Abstract
We propose ‘the moving target hypothesis’ to describe the aetiology of a contemporary coral disease that differs from that of its historical disease state. Hitting the target with coral disease aetiology is a complex pursuit that requires understanding of host and environment, and may lack a single pathogen solution. White pox disease (WPX) affects the Caribbean coral Acropora palmata. Acroporid serratiosis is a form of WPX for which the bacterial pathogen (Serratia marcescens) has been established. We used long-term (1994–2014) photographic monitoring to evaluate historical and contemporary epizootiology and aetiology of WPX affecting A. palmata at eight reefs in the Florida Keys. Ranges of WPX prevalence over time (0–71.4%) were comparable for the duration of the 20-year study. Whole colony mortality and disease severity were high in historical (1994–2004), and low in contemporary (2008–2014), outbreaks of WPX. Acroporid serratiosis was diagnosed for some historical (1999, 2003) and contemporary (2012, 2013) outbreaks, but this form of WPX was not confirmed for all WPX cases. Our results serve as a context for considering aetiology as a moving target for WPX and other coral diseases for which pathogens are established and/or candidate pathogens are identified. Coral aetiology investigations completed to date suggest that changes in pathogen, host and/or environment alter the disease state and complicate diagnosis.
Keywords: coral disease, Acropora palmata, white pox, acroporid serratiosis
1. Introduction
Coral disease diagnostics are changing. Study of coral disease began slowly in the 1970s and 1980s. The early studies were narrow in scope, with reports documenting a disease by describing gross signs and host corals affected [1–4] and in few cases by monitoring affected populations [1] or by classifying a candidate pathogen [4,5]. In the mid-1990s, both the frequency of reports of new coral diseases and efforts to identify causal agents increased [6,7]. There were many early successes. Pathogens were established through fulfillment of Koch's postulates for four coral diseases by the early 2000s [8–12]. Since that time, several candidate pathogens have been described, but few aetiologies have been established (table 1). Today, it is becoming apparent that established pathogens are unlikely to be the sole aetiologic agent responsible for their respective diseases. Our evolving understanding of causal agents may indicate that more than one pathogen causes the same signs on different host colonies at a single point in time or that aetiologies are changing through time. This is similar to the various aetiologies that can cause pneumonia, hepatitis, gastroenteritis or endocarditis in humans [53–56]. To explain temporal changes in the pathogen(s) responsible for disease signs, we propose ‘the moving target hypothesis’ (table 1). ‘The moving target hypothesis’ suggests that the aetiology of a contemporary coral disease differs from that of its historical disease state [25,57,58].
Table 1.
‘The moving target hypothesis' is illustrated by the number of coral diseases for which pathogens have been established (E and bold type) and for which candidate pathogens (C) have been identified. Host is the coral species used for challenge experiments (E) or from which the pathogen was isolated (C). Location and date of each pathogen identification is noted. Diseases are listed in order of date of first pathogen isolation from the reef environment for that disease (bold type). When date of isolation is not included in study methods, date of publication is indicated (*). Unknown (ukn), cyanobacterium (cy), bacterium (ba), fungus (fu), ciliate (ci), virus (vi). For no. of E/C pathogens,+indicates multiple species for a genus (spp.).
| disease | no. of E/C pathogens | E and C pathogen(s) and strain(s) or (accession no.) | type | host(s) | location(s) | date(s) |
|---|---|---|---|---|---|---|
| BBD | 0/7+ | polymicrobial consortium (cy, sulfide-oxidixing ba, sulfate-reducing ba)C | cy, ba |
Colpophyllia natans
Orbicella annularis Montipora capitata |
Florida Hawaii |
1995*[13] 2012 [14] |
| sulfide-oxidizing ba | ||||||
| Beggiatoa sp.C | ba |
Pseudodiploria strigosa
Diploria labyrintheformis Unk Caribbean coral M. capitata |
Bermuda USVI Barbados Hawaii |
1973 [4] 1999–2000 [15] 2012 [14] |
||
| sulfate-reducing ba | ||||||
| Desulfovibrio spp.C | ba |
P. strigosa
D. labyrintheformis Montastraea cavernosa O. annularis Faviid corals |
Bermuda Barbados Curacao Israel |
1973 [4] 2000 [15,16] 2005, 2006 [17] |
||
|
Desulfovibrio sp. strain TBP-1C |
ba |
Pseudodiploria clivosa
Siderastrea siderea |
Florida Dominica |
2000 [18] | ||
| cyanobacteria | ||||||
| Phormidium corallyticumC | cy | ukn Caribbean coral | Belize Bermuda |
1983* [5] | ||
|
Geitlerinema sp. (DQ151461)C (AKA Phormidium corallyticum [15]) |
cy |
M. cavernosa
C. natans P. strigosa S. siderea Porites lutea |
Florida Bahamas Philippines |
1991 [19,20] 2004, 2005 [20] |
||
|
Oscillatoria sp. (AF473936)C |
cy | ukn Caribbean coral M. cavernosa O. annularis P. strigosa Pachyseris speciosa Porites sp. Montipora sp. M. capitata Faviid corals |
USVI Barbados Curacao Palau Hawaii Israel |
1999, 2000 [15] 2000, 2001 [21] 2004 [22] 2005, 2006 [17] 2012 [14] |
||
|
Leptolyngbya sp. strain PCC7375C |
cy |
O. annularis
P. lutea S. siderea |
Florida Philippines USVI |
2004, 2005 [20] | ||
|
Pseudoscillatoria coralii strain BgP10_4SC |
cy |
Favia sp. M. capitata |
Israel Hawaii |
2009* [23] 2012 [14] |
||
| BBL | 2/1 |
Vibrio shiloi strain AK-1E |
ba | Oculina patagonica | Israel | 1995 [11] |
|
Vibrio coralliilyticus strains YB1, YB2, YB3, YB4E strain EM1C |
ba |
Pocillopora damicornis
O. patagonica |
Zanzibar Israel Israel |
1999 [12] 2001 [24] 2011, 2012 [25] |
||
| WP | 1/2 |
Aurantimonas coralicida strain WP1E |
ba | Dichocoenia stokesii | Florida | 1995 [8] |
| small circular single-stranded DNA viruses (SCSDVs)C |
vi | O. annularis | USVI | 2010 [26] | ||
| Philaster lucindaC | ci |
C. natans
O. annularis |
Venezuela Columbia |
2015* [27] | ||
| aspergillosis | 1/0 | Aspergillus sydowii E | fu | Gorgonia ventalina | Bahamas | 1996* [10,28] |
| WBD | 0/5 | Vibrio charchariaeC | ba | Acropora cervicornis | Bahamas Puerto Rico |
1997 [29] 2004 [30] 2014* [31] |
| Rickettsiales-like bacteriumC | ba | A. cervicornis | Florida | 2011 [32] | ||
| Lactobacillus suebicusC | ba | A. cervicornis | not reported | 2014*[31] | ||
| Bacillus sp.C | ba | A. cervicornis | not reported | 2014*[31] | ||
| P. lucindaC | ci | A. cervicornis | Venezuela Columbia |
2014*[31] 2015*[27] |
||
| WPX/acroporid serratiosis | 1/0 |
S. marcescens
strain PDL00E, strain PDR60E |
ba | A. palmata | Florida |
1999 [9] 2003 [33] 2011–2013 [34] |
| WS | 9/12+ | Vibrio sp.E | ba | Montipora aequituberculata | Australia | 2003 [35] |
| Vibrio sp.E | ba | Acropora cytherea | Marshall Islands | 2004 [35] | ||
| three Vibrio spp.E | ba | Pachyseris speciosa | Palau | 2005 [35] | ||
|
Thalassomonos loyonaC strain CBMAI 722T |
ba | Favia favus | Israel | 2005*[36] | ||
| Vibrio harveyiC | ba | Acropora sp. | Indonesia | 2010* [37] | ||
| Porpostoma guamense C | ci | Acropora muricata | Australia | 2012* [38] | ||
| Philaster sp.C | ci | A. muricata | Australia | 2012* [38] | ||
| Arcobacter sp.C | ba | A. muricata | Australia | 2012* [38] | ||
| Aeromonas sp.C | ba | A. muricata | Australia | 2012* [38] | ||
| Vibrio spp.C | ba | A. muricata | Fiji | 2015*[39] | ||
| Reinekea spp.C | ba | A. muricata | Fiji | 2015*[39] | ||
| Verrucomicrobiaceae spp.C | ba | A. muricata | Fiji | 2015*[39] | ||
| P. lucinda C | ci |
A. muricata
Acropora aspera P. damicornis |
Fiji Australia Solomon Islands Maldives |
2015*[27,39] | ||
| Porites ulcerative white spot disease (WS) | Vibrio sp.E | ba | Porites cylindrica | Philippines | 2005–2009 [40] | |
| Porites WS | virus-like particlesC | vi | Porites compressa | Hawaii | 2010 [41] | |
|
Vibrio alginolyticus strain XSBZ03E, strain XSBZ14E |
ba | Porites andrewsi | South China Sea | 2013* [42] | ||
|
Shimia marina NR043300.1C Vibrio hepatarius NR025575.1C |
ba ba |
Porites lutea
P. lutea |
Mayotte Reunion South Africa |
2013* [43] | ||
| Vibrio tubiashi strain P180RE | ba | P. lutea | Mayotte Reunion |
2015* [44] | ||
| P. lucindaC | ci | P. lutea | Mayotte Reunion South Africa |
2015* [27] | ||
| Montipora WS (chronic) |
Vibrio owensii
strain OCN002E |
ba | M. capitata | Hawaii | 2012* [45] | |
| Montipora WS (acute) |
V. coralliilyticus strain OCN008E |
ba | M. capitata | Hawaii | 2013* [46] | |
| BrB | 0/4 | P. guamenseC | ci |
Acropora surculosa
A. muricata Acropora hyacinthus |
Guam Australia |
2005 [47] 2008* [48] 2012 [49] 2015* [27] |
| Philaster sp.C | ci | A. muricata | Australia | 2012* [38] | ||
| Arcobacter sp.C | ba | A. muricata | Australia | 2012* [38] | ||
|
Aeromonas sp.C P. lucindaC |
ba ci |
A. muricata
A. muricata |
Australia Australia |
2012* [38] 2015* [27] |
||
| CYBD | 0/4 | four Vibrio spp.C | ba | Orbicella faveolata | Indonesia | 2008* [50] |
| Porites (white patch) bleaching | 0/1 | virus-like particlesC | vi | Porites australiensis | Australia | 2010 [51] |
| dark spot syndrome | 0/1 | Rhytisma acerinumC | fu | Stephanocoenia intersepta | Venezuela | 2013 [52] |
Today, around 20 coral diseases are described [6,59,60] and 15 pathogens are established for roughly nine diseases, as described by gross signs and host species affected (bacterial bleaching disease (BBL), white plague disease, aspergillosis, acroporid serratiosis and five white syndromes (WS); table 1). Knowledge of coral disease aetiologies is limited due to challenges associated with pathogen identification rather than to a lack of diagnostic effort [29,30,50]. These challenges include (i) different signs used to differentiate diseases may be caused by the same pathogen, (ii) the same signs on the same or multiple species may be caused by more than one pathogen and (iii) an established pathogen may not be found in all cases of the disease.
