/sortilin, which may serve as receptors for NGF and proNGF, respectively (Fig. 3C, D, E). Sortilin was found in cultured GCs but was never seen associated with the cell membrane (Fig. 3E), and hence proNGF may not be able to activate this cell death pathway.
Addition of recombinant NGF (50 ng/ml in serum free media) for 1 h to the GCs significantly increased approx. 2-3-fold the levels of the immediate early gene EGR1, a master transcription factor of the NGF signaling cascade (Fig. 4A, B). Addition of similar amounts of proNGF (50 ng/ml) did not alter EGR1 levels (Fig. 4A, B). We also detected significantly increased (2.5 fold) levels of CHAT, the enzyme responsible for the production of acetylcholine (ACh), after NGF treatment of GCs (serum free media 50 ng/ml NGF for 24 h; Fig. 4C, D), while proNGF (50 ng/ml for 24h) was not effective (Fig. 4C, D). Thus the actions of NGF in ovarian GCs are similar to the ones in neuronal cells.
Figure 4. EGR1 and CHAT level increase in human GCs after stimulation with NGF.
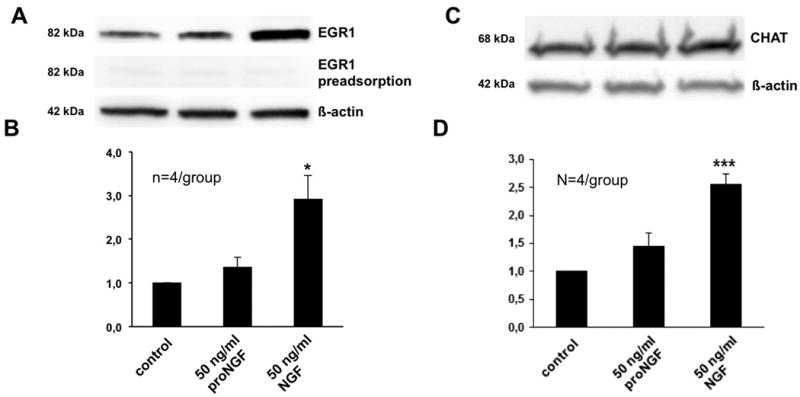
A: Example of a Western blot with human GCs on day 2 of culture; stimulation of human GCs with NGF (50 ng/ml) for 1 h led to an increase in EGR1 levels whereas proNGF stimulation (50 ng/ml) did not. EGR1 pre-adsorption was used as control and β-actin as internal standard.
B: Densitometric analysis of the experiments performed on day 2 of human GC culture. Each column represents the ratio of EGR1 to untreated control (mean ± SEM) of four independent experiments. *, p< 0.05 vs. untreated control.
C: Example of a Western blot with human GCs on day 2-3 of culture; stimulation of human GCs with NGF (50 ng/ml) for 24 h led to an increase in CHAT protein levels whereas proNGF stimulation (50 ng/ml) has no significant effect on the cells. β-actin was used as internal standard.
D: Densitometric analysis of the experiments performed after 24 h stimulation of human GCs on day 2-3 of culture. Each column represents the ratio of CHAT to untreated control (mean ± SEM) of four independent experiments. ***, p < 0.001 vs. untreated control.
Because NGF actions can be witnessed in GCs and NGF can be detected in FF, yet proNGF is mainly produced by GCs, we explored mechanisms that may explain extracellular cleavage of proNGF. We focused on ovarian MMP7, which is an extracellular matrix metalloproteinase, known to process proNGF into its mature form. We detected MMP7 (approx. 25 kDa) in the FF of IVF-patients by Western blotting (n =5; Fig. 5A), however the levels varied. Using the same antibody, MMP7 was found in human and Rhesus monkey antral follicles by immunohistochemistry. Both species showed positive staining in GCs and TCs of large follicles as well as staining of the FF (Fig. 5B). Cultured human GCs express MMP7 as shown by RT-PCR and Western blotting (Fig. 5C), implying that GCs are indeed the source of MMP7 to the FF. This suggests that proNGF is processed by MMP7 in the FF to NGF and that its levels depend on MMP7 levels and activities of this enzyme in FF.
