Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
FCR-treated chronic lymphocytic leukemia patients with mutated IGHV gene achieve long-term PFS, with a plateau on the PFS curve.
MRD-negativity posttreatment is highly predictive of long-term PFS, particularly in patients with mutated IGHV gene.
Abstract
Accurate identification of patients likely to achieve long-progression-free survival (PFS) after chemoimmunotherapy is essential given the availability of less toxic alternatives, such as ibrutinib. Fludarabine, cyclophosphamide, and rituximab (FCR) achieved a high response rate, but continued relapses were seen in initial reports. We reviewed the original 300 patient phase 2 FCR study to identify long-term disease-free survivors. Minimal residual disease (MRD) was assessed posttreatment by a polymerase chain reaction-based ligase chain reaction assay (sensitivity 0.01%). At the median follow-up of 12.8 years, PFS was 30.9% (median PFS, 6.4 years). The 12.8-year PFS was 53.9% for patients with mutated immunoglobulin heavy chain variable (IGHV) gene (IGHV-M) and 8.7% for patients with unmutated IGHV (IGHV-UM). 50.7% of patients with IGHV-M achieved MRD-negativity posttreatment; of these, PFS was 79.8% at 12.8 years. A plateau was seen on the PFS curve in patients with IGHV-M, with no relapses beyond 10.4 years in 42 patients (total follow-up 105.4 patient-years). On multivariable analysis, IGHV-UM (hazard ratio, 3.37 [2.18-5.21]; P < .001) and del(17p) by conventional karyotyping (hazard ratio, 7.96 [1.02-61.92]; P = .048) were significantly associated with inferior PFS. Fifteen patients with IGHV-M had 4-color MRD flow cytometry (sensitivity 0.01%) performed in peripheral blood, at a median of 12.8 years posttreatment (range, 9.5-14.7). All were MRD-negative. The high rate of very long-term PFS in patients with IGHV-M after FCR argues for the continued use of chemoimmunotherapy in this patient subgroup outside clinical trials; alternative strategies may be preferred in patients with IGHV-UM, to limit long-term toxicity.
Introduction
Chemoimmunotherapy (CIT) with fludarabine, cyclophosphamide, and rituximab (FCR) is an established standard-of-care for physically fit patients with chronic lymphocytic leukemia (CLL) requiring therapy,1 with the exception of patients with del(17p) by fluorescence in situ hybridization (FISH)1 and/or TP53 mutations.2,3 Complete remission (CR) rates after FCR are 44% to 72%, with a median progression-free survival (PFS) of 52 to 72 months.1,4 In addition to the impact of TP53 disruption, there is considerable heterogeneity in outcomes according to other pretreatment characteristics, such as immunoglobulin heavy-chain variable (IGHV) gene somatic hypermutation status (IGHV-MS) and β2-microglobulin (B2M) level. There is no accepted cure for CLL, with the exception of allogeneic stem cell transplantation (SCT),5 and it is expected that although treatment with CIT prolongs PFS and survival, all patients will ultimately relapse.
CIT is associated with considerable short-term and long-term toxicity, including acute and prolonged neutropenia and opportunistic infections.1,4 Although CLL is associated with an increased incidence of other cancers,6 a clear relationship with the type of therapy has not been established.7,8 Several small-molecule targeted therapies are now available for CLL; the Bruton tyrosine kinase inhibitor ibrutinib9 and the phosphatidylinositol 3-kinase δ inhibitor idelalisib10 are effective in relapsed/refractory CLL, including patients with high-risk features such as fludarabine-refractory disease and del(17p). Although targeted agents offer the prospect of disease control with reduced toxicity and mutagenicity, including in those patients with high-risk disease, minimal residual disease (MRD)-negative CRs are rare,9-12 and most patients will require indefinite suppression of their disease. Treatment with these novel agents represents a different treatment paradigm to CIT; the intention of CIT is to deliver time-limited therapy, with the aim of achieving deep and durable remission, without the need for ongoing maintenance therapy. Results of a phase 3 study comparing ibrutinib to chlorambucil in the first line setting are expected imminently and important trials comparing ibrutinib plus rituximab to FCR in fit, treatment-naïve patients are accruing. Outside the clinical trial setting, it is critical to accurately identify those groups of patients likely to derive maximal long-term benefit from CIT, when determining a first line treatment paradigm; fit patients likely to achieve long-term remissions with CIT should receive this therapy, whereas patients unlikely to achieve long-term remissions with CIT could potentially receive novel therapies and be spared CIT-associated toxicity.