(a). Different signs, same pathogen
There are limited ways a coral animal exhibits signs of stress: mucus release, tissue discoloration and tissue sloughing. Common names assigned to diseases of corals are derived from these gross signs. White diseases, for example, are named for the gross sign of tissue loss that exposes bright white calcium carbonate coral skeleton. In this respect, white diseases are characterized by the same signs, but the names devised for the white diseases ultimately describe different signs that account for morphology of coral species affected and for geographical range.
In the Caribbean, white diseases are divided into three common names: white band disease (WBD), white pox disease (WPX) and white plague disease (WP). WBD describes signs on just two branching species, Acropora cervicornis and A. palmata. WBD is characterized by a distinct band of tissue loss that progresses across the affected colony from colony base to branch tip or from branch tip to colony base [29]. WPX describes A. palmata colonies with focal to multifocal tissue loss found anywhere on the colony [9]. White signs on non-branching Caribbean corals are called WP. WP signs have been reported for approximately 40 coral species [6,61] and are characterized either by focal tissue loss that begins at the top or side of a coral colony and expands down or across the colony, or by a ring/band of tissue loss that begins at the base of the colony and progresses upward [3,62]. Distinguishing between Caribbean white diseases is complicated by single observations in time that may lump or split these diseases based on tissue loss rate or variation in signs (e.g. WBD type I and type II, WP type I and type II) [6,29,60,62].
Today, white disease signs affecting Indo-Pacific corals fall under a single classification, WS [63,64]. Some cases of WS are further distinguished by the established causal agent, species affected, rate of infection and lesion manifestations [64] (table 1). WS signs have been reported for at least 17 branching and non-branching tropical Pacific species [63,64].
Signs that describe WBD and WP in the Caribbean are similar. The main distinguishing characteristics are that WBD affects branching acroporids and WP affects non-branching corals. Similarly, signs of WS in the Indo-Pacific resemble signs of WBD and WP in the Caribbean [63], and the difference here is limited to geographical range of the hosts. To date, studies of white diseases have lumped the majority of corals with white signs into just two disease conditions: WP in the Caribbean and WS in the Indo-Pacific. It stands to reason that (i) the same disease (WP or WS) on multiple coral species is caused by more than one pathogen or (ii) that different signs attributed to different diseases on the same or multiple species (WBD, WPX, WP or WS) could be caused by the same pathogen.
(b). Same signs, different pathogens
Established pathogens are those for which Koch's postulates have been fulfilled. Multiple pathogens are established for BBL and for WS (table 1). These pathogens are associated with different host species, indicating with a high degree of certainty that the same signs on several different hosts are actually caused by different pathogens. A form of WPX called acroporid serratiosis is diagnosed if and only if classic signs of WPX affecting the A. palmata host co-occur with the presence of the established bacterial pathogen Serratia marcescens [9] (table 1). Other forms of WPX with the same gross signs on the same host species but not associated with S. marcescens also exist [34,65]. Aetiology of the alternative form(s) is unknown. Similarly, WP aetiology has been established for one form of the disease affecting Dichocoenia stokesii, and the identification of candidate pathogens for WP affecting other coral species suggests WP signs are caused by more than one pathogen (table 1).
Candidate pathogens are those that have been found in association with disease signs but have not been established as causal agents. In addition to WP, multiple candidate pathogens have been identified for the same signs for WS, WBD, brown band disease (BrB), black band disease (BBD) and Caribbean yellow band disease (CYBD) (table 1). Candidate pathogens for WS and WBD, for example, each include multiple bacterial species (that differ for each disease) and a ciliate (Philaster lucinda) [27,31,39] (table 1). For both WS and WBD, it is unknown whether each of the candidate bacterial pathogens causes disease independently or as a consortium. Well-controlled pathogen challenge experiments are needed to make this distinction [31,39].
Because it is often difficult to isolate marine pathogens in pure culture (second Koch's postulate), host corals are sometimes challenged with a combination of two or more suspect microorganisms. These microbial cocktails have advanced our understanding of aetiology for WS [42], BBD [66] and CYBD [50], but fall outside the classic cause-and-effect certitude of Koch's postulates studies. Polymicrobial aetiology with multiple pathogens acting synergistically to cause disease signs, as is apparent for BBD [13] and the candidate bacteria-ciliate aetiology in some cases of WS and WBD [31,39], may prove to be common in coral disease diagnostics. Polymicrobial aetiologies may also explain why there has been limited success with establishing disease causation for so many coral diseases.
(c). Established pathogen not found in all cases of a disease
A single pathogen has been established for three coral diseases (acroporid serratiosis, WP and aspergillosis), each in a single host species. A fourth disease, BBL, has been documented in two host species and attributed to a different pathogen in each host (table 1). These five historically established pathogens, identified in the late 1990s and early 2000s, are rare and/or elusive in contemporary diagnostics of their respective diseases. Gross signs associated with these diseases are still observed, but often in the absence of the established causal agent. Aspergillus sydowii first isolated in the mid-1990s, for example, could not be cultured from Gorgonia ventalina with classic signs of aspergillosis in the mid-2000s [67]. Aurantimonas coralicida was identified as a cause of WP in a single coral species (table 1), but has not recently been isolated from WP affected corals [68,69]. Similarly, Vibrio shiloi was shown to cause BBL in Oculina patagonica (table 1), and the mechanisms of infection are well understood for this pathogen, but recent efforts to isolate the bacterium from corals with characteristic signs of this infection have failed [70,71]. Recently, Vibrio coralliilyticus, the established pathogen for BBL affecting Pocillopora damicornis, was identified as a candidate pathogen for BBL affecting O. patagonica [25] (table 1). BBD has been extensively studied since its first description in 1973 and the composition and pathogenicity of the microbial consortium that causes the disease is well characterized [60,66]; however the dominant cyanobacterium and other mat components vary in different disease cases (table 1). Finally, acroporid serratiosis was first diagnosed in 1999 with the isolation of S. marcescens from A. palmata with WPX signs. This form of WPX has been found repeatedly [65] and as recently as 2013 [34], but the absence of S. marcescens from some corals exhibiting WPX signs [34,65,72] suggests that other forms of WPX exist [34,65]. Lack of consistent isolation of established pathogens suggests that the same disease signs can be caused by more than one aetiologic agent and supports the moving target hypothesis that historical and contemporary states of a disease differ.
Coral disease diagnosticians encourage a practice of naming (or renaming) a disease by referencing the established pathogen and/or the affected host rather than using the gross signs alone to supply the disease name [64]. Aspergillosis and acroporid serratiosis are examples of this aetiologic nomenclature. Aspergillosis describes purple multifocal lesions on Gorgonia spp. caused by A. sydowii [10] and acroporid serratiosis describes WPX signs on A. palmata caused by S. marcescens [9]. Contemporary diagnostic efforts are likely to increase the suite of established pathogens that cause the same disease signs on the same or different hosts. Since the mid-2000s, nine pathogens have been established for WS (table 1), indicating a need for specific nomenclature to distinguish the pathogen–host dynamic in each instance [64]. Additional specificity is gained by distinguishing the strain(s) of the pathogen implicated in disease causation and permits distinctions between historical and contemporary cases of a disease (table 1). The strain of the established pathogen for acroporid serratiosis, for example, is different today (S. marcescens strain PDR60) than in the earliest cases of the disease (S. marcescens strain PDL100). This apparent evolution of S. marcescens combined with apparent absence of the pathogen from some A. palmata with WPX signs suggests multiple forms of WPX and aetiology of this disease as a moving target.
Aetiology investigations completed to date (table 1) support the contention that other coral disease aetiologies fit the moving target hypothesis. Here, we address the moving target hypothesis for WPX in the context of a 20-year case study of this disease in the Florida Keys National Marine Sanctuary (FKNMS). We were first to announce [73] and to describe WPX signs affecting A. palmata [9]. We identified the acroporid serratiosis form of WPX and established two strains of S. marcescens as acroporid serratiosis pathogens [9,33]. Since 1994, we have monitored A. palmata populations in the FKNMS for signs of WPX and since 2002, we have concurrently searched a subset of disease lesions and reefs for S. marcescens using culture-based and molecular techniques to confirm acroporid serratiosis [34,65]. This multi-decadal effort highlights the changing disease dynamic of WPX as it transitioned from a severe stressor contributing to whole colony and reef-wide A. palmata mortality approaching 98%, to a recurring condition that causes only partial tissue mortality on affected colonies. This study provides historical and contemporary context for WPX and acroporid serratiosis and highlights the aetiology of WPX as a moving target.
2. Material and methods
To measure WPX prevalence, incidence rate, severity and lethality for A. palmata, we established two kinds of long-term photographic surveys. From 1994 to 2004, we monitored a 13.5 m2 grid consisting of 36 contiguous 0.75 × 0.25 m frames on Eastern Dry Rocks Reef (EDR) (24°27.617′ N; 81° 50.583′ W) [9]. This reef lies southwest of Key West, Florida, within the boundaries of the FKNMS, the Key West National Wildlife Refuge and the EDR Sanctuary Preservation Area (NOAA-SPA). Corners of the grid were demarcated by stainless-steel survey stakes drilled and cemented 1 m into the reef. This georeferenced station was photographed annually in spring (June 1997, May 1998), summer (July 1994, 1995, 1999, 2001–2004, September 1997–1998, October 1996) or winter (December 2000) using an UW Nikonos camera. Slides from the EDR survey were digitally scanned at 600 dpi.