Figure 5. Presence of MMP7 in the follicular fluid, in human and Rhesus monkey ovarian sections and in human GCs.
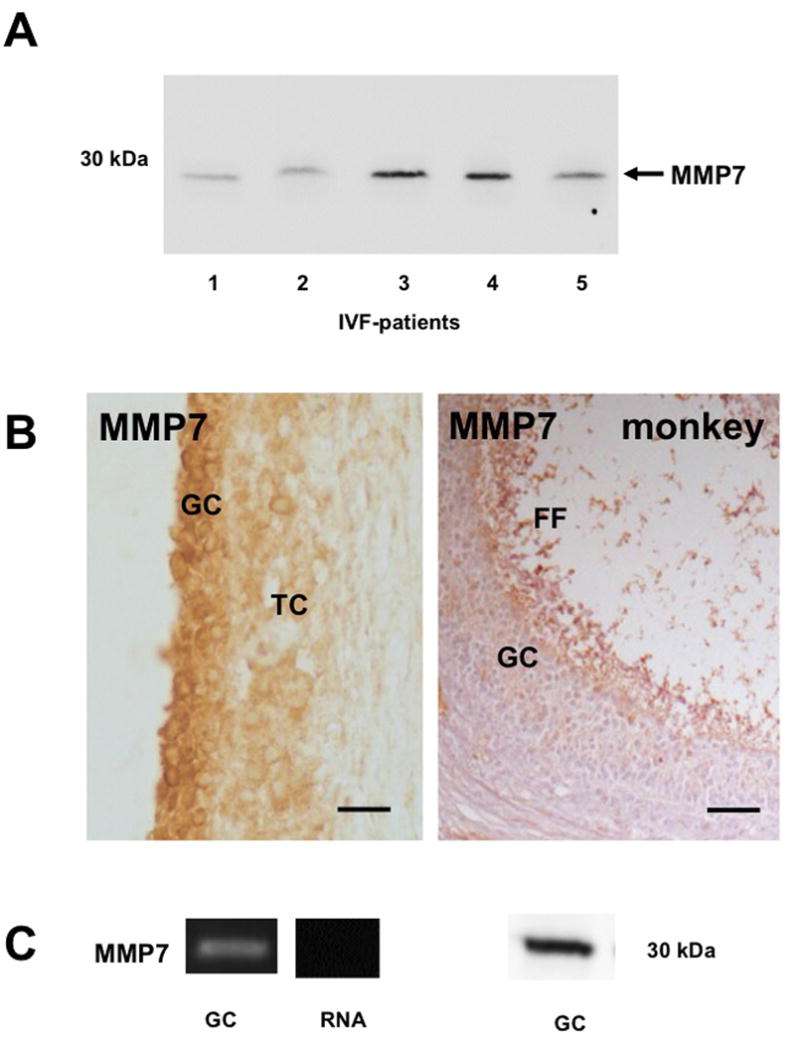
A: Result of a Western blot: MMP7 is present in the FF of 5 IVF-patients.
B: Immunohistochemical staining of MMP7 in paraffin sections of human and monkey ovarian sections. Note that MMP7 can be detected in GCs and TCs. Bars: 50 μm
C: MMP7 is present in human GCs: mRNA (left panel) and protein (right panel). Controls were performed with RNA instead of input cDNA.
Discussion
To our knowledge, there are no previous reports on proNGF in the ovary and ovarian cells. The NGF precursor was unequivocally found in the present study by using a combination of Western blotting and immunohistochemistry in human samples. They allow the conclusion that proNGF is a GC-derived factor. Our immunohistochemical data (not shown) imply that this is also the case in a non-human primate species, the rhesus monkey.