The original phase 2 FCR study at MD Anderson Cancer Center (MDACC)4,13 treated 300 patients with FCR from 1999 to 2003 and led to the randomized German CLL Study Group CLL8 study, which confirmed the superiority of FCR CIT relative to FC, and established FCR as the current standard-of-care for CLL.1 Rossi et al have recently demonstrated that IGHV mutation status, del(17p), and del(11q) are the most important biological factors predicting PFS.14 We present a follow-up of our original study, at a median of 12.8 years posttreatment, with a focus on identifying groups of patients likely to achieve very long-term disease-free survival (DFS).
Methods
Study design
The study design has been reported previously4,13; briefly, patients received fludarabine 25 to 30 mg/m2 and cyclophosphamide 250 to 300 mg/m2 on days 2 to 4 of cycle 1 and days 1 to 3 of cycles 2 to 6; rituximab (375 mg in cycle 1 and 500 mg/m2 in cycles 2 to 6) was administered on day 1. Treatment was given every 4 weeks for a planned total of 6 cycles. Responses were assessed at 3, 6, and 12 months according to the National Cancer Institute-Working Group 1996 criteria15; routine computed tomography (CT) scans for response assessment were not required. This study predated the availability of a highly sensitive 4-color flow cytometry method for MRD determination.16 However, bone marrow (BM) specimens were evaluated at end-of-treatment of MRD by a polymerase chain reaction (PCR)-based ligase assay for patient-specific clonal IGHV, with a validated sensitivity of at least 0.01%,17 equivalent to that achieved with 4-color flow cytometry. Many patients returned to their referring centers for long-term follow-up, but relapse and survival data continued to be collected (see supplemental Methods on the Blood Web site). For the group of patients having long-term follow-up at MDACC, peripheral blood was monitored for MRD using standard European Research Initiative in CLL multicolor flow cytometry assay with a sensitivity of at least 0.01%.16
Statistical considerations
In the current study update, progressive disease was defined according to International Working Group in CLL (IWCLL) 2008 criteria.18 PFS was defined as the time from initiation of therapy until the development of progressive disease, initiation of salvage therapy, or death. A minority (3%) of patients was censored for PFS analysis when they received systemic therapy for another cancer (myelodysplastic syndrome [MDS]/acute myeloid leukemia [AML], n = 8 and T-acute lymphoblastic leukemia, n = 1) while remaining in clinical remission from CLL. No patient received allogeneic SCT in first partial response or CR, although this was subsequently performed in 39 patients following disease progression. Associations between categorical variables and outcomes were evaluated using the Fisher’s exact or χ2 tests, as appropriate, and multivariable analysis (MVA) was performed using logistic regression. Actuarial survival was calculated using the Kaplan–Meier method and comparisons made using the log-rank test; HRs, including 95% confidence intervals (CI) in univariable analysis and MVA were calculated using Cox regression; ζ-associated protein 70 (ZAP70) and lactate dehydrogenase (LDH) were not included in the multivariable model due to very strong associations with IGHV-MS (odds ratio [OR] 6.4 and 3.4, respectively; P < .001 for each). Landmark analyses were performed from the time of MRD assessment (6 months after initiation of treatment) to assess the impact of treatment outcome (including MRD) on PFS and survival.
Cumulative incidence of relapse was calculated using the cumulative incidence method, with death in remission considered a competing risk. In addition, cumulative incidences of death due to CLL, Richter transformation (RT), and other cancers were calculated in a competing risk model; death in remission was again considered a competing risk. The impact of IGHV-MS and baseline B2M on these incidences was assessed.
Statistical analysis and graphical representation was performed with R 3.0.3, SPSS version 22 (IBM Corp, Armonk, NY) and GraphPad Prism 6 software (La Jolla, CA).
Prognostic factor evaluation
IGHV-MS was determined retrospectively from pretreatment formalin-fixed, paraffin-embedded BM clot sections (n = 101) as previously described,19 from pretreatment frozen BM aspirate or peripheral blood specimens (n = 76), or from posttreatment relapse samples (n = 37). Fifteen patients on this study had IGHV-MS determined from both their pretreatment paraffin sample and a fresh pretreatment sample as part of our involvement in the CLL Research Consortium, or tested fresh at the time of relapse. In all 15 cases, the IGHV status was concordant. ZAP-70 expression was determined retrospectively using immunohistochemistry (IHC) performed on baseline BM trephine biopsies.20 FISH results for del(11q) and del(17p) are not available as FISH testing for occult cytogenetic aberrations was not available at MDACC at the time of the study; instead, we performed conventional karyotyping on pretreatment marrow using lipopolysaccharide stimulation.21
Results
Pretreatment patient characteristics
Pretreatment characteristics of the 300 patients are shown in Table 1: median age was 57 years (range, 17-86); 23% were aged 65-74 and 4% were ≥75; 61% had Rai stage I-II and 35% had stage III-IV disease; 4% of patients had Rai stage 0 disease and were treated due to marked constitutional symptoms and/or rapid lymphocyte doubling time. B2M was ≥2× upper limit of normal (ULN) in 43% of patients. IGHV-MS was mutated (IGHV-M) in 88 patients, unmutated (IGHV-UM) in 126, and unknown in 86, mainly due to inadequate pretreatment material availability. Five of 222 patients with conventional karyotyping results had structural alterations resulting in the loss of TP53 (referred to henceforth as del[17p]).