In 2008, we expanded the survey to include seven reefs in the FKNMS and we visited these reefs one to five times per year (table 2). Our visits were grouped according to seasonal changes in water temperature: spring (March–June), summer (July–October) and winter (November–February). During each survey, colonies were photographed digitally with a scale in view. Colonies were relocated by measuring distance and bearing between the coral colony and a single survey stake implanted on each reef. Signs of WPX, WBD, bleaching and predation were recorded in situ.
Table 2.
Reefs and locations included in the contemporary FKNMS-wide survey. Reefs were surveyed in winter (Wi; Nov–Feb), spring (Sp: Mar–June) and summer (Su: July–Oct). Number of times each reef was surveyed per season per year is noted (1, 2 or 3). Disease presence is noted as white pox disease (w) or acroporid serratiosis (ws).
| 2008 |
2009 |
2010 |
2011 |
2012 |
2013 |
2014 |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| reef | location | Wi | Sp | Su | Wi | Sp | Su | Wi | Sp | Su | Wi | Sp | Su | Wi | Sp | Su | Wi | Sp | Su | Wi | Sp | Su |
| Carysfort | 25°13.248′ N, 80°12.594′ W | 1 | 1w | 1w | 1w | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1w | |||||||
| Molasses | 25°00.528′ N, 80°22.590′ W | 1 | 1 | 1w | 1w | 1 | 1 | 1 | 1w | 1 | 1 | 1w | 1 | 1 | 1 | |||||||
| Sombrero | 24°37.518′ N, 81°06.696′ W | 1 | 1w | 1 | 1w | 1 | 1 | 1 | 1ws | 1 | 1 | 1w | 1 | 1 | 1w | |||||||
| Looe Key | 24°32.700′ N, 81°24.400′ W | 1 | 1w | 1w | 3w | 1w | 2w | 1 | 1 | 1 | 1 | 1ws | 1 | 1 | 1ws | 1 | 1 | 1w | ||||
| Western Sambo | 24°28.680′ N, 81°43.026′ W | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| Rock Key | 24°27.270′ N, 81°51.534′ W | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1w | |||||||||
| Palmata Patch | 24°37.243′ N, 82° 52.042′ W | 1 | 1 | 1 | 2 | 2w | 1w | 1w | 1 | 1w | 1w | |||||||||||
Digital images from historical (1994–2004) and contemporary (2008–2014) surveys were analysed using ImageJ software [74]. The whole coral colony and each WPX lesion greater than 1 cm2 in area were traced and numbered. This photographic record allowed us to fate-track the health and survival of 92 A. palmata colonies in the EDR survey and 126 A. palmata colonies at the seven reefs in the FKNMS-wide survey.
From these historical and contemporary surveys, prevalence was calculated as percentage of A. palmata colonies in the population affected with WPX. Incidence rate was calculated for each survey date from the subset of colonies surveyed alive in both the target year and the previous year. From this subset, the incidence rate is given by the number of colonies newly affected with WPX divided by the number of colonies alive and unaffected in the previous survey year. Severity was calculated both as (i) average number of WPX lesions and as (ii) average size of WPX lesions, each per cm2 of living A. palmata tissue. In colonial animals like corals, mortality can be calculated in two ways: either as partial mortality (loss of some living tissue) or as whole mortality (loss of all living tissue). For whole colony mortality, three causes of death could be identified from these surveys (i) DOA (dead skeleton visible; colony died in place), (ii) TKO (colony knocked out of the frame; physically removed) and (iii) FUS (two formerly separate and distinct colonies fuse together). Cause of death was attributed to WPX if and only if the colony was found DOA and showed WPX signs in the previous survey.
Exploratory and formal data analyses were conducted in the R environment [75]. Difference in disease severity of historical and contemporary surveys was established by first logit transforming severity metrics to meet assumptions of normality and then applying analysis of variance to a mixed effects model (lme in package ‘nlme’) [76] with colony identification as a random effect and survey (historical/contemporary) as a fixed effect.
3. Results
(a). Disease severity and coral colony mortality
We have monitored A. palmata populations in the FKNMS for two decades. The decade (1994–2004) of monitoring at EDR provides a historical baseline for comparison to the contemporary (2008–2014) FKNMS-wide survey (figure 1). When monitoring began at EDR in 1994, there were 92 A. palmata colonies within the photostation. This colony number declined to just three colonies by 2000. By the end of the EDR survey in 2004, only one of the original A. palmata colonies remained, representing a 97.8% decline in A. palmata colonies at this reef. WPX affected A. palmata at EDR every year except 2001 and 2003 (figure 1).
Figure 1.
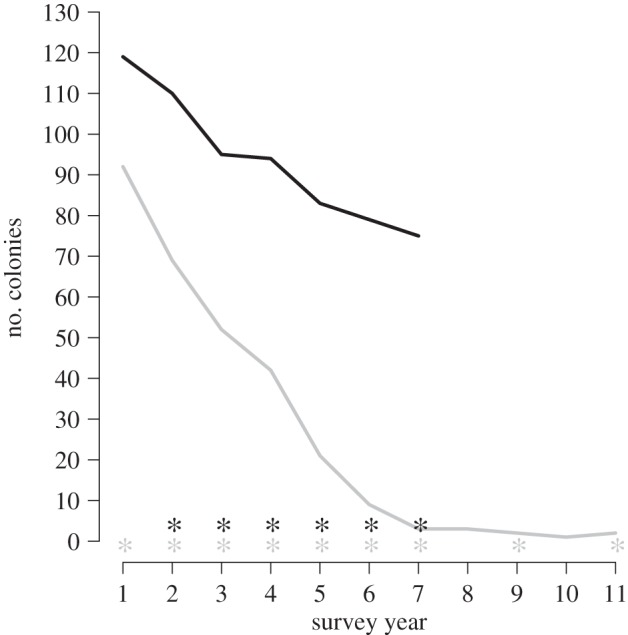
Number of living colonies of A. palmata during each year of the historical survey at EDR (grey) and the contemporary FKNMS-wide survey (black). Surveillance years 1 to 11 are 1994 to 2004 for the historical survey. Surveillance years 1 to 7 are 2008 to 2014 for the contemporary survey. WPX presence is noted (*).
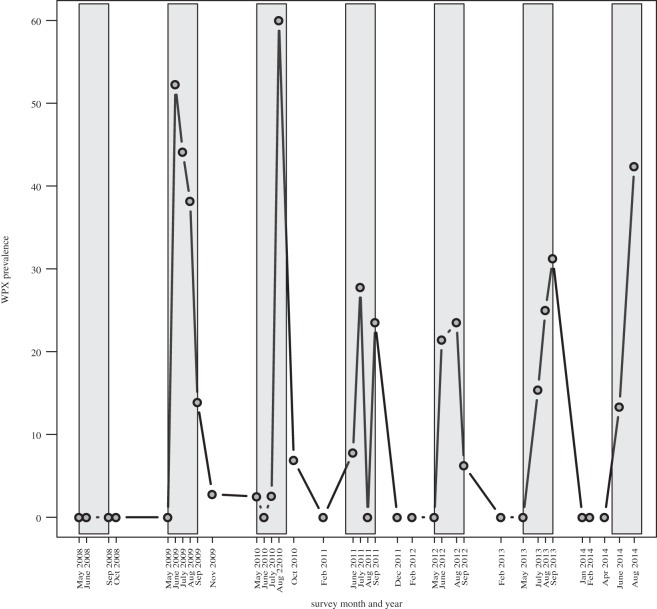
WPX was observed at a minimum of three of the seven monitored reefs in the FKNMS-wide survey every year between 2009 and 2014, and reefs affected varied from year to year (figure 1 and table 2). WPX was most widespread in 2014, affecting five of the seven reefs at that time (table 2). WPX was not observed at any reef in 2008 (figure 2 and table 2). Western Sambo Reef was the only reef where WPX was never observed. The number of A. palmata colonies in the FKNMS-wide survey declined by 44.4% from 126 colonies in 2008 to 70 in 2014 (figure 1).
Figure 2.
Percentage of A. palmata colonies with WPX (prevalence) by month and year for the contemporary FKNMS-wide survey. Vertical grey bars indicate spring and summer.
During the historical survey at EDR, 91 A. palmata colonies were lost. The majority of these losses were whole colony mortality (DOA) attributed to WPX (66 colonies). The remainder was DOA, attributed to factors other than disease (15 colonies) and TKO (10 colonies). In sharp contrast, partial not whole colony mortality, was observed throughout the duration of the contemporary FKNMS-wide survey. The 56 A. palmata colony reduction that occurred between 2008 and 2014 was due to DOA (14 colonies), TKO (40 colonies) and FUS (two colonies). Of the DOA colonies, cause of death was attributed to WPX for only one colony.
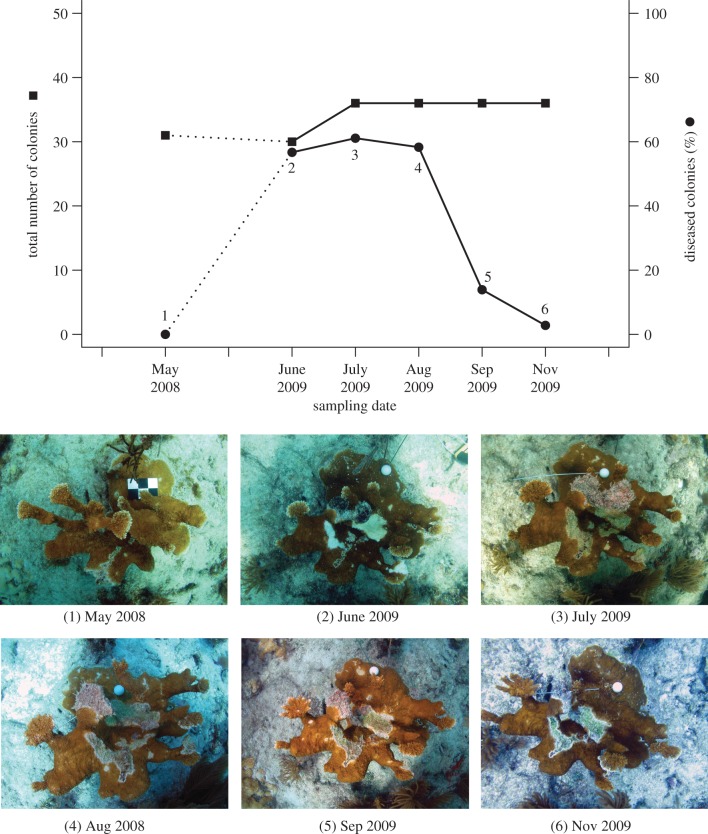
Partial colony mortality observed FKNMS-wide is illustrated with monthly surveys at Looe Key Reef (LK) in summer 2009 (figure 3a). We followed progression of the outbreak at LK with monthly surveys in June, July, August, September and November 2009 (table 2). No whole colony mortality (DOA) due to WPX occurred at LK during the 2009 outbreak. WPX caused only partial mortality, and tissue that was lost during this outbreak was beginning to regrow over affected areas of A. palmata colonies by November 2009 (figure 3b).