What are the actions of proNGF and NGF in human GCs? Some pieces of information for NGF are available (see introduction). In rat PC12 cells NGF, but not proNGF, increased the transcription factor EGR1 [36], a result, which we also obtained in human GCs in the present study. EGR1 is regarded as a master transcription factor and is linked to differentiation and mitosis. Furthermore, a previous study showed that NGF increased levels of CHAT in bovine luteal cells [37]. This enzyme is important for the production of ACh from GCs and was also described in the human ovary and GCs [29, 38-40]. We found that NGF, but not proNGF, elevated the levels of CHAT in human GCs. ACh in the ovary acts as a trophic molecule and regulates ion channel activity in human GCs [40]. In the bovine corpus luteum (CL) [37] it was also reported to exert anti-apoptotic actions. Thus NGF appears to be a trophic factor for human GCs.
In cultured human GCs, however, neither exogenous NGF nor proNGF measurably interfered with cell viability/cell number as indicated by unchanged levels of ATP. This may be due to the existing endogenous production of these factors, which may drive the system and may mask effects of exogenous factors. It may also be related to the limited ability of cultured human GCs cells to proliferate.
Cultured, luteinizing GCs may also differ from follicular GCs especially with regard to susceptibility to cell death and apoptosis and indeed exogenous proNGF did not promote cell death. ATP and caspase assays excluded such actions. Data from our video-imaging studies (results not shown) furthermore supports this conclusion. Actions of proNGF and NGF are initiated upon receptor binding and the receptor molecules linked to proNGF-inducible cell death in brain, namely p75NTR and sortilin, are present in cultured GCs, in addition to TrkA. The cellular localization of sortilin appears to be related to its function, as suggested recently [41]. If so, the intracellular localization, rather than membrane-associated localization of sortilin in GCs seen in our study may provide a possible explanation for a lack of proNGF activity in GCs.
The results obtained suggest that proNGF is solely a biologically inactive precursor of NGF in GCs and the generation of active NGF in FF thus depends on extracellular processing in the FF. In the present study we focused on MMP7, known to cleave proNGF [5, 8, 9]. We found that MMP7 is a human and non-human primate GC product and is present in FF in vivo. Thus we assume that it is involved in the cleavage of proNGF in FF, yet other processing enzymes may also be considered. The serine protease tissue plasminogen activator (tPA) catalyzes the conversion of plasminogen to plasmin, which also is an enzyme able to cleave proNGF [5, 6]. It is a product of GCs but its activity appears to be controlled by inhibitors [17, 18, 25]. To our knowledge, there are no studies on the regulation of MMP7 and plasmin in the human ovary. They and precise methods of determination of proNGF and NGF levels will be required to understand the regulation of the ovarian proNGF/NGF system.
In summary, the study performed with IVF-derived human FF and GCs reveals that human GCs produce proNGF. Based on cellular studies, we suggest that proNGF in FF is an inactive precursor of NGF, and that biologically active NGF can be generated mainly in the extracellular space, e.g. via the GC-product MMP7. The observed effects of NGF on EGR1 and CHAT, also found in neuronal cells, extend the view that NGF is acting as a trophic molecule for GCs. The apparent lack of proNGF actions in GCs, in face of distinct NGF actions, reveals that the human ovarian proNGF/NGF system is more complex than previously known. Transcriptional/translational control mechanisms may determine levels of these factors in the first place, Yet the amounts and the activities of enzymes, which cleave proNGF and NGF in the ovary, may fine-tune the actions of this growth factor system.
Acknowledgments
We gratefully acknowledge the skillful help of Daniel Einwang, Astrid Tiefenbacher, Carola Hermann and the editorial support by Karin Metzrath.
Grant information: DFG MA 1080/17-3 and in part by MA1080/19-1 and NIH grant 8P51OD011092 (G.A.D., S.R.O.) for the operation of the ONPRC.
This work was done in partial fulfillment of the requirements for Dr.rer.nat. projects of SM and JB at the LMU.
Footnotes
Competing interests: There are no competing interests.