Table 1.
Pretreatment characteristics
| Characteristics n = 300 unless stated | n (%) unless stated |
|---|---|
| Age, median (y) (range, y) | 57 (17-86) |
| Age ≥65 | 72 (24) |
| B2M ≥4.0 mg/L, n = 296 | 128/296 (43) |
| Binet stage | |
| A | 76 (25) |
| B | 145 (48) |
| C | 78 (26) |
| Rai stage III-IV | 107 (36) |
| Male gender | 211 (70) |
| ECOG PS | |
| 0 | 119 (40) |
| 1 | 171 (57) |
| 2 | 10 (3) |
| IGHV unmutated, n = 214 | 126 (59) |
| WCC ≥200 × 109/L | 27 (9) |
| LDH > ULN | 108 (36) |
| ZAP70 positive by IHC, n = 209 | 126 (60) |
| Del(17p) by karyotyping, n = 222 | 5 (2) |
ECOG, Eastern Co-operative Oncology Group; PS, performance status; WCC, white cell count.
Response rates and MRD-negativity
Overall response rate was 95% with 72% CR. Two patients died of sepsis prior to response assessment and 13 failed to respond. A total of 246 patients had posttreatment MRD analysis from BM by PCR-based assay; of these, 43.1% achieved MRD-negativity. Associations between pretreatment characteristics, CR rate, and MRD-negativity by PCR are shown in Table 2. There was no significant difference in CR rate according to IGHV-MS. On MVA, only a baseline B2M ≥4.0 (OR, 0.32 [0.13-0.79]; P = .013) was significantly associated with an inferior likelihood of achieving CR; the only pretreatment characteristics on MVA associated with an inferior likelihood of achieving MRD-negativity were male gender (OR, 0.33 [0.16-0.68]; P = .003) and IGHV-UM (OR, 0.45 [0.26-0.99]; P = .045). In the 5 patients with del(17p), CR was achieved in 1 patient. Patients who completed 6 cycles of therapy were more likely to achieve CR and MRD-negativity (Table 2).
Table 2.
Associations between pretreatment characteristics and achievement of CR and MRD-negativity
| Characteristics n = 300 unless stated | CR n (%) | P | MRD-negative | P |
|---|---|---|---|---|
| Age, median (range); 57 y (17-86 y) | ||||
| Age category | ||||
| Age <65, n = 228 | 173 (75.9) | |||
| Age 65-74, n = 61 | 42 (68.9) | 104/239 (43.5) | ||
| Age ≥75, n = 11 | 4 (36.4) | .011 | 2/7 (28.6) | .43 |
| B2M (mg/L), n = 296 | ||||
| <4.0, n = 168 | 143 (85.1) | 69/141 (48.9) | ||
| ≥4.0, n = 128 | 73 (57.0) | <.001 | 35/101 (34.7) | .027 |
| Gender | ||||
| Male, n = 211 | 155 (70.8) | 63/173 (36.4) | ||
| Female, n = 89 | 64 (71.9) | .782 | 43/73 (58.9) | .001 |
| ECOG PS | ||||
| 0, n = 119 | 96 (80.7) | 43/95 (45.3) | ||
| 1, n = 171 | 118 (69.0) | 60/143 (42.0) | ||
| 2, n = 10 | 5 (50.0) | .022 | 3/5 (37.5) | .84 |
| IGHV mutation status, n = 214 | ||||
| Mutated, n = 88 | 73 (83.0) | 35/69 (50.7) | ||
| Unmutated, n = 126 | 91 (72.2) | .068 | 35/106 (33.0) | .019 |
| Rai stage | ||||
| 0-II,* n = 193 | 147 (76.2) | 76/165 (46.1) | ||
| III-IV, n = 107 | 72 (67.3) | .097 | 30/81 (37.0) | .18 |
| WCC (×109/L) | ||||
| <200, n = 273 | 207 (75.8) | 100/221 (45.1) | ||
| ≥200, n = 27 | 12 (44.4) | <.001 | 6/25 (24.0) | .042 |
| LDH | ||||
| Normal, n = 192 | 145 (75.5) | 69/157 (43.9) | ||
| > Normal, n = 108 | 74 (68.5) | .190 | 37/89 (41.6) | .72 |
| ZAP70 by IHC, n = 209 | ||||
| Negative, n = 83 | 63 (75.9) | 34/66 (51.5) | ||
| Positive, n = 126 | 95 (75.4) | .933 | 39/102 (38.2) | .09 |
| Del(17p) by karyotyping, n = 222 | ||||
| Del(17p), n = 5 | 1 (20.0) | 0/4 (0) | ||
| No del(17p), n = 217 | 163 (75.1) | .006 | 77/175 (44.0) | .079 |
| Number of cycles received | ||||
| 1-3, n = 33 | 10 (30.3) | 2/19 (10.5) | ||
| 4-5, n = 44 | 28 (63.6) | 12/31 (38.7) | ||
| 6, n = 223 | 181 (81.2) | <.001 | 92/196 (46.9) | .008 |
A total of 11 patients had Rai stage 0 disease and were treated due to disease-associated symptoms.
