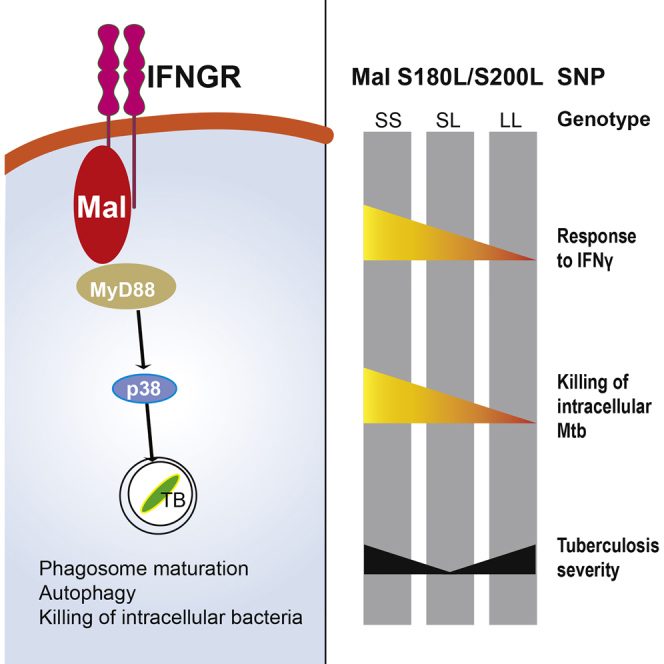
Summary
Humans that are heterozygous for the common S180L polymorphism in the Toll-like receptor (TLR) adaptor Mal (encoded by TIRAP) are protected from a number of infectious diseases, including tuberculosis (TB), whereas those homozygous for the allele are at increased risk. The reason for this difference in susceptibility is not clear. We report that Mal has a TLR-independent role in interferon-gamma (IFN-γ) receptor signaling. Mal-dependent IFN-γ receptor (IFNGR) signaling led to mitogen-activated protein kinase (MAPK) p38 phosphorylation and autophagy. IFN-γ signaling via Mal was required for phagosome maturation and killing of intracellular Mycobacterium tuberculosis (Mtb). The S180L polymorphism, and its murine equivalent S200L, reduced the affinity of Mal for the IFNGR, thereby compromising IFNGR signaling in macrophages and impairing responses to TB. Our findings highlight a role for Mal outside the TLR system and imply that genetic variation in TIRAP may be linked to other IFN-γ-related diseases including autoimmunity and cancer.
Keywords: Mal, TIRAP, interferon gamma, tuberculosis, phagolysosome maturation, autophagy
Graphical Abstract
Highlights
-
•
Mal has a TLR-independent role in IFNGR signaling
-
•
IFNGR signaling via Mal leads to p38 phosphorylation, autophagy, and killing of TB
-
•
The S180L mutation attenuates responses to IFN-γ stimulation
-
•
S180L mutations impair in vitro and in vivo responses to TB
Mal (encoded by TIRAP) is a signaling adaptor in the TLR pathway. Ní Cheallaigh and colleagues demonstrate an additional role for Mal in IFN-γ signaling and find that it is required to kill intracellular M. tuberculosis. The common human Mal S180L polymorphism attenuates IFN-γ signaling and impairs responses to tuberculosis infection.
Introduction
Genetic variation in proteins involved in innate immunity, particularly Toll-like receptors (TLRs) and their signaling adaptor proteins, has been proposed to account for variation in susceptibility to infectious pathogens. MyD88 adaptor-like (Mal), encoded by the gene Toll-interleukin 1 receptor (TIR) domain-containing adaptor protein (TIRAP), was initially described as a signaling adaptor protein leading to nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation downstream of TLR4 (Fitzgerald et al., 2001, Horng et al., 2001) and TLR2 (Horng et al., 2002, Yamamoto et al., 2002). A role for Mal as a “bridging adaptor” has since been established with Mal recruited to the plasma membrane, where it facilitates myeloid differentiation primary response gene 88 (MyD88) delivery to activated TLRs to initiate signal transduction in a structure called the Myddosome (Bonham et al., 2014, Kagan and Medzhitov, 2006). Mal has also been reported to function as a signaling adaptor for endosomal TLR signaling (Bonham et al., 2014).
Two non-synonymous single nucleotide polymorphisms (SNPs) in TIRAP with functional consequences have been identified, D96N and S180L. The S180L SNP is common in Indian and European (approximately 15%–20% carrying the L allele and 2%–3% being homozygous for the L allele) populations (Ferwerda et al., 2009) and results in alteration of a potential binding site near D96, leading to steric occlusion (Valkov et al., 2011). S180L has been associated with altered susceptibility to a number of infectious diseases including severe sepsis, severe pneumococcal disease, Haemophilus influenzae, and malaria (Ferwerda et al., 2009, Khor et al., 2007, Ladhani et al., 2010). An association has been reported between the S180L TIRAP polymorphism and TB susceptibility with heterozygotes for the mutation showing protection from disease and homozygotes showing increased susceptibility (Capparelli et al., 2013, Castiblanco et al., 2008, Khor et al., 2007, Selvaraj et al., 2010), although other studies have failed to replicate these findings (Dissanayeke et al., 2009, Nejentsev et al., 2008). A recent meta-analysis of the data confirms the association (Liu et al., 2014). The mechanism underlying the effect of the S180L SNP has not yet been elucidated.
Macrophages are key phagocytic cells that can eliminate or harbor intracellular bacteria, such as Mtb and also play a key role in secreting cytokines, which polarize subsequent adaptive immunity to a beneficial T helper 1 (Th1) or deleterious Th2 type response. Macrophages carry out a number of key antimicrobial functions including autophagy and phagosomal maturation, which if successful can kill intracellular mycobacteria (Deretic et al., 2006, Harris et al., 2009). IFN-γ plays a critical role in promoting antimicrobial functions. It activates macrophages, leading to production of nitric oxide (NO) and reactive oxygen species (ROS), phagosomal maturation, autophagy, and bactericidal activity (Gutierrez et al., 2004, MacMicking, 2012, Matsuzawa et al., 2014). Individuals with partial or complete defects in the IFN-γ signaling pathway have increased susceptibility to Mtb, as well as to other mycobacterial species (Bogunovic et al., 2012, Filipe-Santos et al., 2006).
In this study, we report that the murine equivalent of S180L, S200L, replicated human findings with homozygotes displaying increased severity of tuberculous disease. In vitro, the S200L mutation resulted in impaired phagosome maturation and killing of Mtb. We demonstrate that, unlike S180L, S200L did not affect TLR signaling. The impaired TB immunity seen with S200L was due to its effect on a Mal-dependent, TLR-independent, IFNGR signaling pathway. Mal-dependent responses to IFN-γ included p38 MAPK phosphorylation, autophagy, and phagosomal maturation, but not the canonical signal transducer and activator of transcription-1 (STAT-1) phosphorylation pathway. The S180L polymorphism reduced the affinity of Mal for the IFNGR, thereby compromising human macrophage responses to IFN-γ. This Mal-dependent IFNGR signaling pathway, modulated by the S180L mutation, might affect susceptibility to infectious diseases, inflammatory diseases, and cancer.
Results
The S200L Mutation Is Associated with Increased Severity of TB Disease in Vivo
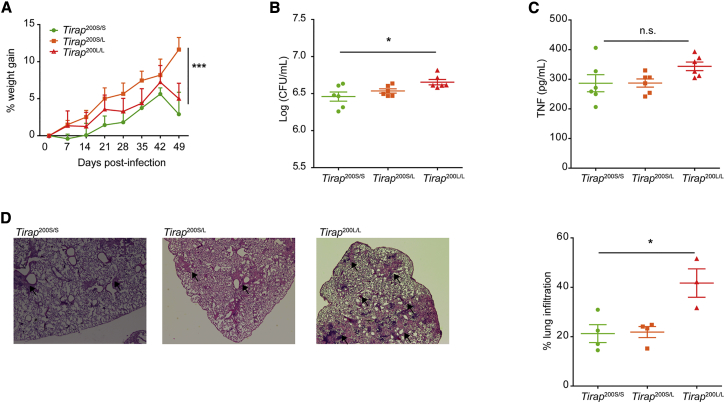
Mice with the equivalent of the human S180L mutation in TIRAP (Tirap 200L) were generated to provide an in vivo model of Mtb infection (Figure S1). Wild-type (SS), heterozygote (SL), and homozygote (LL) mice were infected with a high dose of Mtb H37Rv (a laboratory strain of virulent Mtb) via aerosol and weighed weekly. SL mice were protected against weight loss (Figure 1A). Mice were sacrificed at 8 weeks post-infection and lung lysates were analyzed. LL mice showed increased severity of TB disease, with an increase in bacterial burden (Figure 1B), despite similar levels of lung tumor necrosis factor alpha (TNF-α) production (Figure 1C). Most markedly however, we observed increased lung inflammation in LL mice (Figure 1D) compared to SL and SS mice. This correlates with the protection for heterozygotes and the increased susceptibility seen in homozygotes for the mutation in human studies. The phenotype of TB infection seen with the S200L mutation in mice replicated the human phenotype with S180L.