Figure 3.
Monthly survey of A. palmata at LK, Florida, in summer 2009. (a) Total number of A. palmata colonies (left axis) and percentage of A. palmata colonies with WPX (prevalence) in May 2008 and June, July, August, September and November 2009 (right axis). (b) Onset and progression of WPX in a single A. palmata colony for all six dates plotted in (a). WPX is present June to September 2009. (Online version in colour.)
WPX severity, calculated as average size and as average number of WPX lesions per cm2 of living coral tissue, was significantly greater for the historical survey at EDR than for the contemporary FKNMS-wide survey (figure 4; ANOVA applied to a fitted mixed effect model using each severity metric, p < 0.0001). Average number of lesions per projected cm2 of living coral tissue was 0.0336 per cm2 and 0.0010 per cm2, in historical and contemporary surveys, respectively. The projected surface area of A. palmata affected by lesions averaged 8.9% in the historical survey and just 1.1% in the contemporary survey. Though decline in projected surface area of living A. palmata tissue was recorded in 2010 and 2012, growth exceeded tissue loss in 2011, 2013 and 2014. Overall change in A. palmata surface area favoured growth. Projected surface area of live A. palmata increased from 117 276 cm2 in 2009 to 136 295 cm2 in 2014.
Figure 4.
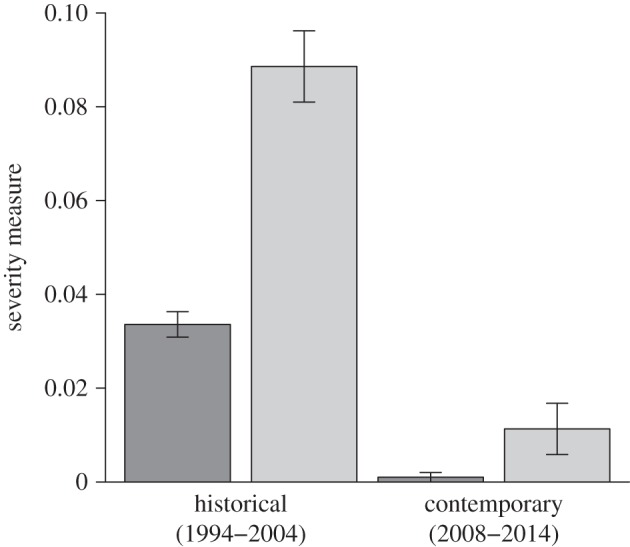
WPX severity during the historical survey at EDR and the contemporary FKNMS-wide survey. Severity is presented both as average number (dark grey) and as average size (light grey) of WPX lesions per projected cm2 of living A. palmata tissue. Bars indicate standard error.
(b). Disease prevalence and incidence rate
WPX prevalence at EDR ranged from 0% to 71.4%. Prevalence was 30.4% (n = 92 A. palmata colonies in the population) in 1994 and increased thereafter as colony number declined to 50.7% (n = 69, 1995), 57.7% (n = 52, 1996), 60.9% (n = 46, June 1997) and 64.3% (n = 42, September 1997). In May and September 1998, prevalence was 62.1% (n = 29) and 71.4% (n = 21), respectively. Incidence rate at EDR was 25.0% (n = 64 colonies alive and unaffected in the previous survey year) in 1995, 17.6% (n = 34) in 1996, 27.3% (n = 22) in June 1997, 38.9% (n = 18) in September 1997, 13.3% (n = 15) in May 1998 and 36.4% (n = 11) September 1998. The number of A. palmata colonies at EDR declined to nine by 1999 and to three by 2000 (figure 1), and WPX prevalence in these years was 44.4% and 33.3%, respectively. Incidence rate was 0% in 1999 (n = 6), 2000 (n = 5) and 2001 (n = 2). In both 2002 and 2004 when only two colonies occupied the EDR photostation, WPX prevalence was 50%. Incidence rate was 33.3% (n = 3) in 2002 and 100% (n = 1) in 2004. No WPX signs were observed in 2001 (n = 3 colonies in the population) and 2003 (n = 1) (figure 1). The one A. palmata colony added to EDR between 2003 and 2004 was not a recruit, but a colony outside the photostation that had grown into the frame.
WPX prevalence in the FKNMS-wide survey ranged from 0% to 60.0%. No WPX signs were observed in 2008 (n = 126 colonies in the population). Annual prevalence/incidence rate was 37.6% (n = 117 colonies in the population)/22.4% (n = 58 colonies alive and unaffected in the previous survey year) in 2009, 10.6% (n = 94)/0% (n = 33) in 2010, 21.8% (n = 87)/25.6% (n = 39) in 2011, 23.2% (n = 82)/14.3% (n = 21) in 2012, 19.5% (n = 77)/8.3% (n = 12) in 2013 and 38.6% (n = 70)/25.0% (n = 20) in 2014. Peak prevalence in each year of the FKNMS-wide survey occurred in warmer months (figure 2). In 2009, this peak occurred in spring (June) with 52.3% of A. palmata colonies (n = 117) affected. From 2010 to 2014, peak prevalence occurred in summer: August 2010 (60.0% of A. palmata affected, (n = 94), July 2011 (27.8%, n = 87), August 2012 (23.5%, n = 82), September 2013 (31.2%, n = 77) and August 2014 (42.4%, n = 70). Prevalence returned to 0% of colonies affected during winter in 2011 (February, December) and during winter and spring in 2012, 2013 (February and May) and 2014 (January, February, April). No WPX was observed in August 2011, but signs were present in July and September of that year (figure 2).
Monthly surveys at LK in summer 2009 demonstrate progression of WPX (figure 3a). We first observed WPX affecting 56.7% of the A. palmata colonies (n = 30) at this reef during our June 2009 routine survey. Large active WPX lesions affected the colonies in June, and by July and August the disease progressed from lesion margins (figure 3b). WPX prevalence during this outbreak peaked in July, with 61.1% of A. palmata colonies (n = 36) affected. By November 2009, just 2.8% of colonies (n = 36) exhibited WPX.
WPX was observed at Palmata Patch Reef in the Dry Tortugas National Park (DTNP) annually between 2011 and 2014 (table 2). The 2011 DTNP outbreak was documented in July (27.7% of A. palmata colonies affected, n = 18) and preceded and then coincided with a bleaching event in September. In September, 100% of A. palmata colonies at Palmata Patch Reef (n = 18) were bleached and WPX lesions could not be distinguished or quantified on bleached colonies. By December 2011, no WPX was observed at Palmata Patch and all A. palmata colonies had recovered their pigmentation.
4. Discussion
Host(s), environment(s) and pathogen(s) all affect aetiology [77]. Our historical and contemporary surveys of A. palmata populations in the FKNMS exemplify the changing disease state of WPX and support coral disease aetiology as a moving target. WPX is a then (1994–2004) and a now (2008–2014) disease in terms of severity, aetiology and contribution to whole colony mortality. The range of WPX prevalence values through time was comparable for historical and contemporary A. palmata populations.
(a). Disease severity and coral colony mortality
Unlike historical (1994–2004) WPX outbreaks, which were more severe and were coincident with high whole colony mortality, less severe contemporary (2008–2014) outbreaks caused, with the exception of one whole colony death, only partial colony mortality (figure 4). The number of colonies at EDR declined from 92 in 1994 to two in 2004 and was coincident with WPX signs in nine years of this decade-long survey (figure 1). A large majority (96.7%) of losses (89 colonies) occurred within the first seven years (1994–2000) of the survey and represent a 97% decline in percentage living cover of A. palmata at this reef during the same time period [78]. The seven-year contemporary FKNMS-wide survey showed lower (44.4%) whole colony mortality (from 126 to 70 colonies) than the EDR survey. Though WPX was detected every year from 2009 to 2014, partial but not whole colony mortality of WPX-affected A. palmata was most often observed (figures 1 and 4). Deaths of just 14 colonies (11.1%) were DOA and only one of these colony losses (1.8%) was attributed to WPX. The historical survey was based on once-per-year monitoring (except in 1997 and 1998), making confirmation of cause of death difficult, but our method of attributing mortality to WPX when disease signs were present in the previous annual survey attributed 87.9% of DOA to WPX. Consistent presence of WPX coincident with a 97.8% colony loss at EDR supports high whole colony mortality caused by WPX; however, stressors other than annual presence of WPX signs including elevated seawater temperature, predation and storms [79–82] may also have contributed to the historical loss of the A. palmata population at EDR.
Williams & Miller [79] surveyed A. palmata in the upper Florida Keys between 2004 and 2010. Their study overlaps our contemporary survey both spatially (Carysfort Reef and Molasses Reef) and temporally (2008–2010). They quantified 53% whole colony mortality (n = 210 colonies) with cause of death divided into the equivalent of DOA (32%) and TKO (21%). Living A. palmata cover declined by approximately 50% over the duration of their seven-year survey, primarily due to partial not whole colony mortality. This partial mortality was caused by disease, including WPX, WBD and rapid tissue loss disease (30%), predation by snails (Coralliophila abbreviata) (29%), and hurricane damage (24%) [79]. Williams & Miller [79] noted that WBD was rare and WPX was more common, a finding that is frequently reported for contemporary A. palmata populations [80,81,83–85]. WBD was never observed affecting A. palmata colonies tracked in our historical and contemporary surveys.
Acropora palmata populations today are affected more by WPX than by WBD. Though unrecognized, WPX may have played a primary role in historical losses of this species. This examination of our historical data for EDR revealed that WPX signs were first apparent at this reef as early as 1994 rather than in 1996, as previously reported [9], making 1994 the earliest confirmed case of WPX in the Florida Keys. It is suspected that WPX emerged prior to the mid-1990s and probable cases were documented as early as 1970 [86]. Though WBD signs are well described for the first report of WBD in 1977 [1], it is possible that other historical reports of WBD on A. palmata actually describe WPX [86]. The similar signs and same host for WPX and WBD, combined with limited quantitative data linking A. palmata decline to WBD, complicate our understanding of the role of each of these diseases in Caribbean-wide collapse of A. palmata populations [9,85,87]. The role of WPX in A. palmata decline is well documented [9,79,80,88], while the widely reported implication that WBD drove the historical loss of this species is largely anecdotal. The limited epizootiological data for WBD highlights the necessity of diagnostic plans to fully assess modern marine disease outbreaks [77].