PFS
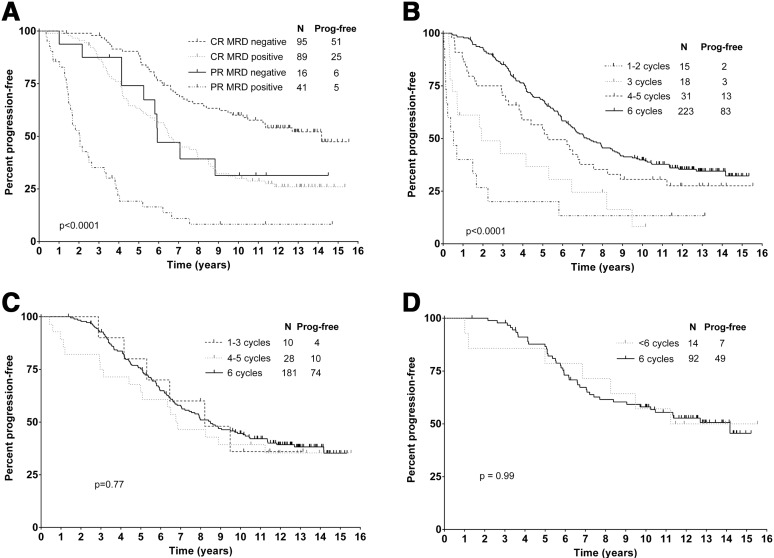
At a median follow-up of 12.8 years in surviving patients, median PFS was 6.4 years and 12.8-year PFS was 30.9% (Figure 1A). Univariable associations between baseline characteristics, PFS, and survival are shown in Table 3. On MVA, only IGHV-UM (hazard ratio [HR], 3.37 [2.18-5.21]; P < .001) and del(17p) (HR, 7.96 [1.02-61.92]; P = .048) were significantly associated with inferior PFS. Median PFS was not reached (NR) for patients with IGHV-M and 4.2 years for patients with IGHV-UM (P < .001) (Figure 1B); 12.8-year PFS was 53.9% for patients with IGHV-M and 8.9% for patients with IGHV-UM. A striking feature of the PFS curves is the emergence of a plateau in patients with IGHV-M, a feature not seen in previous prospective studies of therapy in CLL. Specifically, the last relapse occurred at 10.4 years. A total of 42 patients were followed beyond this point for a median of 2.5 years (range, 0.1-5.0), with no further relapses seen during a total of 105.4 patient-years of follow-up. Moreover, 15 patients with IGHV-M having long-term follow-up at MDACC had peripheral blood MRD flow cytometry (with a sensitivity of <0.01%) performed, at a median time of 12.8 years posttreatment (range, 9.5-14.7); all were MRD-negative. Achievement of MRD-negativity by PCR at completion of treatment was associated with marked prolongation of PFS relative to patients who remained MRD-positive (median, 13.7 years for PCR-negative vs 4.0 years). This benefit was particularly seen in those patients with IGHV-M: IGHV-M patients who were PCR-negative at end-of-treatment had a 12.8-year PFS of 79.8% (compared with 36.9% if MRD-positive) (Figure 1D); in contrast, there were few long-term disease-free survivors in IGHV-UM patients, even those who achieved MRD-negative CR (median PFS, 5.5 years; 12.8-year PFS, 16.3%). Pretreatment B2M ≥4.0 mg/L was associated with inferior PFS for patients with IGHV-M (median PFS, NR vs 7.3 years; 12.8-year PFS, 61.6% vs 38.8%), P < .001, but not IGHV-UM (P = .11) (Figure 1C). MRD-negativity was associated with superior PFS, independent of IWCLL response category (Figure 2A). There was also a direct correlation between the number of cycles of treatment received and PFS (Figure 2B). However, when only patients who achieved CR (Figure 2C) or MRD-negativity (Figure 2D) were assessed, there was no difference in PFS according to number of cycles of treatment received.