Figure 1.
Mice Homozygous for Mal S200L, the Equivalent of Mal S180L, Develop More Severe Lung Inflammation in Response to In Vivo Infection with Mycobacterium tuberculosis
Age- and sex-matched WT (Mal200S/S), heterozygote (Mal200S/L), and homozygote (Mal200L/L) mice were infected with 500 cfu of Mycobacterium tuberculosis (Mtb) H37Rv by aerosol.
(A) Weights of eight mice in each group were measured weekly.
(B) Mice were sacrificed at 8 weeks post-infection, and lung homogenates from five or six mice per group were plated for measurement of bacterial burden.
(C) TNF-α in lung homogenates from five or six mice per group was measured by ELISA.
(D) Lungs from three to four mice per group were fixed in formalin, stained with haemotoxylin and eosin, and area of inflammation assessed by microscopy with representative images and quantifications are shown. All data are means ± SD. A two-way ANOVA was used to analyze data in (A). A one-way ANOVA (non-parametric, Kruskal-Wallis) was used to analyze data in (B)–(D). ∗p < 0.05, ∗∗∗p < 0.001) for all experiments.
S200L Affects Macrophage Function Independently of Cytokine Production
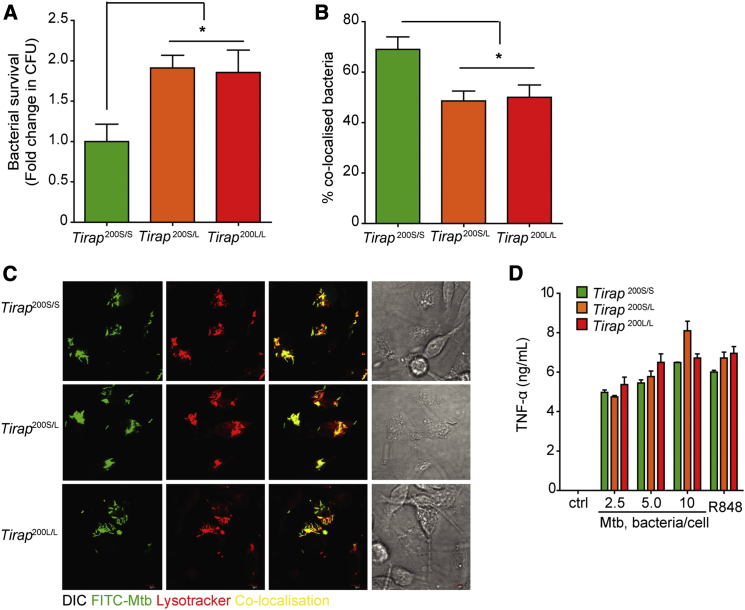
Mycobacterial survival in macrophages is an in vitro read-out of macrophage function (Watson et al., 2012). Macrophages from mice with the S200L mutation displayed a defect in killing of intracellular mycobacteria (Figure 2A). Macrophages from mice with the S200L mutation displayed a defect in phagosome maturation, corresponding with the defect seen in bactericidal activity (Figures 2B and 2C). Unlike Tirap−/− macrophages described later, the S200L macrophages displayed no impairment in production of these cytokines (Figures 2D and S2A), indicating that the defect seen in mycobactericidal activity was not due to an impairment of cytokine production. In addition, S200L homozygote macrophages, unlike Tirap−/− macrophages, did not exhibit attenuated cytokine responses to TLR2 and TLR4 ligands (Figures S2B and S2C), indicating that S200L did not affect TLR2 or TLR4 signaling.
Figure 2.
The S200L Mutation Impairs Macrophage Phagosome Maturation and Killing of Intracellular Mtb
(A) Primary BMM were infected with Mtb H37Rv and lysed at 72 hr. Serial dilutions of lysates were plated out to determine bacterial numbers.
(B and C) Cells were infected with FITC-stained Mtb H37Rv for 2 hr, fixed and stained with Lysotracker (LT, Life Technologies DND-99), and co-localization of Mtb with LT+ phagolysosomes was assessed by confocal microscopy and quantified in (B) with representative images in (C).
(D) Primary BMM were infected overnight with Mtb H37Rv and supernatants analyzed for TNF-α production by ELISA. All experiments show mean ± SD pooled from three separate experiments with macrophages from one mouse per group in each experiment ∗p < 0.05 (one-way ANOVA used to analyze all experiments).
Mal and MyD88, but Not TLRs 2 and 4, Are Required for Macrophage Killing of Virulent Mtb
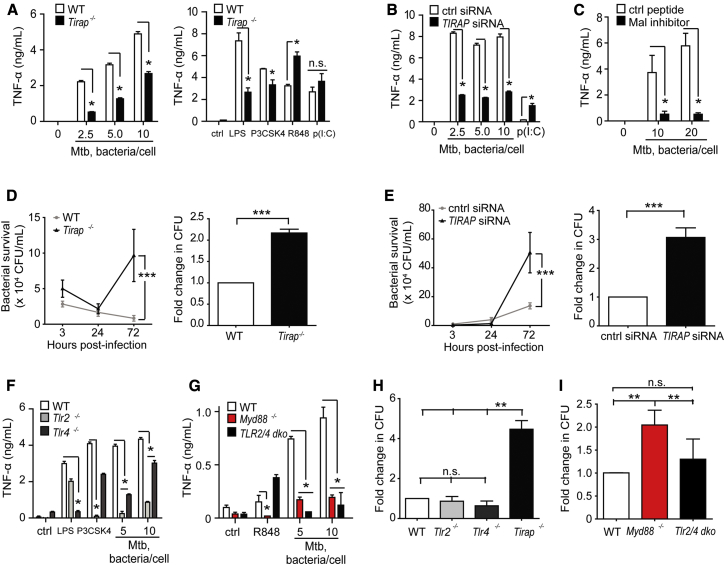
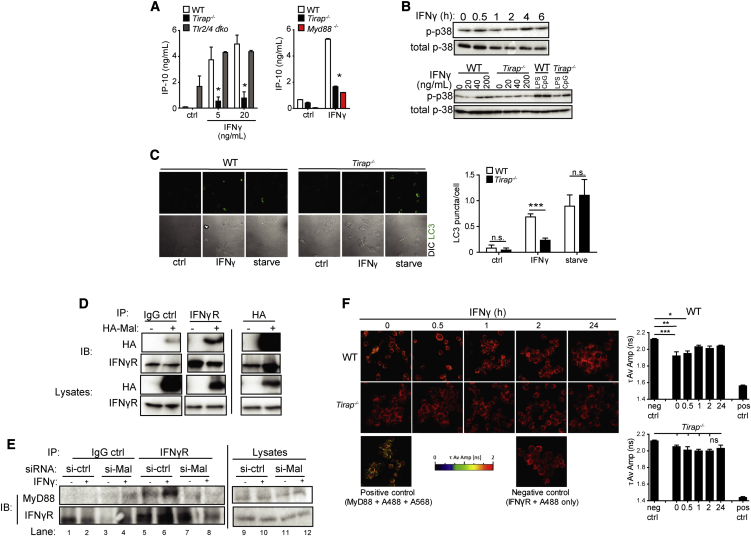
We proceeded to assess whether Mal-deficient macrophages showed a similar in vitro phenotype to S200L macrophages in response to Mtb infection. Immortalized and primary murine bone marrow macrophages were used, as well as TIRAP-silenced THP-1 cells. In contrast to our observations in S200L macrophages (Figure 2D), we observed that production of the key anti-mycobacterial cytokine TNF-α in response to Mtb was greatly reduced in Mal-deficient cells (Figures 3A–3C). Production of IL-1α, IL-1β, IL-6, and IL-12p40, but not IL-27 or IL-10, was also impaired in the absence of Mal (Figures S3A and S3B, with confirmation of TIRAP silencing in Figure S3C). Mal-deficient macrophages (Figures 3D and 3E and S3D) showed a marked inability to kill intracellular Mtb, similar to that seen in the S200L macrophages. We also assessed the role of TLRs 2 and 4 in our model. Tlr2−/−, Tlr2/4 double knockout (dko) and Myd88−/− macrophages replicated the defect in pro-inflammatory cytokine production seen in Mal deficient cells. Tlr4−/− macrophages showed a smaller, but still significant, impairment in cytokine responses (Figures 3F and 3G and S3E and S3F). Notably, whereas Myd88−/− macrophages replicated the defect in bactericidal activity seen in Tirap−/− cells, Tlr2−/−, Tlr4−/−, and Tlr2/4 dko murine macrophages and THP-1 macrophages treated with an anti-TLR2 antibody did not show a similar defect despite impairments in cytokine induction (Figures 3H and 3I and S3G). Consistent with our findings is a previous report of unimpaired bactericidal activity of Tlr2/4/9 triple knockout macrophages and impaired restriction of Mtb growth by Myd88−/− macrophages (Hölscher et al., 2008). These data indicated that Mal and MyD88 had a function in killing of Mtb, and that this was distinct from the known role of Mal downstream of TLRs 2 and 4. This defect in mycobactericidal activity seen in the absence of Mal, but not in the absence of TLRs 2 and 4, was not due to defects in cytokine production.