Regrowth of living tissue over WPX lesions was observed in our 2009 monthly summer survey at LK (figure 3b). Cessation of disease signs followed by regrowth of tissue over the lesion [9,80] is a positive indicator of the ability of an A. palmata host to survive and recover from the partial mortality caused by contemporary WPX (figure 3a). Though tissue regeneration was occasionally evident in historical outbreaks of WPX at our EDR site and at sites elsewhere in the Caribbean, the rapid rate of disease lesion progression [9,88] and disease severity (figure 4) in these early outbreaks effectively prevented recovery for most colonies.
Whole colony mortality following historical WPX outbreaks may have removed the most susceptible host individuals from affected populations. Under this scenario, contemporary host colonies would be expected to be more resistant to WPX. WPX is consistently present (figure 1) and WPX prevalence is high (figures 2 and 3) throughout the 20-year study. While historical and contemporary A. palmata populations were equally susceptible to WPX, contemporary infections were less severe (figure 4) and infrequently resulted in whole colony mortality [79,81,85]. The contemporary survey documented only one colony death caused by WPX. In addition, partial tissue loss was regained entirely by colony growth by the end of the survey. We measured a 16.2% increase in projected living surface area of A. palmata between 2009 and 2014. Similarly, a contemporary (2004–2010) survey of A. palmata in the US Virgin Islands (USVI), showed that colonies increased in live tissue area even when affected by WPX, bleaching and hurricane damage [84]. Though tissue growth exceeded tissue loss in our contemporary survey, the projected surface area of live A. palmata measured in the FKNMS in 2014 (136 295.4 cm2) was low compared with historical values [89] and is not evidence of regional population recovery.
Acropora palmata colony survival following contemporary WPX outbreaks is likely aided by regeneration of tissue over areas denuded by infection; however, tissue regrowth is often incomplete, leaving calcium carbonate skeleton exposed and reducing percentage live cover of A. palmata. Tissue lost from a coral colony will affect sexual reproduction and net-growth [90], increase reef erosion and decrease reef calcification [91,92]. Whole and partial colony mortality of A. palmata and A. cervicornis quantified since the 1970s [9,85,87] has altered the three-dimensional structure and complexity of Caribbean coral reefs [91,92]. Partial tissue loss may increase susceptibility of A. palmata colonies to hurricane breakage, predators, bleaching and disease [78,79,90,93]. Thus the annual occurrence of WPX and the associated partial mortality recorded in our study and by others [79,81,85] threatens the recovery of A. palmata populations in the FKNMS.
(b). Disease prevalence and incidence rate
During historical EDR and contemporary FKNMS-wide surveys, WPX prevalence ranged from 0% to approximately 70%. This prevalence is comparable to that reported for the USVI (0–53%) between 2004 and 2010 [84]. WPX prevalence showed a pronounced seasonal pattern, increasing during warmer late spring and summer months and declining in winter and early spring periods. Disease prevalence and incidence rate indicated high persistence or recurrence of disease signs in the same A. palmata colonies throughout the historical survey at EDR and in 2010, 2012 and 2013 in the FKNMS-wide contemporary survey, where change in prevalence in those years was slight and amount of new infections were low. In 2009, 2011 and 2014 disease prevalence and incidence rates showed that many colonies unaffected by disease in the previous survey year developed disease signs. Peak prevalence in each year of the FKNMS-wide survey ranged from 23.5% to 60.0% of colonies affected (figure 2). WPX prevalence is documented to increase seasonally [79,88], including at EDR [9], and in association with bleaching [80]. During our historical survey at EDR, simultaneous signs of WPX and bleaching occurred in September 1997 and September 1998. The only A. palmata bleaching observed during our FKNMS-wide survey occurred in summer 2011 at Rock Key Reef, Molasses Reef (August) and Palmata Patch Reef (September). The 1997, 1998 and 2011 A. palmata bleaching events may have contributed to the whole colony mortality and partial colony mortality quantified, respectively, in our historical and contemporary surveys.
Palmata Patch Reef in DTNP is located 112.5 km (70 miles) from any significant anthropogenic sources of wastewater pollution. A contemporary established acroporid serratiosis pathogen, S. marcescens strain PDR60 (table 1) is linked to a wastewater source [33] and was cultured from wastewater in the Florida Keys as recently as 2013 [34]. For this reason, we hypothesized that WPX signs at Palmata Patch would be less prevalent than at reefs closer to the more populated Florida Keys. This hypothesis was supported from 2008 to 2010. However, beginning in 2011, we documented A. palmata with WPX signs in the DTNP annually in spring (June 2012, 2014) and/or summer (July 2011, September 2012, 2013) (table 2). WPX prevalence was greatest at this reef in September 2013 with 31.3% of colonies affected (n = 16). Palmata Patch was the only reef where A. palmata simultaneously exhibited signs of bleaching and WPX. While bleaching of A. palmata was not widespread during our surveys, WPX was common and most prevalent during warmer months. These data suggest that either the seasonal pattern of WPX prevalence is unrelated to temperature stress or that temperature stress was sufficient to promote WPX but insufficient to produce signs of bleaching on A. palmata host colonies.
(c). Disease aetiology
An established WPX pathogen, S. marcescens, was cultured from lesions during historical outbreaks in 1999 and 2003, confirming acroporid serratiosis for these cases of WPX [9,33] (table 1). Aetiological investigations for WPX began mid-way through the historical survey (1999) and were not systematic until 2011. Between 2011 and 2013, culture and quantitative real-time PCR (qPCR) techniques were employed to detect S. marcescens in the surface mucus layer (SML) of corals with and without WPX signs at six of the reefs within our FKNMS-wide survey [34]. Joyner et al. [34] collected SML from WPX-affected A. palmata for qPCR assays at Carysfort Reef (2011), Sombrero Reef (2011, 2012, 2013) and LK (2012, 2013), and for culture at Molasses Reef (2011, 2012, 2013) and LK (2012, 2013). Acroporid serratiosis was confirmed with qPCR at Sombrero in 2012 and at LK in 2012 and 2013 (table 2) [34]. Serratia marcescens was not detected from WPX lesions with culture methods. Culture did, however, detect the established pathogen S. marcescens strain PDR60 (table 1) in SML of apparently healthy non-host corals Porites porites and Porites astreoides at LK in 2012 [34].
WPX aetiology is a moving target for which we have hit the mark once with establishment of S. marcescens as one pathogen, but evidence suggests other pathogens or conditions also contribute to WPX signs. This initial success has much to do with the culturability of S. marcescens. Efforts to isolate pathogens for other coral diseases are likely hindered by the presence of unculturable pathogens, making not only isolation, but ultimately establishment with Koch's postulates, impossible. Ongoing efforts to detect S. marcescens (with culture and/or molecular methods) have been successful for (i) WPX lesions in some cases of the disease including outbreaks in 1999, 2003, 2012 and 2013, (ii) non-host corals in 2002 (Siderastrea siderea), 2003 (Solenastrea bournoni) and 2012 (P. astreoides, P. porites), (iii) the corallivorous snail C. abbreviata in 2003 and 2006, and (iv) seawater in 2003, 2012 and 2013 [9,34,65]. Presence of WPX signs during all years from 2009 to 2014 of the FKNMS-wide survey and the lack of consistent confirmation of acroporid serratiosis support the hypothesis that WPX signs are caused by more than one pathogen. WPX may have always been caused by a variety of pathogens (including S. marcescens) on different host colonies, or WPX may be in transition from acroporid serratiosis to a disease with the same signs, but different aetiology.
Aetiological investigations of BBL-affected Oculina patogonica suggest a pathogen transition from V. shiloi to V. coralliilyticus [25] (table 1). Vibrio coralliilyticus is also the only pathogen established for more than one disease: BBL in the Red Sea and acute Montipora WS in Hawaii (table 1). The same candidate pathogens are associated with both BrB and WS affecting Acropora muricata on the Great Barrier Reef (GBR), Australia [38]. These candidate pathogens include two ciliates (Porpostoma guamense and P. lucinda) and two bacteria (Arcobacter sp. and Aeromonas sp.) [27,38]. Philaster lucinda has also been linked to the aetiologies of WS in the Indo-Pacific and WBD and WP in the Caribbean, providing the first diagnostic links between these formerly geographically distinct diseases (table 1) [27,31,39]. Ciliates are candidate secondary pathogens that consume coral tissue as it is sloughed from the coral skeleton, likely following primary infection by one or more bacteria or viruses [27,31,39]. It is this tissue consumption that is chiefly responsible for generating the distinct line of tissue loss that characterizes WS, WBD, WP and BrB [31,38,39]. Identification of candidate ciliate pathogens (P. guamense for WS and BrB and P. lucinda for WS, WBD and WP) supports the different signs, same pathogen hypothesis and recommends an investigation of the role of ciliates in the aetiology of WPX [94].
5. Conclusion
This 20-year case study investigating A. palmata with WPX signs is the story of a changing disease and exemplifies coral disease aetiology as a moving target. Historical WPX was a severe stressor that contributed to 97.8% whole colony mortality. Contemporary WPX is a less severe recurring disease that causes only partial colony mortality. WPX is an example of an early success in the field of coral disease diagnostics. Gross signs are well described and affected host populations have been monitored extensively since the mid-1990s [9,80,81,85,88,95]. A pathogen was established for the acroporid serratiosis form of this disease by the early 2000s [9] and today this form is diagnosed for some, but not all, cases of WPX-affected A. palmata [34]. WPX signs resemble those of WBD and WP in the Caribbean and WS in the Indo-Pacific. The P. lucinda ciliate is a candidate pathogen for WBD, WP and WS [27,31,39], suggesting that this or another species of ciliate may contribute to WPX aetiology in the presence or absence of bacterial pathogen(s) such as S. marcescens.
Aetiology is advanced, but not resolved, by establishing a pathogen for a single host. As host genotypes and environments change, so may the pathogen(s) that cause disease (table 1). Disease-associated coral mortality is attributed to a combination of stressors to the host including temperature-induced changes in environment [80,81,96], injury caused by predators and humans [49,97,98], and run-off of sediment, nutrients and sewage into near shore waters [98–100]. Pathogens may be delivered to reefs via these stressors [10,33].