Figure 1.
Estimates of PFS and overall survival in the total cohort and PFS according to mutation status, B2M, and posttreatment MRD. (A) PFS and survival in the total cohort. (B) PFS according to mutation status. (C) PFS according to mutation status and baseline B2M. (D) Six-month landmark PFS according to mutation status and achievement of posttreatment MRD-negativity.
Table 3.
HRs for PFS and survival according to baseline characteristics on univariable analysis
| Baseline characteristics | PFS HR (95% CI) | P | OS HR (95% CI) | P |
|---|---|---|---|---|
| Age ≥65, n = 72 | 1.59 (1.17-2.17) | .003 | 2.66 (1.90-3.72) | <.001 |
| Male gender, n = 211 | 1.24 (0.91-1.69) | .179 | 1.29 (0.89-1.88) | .177 |
| B2M ≥4.0 (mg/L), n = 128 | 1.97 (1.49-2.61) | <.001 | 2.51 (1.80-3.49) | <.001 |
| Unmutated IGHV, n = 126 | 3.62 (2.50-5.24) | <.001 | 2.62 (1.71-4.01) | <.001 |
| ECOG PS >0, n = 181 | 1.25 (0.94-1.67) | .123 | 1.32 (0.97-1.78) | .076 |
| Rai stage III-IV, n = 107 | 1.27 (0.95-1.69) | .101 | 1.34 (0.96-1.87) | .081 |
| WCC >200 × 109/L, n = 27 | 2.51 (1.63-3.86) | <.001 | 1.90 (1.16-3.11) | .011 |
| LDH > normal, n = 108 | 1.53 (1.15-2.03) | .003 | 1.64 (1.18-2.27) | .003 |
| ZAP70 IHC pos, n = 126 | 2.44 (1.68-3.54) | <.001 | 1.84 (1.22-2.79) | .004 |
| Karyotype: del(17p), n = 5 | 4.18 (1.70-10.24) | .002 | 4.52 (1.66-12.37) | .003 |
Figure 2.
Estimates of PFS according to depth of response and number of cycles received. (A) PFS according to IWCLL response criteria and MRD. (B) PFS according to number of FCR cycles received. (C) PFS according to number of FCR cycles received in patients who achieved CR. (D) PFS according to number of FCR cycles received in patients who achieved MRD-negativity posttreatment.
Overall survival (OS)
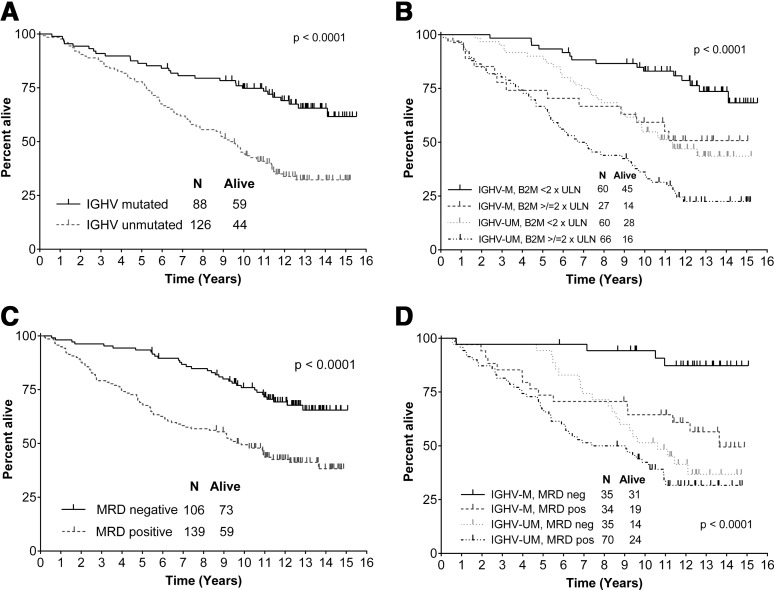
Median OS for the total cohort was 12.7 years. Univariable associations between pretreatment characteristics and survival are shown in Table 3. On MVA, the following were significantly associated with inferior survival: age ≥65 (HR, 3.0 [1.86-4.80]; P = .001), IGHV-UM (HR, 2.87 [1.70-4.84]; P < .001), B2M ≥4.0 (1.73 [1.09-2.75]; P = .020), and del(17p) (HR, 10.29 [1.29-81.99]; P = .028). Survival was markedly inferior for patients with IGHV-UM compared with IGHV-M (median, 9.4 years vs NR and 12.8-years, 32.2% vs 65.5%; P < .001) and B2M ≥4.0 predicted inferior survival in both IGHV-UM and IGHV-M patients (P = .002 and P = .011, respectively) (Figure 3). Achieving MRD-negativity predicted superior long-term survival in patients with IGHV-M only; 12.8-year survival was 87.2% vs 56.5% for MRD-negative and MRD-positive patients, respectively (P = .003) (Figure 3). In contrast, there was no difference in survival for IGHV-UM patients according to MRD status (P = .146).