Figure 3.
Mal and MyD88, but Not TLR2 or TLR4, Are Required for Macrophage Killing of Virulent Mycobacterium tuberculosis
(A) TNF-α secretion by murine wild-type (WT) and Tirap−/− immortalized bone marrow-derived macrophages (iBMM) (1 × 106/ml) infected with Mtb H37Rv (left panel) or treated with the indicated TLR ligands (right panel), was measured in supernatants collected after 20 hr stimulation and analyzed by ELISA.
(B) Cytokine secretion by PMA-differentiated THP-1 (5 × 105/ml) cells transfected with siRNA against Mal or scrambled control in response to infection with Mtb H37Rv (20 hr) was measured by ELISA.
(C) ELISA of TNF-α secretion by PMA-differentiated THP-1 cells (5 × 105/ml) treated with a Mal inhibitor peptide (TIRAP inhibitory peptide, Calbiochem, 613571) before infection with Mtb H37Rv (20 hr).
(D) WT and Tirap−/− iBMM were infected with Mtb H37Rv at a multiplicity of infection of 10 bacteria/cell and lysed at 3, 24, and 72 hr. Serial dilutions of lysates were plated out to determine bacterial numbers. Left panels show data representative of at least three separate experiments, right panels are mean ± SD pooled from bacterial counts at 72 hr from three separate experiments.
(E) PMA-differentiated THP-1 cells transfected with siRNA against TIRAP (Dharmacon/Thermoscientific) or scrambled control siRNA, were infected with Mtb H37Rv and bacterial numbers determined as above. Left panel shows data representative of at least three separate experiments, and right panel is mean ± SD pooled from three separate experiments.
(F and G) TNF-α secretion by iBMM (1 × 106/ml) infected with Mtb H37Rv (20 hr) was measured by ELISA.
(H and I) iBMM were infected with Mtb H37Rv and bacterial numbers determined as above. Data are means ± SD of data pooled from three separate experiments. ∗p < 0.05 (Mann-Whitney) for all experiments.
Mal Is Required for Autophagy and IFN-γ Induced Phagosome Maturation
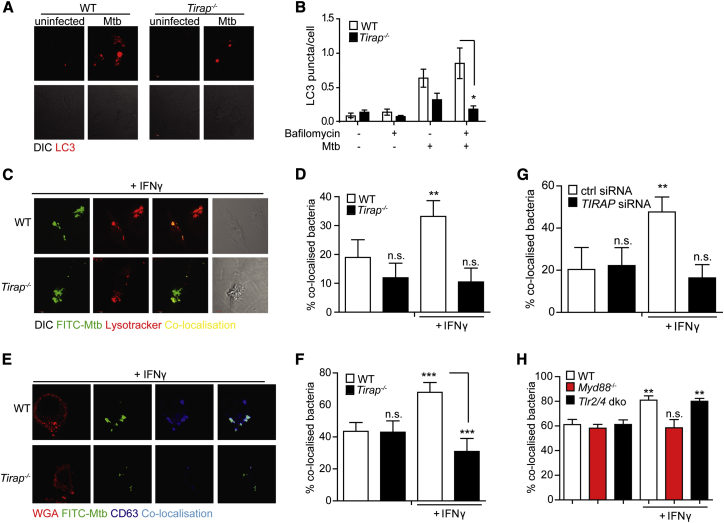
We sought to identify Mal-dependent but TLR-independent macrophage effector mechanisms. Autophagy provides a mechanism of killing and removing intracellular pathogens and contributes to a number of critical host immune responses to Mtb (Ní Cheallaigh et al., 2011). To determine whether Mtb-induced autophagic flux was impaired in the absence of Mal, we infected primary macrophages with Mtb in the presence or absence of bafilomycin, which blocks autophagosome fusion with lysosomes and thereby completion of autophagy and breakdown of autophagosomes (Yamamoto et al., 1998). Mtb-induced autophagic flux was reduced in the absence of Mal (Figure 4A-B). When autophagy was impaired in macrophages using knockdown of the key effector proteins ATG7 or BECLIN-1 by siRNA, a similar defect in bactericidal activity and phagosomal maturation to that seen in Mal-deficient cells was observed (Figures S4A–S4C).
Figure 4.
Mal Regulates IFN-γ Induced Maturation of Mtb Containing Phagosomes Independently of TLR2 and TLR4
(A and B) Primary WT and Tirap−/− bone-marrow-derived macrophages (BMM) were stimulated for 16 hr with Mtb in the presence or absence of bafilomycin. Cells were stained with anti-LC3 antibody (Invitrogen L10352) (representative images in (A) and LC3+ puncta per cell quantified by confocal microscopy (B).
(C–F) WT and Tirap−/− iBMM were stimulated overnight with rmIFN-γ (20 ng/ml) prior to infection with FITC-labeled Mtb H37Rv. Cells were stained with Lysotracker (LT) (C and D) and co-localization of Mtb with LT+ phagolysosomes was assessed by confocal microscopy, (representative images in C, quantified in D). Alternatively, cells were stained with anti-CD63 antibody (Santa Cruz, H-193) (E and F) and co-localization of Mtb with CD63-positive phagolysosomes was assessed by confocal microscopy, (representative images in E, quantified in F).
(G) THP-1 cells were transfected with siRNA against TIRAP or scrambled control prior to differentiation with PMA. Cells were stimulated overnight with recombinant human (rh)IFN-γ (20 ng/ml) prior to infection with FITC-labeled Mtb H37Rv and stained with LT. Co-localization of Mtb with LT+ phagolysosomes was quantified by confocal microscopy.
(H) iBMM were infected with FITC-labeled Mtb H37Rv and stained with LT. Co-localization of Mtb with LT+ phagolysosomes was quantified by confocal microscopy. Data shown are mean ± SD from a single experiment representative of three separate experiments are shown for (B) and mean ± SD of data pooled from three separate experiments for all other experiments (D, F–H). ∗p < 0.05 (Mann-Whitney) for all experiments.
We assessed whether Mal was required for phagosomal maturation by pretreating macrophages with IFN-γ or RPMI control and infecting the macrophages with FITC-labeled live Mtb. We identified phagolysosomes using anti-CD63 antibody or LysoTracker Red and assessed co-localization of bacteria and phagolysosomes. IFN-γ pre-treatment increased co-localization of bacteria and phagolysosomes in WT, but not in Tirap−/− macrophages (Figures 4C and 4D [LysoTracker] and Figures 4E and 4F [CD63]) a trend which persisted up to 24 hr post IFN-γ treatment (Figure S4D). Similar results were obtained in differentiated THP-1 cells treated with siRNA against Mal and stained with LysoTracker or the mature endolysosomal marker LAMP-1 (Figures 4G and S4E). Notably, IFN-γ did increase phagosomal maturation in Tlr2/4 dko, but not Myd88−/− macrophages (Figure 4H). Thus, Mal and MyD88, but not TLRs 2 and 4, were required for IFN-γ-induced phagosome maturation.