References
- 1.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 2.Le AP, Friedman WJ. Matrix metalloproteinase-7 regulates cleavage of pro-nerve growth factor and is neuroprotective following kainic acid-induced seizures. J Neurosci. 2012;32:703–712. doi: 10.1523/JNEUROSCI.4128-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siao CJ, Lorentz CU, Kermani P, Marinic T, Carter J, McGrath K, Padow VA, Mark W, Falcone DJ, Cohen-Gould L, Parrish DC, Habecker BA, Nykjaer A, Ellenson LH, Tessarollo L, Hempstead BL. ProNGF, a cytokine induced after myocardial infarction in humans, targets pericytes to promote microvascular damage and activation. J Exp Med. 2012;209:2291–2305. doi: 10.1084/jem.20111749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng S, Wuu J, Mufson EJ, Fahnestock M. Increased proNGF levels in subjects with mild cognitive impairment and mild Alzheimer disease. J Neuropathol Exp Neurol. 2004;63:641–649. doi: 10.1093/jnen/63.6.641. [DOI] [PubMed] [Google Scholar]
- 5.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 6.Bruno MA, Cuello AC. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc Natl Acad Sci U S A. 2006;103:6735–6740. doi: 10.1073/pnas.0510645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spinnler K, Frohlich T, Arnold GJ, Kunz L, Mayerhofer A. Human tryptase cleaves pro-nerve growth factor (pro-NGF): hints of local, mast cell-dependent regulation of NGF/pro-NGF action. J Biol Chem. 2011;286:31707–31713. doi: 10.1074/jbc.M111.233486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith MM, Shi L, Navre M. Rapid identification of highly active and selective substrates for stromelysin and matrilysin using bacteriophage peptide display libraries. J Biol Chem. 1995;270:6440–6449. doi: 10.1074/jbc.270.12.6440. [DOI] [PubMed] [Google Scholar]
- 9.Kendall TJ, Hennedige S, Aucott RL, Hartland SN, Vernon MA, Benyon RC, Iredale JP. p75 Neurotrophin receptor signaling regulates hepatic myofibroblast proliferation and apoptosis in recovery from rodent liver fibrosis. Hepatology. 2009;49:901–910. doi: 10.1002/hep.22701. [DOI] [PubMed] [Google Scholar]
- 10.Linher-Melville K, Li J. The roles of glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor and nerve growth factor during the final stage of folliculogenesis: a focus on oocyte maturation. Reproduction. 2013;145:R43–54. doi: 10.1530/REP-12-0219. [DOI] [PubMed] [Google Scholar]
- 11.Abir R, Fisch B, Jin S, Barnnet M, Ben-Haroush A, Felz C, Kessler-Icekson G, Feldberg D, Nitke S, Ao A. Presence of NGF and its receptors in ovaries from human fetuses and adults. Mol Hum Reprod. 2005;11:229–236. doi: 10.1093/molehr/gah164. [DOI] [PubMed] [Google Scholar]
- 12.Raffioni S, Bradshaw RA, Buxser SE. The receptors for nerve growth factor and other neurotrophins. Annu Rev Biochem. 1993;62:823–850. doi: 10.1146/annurev.bi.62.070193.004135. [DOI] [PubMed] [Google Scholar]
- 13.Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 14.Romero C, Paredes A, Dissen GA, Ojeda SR. Nerve growth factor induces the expression of functional FSH receptors in newly formed follicles of the rat ovary. Endocrinology. 2002;143:1485–1494. doi: 10.1210/endo.143.4.8711. [DOI] [PubMed] [Google Scholar]
- 15.Salas C, Julio-Pieper M, Valladares M, Pommer R, Vega M, Mastronardi C, Kerr B, Ojeda SR, Lara HE, Romero C. Nerve growth factor-dependent activation of trkA receptors in the human ovary results in synthesis of follicle-stimulating hormone receptors and estrogen secretion. J Clin Endocrinol Metab. 2006;91:2396–2403. doi: 10.1210/jc.2005-1925. [DOI] [PubMed] [Google Scholar]