Coral disease diagnostics mirrors medical research. Fever in humans and tissue loss in corals are aetiology-independent signs of infection. Diagnoses of aetiologies are complicated when diseases share the same signs, manifested by different pathogens [54,101] (table 1). Culture methods are often unreliable and fail to detect the established pathogen even when its presence is confirmed with molecular methods [34,102]. Due to the universal nature of clinical disease signs, medical professionals caution patients against self-diagnosis with symptom checker websites [103]. Likewise, accurate diagnosis of coral disease aetiology requires well-informed analyses of not only gross disease signs and host coral affected, but long-term monitoring of affected host populations that includes assessment of environmental parameters including temperature [104] and water quality [105].
Early successes that established single pathogens for gross coral disease signs in a single host species (table 1) have advanced the field and informed our current understanding of the intricacies of coral disease aetiology. Given that losses of living coral cover in the Caribbean and on the GBR exceed 50% since the 1970s [106,107] and satisfaction of Koch's postulates may establish one of many pathogens that cause the same disease signs, an accurate diagnosis might have limited explanatory power. We propose that rather than chase multiple pathogens, efforts and resources to conserve coral reefs be allocated to understanding host susceptibility under different environment scenarios [108].
Supplementary Material
Acknowledgements
Fieldwork was conducted at the Mote Tropical Research Laboratory on Summerland Key and at the Keys Marine Lab on Key Largo.
Ethics
Research was conducted under permits FKNMS-2010-131-A1, DRTO-2011-SCI-0015 and DRTO-2012-SCI-0014.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
K.S. designed study, collected field data, completed data analysis, drafted manuscript; B.B. completed image and data analysis, helped draft manuscript; A.P. designed study, coordinated image and data analysis, helped draft manuscript; D.K. designed study, collected field data, helped draft manuscript; K.K. collected field data, helped draft manuscript; E.L. designed study, helped draft manuscript; J.P. designed study, collected field data, coordinated image and data analysis, helped draft manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
Funding provided by NSF-NIH Ecology of Infectious Disease program grant nos. EF1015032 (to K.S.) and EF1015342 (to E.L., J.P., A.P.) and by a Rollins College Critchfield Research Grant (to K.S.).
References
- 1.Gladfelter WB. 1982. White-band disease in Acropora palmata: implications for the structure and growth of shallow reefs. Bull. Mar. Sci. 32, 639–643. [Google Scholar]
- 2.Antonius A. 1985. Coral diseases in the Indo-Pacific: a first record. Mar. Ecol. 6, 197–218. ( 10.1111/j.1439-0485.1985.tb00322.x) [DOI] [Google Scholar]
- 3.Dustan P. 1977. Vitality of reef coral populations off Key Largo, Florida: recruitment and mortality. Environ. Geol. 2, 51–58. ( 10.1007/BF02430665) [DOI] [Google Scholar]
- 4.Garrett P, Ducklow H. 1975. Coral diseases in Bermuda. Nature 253, 349–350. ( 10.1038/253349a0) [DOI] [Google Scholar]
- 5.Rutzler K, Santavy DL. 1983. The black band disease of Atlantic reef corals. I. Description of a cyanophyte pathogen. Mar. Ecol. 4, 301–319. ( 10.1111/j.1439-0485.1983.tb00116.x) [DOI] [Google Scholar]
- 6.Sutherland KP, Porter JW, Torres C. 2004. Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar. Ecol. Prog. Ser. 266, 273–302. ( 10.3354/meps266273) [DOI] [Google Scholar]
- 7.Ward JR, Lafferty KD. 2004. The elusive baseline of marine disease: are diseases in ocean ecosystems increasing? PLoS Biol. 2, 542–547. ( 10.1371/journal.pbio.0020120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denner EBM, Smith GW, Busse HJ, Schumann P, Narzt T, Polson SW, Lubitz W, Richardson LL. 2003. Aurantimonas coralicida gen. nov., sp. nov., the causative agent of white plague type II on Caribbean scleractinian corals. Int. J. Syst. Evol. Microbiol. 53, 1115–1122. ( 10.1099/ijs.0.02359-0) [DOI] [PubMed] [Google Scholar]
- 9.Patterson KL, Porter JW, Ritchie KB, Polson SW, Mueller EM, Peters EC, Santavy DL, Smith GW. 2002. The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc. Natl Acad. Sci. USA 99, 8725–8730. ( 10.1073/pnas.092260099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith GW, Ives LD, Nagelkerken IA, Ritchie KB. 1996. Caribbean sea-fan mortalities. Nature 383, 487 ( 10.1038/383487a0) [DOI] [Google Scholar]
- 11.Kushmaro A, Banin E, Loya Y, Stackebrandt E, Rosenberg E. 2001. Vibrio shiloi sp. nov., the causative agent of bleaching of the coral Oculina patagonica. Int. J. Syst. Evol. Microbiol. 51, 1383–1388. ( 10.1099/00207713-51-4-1383) [DOI] [PubMed] [Google Scholar]
- 12.Ben-Haim Y, Thompson FL, Thompson CC, Cnockaert MC, Hoste B, Swings J, Rosenberg E. 2003. Vibrio coralliilyticus sp. nov., a temperature-dependent pathogen of the coral Pocillopora damicornis. Int. J. Syst. Evol. Microbiol. 53, 309–315. ( 10.1099/ijs.0.02402-0) [DOI] [PubMed] [Google Scholar]
- 13.Carlton RG, Richardson LL. 1995. Oxygen and sulfide dynamics in a horizontally migrating cyanobacterial mat: black band disease of corals. FEMS Microbiol. Ecol. 18, 155–162. ( 10.1111/j.1574-6941.1995.tb00173.x) [DOI] [Google Scholar]
- 14.Aeby GS, Work TM, Runyon CM, Shore-Maggio A, Ushijima B, Videau P, Beurmann S, Callahan SM. 2015. First record of black band disease in the Hawaiian archipelago: response, outbreak status, virulence, and a method of treatment. PLoS ONE 10, e0120853 ( 10.1371/journal.pone.0120853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooney RP, Pantos O, Le Tissier MDA, Barer MR, O'Donnell AG, Bythell JC. 2002. Characterization of the bacterial consortium associated with black band disease in coral using molecular microbiological techniques. Environ. Microbiol. 4, 401–413. ( 10.1046/j.1462-2920.2002.00308.x) [DOI] [PubMed] [Google Scholar]
- 16.Frias-Lopez J, Zerkle AL, Bonheyo GT, Fouke BW. 2002. Partitioning of bacterial communities between seawater and healthy, black band diseased, and dead coral surfaces. Appl. Environ. Microbiol. 68, 2214–2228. ( 10.1128/AEM.68.5.2214-2228.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barneah O, Ben-Dov E, Kramarsky-Winter E, Kushmaro A. 2007. Characterization of black band disease in Red Sea stony corals. Environ. Microbiol. 9, 1995–2006. ( 10.1111/j.1462-2920.2007.01315.x) [DOI] [PubMed] [Google Scholar]
- 18.Viehman S, Mills DK, Meichel GW, Richardson LL. 2006. Culture and identification of Desulfovibrio spp. from corals infected by black band disease on Dominican and Florida Keys reefs. Dis. Aquat. Organ. 69, 119–127. ( 10.3354/dao069119) [DOI] [PubMed] [Google Scholar]
- 19.Richardson LL, Kuta KG. 2003. Ecological physiology of the black band disease cyanobacterium Phormidium corallyticum. FEMS Microbiol. Ecol. 43, 287–298. ( 10.1016/S0168-6496(03)00025-4) [DOI] [PubMed] [Google Scholar]
- 20.Myers JL, Sekar R, Richardson LL. 2007. Molecular detection and ecological significance of the cyanobacterial genera Geitlerinema and Leptolyngbya in black band disease of corals. Appl. Environ. Microbiol. 73, 5173–5182. ( 10.1128/AEM.00900-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frias-Lopez J, Bonheyo GT, Jin Q, Fouke BW. 2003. Cyanobacteria associated with coral black band disease in Caribbean and Indo-Pacific reefs. Appl. Environ. Microbiol. 69, 2409–2413. ( 10.1128/AEM.69.4.2409-2413.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sussman M, Bourne DG, Willis BL. 2006. A single cyanobacterial ribotype is associated with both red and black bands on diseased corals from Palau. Dis. Aquat. Organ. 69, 111–118. ( 10.3354/dao069111) [DOI] [PubMed] [Google Scholar]
- 23.Rasoulouniriana D, Siboni N, Eitan BD, Esti KW, Loya Y, Kushmaro A. 2009. Pseudoscillatoria coralii gen. nov., sp. nov., a cyanobacterium associated with coral black band disease (BBD). Dis. Aquat. Organ. 87, 91–96. ( 10.3354/dao02089) [DOI] [PubMed] [Google Scholar]
- 24.Ben-Haim Y, Zicherman-Keren M, Rosenberg E. 2003. Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. Appl. Environ. Microbiol. 69, 4236–4241. ( 10.1128/AEM.69.7.4236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills E, Shechtman K, Loya Y, Rosenberg E. 2013. Bacteria appear to play important roles in both causing and preventing the bleaching of the coral Oculina patagonica. Mar. Ecol. Prog. Ser. 489, 155–162. ( 10.3354/meps10391) [DOI] [Google Scholar]
- 26.Soffer N, Brandt ME, Correa AMS, Smith TB, Thurber RV. 2014. Potential role of viruses in white plague coral disease. ISME J. 8, 271–283. ( 10.1038/ismej.2013.137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sweet MJ, Séré MG. In press. Ciliate communities consistently associated with coral diseases. J. Sea Res. ( 10.1016/j.seares.2015.06.008) [DOI] [Google Scholar]
- 28.Geiser DM, Taylor JW, Ritchie KB, Smith GW. 1998. Cause of sea fan death in the West Indies. Nature. 394, 137–138. ( 10.1038/28079)9671296 [DOI] [Google Scholar]
- 29.Ritchie KB, Smith GW. 1998. Type II white-band disease. Rev. Biol. Trop. 46, 199–203. [Google Scholar]
- 30.Gil-Agudelo DL, Smith GW, Weil E. 2006. The white band disease type II pathogen in Puerto Rico. Rev. Biol. Trop. 54, 59–67.18457175 [Google Scholar]