Mal and MyD88, but Not TLRs 2 and 4, Are Required for Interferon-Inducible Protein 10 Production, p38 Phosphorylation, and Autophagy in Response to IFN-γ
Given our findings of impaired IFN-γ induced phagosomal maturation in the absence of Mal and MyD88 but not TLRs 2 and 4, we hypothesized that the Mal-dependent but TLR-independent pathway required for killing of intracellular Mtb might be explained by Mal participating in MyD88-dependent IFNGR signaling. Previously, normal STAT-1 phosphorylation but impaired IFN-inducible protein 10 (IP-10) secretion was reported in MyD88-deficient macrophages (Sun and Ding, 2006). We identified a profound defect in IFN-γ-induced IP-10 secretion in Tirap−/− and Myd88−/− macrophages, but not in Tlr2/4 dko macrophages or Tram−/− macrophages (Figure 5A and S5A). IP-10 secretion was also reduced in TIRAP-silenced THP-1 cells (Figure S5B). IFN-γ-induced STAT-1 phosphorylation remained intact in Mal-deficient cells (Figure S5C). We observed a reduction in Cxcl10/Ip10 mRNA and Tnfa mRNA in Tirap−/− macrophages after IFN-γ treatment—a trend not observed for the STAT-1 target gene, Nos2, or for Arg1 mRNA (Figure S5D).
Figure 5.
Mal Associates with the IFN-γR and Is Required for IFN-γ Induced p38 Phosphorylation and Autophagy
(A) iBMM were treated with rmIFN-γ for 20 hr and secretion of IP-10 was measured by ELISA. Data are means ± SD from a single experiment representative of three separate experiments ∗p < 0.05, (Mann-Whitney).
(B) Primary BMM were treated for the indicated times (0–6 hr) at 20 ng/mL (top panel) and for 4 hr with rmIFN-γ at the indicated concentrations (0–100 ng/mL, bottom panel) alongside LPS or R848 (10 min). Lysates were prepared and analyzed for phosphorylation of p38 MAP-kinase by immunoblotting with anti-p-p38 antibody (Cell Signaling, 9211). Blots were stripped and re-probed for total p38 (bottom panels). Data shown are representative of three separate experiments.
(C) Primary BMM were stimulated for 16 hr with rmIFN-γ (20 ng/ml) and bafilomycin (100nM) or for 2 hr with starvation medium. Cells were stained with anti-LC3 antibody and LC3 puncta per cell were quantified with confocal microscopy. Data are means ± SD from a single experiment representative of three separate experiments, ∗p < 0.05, (Mann-Whitney).
(D) HEK293 cells were transfected with HA-tagged Mal and immunoprecipitation was performed with antibodies to HA (Sigma, H6908) and interferon gamma receptor (Santa Cruz, sc-700), along with a control non-specific rabbit IgG, on cell lysates as indicated. Lysates were then blotted with anti-HA antibody. Data shown are representative of three independent experiments.
(E) RAW264.7 cells were transfected with the indicated siRNAs (50 nM) for 72 hr prior to treatment with IFN-γ (100 ng/ml, 10 min) and immunoprecipitation was performed with antibodies to IFN-γR1 or an IgG control on cell lysates as indicated. IP-samples were then analyzed for MyD88 expression by immunoblotting with anti-MyD88 (Millipore, 16527) (top panel) alongside IFN-γR1 expression (bottom panels). Data shown are representative of three independent experiments.
(F) iBMM were treated with IFN-γ (10 ng/ml) for 0–24 hr. Cells were fixed and stained with antibodies against MyD88 and IFNGR2 followed by fluorescent secondary antibodies (Alexa Fluor 568 and Alexa Fluor 488). Changes in the amplitude weighted average lifetime (τ Av Amp) of the donor (A488) due to proximity with the acceptor (A568) were measured. A decrease in τ Av Amp indicates interaction between the molecules and is quantified on right. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005; one-way ANOVA.
P3 mitogen-activated protein kinases (MAPK) are a class of MAPK that respond to stress stimuli including cytokines and are involved in apoptosis and autophagy. An IFNγ-induced p38 MAPK signaling pathway, which culminates in autophagy and killing of intracellular bacteria, was recently reported (Matsuzawa et al., 2014). We hypothesized that Mal might be required for this pathway. P38 MAPK was phosphorylated in response to IFN-γ, peaking at 4 hr post-treatment (Figures 5B, top panel), and this was impaired in Tirap−/− macrophages (Figure 5B, bottom panel and S5E). IFN-γ induced autophagy, but not starvation-induced autophagy, was reduced in Tirap−/− macrophages, (Figures 5C and S6A and S6B). These data demonstrate that Mal was required for IFN-γ induced P38 MAPK phosphorylation, autophagy and IP-10 secretion.
Mal Interacts Directly with the IFNGR
We hypothesized that Mal might act as a bridging adaptor for MyD88 and the IFNGR. Immunoprecipitation of endogenous IFNGR resulted in co-immunoprecipitation of overexpressed Mal (Figure 5D, left panel). This interaction required full-length Mal, as a mutant construct consisting solely of the TIR domain did not immunoprecipitate with the IFNGR (Figure S7A). Immunoprecipitation of full-length overexpressed HA-Mal resulted in co-immunoprecipitation of IFNGR, confirming that Mal can bind IFNGR directly (Figure 5D, right panel). Mal did not precipitate with other proteins including Beclin-1 and BCL-2 (Figure S7B).
We then hypothesized that if Mal functions as a bridging adaptor between MyD88 and the IFNGR, then the previously reported interaction between MyD88 and the IFNGR (Sun and Ding, 2006) would be reduced in the absence of Mal. We observed increased recruitment of MyD88 to IFNGR in IFNγ-treated control cells (Figure 5E, lanes 5/6); however, in TIRAP-silenced or Mal-deficient cells (knockdown shown in Figure S7C) this interaction was reduced to background levels (Figure 5E, lanes 7/8 and Figure S7D).
We then confirmed this finding using fluorescence lifetime imaging microscopy-fluorescence resonance energy transfer (FLIM FRET) technology. FRET occurred between IFNGR and MyD88, indicating that they are interacting basally and for up to 0.5 hr post IFN-γ treatment (Figure 5F) in WT cells. This steady-state interaction between MyD88 and IFNGR was disrupted after initial signal transduction. In Tirap−/− cells, there was no evidence of such a basal interaction, a pattern not altered by subsequent IFN-γ treatment. These data demonstrated that Mal bound to IFNGR and was required for the interaction between MyD88 and IFNGR.
Interferon Gamma Is Required for Autophagy and Phagosome Maturation in Response to Mtb
Our data had established that Mal is required for an IFN-γ signaling pathway, culminating in p38 MAPK phosphorylation and autophagy. We hypothesized that the observed defect in autophagy and killing of intracellular Mtb was due to the defect in the IFN-γ induced p38 MAPK phosphorylation pathway. However, we observed the deficits in autophagy and killing in a monoculture of macrophages in the absence of exogenous IFN-γ. We therefore hypothesized that macrophages secrete small quantities of IFN-γ that are functionally relevant in our model. Immortalized and primary macrophages and differentiated THP-1 cells produced small but detectable amounts of IFN-γ when infected with Mtb (Figures S8A–S8C). IFN-γ production was reduced in Tirap−/− immortalized bone-marrow-derived macrophages (iBMM) (Figure S8A)—this may be due to impaired TLR2 and/or TLR4 signaling or impaired IFN-γ signaling, because IFN-γ can upregulate its own production in a positive feedback loop. We also observed IFN-γ production by immortalized and primary macrophages using intracellular staining and flow cytometry (Figures S8D–S8F). Macrophages produced a considerable amount of IFN-γ if allowed to recover from LPS tolerization and restimulated for 4 hr (Figures S8E–S8G). Although our data show that Mtb can induce IFN-γ production by macrophages, the levels produced are extremely low compared to those produced by T cells or NK cells. We therefore sought to assess whether the low levels of IFN-γ present in our macrophage cultures were functionally signficant for our key endpoints. Macrophages treated with a blocking antibody against IFN-γ (Figures S9A and S9B) and Ifng–/− macrophages (Figures S9C and S9D) showed reduced maturation of Mtb containing phagosomes. In the case of Ifng−/− macrophages, maturation was restored by adding exogenous IFN-γ (Figure S9D). Ifng−/− macrophages also showed reduced autophagy in response to Mtb (Figures S9E and S9F). Finally, Ifng−/− macrophages showed a defect in bactericidal activity as assessed by intracellular bacterial burden at 72 hr, similar to that seen in Tirap−/− macrophages (Figure S9G). These data demonstrated that macrophages secrete small but functionally relevant amounts of IFN-γ.