- 16.Julio-Pieper M, Lozada P, Tapia V, Vega M, Miranda C, Vantman D, Ojeda SR, Romero C. Nerve growth factor induces vascular endothelial growth factor expression in granulosa cells via a trkA receptor/mitogen-activated protein kinase-extracellularly regulated kinase 2-dependent pathway. J Clin Endocrinol Metab. 2009;94:3065–3071. doi: 10.1210/jc.2009-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ambekar AS, Nirujogi RS, Srikanth SM, Chavan S, Kelkar DS, Hinduja I, Zaveri K, Prasad TS, Harsha HC, Pandey A, Mukherjee S. Proteomic analysis of human follicular fluid: a new perspective towards understanding folliculogenesis. J Proteomics. 2013;87:68–77. doi: 10.1016/j.jprot.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Twigt J, Steegers-Theunissen RP, Bezstarosti K, Demmers JA. Proteomic analysis of the microenvironment of developing oocytes. Proteomics. 2012;12:1463–1471. doi: 10.1002/pmic.201100240. [DOI] [PubMed] [Google Scholar]
- 19.Seifer DB, Feng B, Shelden RM. Immunocytochemical evidence for the presence and location of the neurotrophin-Trk receptor family in adult human preovulatory ovarian follicles. Am J Obstet Gynecol. 2006;194:1129–1134. doi: 10.1016/j.ajog.2005.12.022. discussion 1134-1126. [DOI] [PubMed] [Google Scholar]
- 20.Buyuk E, Seifer DB. Follicular-fluid neurotrophin levels in women undergoing assisted reproductive technology for different etiologies of infertility. Fertil Steril. 2008;90:1611–1615. doi: 10.1016/j.fertnstert.2007.08.085. [DOI] [PubMed] [Google Scholar]
- 21.Buyuk E, Santoro N, Cohen HW, Charron MJ, Jindal S. Reduced neurotrophin receptor tropomyosin-related kinase A expression in human granulosa cells: a novel marker of diminishing ovarian reserve. Fertil Steril. 2011;96:474–478. doi: 10.1016/j.fertnstert.2011.05.017. e474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadeu JC, Doedee AM, Neal MS, Hughes EG, Foster WG. Neurotrophins (BDNF and NGF) in follicular fluid of women with different infertility diagnoses. Reprod Biomed Online. 2012;24:174–179. doi: 10.1016/j.rbmo.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Dissen GA, Garcia-Rudaz C, Paredes A, Mayer C, Mayerhofer A, Ojeda SR. Excessive ovarian production of nerve growth factor facilitates development of cystic ovarian morphology in mice and is a feature of polycystic ovarian syndrome in humans. Endocrinology. 2009;150:2906–2914. doi: 10.1210/en.2008-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuello AC, Bruno MA, Bell KF. NGF-cholinergic dependency in brain aging, MCI and Alzheimer's disease. Curr Alzheimer Res. 2007;4:351–358. doi: 10.2174/156720507781788774. [DOI] [PubMed] [Google Scholar]
- 25.Liu YX, Liu XM, Nin LF, Shi L, Chen SR. Serine protease and ovarian paracrine factors in regulation of ovulation. Front Biosci (Landmark Ed) 2013;18:650–664. doi: 10.2741/4128. [DOI] [PubMed] [Google Scholar]
- 26.Mayerhofer A, Fohr KJ, Sterzik K, Gratzl M. Carbachol increases intracellular free calcium concentrations in human granulosa-lutein cells. J Endocrinol. 1992;135:153–159. doi: 10.1677/joe.0.1350153. [DOI] [PubMed] [Google Scholar]
- 27.Mayerhofer A, Sterzik K, Link H, Wiemann M, Gratzl M. Effect of oxytocin on free intracellular Ca2+ levels and progesterone release by human granulosa-lutein cells. J Clin Endocrinol Metab. 1993;77:1209–1214. doi: 10.1210/jcem.77.5.8077313. [DOI] [PubMed] [Google Scholar]
- 28.Bulling A, Berg FD, Berg U, Duffy DM, Stouffer RL, Ojeda SR, Gratzl M, Mayerhofer A. Identification of an ovarian voltage-activated Na+-channel type: hints to involvement in luteolysis. Mol Endocrinol. 2000;14:1064–1074. doi: 10.1210/mend.14.7.0481. [DOI] [PubMed] [Google Scholar]