- 31.Sweet MJ, Croquer A, Bythell JC. 2014. Experimental antibiotic treatment identifies potential pathogens of white band disease in the endangered Caribbean coral Acropora cervicornis. Proc. R. Soc. B 281, 20140094 ( 10.1098/rspb.2014.0094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller MW, Lohr KE, Cameron CM, Williams DE, Peters EC. 2014. Disease dynamics and potential mitigation among restored and wild staghorn coral, Acropora cervicornis. PeerJ 2, e541 ( 10.7717/peerj.541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutherland KP, Shaban S, Joyner JL, Porter JW, Lipp EK. 2011. Human pathogen shown to cause disease in the threatened elkhorn coral Acropora palmata. PLoS ONE 6, e23468 ( 10.1371/journal.pone.0023468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joyner JL, Sutherland KP, Kemp D, Berry B, Griffin A, Porter J, Amador MHB, Noren HKG, Lipp EK. 2015. Systematic analysis of white pox disease in Acropora palmata of the Florida Keys and the role of Serratia marcescens. Appl. Environ. Microbiol. 81, 4451–4457. ( 10.1128/AEM.00116-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sussman M, Willis BL, Victor S, Bourne DG. 2008. Coral pathogens identified for white syndrome (WS) epizootics in the Indo-Pacific. PLoS ONE 3, e2393 ( 10.1371/journal.pone.0002393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson FL, Barash Y, Sawabe T, Sharon G, Swings J, Rosenberg E. 2006. Thalassomonas loyana sp. nov., a causative agent of the white plague-like disease of corals on the Eilat coral reef. Int. J. Syst. Evol. Microbiol. 56, 365–368. ( 10.1099/ijs.0.63800-0) [DOI] [PubMed] [Google Scholar]
- 37.Luna GM, Bongiorni L, Gili C, Biavasco F, Danovaro R. 2010. Vibrio harveyi as a causative agent of the white syndrome in tropical stony corals. Environ. Microbiol. Rep. 2, 120–127. ( 10.1111/j.1758-2229.2009.00114.x) [DOI] [PubMed] [Google Scholar]
- 38.Sweet M, Bythell J. 2012. Ciliate and bacterial communities associated with white syndrome and brown band disease in reef-building corals. Environ. Microbiol. 14, 2184–2199. ( 10.1111/j.1462-2920.2012.02746.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sweet MJ, Bythell J. 2015. White syndrome in Acropora muricata: non-specific bacterial infection and ciliate histophagy. Mol. Ecol. 24, 1150–1159. ( 10.1111/mec.13097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arboleda MDM, Reichardt WT. 2010. Vibrio sp. causing Porites ulcerative white spot disease. Dis. Aquat. Organ. 90, 93–104. ( 10.3354/dao02222) [DOI] [PubMed] [Google Scholar]
- 41.Lawrence SA, Davy JE, Aeby GS, Wilson WH, Davy SK. 2014. Quantification of virus-like particles suggests viral infection in corals affected by Porites tissue loss. Coral Reefs 33, 687–691. ( 10.1007/s00338-014-1168-8) [DOI] [Google Scholar]
- 42.Zhenyu X, Shaowen K, Chaoqun H, Zhixiong Z, Shifeng W, Yongcan Z. 2013. First characterization of bacterial pathogen, Vibrio alginolyticus, for Porites andrewsi white syndrome in the South China Sea. PLoS ONE 8, e75425 ( 10.1371/journal.pone.0075425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Séré MG, Tortosa P, Chabanet P, Turquet J, Quod JP, Schleyer MH. 2013. Bacterial communities associated with Porites white patch syndrome (PWPS) on three western Indian Ocean (WIO) coral reefs. PLoS ONE 8, e83746 ( 10.1371/journal.pone.0083746) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sere MG, Tortosa P, Chabanet P, Quod JP, Sweet MJ, Schleyer MH. 2015. Identification of a bacterial pathogen associated with Porites white patch syndrome in the Western Indian Ocean. Mol. Ecol. 24, 4570–4581. ( 10.1111/mec.13326) [DOI] [PubMed] [Google Scholar]
- 45.Ushijima B, Smith A, Aeby GS, Callahan SM. 2012. Vibrio owensii induces the tissue loss disease Montipora white syndrome in the Hawaiian reef coral Montipora capitata. PLoS ONE 7, e46717 ( 10.1371/journal.pone.0046717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ushijima B, Videau P, Burger AH, Shore-Maggio A, Runyon CM, Sudek M, Aeby GS, Callahan SM. 2014. Vibrio coralliilyticus strain OCN008 is an etiological agent of acute montipora white syndrome. Appl. Environ. Microbiol. 80, 2102–2109. ( 10.1128/AEM.03463-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lobban CS, Raymundo LM, Montagnes DJS. 2011. Porpostoma guamensis n. sp., a Philasterine scuticociliate associated with brown-band disease of corals. J. Eukaryot. Microbiol. 58, 103–113. ( 10.1111/j.1550-7408.2010.00526.x) [DOI] [PubMed] [Google Scholar]
- 48.Bourne DG, Boyett HV, Henderson ME, Muirhead A, Willis BL. 2008. Identification of a ciliate (Oligohymenophorea: Scuticociliatia) associated with brown band disease on corals of the Great Barrier Reef. Appl. Environ. Microbiol. 74, 883–888. ( 10.1128/AEM.01124-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katz SM, Pollock FJ, Bourne DG, Willis BL. 2014. Crown-of-thorns starfish predation and physical injuries promote brown band disease on corals. Coral Reefs 33, 705–716. ( 10.1007/s00338-014-1153-2) [DOI] [Google Scholar]
- 50.Cervino JM, et al. 2008. The Vibrio core group induces yellow band disease in Caribbean and Indo-Pacific reef-building corals. J. Appl. Microbiol. 105, 1658–1671. ( 10.1111/j.1365-2672.2008.03871.x) [DOI] [PubMed] [Google Scholar]
- 51.Lawrence SA, Davy JE, Wilson WH, Hoegh-Guldberg O, Davy SK. 2015. Porites white patch syndrome: associated viruses and disease physiology. Coral Reefs 34, 249–257. ( 10.1007/s00338-014-1218-2) [DOI] [Google Scholar]
- 52.Sweet M, Burn D, Croquer A, Leary P. 2013. Characterisation of the bacterial and fungal communities associated with different lesion sizes of dark spot syndrome occurring in the coral Stephanocoenia intersepta. PLoS ONE 8, e62580 ( 10.1371/journal.pone.0062580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathew JL, et al. 2015. Etiology of community acquired pneumonia among children in India: prospective, cohort study. J. Glob. Health 5, 020418 ( 10.7189/jogh.05.020418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siciliano RF, Mansur AJ, Castelli JB, Arias V, Grinberg M, Levison ME, Strabelli TMV. 2014. Community-acquired culture-negative endocarditis: clinical characteristics and risk factors for mortality. Int. J. Infect. Dis. 25, 191–195. ( 10.1016/j.ijid.2014.05.005) [DOI] [PubMed] [Google Scholar]
- 55.McMahon B, Block J, Block T, Cohen C, Evans AA, Hosangadi A, London WT, Sherman M. 2016. Hepatitis-associated liver cancer: gaps and opportunities to improve care. J. Natl Cancer Inst. 108, djv359. ( 10.1093/jnci/djv359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Reilly CE, et al. 2007. A waterborne outbreak of gastroenteritis with multiple etiologies among resort island visitors and residents: Ohio, 2004. Clin. Infect. Dis. 44, 506–512. ( 10.1086/511043) [DOI] [PubMed] [Google Scholar]
- 57.Bruno JF, Ellner SP, Vu I, Kim K, Harvell CD, Ellner P. 2011. Impacts of aspergillosis on sea fan coral demography: modeling a moving target. Ecol. Monogr. 81, 123–139. ( 10.1890/09-1178.1) [DOI] [Google Scholar]
- 58.Randall CJ, van Woesik R. 2015. Contemporary white-band disease in Caribbean corals driven by climate change. Nat. Clim. Change 5, 375–379. ( 10.1038/nclimate2530) [DOI] [Google Scholar]
- 59.Woodley CM, Downs CA, Bruckner AW, Porter JW, Galloway S. 2015. Diseases of coral. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 60.Weil E, Rogers CS. 2011. Coral reef diseases in the Atlantic-Caribbean. In Coral reefs: an ecosystem in transition (eds Dubinsky Z, Stambler N), pp. 465–491. New York, NY: Springer. [Google Scholar]
- 61.Weil E, Smith G, Gil-Agudelo DL. 2006. Status and progress in coral disease research. Dis. Aquat. Organ. 69, 1–7. ( 10.3354/dao069001) [DOI] [PubMed] [Google Scholar]
- 62.Richardson LL, Goldberg WM, Carlton RG, Halas JC. 1998. Coral disease outbreak in the Florida Keys: plague type II. Rev. Biol. Trop. 46, 187–198. [Google Scholar]
- 63.Willis BL, Page CA, Dinsdale EA. 2004. Coral disease on the Great Barrier Reef. In Coral health and disease, (eds E Rosenberg, Y Loya), pp. 69–104. Heidelberg, Germany: Springer. [Google Scholar]
- 64.Bourne DG, Ainsworth TD, Pollock FJ, Willis BL. 2015. Towards a better understanding of white syndromes and their causes on Indo-Pacific coral reefs. Coral Reefs 34, 233–242. ( 10.1007/s00338-014-1239-x) [DOI] [Google Scholar]
- 65.Sutherland KP, Porter JW, Turner JW, Thomas BJ, Looney EE, Luna TP, Meyers MK, Futch JC, Lipp EK. 2010. Human sewage identified as likely source of white pox disease of the threatened Caribbean elkhorn coral, Acropora palmata. Environ. Microbiol. 12, 1122–1131. ( 10.1111/j.1462-2920.2010.02152.x) [DOI] [PubMed] [Google Scholar]
- 66.Richardson LL. 2004. Black band disease. In Coral health and disease (eds Rosenberg E, Loya Y), pp. 325–335. Heidelberg, Germany: Springer. [Google Scholar]
- 67.Toledo-Hernández C, Zuluaga-Montero A, Bones-González A, Rodríguez JA, Sabat AM, Bayman P. 2008. Fungi in healthy and diseased sea fans (Gorgonia ventalina): Is Aspergillus sydowii always the pathogen? Coral Reefs 27, 707–714. ( 10.1007/s00338-008-0387-2) [DOI] [Google Scholar]
- 68.Roder C, Arif C, Bayer T, Aranda M, Daniels C, Shibl A, Chavanich S, Voolstra CR. 2014. Bacterial profiling of white plague disease in a comparative coral species framework. ISME J. 8, 31–39. ( 10.1038/ismej.2013.127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sunagawa S, DeSantis TZ, Piceno YM, Brodie EL, DeSalvo MK, Voolstra CR, Weil E, Andersen GL, Medina M. 2009. Bacterial diversity and white plague disease-associated community changes in the Caribbean coral Montastraea faveolata. ISME J. 3, 512–521. ( 10.1038/ismej.2008.131) [DOI] [PubMed] [Google Scholar]
- 70.Ainsworth TD, Fine M, Roff G, Hoegh-Guldberg O. 2008. Bacteria are not the primary cause of bleaching in the Mediterranean coral Oculina patagonica. ISME J. 2, 67–73. ( 10.1038/ismej.2007.88) [DOI] [PubMed] [Google Scholar]
- 71.Rosenberg E, Kushmaro A, Kramarsky-Winter E, Banin E, Yossi L. 2009. The role of microorganisms in coral bleaching. ISME J. 3, 139–146. ( 10.1038/ismej.2008.104) [DOI] [PubMed] [Google Scholar]
- 72.Lesser MP, Jarett JK. 2014. Culture-dependent and culture-independent analyses reveal no prokaryotic community shifts or recovery of Serratia marcescens in Acropora palmata with white pox disease. FEMS Microbiol. Ecol. 88, 457–467. ( 10.1111/1574-6941.12311) [DOI] [PubMed] [Google Scholar]
- 73.Holden C. 1996. Coral disease hotspot in the Florida Keys. Science 274, 2017 ( 10.1126/science.274.5295.2017a) [DOI] [Google Scholar]
- 74.Rasband WS.2015. ImageJ. See http://imagej.nih.gov/ij/ .