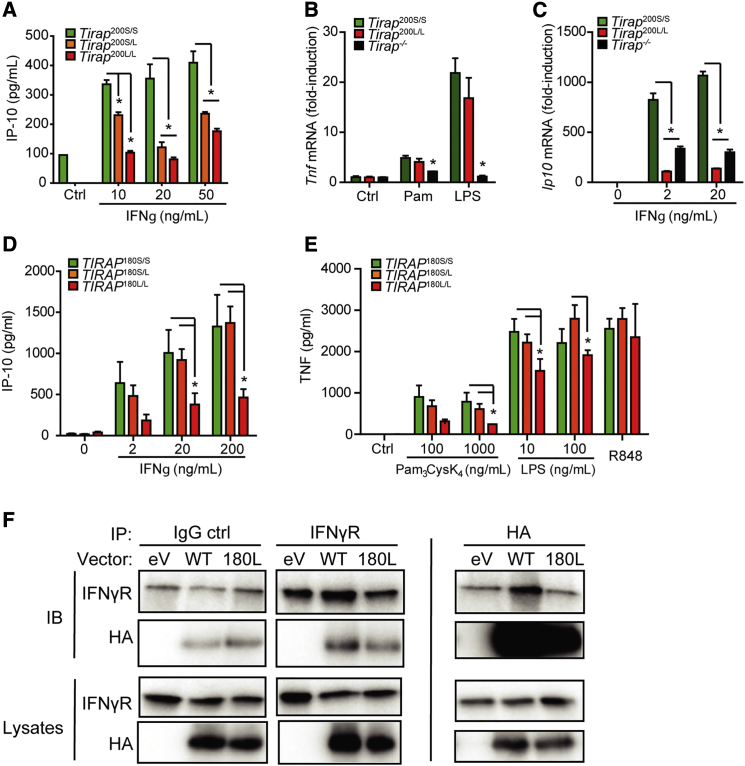
The S200L/S180L Mal Mutation Reduces IFN-γ Signaling
Given the new role identified for Mal in IFN-γ signaling, we then proceeded to look at the effect of the S200L polymorphism on TLR2, TLR4, and IFN-γ signaling in macrophages. As noted above, the S200L mutation did not affect secretion of the pro-inflammatory cytokine TNF-α in response to the TLR2 ligands Malp-2 and Pam3CysK4 or the TLR4 ligand LPS (Figures S2A–S2C) or Tnfa mRNA levels in response to TLR2 and TLR4 ligands (Figure 6B). However, macrophages from LL mice and, to a lesser extent, SL mice, showed a decreased IP-10 response to IFN-γ stimulation (Figures 6A and 6C), indicating that carriage of the 200L allele in mice, impairs IFN-γ, but not TLR, responses.
Figure 6.
The S180L/S200L Mutation Reduces Affinity for IFNγR1 and Impairs Responses to IFN-γ
(A) IP-10 secretion by primary murine BMM stimulated for 20 hr with rmIFN-γ and supernatants analyzed by ELISA, mean ± SD pooled from three separate experiments with macrophages from one mouse per group in each experiment.
(B and C) Tnfa and Ip-10 mRNA levels from iBMM stimulated for 4 hr with Pam3Cys4K (100 μg/mL), LPS (100 ng/ml) or rmIFN-γ at the concentrations indicated.
(D and E) Monocyte-derived macrophages from human volunteers genotyped for the S180L SNP (n = 12 for TIRAP 180S/S, n = 12 for TIRAP 180S/L, and n = 4 for TIRAP 180L/L) were stimulated with rhIFN-γ (D) or TLR ligands (E) for 16 hr. Supernatants were analyzed for IP-10 production (D) or TNF (E) by ELISA. Mean ± SD shown in graph, analyzed using two-way ANOVA.
(F) HEK293 cells were transfected with HA-tagged wild-type, HA-tagged S180L variant Mal, or HA-tagged empty vector and an immunoprecipitation was performed with antibodies to HA and IFNGR1 on cell lysates as indicated prior to Western blotting.
We then sought to determine the effect of the S180L mutation in human macrophages. We derived macrophages from peripheral blood monocytes (MDMs) from donors genotyped using allelic discrimination. MDMs were stimulated with TLR ligands and TNF-α secretion was measured by ELISA. We observed decreased responses to TLR2 and TLR4 ligands in human MDMs (Figure 6E). We observed a decrease in IFN-γ-driven IP-10 secretion in MDMs from individuals who were homozygous for the S180L mutation (Figure 6D). We proceeded to assess whether the S180L mutation altered the affinity of Mal for the IFNGR. Using site-directed mutagenesis, we synthesized HA-tagged human Mal with the S180L mutation (180L). S180L variant human Mal shows decreased affinity for the IFNGR relative to HA-tagged wild-type Mal (Figure 6F). The S180L mutation reduces the affinity of Mal for the IFNGR and reduces IP-10 secretion in response to IFN- γ stimulation.
Discussion
Here we show that the murine equivalent of the S180L mutation in Mal, Mal S200L, replicated the phenotype previously reported from humans with the S180L polymorphism: it conferred protection from tuberculous disease on heterozygotes for the mutation and increased susceptibility on homozygotes. In vitro, Mal S200L impaired phagosome maturation and killing of intracellular mycobacteria. However, unlike S180L, S200L did not affect TLR signaling. We demonstrated a mechanism for these observations: the S200L polymorphism affected a TLR-independent, Mal-dependent, IFNGR signaling pathway. Mal-dependent IFNGR signaling was required for p38 phosphorylation, autophagy, phagosome maturation, and killing of intracellular mycobacteria. This IFNGR signaling pathway was attenuated by the human mutation S180L. This offers an explanation for how the common S180L mutation affects host innate immune responses to Mtb. The fact that this common polymorphism attenuates IFNGR signaling might have relevance for host susceptibility to a number of IFN-γ-related conditions including autoimmunity and cancers.
A number of publications regarding the effect of the S180L SNP on infectious disease susceptibility in humans have reported a heterozygote advantage with increased susceptibility seen in homozygotes (Capparelli et al., 2013, Castiblanco et al., 2008, Khor et al., 2007, Selvaraj et al., 2010). A murine model using the equivalent of the S180L mutation, S200L, replicated these findings, with heterozygotes protected from weight loss (a cardinal clinical feature of human tuberculosis) and homozygotes displaying increased bacterial burden and lung inflammation.
In vitro, macrophages from mice carrying the L allele displayed impaired phagosome maturation and killing of Mtb but did not show evidence of impaired cytokine production. In contrast, macrophages from TLR-deficient mice displayed impaired cytokine production but preserved phagosome maturation and killing of Mtb. Mal-deficient macrophages displayed impairment of cytokine production in addition to the phenotype seen in the S180L macrophages of impaired phagosome maturation and killing.
These findings led us to search for a TLR-independent function for Mal. We show here a TLR2- and TLR4-independent role for Mal in the IFNGR signaling pathway. Mal bound to the IFNGR and MyD88 and acted as a bridging adaptor between these proteins. Mal was required for IP-10 production in response to IFN-γ and for a pathway involving p38 MAPK phosphorylation, culminating in autophagy and killing of intracellular bacteria. Autophagy is a key means of killing intracellular bacteria, including Mtb, and also plays a role in regulation of cytokine secretion (Ní Cheallaigh et al., 2011, Peral de Castro et al., 2012). IFN-γ-induced phagosome maturation is dependent on Beclin-1 and might indeed be autophagy-dependent (Harris et al., 2007). The defect in autophagy seen in the absence of Mal-dependent IFNGR signaling provides an explanation for why Mal and MyD88, but not TLRs 2 and 4, are required for macrophage killing of Mtb.
IFN-γ is a canonical macrophage activator and is known to play a critical role in immune responses to Mtb (Bogunovic et al., 2012, Cooper et al., 1993, Filipe-Santos et al., 2006, Flesch and Kaufmann, 1991, Flynn et al., 1993). IFN-γ is secreted in large quantities by activated Th1 cells (Mosmann and Coffman, 1989), activated CD8+ cytotoxic cells (Sad et al., 1995), and NK cells (Perussia, 1991). However, our model for bactericidal activity and autophagy consisted of a monoculture of macrophages. There are numerous reports of macrophages secreting IFN-γ (Darwich et al., 2009, Di Marzio et al., 1994, Fenton et al., 1997, Fultz et al., 1993), albeit in limited quantities, although some authors have highlighted the possible effect of contaminating cells in producing IFN-γ (Schleicher et al., 2005). The increased proportion of IFN-γ producing cells in macrophages pre-stimulated with LPS and then “recovered” (O’Carroll et al., 2014) provides compelling evidence that macrophages can produce IFN-γ given appropriate stimuli.