Figure 3.
Estimates of overall survival according to pretreatment mutation status, B2M, and posttreatment MRD. (A) OS according to mutation status. (B) OS according to mutation status and baseline B2M. (C) Six-month landmark OS according to the achievement of posttreatment MRD-negativity. (D) Six-month landmark OS from end-of-therapy according to mutation status and the achievement of posttreatment MRD-negativity.
Causes of death and incidence of second cancers
CLL remained the most common cause of death (58.1%), followed by other cancers (18.4%), RT (15.4%), and infection in remission (6.6%). A total of 101 other cancers occurred during follow-up (Table 4), most commonly MDS/AML, RT, and non-melanoma skin cancer. Pretreatment biologic characteristics of patients who developed RT, MDS/AML, and other hematologic and nonhematologic tumors are shown in supplemental Table 1. Only 4 of 14 patients who developed MDS/AML had received salvage therapy for CLL. We have examined the relationship between CLL, treatment, and other cancers in a separate group of 797 patients.6
Table 4.
Other cancers occurring during follow-up
| Cancer | Number |
|---|---|
| Secondary hematologic | |
| AML/myelodysplasia | 14 |
| Myeloproliferative disorder, unclassified | 1 |
| T-acute lymphoblastic leukemia | 1 |
| Mature T-cell lymphoproliferative disorders | 3 |
| RT | |
| Diffuse large B-cell lymphoma | 20 |
| Hodgkin lymphoma | 3 |
| Burkitt lymphoma | 1 |
| Solid tumors | |
| Non-melanoma skin cancer | 28 |
| Prostate | 9 |
| Breast cancer | 4 |
| Melanoma | 4 |
| Lung cancer | 3 |
| Ovarian cancer | 3 |
| Renal cell carcinoma | 3 |
| Papillary thyroid cancer | 2 |
| Esophageal adenocarcinoma | 1 |
| Merkel cell tumor | 1 |
| Brain tumor | 1 |
| Colorectal cancer | 1 |
| Other | 3 |
Cumulative incidences of relapse, death in remission, death from other cancers, and RT
Two separate competing risk analyses were performed: firstly, cumulative incidence of relapse vs non–CLL-related death; secondly, cumulative incidence of CLL-related death (including post-allogeneic SCT or from sepsis during treatment) vs death due to RT, or other cancers (Figure 4). Cumulative incidences of relapse at the median follow-up of 12.8 years were 34.5% and 85.3% in IGHV-M and IGHV-UM patients, respectively (P < .001); no relapses were seen in IGHV-M beyond 10.4 years and, if MRD-negative posttreatment, the last relapse was seen at 7.2 years; 3 relapses in 14 patients with a total 28.6 patient-years of follow-up beyond 10 years were seen in patients with IGHV-UM. The cumulative incidence at 12.8 years of CLL-related death was significantly higher in patients with IGHV-UM than IGHV-M (49.1% vs 12.4%; P < .001) and for patients with B2M ≥2× ULN than <2× ULN (42.3% vs 18.2%; P < .001). On MVA, both IGHV-MS and B2M were significantly associated with increased risk of CLL-related death (P < .001 and P = .003, respectively), whereas only IGHV-MS was associated with a higher risk of relapse (P < .001), suggesting that B2M ≥4.0 at baseline may be associated with inferior salvageability at relapse. Cumulative incidence of RT was higher in patients with baseline B2M ≥2× ULN compared with those with baseline B2M <2× ULN (13.3% vs 3.7% at 12.8 years; P = .002) (data not shown). Cumulative incidence of death at 12.8 years due to other cancers was not different according to IGHV-MS or baseline B2M (9.0% in IGHV-UM and 7.4% in IGHV-M [P = .24], and was 9.2% and 8.9% in patients with B2M ≥4.0 vs <4.0, respectively [P = .77]).
Figure 4.
Cumulative incidences of relapse, non–CLL-related death, CLL-related death, and RT. (A) Cumulative incidence of relapse and death in remission. (B) Cumulative incidence of death due to CLL-related causes, second cancers, and RT. (C) Cumulative incidences of relapse and death in remission according to mutation status. (D) Cumulative incidences of death due to CLL, other cancers, or RT according to mutation status.