- 29.Mayerhofer A, Kunz L, Krieger A, Proskocil B, Spindel E, Amsterdam A, Dissen GA, Ojeda SR, Wessler I. FSH regulates acetycholine production by ovarian granulosa cells. Reprod Biol Endocrinol. 2006;4:37. doi: 10.1186/1477-7827-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saller S, Merz-Lange J, Raffael S, Hecht S, Pavlik R, Thaler C, Berg D, Berg U, Kunz L, Mayerhofer A. Norepinephrine, active norepinephrine transporter, and norepinephrine-metabolism are involved in the generation of reactive oxygen species in human ovarian granulosa cells. Endocrinology. 2012;153:1472–1483. doi: 10.1210/en.2011-1769. [DOI] [PubMed] [Google Scholar]
- 31.Albrecht M, Ramsch R, Kohn FM, Schwarzer JU, Mayerhofer A. Isolation and cultivation of human testicular peritubular cells: a new model for the investigation of fibrotic processes in the human testis and male infertility. J Clin Endocrinol Metab. 2006;91:1956–1960. doi: 10.1210/jc.2005-2169. [DOI] [PubMed] [Google Scholar]
- 32.Mayerhofer A, Frungieri MB, Fritz S, Bulling A, Jessberger B, Vogt HJ. Evidence for catecholaminergic, neuronlike cells in the adult human testis: changes associated with testicular pathologies. J Androl. 1999;20:341–347. [PubMed] [Google Scholar]
- 33.Rey-Ares V, Lazarov N, Berg D, Berg U, Kunz L, Mayerhofer A. Dopamine receptor repertoire of human granulosa cells. Reprod Biol Endocrinol. 2007;5:40. doi: 10.1186/1477-7827-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saller S, Kunz L, Dissen GA, Stouffer R, Ojeda SR, Berg D, Berg U, Mayerhofer A. Oxytocin receptors in the primate ovary: molecular identity and link to apoptosis in human granulosa cells. Hum Reprod. 2010;25:969–976. doi: 10.1093/humrep/dep467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adam M, Saller S, Ströbl S, Hennebold JD, Dissen GA, Ojeda SR, Stouffer RL, Berg D, Berg U, Mayerhofer A. Decorin is a part of the ovarian extracellular matrix in primates and may act as a signaling molecule. Hum Reprod. 2012;27:3249–3258. doi: 10.1093/humrep/des297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D'Onofrio M, Paoletti F, Arisi I, Brandi R, Malerba F, Fasulo L, Cattaneo A. NGF and proNGF regulate functionally distinct mRNAs in PC12 cells: an early gene expression profiling. PLoS One. 2011;6:e20839. doi: 10.1371/journal.pone.0020839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Zi'abi MO, Bowolaksono A, Okuda K. Survival role of locally produced acetylcholine in the bovine corpus luteum. Biol Reprod. 2009;80:823–832. doi: 10.1095/biolreprod.108.069203. [DOI] [PubMed] [Google Scholar]
- 38.Fritz S, Fohr KJ, Boddien S, Berg U, Brucker C, Mayerhofer A. Functional and molecular characterization of a muscarinic receptor type and evidence for expression of choline-acetyltransferase and vesicular acetylcholine transporter in human granulosa-luteal cells. J Clin Endocrinol Metab. 1999;84:1744–1750. doi: 10.1210/jcem.84.5.5648. [DOI] [PubMed] [Google Scholar]
- 39.Fritz S, Kunz L, Dimitrijevic N, Grunert R, Heiss C, Mayerhofer A. Muscarinic receptors in human luteinized granulosa cells: activation blocks gap junctions and induces the transcription factor early growth response factor-1. J Clin Endocrinol Metab. 2002;87:1362–1367. doi: 10.1210/jcem.87.3.8326. [DOI] [PubMed] [Google Scholar]
- 40.Mayerhofer A, Kunz L. A non-neuronal cholinergic system of the ovarian follicle. Ann Anat. 2005;187:521–528. doi: 10.1016/j.aanat.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Campagnolo L, Costanza G, Francesconi A, Arcuri G, Moscatelli I, Orlandi A. Sortilin expression is essential for pro-nerve growth factor-induced apoptosis of rat vascular smooth muscle cells. PLoS One. 2014;9:e84969. doi: 10.1371/journal.pone.0084969. [DOI] [PMC free article] [PubMed] [Google Scholar]