We show here that the murine immortalized and primary macrophages used in our Mtb infection model secreted small but functionally decisive quantities of IFN-γ in response to infection with Mtb and that Mal played a critical role in macrophage responses to Mtb, which are IFN-γ dependent.
Given our identification of a role for Mal in IFNGR signaling and its established role in TLR2 and TLR4 signaling, we examined the effect of the S180L polymorphism and the murine equivalent of the S180L polymorphism, S200L, on these pathways. We show that responses to IFN-γ are reduced in human cells with the 180L variant. Human 180L variant Mal showed a reduced affinity for the IFNGR. Cells from mice with the equivalent mutation also showed impairment in IFNGR signaling.
Human S180L reduced responses to TLR2 and TLR4 ligands, consistent with the known role of Mal as a signaling adaptor protein downstream of TLRs 2 and 4, and with published data on the role of TLR2 as a pattern-recognition receptor involved in pro-inflammatory cytokine production by Mtb-infected macrophages. Previously published data have shown that Mal is not required for responses to high doses of TLR2 ligands (Kenny et al., 2009); however, we hypothesize that the doses of Mtb used in this study correspond to the lower doses of TLR2 ligands for which Mal is required. Our findings that S180L reduced responses to TLR 2 and 4 ligands are consistent with a previous report using L-variant Mal transfected into murine MEFs (Khor et al., 2007) and with a recent study where PBMCs from individuals with the Mal S180L allele were stimulated with heat-killed Mtb (Capparelli et al., 2013). In contrast, another publication reported no difference between TLR2 and TLR4 signaling in vitro in PBMCs from individuals with the SS and SL genotypes but did show an increase in response to low doses of TLR2 ligand in PBMCs from a single individual with the LL genotype (Ferwerda et al., 2009). This finding might have been caused by the presence of polymorphisms in MyD88 which interact with the Mal SNP (Capparelli et al., 2013).
Macrophages from mice homozygous for the S200L mutation, the murine equivalent of S180L, did not show an impairment in TLR signaling but did show an impairment in IFN-γ signaling. Murine Mal differs from human Mal in that it has 20 more amino acids at the N terminus. Mutations in Mal might selectively impair certain signaling pathways only, as demonstrated by a recent report on a form of Mal with an altered lipid binding domain, which selectively impaired responses to the TLR9 ligand CpG but not the TLR4 ligand LPS (Bonham et al., 2014).
Importantly, the selective impairment of IFN-γ but not TLR signaling in S200L macrophages meant that the reduced phagosomal maturation and bacterial killing in macrophages with the L allele and the increased bacterial burden and increased inflammatory response seen in homozygote S200L mice could be the result of aberrant IFN-γ signaling associated with the S180L SNP rather than the result of impairment in TLR signaling. The increased inflammation seen in the homozygote mutant mice might reflect the increase in bacterial burden in the homozygote mice or might reflect a loss of IFN-γ-mediated regulation of neutrophil recruitment and inflammation (Mishra et al., 2013, Nandi and Behar, 2011). The increase in bacterial burden and inflammation is not as marked as the defect seen in mice entirely deficient in IFN-γ (Cooper et al., 1993, Flynn et al., 1993), but could be consistent with a partial defect in IFN-γ signaling.
Macrophage in vitro assays and assessment of bacterial burden and inflammation following infection demonstrated a disadvantage for homozygote mutants, whereas serial weights demonstrated increased severity of disease in both WT and mutant homozygote individuals. A number of publications on the S180L SNP and TB susceptibility in humans have reported a heterozygote advantage and/or increased susceptibility in mutant homozygotes. A heterozygote advantage in vivo has also been reported for a SNP in leukotriene A4 hydrolase (LTA4), with impaired in vitro responses seen in homozygotes for the mutation. This effect has been attributed to the effect of the SNP on mitochondrial reactive oxygen species production and cell necrosis (Roca and Ramakrishnan, 2013). As we face an era of drug-resistant tuberculosis, improved understanding of common mutations, which affect inflammatory responses to pathogens such as Mal S180L, might guide us to improved use of immunomodulatory treatment. Tobin and colleagues have highlighted the potential of using LTA4H host genotypes to guide choice of treatment for tuberculosis: individuals with tuberculous meningitis with a LTA4 genotype associated with an excessive inflammatory response benefited from adjunctive immunomodulatory steroid treatment, whereas this was harmful in those with a LTA4 genotype associated with an inadequate inflammatory response (Tobin et al., 2012). It is tempting to speculate that the Mal S180L genotype could be used to inform treatment decisions in a similar manner.
The S180L SNP in Mal has been reported to be associated with altered susceptibility to not only mycobacterial disease, but also to other infectious diseases including pneumococcal disease, malaria, and Chaga’s disease. IFN-γ signaling also plays an important role in immune responses to these pathogens. Hitherto, the association of systemic lupus erythematosus (SLE) with S180L has been unexplained. TLR2 and TLR4 are not thought to play an important role in SLE (Kim et al., 2009), although TLR 9 has been implicated (Yang et al., 2012). Our data suggest that the increased susceptibility to SLE associated with S180L might be due to defects in the Mal-dependent IFN-γ signaling pathway rather than alterations in TLR2 and 4 signaling (Pollard et al., 2013).
Indeed, IFN-γ has a critical role in immunity, including promoting differentiation of macrophages to a classically activated phenotype (Martinez and Gordon, 2014). IFN-γ plays a role across a spectrum of non-infectious diseases including atherogenesis, autoimmune diseases such as chronic atopic dermatitis, and cancer (Feingold, 2014, Ikeda et al., 2002, Schroecksnadel et al., 2006). Given the frequency of the Mal S180L SNP, it might be fruitful to assess whether the Mal-dependent IFN-γ signaling pathway plays a role in these diseases.
Experimental Procedures
Cell Lines and Culture
Primary bone-marrow-derived macrophages were derived from the femurs of Tirap−/−, Ifng−/−, and WT mice and differentiated for 7 days with medium containing MCSF. THP-1 cells (ATCC) were transfected with siRNA against Mal (Dharmacon) and scrambled control prior to being differentiated into macrophage-like cells with phorbol myristate acetate (100 nmol/L).
Assessment of Bacterial Growth
Macrophages were grown at 1 × 105 cells/ml in 12-well plates in antibiotic-free RPMI supplemented with 10% fetal calf serum. A suspension of Mtb H37Rv was prepared as described in Supplemental Experimental Procedures, and macrophages were infected with Mtb at a MOI of 10 bacteria/macrophage. Extracellular bacteria were washed off at 3 hr post-infection. Cells were lysed at the indicated time points and bacteria were harvested and colonies were counted approximately 21 days later.
Phagosome Maturation Assays
Mtb H37Rv was labeled with FITC (1 mg/ml, Sigma). Bacteria were incubated with cells for 1 hr prior to the addition of LysoTracker Red DND-99 (Invitrogen) (100 nmol/L) for 1 hr prior to fixation. Alternatively, cells were fixed, permeabilized, and with anti-CD63 /LAMP-3 (Santa Cruz Biotechnology) (1 μg/mL) followed by secondary antibody. Images were recorded on an Olympus FluoView 1000 and a Zeiss LSM 510 laser scanning confocal microscope.
Autophagy Analysis
Autophagosome formation was measured by LC3 punctate staining using LC3 antibody (Invitrogen). Autophagic flux was inhibited using either bafilomycin (100mM) or a combination of E64d and pepstatin.
Cytokine Measurements
Cytokine measurements were performed in supernatants using commercial ELISA kits.
Co-Immunoprecipitation
HEK293 cells were incubated for 24 hr with DNA encoding various proteins in the presence of Genejuice. Immunoprecipitation was initiated by incubation of lysates for 2 hr with protein A/G sepharose beads (Amersham) plus control antibodies. Precleared lysates were then incubated at 4°C for at least 2 hr with various antibodies and protein G beads (Amersham) prior to separation by SDS- PAGE and visualization with an enhanced chemiluminescence system (Li-Cor).
Plasmids
HA-Mal has been previously described (Valkov et al., 2011). Site-directed mutagensis was carried out to generate HA-tagged S180L variant Mal which was amplified using Miniprep (QIAGEN). The sequences of both HA-Mal and HA-S180L Mal were confirmed by seqencing (Eurofins).
Fluorescence Lifetime Imaging Microscopy-Fluorescence Resonance Energy Transfer
Macrophages were stained with primary antibodies against IFNGR2 (MyBioSource) and MyD88 (Abcam) and secondary antibodies Alexa Fluor A488 (donor) and A568 (acceptor) antibodies. An Olympus FV1000 microscope equipped with a PicoHarp300 FLIM extension and a 485 nm pulsed laser diode from PicoQuant was used to record FLIM data.