Discussion
This study has the longest follow-up of any clinical trial published to date in CLL. This gave us a unique capacity to examine the natural history of the disease in the context of a uniformly treated cohort of patients who received current gold-standard treatment, to determine if there were patients who achieved very long-term DFS. Patients with IGHV-M represent 37% to 41% of patients with IGHV-MS results, treated in this and other CIT studies.1 In our series, patients with IGHV-M had a highly favorable outcome following primary therapy with FCR, with a plateau on the PFS curve, and persistent MRD-negativity in all long-term survivors who were tested. In contrast, there were few long-term disease-free survivors among patients with IGHV-UM, who have a median PFS of only 4.2 years; only 9 of 126 patients with IGHV-UM remained in clinical remission beyond 10 years. The difference in PFS translated into a significant survival advantage for patients with IGHV-M.
Our data confirm the importance of achieving MRD-negativity after CIT. In particular, patients with IGHV-M who achieved MRD-negativity had excellent outcomes, with ∼80% remaining in ongoing remission on extended follow-up. The reasons for almost universal relapse in IGHV-UM patients despite achieving MRD-negativity are unclear. This discrepancy may represent a limitation of test sensitivity; ie, patients with IGHV-UM may have achieved a lesser degree of disease debulking; residual disease in apparently MRD-negative patients could therefore potentially have been detected using a more sensitive assay. Posttreatment MRD-negativity was prognostic independent of IWCLL response category.
Two elements of the study methodology warrant discussion: firstly, standard response assessment at the time of the study was based on clinical rather than CT criteria15; although the use of CT scans for response assessment and in surveillance may increase sensitivity for detecting subclinical disease, these advantages are largely negated by our long follow-up period. Secondly, patients in our study were younger (57 vs 61 years), and more likely to have early stage CLL (Binet stage A, 25% vs 5%) than the CLL8 study (supplemental Table 2); these patients were treated according to existing guidelines due to progressive disease and/or symptoms. Although this difference is worth noting, neither the CLL8 study1 nor the study by Rossi et al14 showed differences in PFS according to Binet stage; instead, biological characteristics of the disease, particularly IGHV-MS, del(17p), and del(11q) were key determinants of PFS, corroborating our data.
The strengths of our study are the prospective nature of the study, the uniformity of treatment, and the very long follow-up. Unfortunately, information regarding specific cytogenetic subgroups by FISH22 and of important molecular alterations, such as TP53, NOTCH1, and SF3B1 mutations2,23-26 was not available. These data were provided by the CLL81 and Rossi studies.14 Importantly, both studies showed an apparent PFS plateau in IGHV-M patients without adverse FISH features, and the survival for such patients in the Rossi series was similar to that of the matched normal population. Thus, our study provides prospective, long-term confirmation of the apparent PFS plateaus emerging after FCR therapy reported in the two other cohorts.
Important studies are underway to determine whether ibrutinib plus rituximab is a superior first line therapy to FCR in the United States (#NCT02048813) and the United Kingdom (MRC FLAIR study). Our results, which demonstrate a PFS plateau in patients with IGHV-M, suggest that future investigation of the addition of novel therapies to FCR, to further improve the fraction of patients on this plateau, is also warranted; one such study of ibrutinib added to FCR (#NCT02251548) is currently accruing. Very prolonged follow-up will be required to detect any improvement in PFS, and especially survival, achieved by the addition of novel therapies to FCR. However, we have shown here that MRD-negativity is highly predictive of long-term DFS in IGHV-M CLL after FCR; achievement of MRD-negativity (assessed by an assay with a sensitivity of at least 0.01%) will therefore be a useful primary end point for such studies and serve as a surrogate for PFS and OS.
The existence of PFS plateaus in IGHV-M patients treated with FCR across multiple independent cohorts, as well as persistent MRD-negativity in all tested patients in our series, raises the tantalizing prospect that many of these patients may be cured of their CLL. Further follow-up of the patients on this plateau in the coming years, as well as systematic testing of all patients for MRD, is planned to determine whether this prospect is indeed a reality.
Acknowledgments
This study is in memory of Susan Lerner, who lovingly cared for our CLL database and without whom much of this work would not have been possible.
P.A.T. received funding from the CLL Global Research Foundation and the Haematology Society of Australia and New Zealand.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.A.T. and C.S.T. collected and analyzed data, performed statistical analysis, and wrote the paper; S.M.O., W.G.W., H.M.K., and E.J.F. provided clinical care to patients, developed critical themes, and co-wrote the paper; S.C.S. collected and analyzed data and co-wrote the paper; F.S. performed statistical analysis and co-wrote the paper; W.P. designed the clinical protocol and co-wrote the paper; M.J.K. designed the clinical protocol, provided clinical care to patients, developed critical themes, and co-wrote the paper.
Correspondence: Michael J. Keating, Professor of Medicine, Department of Leukemia, MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: mkeating@mdanderson.org.