- 75.R Core Team. 2008. R: a language and environment for statistical computing. Vienna, Austria: The R Foundation for Statistical Computing. [Google Scholar]
- 76.Pinheiro J, Bates D, DebRoy S, Sarkar D, orpR Core Team 2015. nlme: linear and nonlinear mixed effects models. See http://cran.r-project.org/package=nlme.
- 77.Burge CA, et al. 2016. Complementary approaches to diagnosing marine diseases: a union of the modern and the classic. Phil. Trans. R. Soc. B 371, 20150207 ( 10.1098/rstb.2015.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sutherland KP, Ritchie KB. 2004. White pox disease of the Caribbean elkhorn coral. In Coral health and disease (eds Rosenberg E, Loya Y), pp. 289–300. Heidelberg, Germany: Springer. [Google Scholar]
- 79.Williams DE, Miller MW. 2012. Attributing mortality among drivers of population decline in Acropora palmata in the Florida Keys (USA). Coral Reefs 31, 369–382. ( 10.1007/s00338-011-0847-y) [DOI] [Google Scholar]
- 80.Rogers CS, Muller EM. 2012. Bleaching, disease and recovery in the threatened scleractinian coral Acropora palmata in St. John, US Virgin Islands: 2003–2010. Coral Reefs 31, 807–819. ( 10.1007/s00338-012-0898-8) [DOI] [Google Scholar]
- 81.Muller EM, Rogers CS, Spitzack AS, Van Woesik R. 2008. Bleaching increases likelihood of disease on Acropora palmata (Lamarck) in Hawksnest Bay, St John, US Virgin Islands. Coral Reefs 27, 191–195. ( 10.1007/s00338-007-0310-2) [DOI] [Google Scholar]
- 82.Muller EM, Van Woesik R. 2012. Caribbean coral diseases: primary transmission or secondary infection? Glob. Chang. Biol. 18, 3529–3535. ( 10.1111/gcb.12019) [DOI] [Google Scholar]
- 83.Mayor PA, Rogers CS, Hillis-Starr ZM. 2006. Distribution and abundance of elkhorn coral, Acropora palmata, and prevalence of white-band disease at Buck Island Reef National Monument, St. Croix, US Virgin Islands. Coral Reefs 25, 239–242. ( 10.1007/s00338-006-0093-x) [DOI] [Google Scholar]
- 84.Muller EM, Rogers CS, van Woesik R. 2014. Early signs of recovery of Acropora palmata in St. John, US Virgin Islands. Mar. Biol. 161, 359–365. ( 10.1007/s00227-013-2341-2) [DOI] [Google Scholar]
- 85.Rodríguez-Martínez RE, Banaszak AT, McField MD, Beltrán-Torres AU, Álvarez-Filip L. 2014. Assessment of Acropora palmata in the mesoamerican reef system. PLoS ONE 9, e96140 ( 10.1371/journal.pone.0096140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rogers CS, Sutherland KP, Porter JW. 2005. Has white pox disease been affecting Acropora palmata for over 30 years? Coral Reefs 24, 194 ( 10.1007/s00338-004-0470-2) [DOI] [Google Scholar]
- 87.Aronson RB, Precht WF. 2001. White band diseases and the changing face of Caribbean coral reefs. Hydrobiologia 460, 25–38. ( 10.1007/978-94-017-3284-0_2) [DOI] [Google Scholar]
- 88.Rodríguez-Martínez RE, Banaszak AT, Jordán-Dahlgren E. 2001. Necrotic patches affect Acropora palmata (Scleractinia: Acroporidae) in the Mexican Caribbean. Dis. Aquat. Organ. 47, 229–234. ( 10.3354/dao047229) [DOI] [PubMed] [Google Scholar]
- 89.Porter JW, Meier OW. 1992. Quantification of loss and change in Floridian reef coral populations. Integr. Comp. Biol. 32, 625–640. ( 10.1093/icb/32.6.625) [DOI] [Google Scholar]
- 90.Williams DE, Miller MW, Kramer KL. 2008. Recruitment failure in Florida Keys Acropora palmata, a threatened Caribbean coral. Coral Reefs 27, 697–705. ( 10.1007/s00338-008-0386-3) [DOI] [Google Scholar]
- 91.Alvarez-Filip L, Carricart-Ganivet JP, Horta-Puga G, Iglesias-Prieto R. 2013. Shifts in coral-assemblage composition do not ensure persistence of reef functionality. Sci. Rep. 3, 3486 ( 10.1038/srep03486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kennedy EV, et al. 2013. Avoiding coral reef functional collapse requires local and global action. Curr. Biol. 23, 912–918. ( 10.1016/j.cub.2013.04.020) [DOI] [PubMed] [Google Scholar]
- 93.Porter JW, Meyers MK, Ruzicka R, Callahan M, Colella M, Rathbun S, Sutherland KP. 2012. Catastrophic loss of Acropora palmata in the Florida Keys: failure of the ‘sorcerer's apprentice effect’ to aid recovery following the 2005 Atlantic hurricane season. In Proc. 12th Int. Coral Reef Symp., Cairns, Australia, 9–13 July 2012. See http://www.icrs2012.com/proceedings/manuscripts/ICRS2012_19B_1.pdf.
- 94.Randall CJ, Jordán-Garza AG, van Woesik R. 2015. Ciliates associated with signs of disease on two Caribbean corals. Coral Reefs 34, 243–247. ( 10.1007/s00338-014-1212-8) [DOI] [Google Scholar]
- 95.Miller J, Waara R, Muller E, Rogers CS. 2006. Coral bleaching and disease combine to cause extensive mortality on reefs in US Virgin Islands. Coral Reefs 25, 418 ( 10.1007/s00338-006-0125-6) [DOI] [Google Scholar]
- 96.Muller EM, van Woesik R. 2014. Genetic susceptibility, colony size, and water temperature drive white-pox disease on the coral Acropora palmata. PLoS ONE 9, e110759 ( 10.1371/journal.pone.0110759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gignoux-Wolfsohn SA, Marks CJ, Vollmer SV. 2012. White band disease transmission in the threatened coral, Acropora cervicornis. Sci. Rep. 2, 804 ( 10.1038/srep00804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lamb JB, True JD, Piromvaragorn S, Willis BL. 2014. Scuba diving damage and intensity of tourist activities increases coral disease prevalence. Biol. Conserv. 178, 88–96. ( 10.1016/j.biocon.2014.06.027) [DOI] [Google Scholar]
- 99.Pollock FJ, Lamb JB, Field SN, Heron SF, Schaffelke B, Shedrawi G, Bourne DG, Willis BL. 2014. Sediment and turbidity associated with offshore dredging increase coral disease prevalence on nearby reefs. PLoS ONE 9, e102498 ( 10.1371/journal.pone.0102498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vega Thurber RL, Burkepile DE, Fuchs C, Shantz AA, Mcminds R, Zaneveld JR. 2014. Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Glob. Chang. Biol. 20, 544–554. ( 10.1111/gcb.12450) [DOI] [PubMed] [Google Scholar]
- 101.Denno DM, et al. 2012. Diarrhea etiology in a pediatric emergency department: a case control study. Clin. Infect. Dis. 55, 897–904. ( 10.1093/cid/cis553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alam M, et al. 2010. Diagnostic limitations to accurate diagnosis of cholera. J. Clin. Microbiol. 48, 3918–3922. ( 10.1128/JCM.00616-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schembri G, Schober P. 2009. The Internet as a diagnostic aid: the patients’ perspective. Int. J. STD AIDS 20, 231–233. ( 10.1258/ijsa.2008.008339) [DOI] [PubMed] [Google Scholar]
- 104.Maynard J, et al. 2016. Improving marine disease surveillance through sea temperature monitoring, outlooks and projections. Phil. Trans. R. Soc. B 371, 20150208 ( 10.1098/rstb.2015.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lamb JB, Wenger AS, Devlin MJ, Ceccarelli DM, Williamson DH, Willis BL. 2016. Reserves as tools for alleviating impacts of marine disease. Phil. Trans. R. Soc. B 371, 20150210 ( 10.1098/rstb.2015.0210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.De'ath G, Fabricius KE, Sweatman H, Puotinen M. 2012. From the cover: the 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl Acad. Sci. USA 109, 17 995–17 999. ( 10.1073/pnas.1208909109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR. 2003. Long-term region-wide declines in Caribbean corals. Science 301, 958–960. ( 10.1126/science.1086050) [DOI] [PubMed] [Google Scholar]
- 108.Casadevall A, Pirofski L. 2014. Ditch the term pathogen. Nature 516, 165 ( 10.1038/516165a) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.