Genotyping
Mal genotype was determined on DNA extracted from buccal swabs (Isohelix, Cell Product). Genotyping of the Mal S180L and polymorphism was performed using the TaqMan Allelic Discrimination System (PE Biosystems).
FACS Analysis
Immortalized macrophages were stimulated, fixed, and stained with anti-pSTAT1 (Y701) antibody conjugated to AlexaFluor 488 before analysis on a BD FACSCanto II analyzer. For analysis of intracellular IFN-γ production, macrophages were infected Mtb H37Rv and then incubated with Brefeldin A. Cells were permeabilized and stained with an anti-mouse IFN-γ antibody (BD) or isotype control.
Mice
Mal S200L heterozygote and homozygote mice (C57BL/6 background) were generated as described in Figure S1. S200L mice were generated with C57BL/6 embryonic stem cells and C57BL/6 blastocysts. Mice were age and sex matched. Mice were infected with Mtb via aerosol (Martens et al., 2012) at approximately 8 weeks of age.
Bacterial Load
At 8 weeks, mice were sacrificed. Lung homogenates from six mice were plated to measure bacterial burden.
Lung Histology
Lungs were inflated, fixed, and stained (H&E). Lung surface area of inflammation was measured with a Nikon Eclipse E400. Percent total lung area involved with inflammation was calculated by dividing the cumulative area of inflammation by the total lung surface area examined for each lung studied.
Lung Cytokine Expression
Lung lysates were assayed for TNF-α by ELISA (R&D Systems).
Statistical Analysis
A one-way ANOVA was performed to assess for statistically significant difference of the means between groups. Chi-square analysis was used to assess statistically significant proportions of co-localization between groups. p values < 0.05 were considered significant. Error bars represent SD of the mean.
Author Contributions
Conceptualization: C.N.C., F.J.S., J.H., N.M.-W., S.V.G., L.A.J.O.N., E.C.L., and J.K.; Methodology: C.N.C., F.J.S., J.H., J.L., N.M.-W., E.P.M., M.M.H., S.A.J., and S.V.G.; Investigation: C.N.C., F.J.S., J.H., N.M.-W., J.L., K.W., E.P.M., A.S., L.E.G., M.C., N.M., C.H.A.H., G.A.T., E.C.C., S.A.J., M.M.H., S.C.C., M.O.S., and C.M.F.; Formal Analysis: C.N.C., F.J.S., J.H., N.M.-W., and S.A.J.; Resources: S.C.C., N.J.B., D.G., H.K., S.V.G., and L.A.J.O.N.; Writing – Original Draft: C.N.C.; Writing – Review & Editing: C.N.C., F.J.S., J.H., H.K., E.C.L., and J.K.; Funding Acquisition: C.N.C., L.A.J.O.N., E.C.L., and J.K.; Supervision: H.K., S.V.G., L.A.J.O.N., E.C.L., and J.K. F.J.S. and J.H. contributed equally to this work.
Acknowledgments
Supported in part by Health Research Board Ireland by grant NSAFP 2009/2 (C.N.C.), CSA/2004/7 and CSA/2012/16 (J.K.), The Royal City of Dublin Hospital Trust (J.K.), NIH grant HL081149 (H.K.), Wellcome Trust PhD award 102395/Z/13/Z (A.S.), Science Foundation Ireland Investigator Awards 08/IN.1/B2038 (S.G.) and 12/IA/1421 (E.C.L.), and the Science Foundation Ireland Strategic Research Cluster 07/SRC/B1144 (E.C.L.).
Published: February 16, 2016
Footnotes
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Supplemental Information includes six figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2016.01.019.
Contributor Information
Clíona Ní Cheallaigh, Email: nicheacm@tcd.ie.
Ed C. Lavelle, Email: lavellee@tcd.ie.
Supplemental Information
References
- Bogunovic D., Byun M., Durfee L.A., Abhyankar A., Sanal O., Mansouri D., Salem S., Radovanovic I., Grant A.V., Adimi P. Mycobacterial disease and impaired IFN-γ immunity in humans with inherited ISG15 deficiency. Science. 2012;337:1684–1688. doi: 10.1126/science.1224026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham K.S., Orzalli M.H., Hayashi K., Wolf A.I., Glanemann C., Weninger W., Iwasaki A., Knipe D.M., Kagan J.C. A promiscuous lipid-binding protein diversifies the subcellular sites of toll-like receptor signal transduction. Cell. 2014;156:705–716. doi: 10.1016/j.cell.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capparelli R., De Chiara F., Di Matteo A., Medaglia C., Iannelli D. The MyD88 rs6853 and TIRAP rs8177374 polymorphic sites are associated with resistance to human pulmonary tuberculosis. Genes Immun. 2013;14:504–511. doi: 10.1038/gene.2013.48. [DOI] [PubMed] [Google Scholar]
- Castiblanco J., Varela D.-C., Castaño-Rodríguez N., Rojas-Villarraga A., Hincapié M.-E., Anaya J.-M. TIRAP (MAL) S180L polymorphism is a common protective factor against developing tuberculosis and systemic lupus erythematosus. Infect. Genet. Evol. 2008;8:541–544. doi: 10.1016/j.meegid.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Cooper A.M., Dalton D.K., Stewart T.A., Griffin J.P., Russell D.G., Orme I.M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwich L., Coma G., Peña R., Bellido R., Blanco E.J., Este J.A., Borras F.E., Clotet B., Ruiz L., Rosell A. Secretion of interferon-gamma by human macrophages demonstrated at the single-cell level after costimulation with interleukin (IL)-12 plus IL-18. Immunology. 2009;126:386–393. doi: 10.1111/j.1365-2567.2008.02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V., Singh S., Master S., Harris J., Roberts E., Kyei G., Davis A., de Haro S., Naylor J., Lee H.H., Vergne I. Mycobacterium tuberculosis inhibition of phagolysosome biogenesis and autophagy as a host defence mechanism. Cell. Microbiol. 2006;8:719–727. doi: 10.1111/j.1462-5822.2006.00705.x. [DOI] [PubMed] [Google Scholar]
- Di Marzio P., Puddu P., Conti L., Belardelli F., Gessani S. Interferon gamma upregulates its own gene expression in mouse peritoneal macrophages. J. Exp. Med. 1994;179:1731–1736. doi: 10.1084/jem.179.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayeke S.R., Levin S., Pienaar S., Wood K., Eley B., Beatty D., Henderson H., Anderson S., Levin M. Polymorphic variation in TIRAP is not associated with susceptibility to childhood TB but may determine susceptibility to TBM in some ethnic groups. PLoS ONE. 2009;4:e6698. doi: 10.1371/journal.pone.0006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold K.R. The adverse effect of IFN gamma on stratum corneum structure and function in psoriasis and atopic dermatitis. J. Invest. Dermatol. 2014;134:597–600. doi: 10.1038/jid.2013.440. [DOI] [PubMed] [Google Scholar]
- Fenton M.J., Vermeulen M.W., Kim S., Burdick M., Strieter R.M., Kornfeld H. Induction of gamma interferon production in human alveolar macrophages by Mycobacterium tuberculosis. Infect. Immun. 1997;65:5149–5156. doi: 10.1128/iai.65.12.5149-5156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferwerda B., Alonso S., Banahan K., McCall M.B., Giamarellos-Bourboulis E.J., Ramakers B.P., Mouktaroudi M., Fain P.R., Izagirre N., Syafruddin D. Functional and genetic evidence that the Mal/TIRAP allele variant 180L has been selected by providing protection against septic shock. Proc. Natl. Acad. Sci. USA. 2009;106:10272–10277. doi: 10.1073/pnas.0811273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe-Santos O., Bustamante J., Chapgier A., Vogt G., de Beaucoudrey L., Feinberg J., Jouanguy E., Boisson-Dupuis S., Fieschi C., Picard C., Casanova J.L. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin. Immunol. 2006;18:347–361. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Fitzgerald K.A., Palsson-McDermott E.M., Bowie A.G., Jefferies C.A., Mansell A.S., Brady G., Brint E., Dunne A., Gray P., Harte M.T. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- Flesch I.E., Kaufmann S.H. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect. Immun. 1991;59:3213–3218. doi: 10.1128/iai.59.9.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J.L., Chan J., Triebold K.J., Dalton D.K., Stewart T.A., Bloom B.R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz M.J., Barber S.A., Dieffenbach C.W., Vogel S.N. Induction of IFN-gamma in macrophages by lipopolysaccharide. Int. Immunol. 1993;5:1383–1392. doi: 10.1093/intimm/5.11.1383. [DOI] [PubMed] [Google Scholar]