References
- 1.Hallek M, Fischer K, Fingerle-Rowson G, et al. International Group of Investigators; German Chronic Lymphocytic Leukaemia Study Group. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 2.Zenz T, Eichhorst B, Busch R, et al. TP53 mutation and survival in chronic lymphocytic leukemia. J Clin Oncol. 2010;28(29):4473–4479. doi: 10.1200/JCO.2009.27.8762. [DOI] [PubMed] [Google Scholar]
- 3.Rossi D, Khiabanian H, Spina V, et al. Clinical impact of small TP53 mutated subclones in chronic lymphocytic leukemia. Blood. 2014;123(14):2139–2147. doi: 10.1182/blood-2013-11-539726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tam CS, O’Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112(4):975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dreger P. Allotransplantation for chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2009;2009(1):602–609. doi: 10.1182/asheducation-2009.1.602. [DOI] [PubMed] [Google Scholar]
- 6.Falchi L, Keating M, Lerner S, et al. Other cancers in long-term survivors of chronic lymphocytic leukemia: incidence and prognostic relevance. Blood. 2014;124(21):3323. [Google Scholar]
- 7.Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27(6):904–910. doi: 10.1200/JCO.2008.17.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheson BD, Vena DA, Barrett J, Freidlin B. Second malignancies as a consequence of nucleoside analog therapy for chronic lymphoid leukemias. J Clin Oncol. 1999;17(8):2454–2460. doi: 10.1200/JCO.1999.17.8.2454. [DOI] [PubMed] [Google Scholar]
- 9.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrd JC, Brown JR, O’Brien S, et al. RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15(1):48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 14.Rossi D, Terzi-di-Bergamo L, De Paoli L, et al. Molecular prediction of durable remission after first-line fludarabine-cyclophosphamide-rituximab in chronic lymphocytic leukemia. Blood. 2015;126(16):1921–1924. doi: 10.1182/blood-2015-05-647925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87(12):4990–4997. [PubMed] [Google Scholar]
- 16.Rawstron AC, Villamor N, Ritgen M, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia. 2007;21(5):956–964. doi: 10.1038/sj.leu.2404584. [DOI] [PubMed] [Google Scholar]
- 17.Jilani I, Keating M, Day A, et al. Simplified sensitive method for the detection of B-cell clonality in lymphoid malignancies. Clin Lab Haematol. 2006;28(5):325–331. doi: 10.1111/j.1365-2257.2006.00813.x. [DOI] [PubMed] [Google Scholar]
- 18.Hallek M, Cheson BD, Catovsky D, et al. International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin KI, Tam CS, Keating MJ, et al. Relevance of the immunoglobulin VH somatic mutation status in patients with chronic lymphocytic leukemia treated with fludarabine, cyclophosphamide, and rituximab (FCR) or related chemoimmunotherapy regimens. Blood. 2009;113(14):3168–3171. doi: 10.1182/blood-2008-10-184853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Admirand JH, Knoblock RJ, Coombes KR, et al. Immunohistochemical detection of ZAP70 in chronic lymphocytic leukemia predicts immunoglobulin heavy chain gene mutation status and time to progression. Mod Pathol. 2010;23(11):1518–1523. doi: 10.1038/modpathol.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castoldi GL, Lanza F, Cuneo A. Cytogenetic aspects of B-cell chronic lymphocytic leukemia: their correlation with clinical stage and different polyclonal mitogens. Cancer Genet Cytogenet. 1987;26(1):75–84. doi: 10.1016/0165-4608(87)90135-x. [DOI] [PubMed] [Google Scholar]
- 22.Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Lawrence MS, Wan Y, et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N Engl J Med. 2011;365(26):2497–2506. doi: 10.1056/NEJMoa1109016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossi D, Cerri M, Deambrogi C, et al. The prognostic value of TP53 mutations in chronic lymphocytic leukemia is independent of Del17p13: implications for overall survival and chemorefractoriness. Clin Cancer Res. 2009;15(3):995–1004. doi: 10.1158/1078-0432.CCR-08-1630. [DOI] [PubMed] [Google Scholar]
- 25.Rossi D, Rasi S, Fabbri G, et al. Mutations of NOTCH1 are an independent predictor of survival in chronic lymphocytic leukemia. Blood. 2012;119(2):521–529. doi: 10.1182/blood-2011-09-379966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zenz T, Kröber A, Scherer K, et al. Monoallelic TP53 inactivation is associated with poor prognosis in chronic lymphocytic leukemia: results from a detailed genetic characterization with long-term follow-up. Blood. 2008;112(8):3322–3329. doi: 10.1182/blood-2008-04-154070. [DOI] [PubMed] [Google Scholar]