- Gutierrez M.G., Master S.S., Singh S.B., Taylor G.A., Colombo M.I., Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Harris J., De Haro S.A., Master S.S., Keane J., Roberts E.A., Delgado M., Deretic V. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity. 2007;27:505–517. doi: 10.1016/j.immuni.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Harris J., Hope J.C., Lavelle E.C. Autophagy and the immune response to TB. Transbound. Emerg. Dis. 2009;56:248–254. doi: 10.1111/j.1865-1682.2009.01069.x. [DOI] [PubMed] [Google Scholar]
- Hölscher C., Reiling N., Schaible U.E., Hölscher A., Bathmann C., Korbel D., Lenz I., Sonntag T., Kröger S., Akira S. Containment of aerogenic Mycobacterium tuberculosis infection in mice does not require MyD88 adaptor function for TLR2, -4 and -9. Eur. J. Immunol. 2008;38:680–694. doi: 10.1002/eji.200736458. [DOI] [PubMed] [Google Scholar]
- Horng T., Barton G.M., Medzhitov R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat. Immunol. 2001;2:835–841. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- Horng T., Barton G.M., Flavell R.A., Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420:329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Old L.J., Schreiber R.D. The roles of IFN γ in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- Kagan J.C., Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Kenny E.F., Talbot S., Gong M., Golenbock D.T., Bryant C.E., O’Neill L.A. MyD88 adaptor-like is not essential for TLR2 signaling and inhibits signaling by TLR3. J. Immunol. 2009;183:3642–3651. doi: 10.4049/jimmunol.0901140. [DOI] [PubMed] [Google Scholar]
- Khor C.C., Chapman S.J., Vannberg F.O., Dunne A., Murphy C., Ling E.Y., Frodsham A.J., Walley A.J., Kyrieleis O., Khan A. A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nat. Genet. 2007;39:523–528. doi: 10.1038/ng1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.U., Sreih A., Bucala R. Toll-like receptors in systemic lupus erythematosus; prospects for therapeutic intervention. Autoimmun. Rev. 2009;8:204–208. doi: 10.1016/j.autrev.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladhani S.N., Davila S., Hibberd M.L., Heath P.T., Ramsay M.E., Slack M.P., Pollard A.J., Booy R. Association between single-nucleotide polymorphisms in Mal/TIRAP and interleukin-10 genes and susceptibility to invasive haemophilus influenzae serotype b infection in immunized children. Clin. Infect. Dis. 2010;51:761–767. doi: 10.1086/656236. [DOI] [PubMed] [Google Scholar]
- Liu Q., Li W., Li D., Feng Y., Tao C. TIRAP C539T Polymorphism Contributes to Tuberculosis Susceptibility: Evidence from a Meta-Analysis. Infect Genet Evol. 2014 doi: 10.1016/j.meegid.2014.06.025. [DOI] [PubMed] [Google Scholar]
- MacMicking J.D. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat. Rev. Immunol. 2012;12:367–382. doi: 10.1038/nri3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens G.W., Vallerskog T., Kornfeld H. Hypercholesterolemic LDL receptor-deficient mice mount a neutrophilic response to tuberculosis despite the timely expression of protective immunity. J. Leukoc. Biol. 2012;91:849–857. doi: 10.1189/jlb.0311164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa T., Fujiwara E., Washi Y. Autophagy activation by interferon-γ via the p38 mitogen-activated protein kinase signalling pathway is involved in macrophage bactericidal activity. Immunology. 2014;141:61–69. doi: 10.1111/imm.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra B.B., Rathinam V.A., Martens G.W., Martinot A.J., Kornfeld H., Fitzgerald K.A., Sassetti C.M. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β. Nat. Immunol. 2013;14:52–60. doi: 10.1038/ni.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T.R., Coffman R.L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Nandi B., Behar S.M. Regulation of neutrophils by interferon-γ limits lung inflammation during tuberculosis infection. J. Exp. Med. 2011;208:2251–2262. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejentsev S., Thye T., Szeszko J.S., Stevens H., Balabanova Y., Chinbuah A.M., Hibberd M., van de Vosse E., Alisjahbana B., van Crevel R. Analysis of association of the TIRAP (MAL) S180L variant and tuberculosis in three populations. Nat. Genet. 2008;40:261–262. doi: 10.1038/ng0308-261. author reply 262–263. [DOI] [PubMed] [Google Scholar]
- Ní Cheallaigh C., Keane J., Lavelle E.C., Hope J.C., Harris J. Autophagy in the immune response to tuberculosis: clinical perspectives. Clin. Exp. Immunol. 2011;164:291–300. doi: 10.1111/j.1365-2249.2011.04381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Carroll C., Fagan A., Shanahan F., Carmody R.J. Identification of a unique hybrid macrophage-polarization state following recovery from lipopolysaccharide tolerance. J. Immunol. 2014;192:427–436. doi: 10.4049/jimmunol.1301722. [DOI] [PubMed] [Google Scholar]
- Peral de Castro C., Jones S.A., Ní Cheallaigh C., Hearnden C.A., Williams L., Winter J., Lavelle E.C., Mills K.H.G., Harris J. Autophagy regulates IL-23 secretion and innate T cell responses through effects on IL-1 secretion. J. Immunol. 2012;189:4144–4153. doi: 10.4049/jimmunol.1201946. [DOI] [PubMed] [Google Scholar]
- Perussia B. Lymphokine-activated killer cells, natural killer cells and cytokines. Curr. Opin. Immunol. 1991;3:49–55. doi: 10.1016/0952-7915(91)90076-d. [DOI] [PubMed] [Google Scholar]
- Pollard K.M., Cauvi D.M., Toomey C.B., Morris K.V., Kono D.H. Interferon-γ and systemic autoimmunity. Discov. Med. 2013;16:123–131. [PMC free article] [PubMed] [Google Scholar]
- Roca F.J., Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. 2013;153:521–534. doi: 10.1016/j.cell.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sad S., Marcotte R., Mosmann T.R. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–279. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- Schleicher U., Hesse A., Bogdan C. Minute numbers of contaminant CD8+ T cells or CD11b+CD11c+ NK cells are the source of IFN-gamma in IL-12/IL-18-stimulated mouse macrophage populations. Blood. 2005;105:1319–1328. doi: 10.1182/blood-2004-05-1749. [DOI] [PubMed] [Google Scholar]
- Schroecksnadel K., Frick B., Winkler C., Fuchs D. Crucial role of interferon-gamma and stimulated macrophages in cardiovascular disease. Curr. Vasc. Pharmacol. 2006;4:205–213. doi: 10.2174/157016106777698379. [DOI] [PubMed] [Google Scholar]
- Selvaraj P., Harishankar M., Singh B., Jawahar M.S., Banurekha V.V. Toll-like receptor and TIRAP gene polymorphisms in pulmonary tuberculosis patients of South India. Tuberculosis (Edinb.) 2010;90:306–310. doi: 10.1016/j.tube.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Sun D., Ding A. MyD88-mediated stabilization of interferon-gamma-induced cytokine and chemokine mRNA. Nat. Immunol. 2006;7:375–381. doi: 10.1038/ni1308. [DOI] [PubMed] [Google Scholar]
- Tobin D.M., Roca F.J., Oh S.F., McFarland R., Vickery T.W., Ray J.P., Ko D.C., Zou Y., Bang N.D., Chau T.T. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148:434–446. doi: 10.1016/j.cell.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkov E., Stamp A., Dimaio F., Baker D., Verstak B., Roversi P., Kellie S., Sweet M.J., Mansell A., Gay N.J. Crystal structure of Toll-like receptor adaptor MAL/TIRAP reveals the molecular basis for signal transduction and disease protection. Proc. Natl. Acad. Sci. USA. 2011;108:14879–14884. doi: 10.1073/pnas.1104780108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R.O., Manzanillo P.S., Cox J.S. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell. 2012;150:803–815. doi: 10.1016/j.cell.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Tagawa Y., Yoshimori T., Moriyama Y., Masaki R., Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct. Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Sato S., Hemmi H., Sanjo H., Uematsu S., Kaisho T., Hoshino K., Takeuchi O., Kobayashi M., Fujita T. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–329. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- Yang Z., Liang Y., Qin B., Li C., Zhong R. TLR9 polymorphisms and systemic lupus erythematosus risk in Asians: a meta-analysis study. Cytokine. 2012;57:282–289. doi: 10.1016/j.cyto.2011.11.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.