Abstract
Past theoretical models suggest fishing disease-impacted stocks can reduce parasite transmission, but this is a good management strategy only when the exploitation required to reduce transmission does not overfish the stock. We applied this concept to a red abalone fishery so impacted by an infectious disease (withering syndrome) that stock densities plummeted and managers closed the fishery. In addition to the non-selective fishing strategy considered by past disease-fishing models, we modelled targeting (culling) infected individuals, which is plausible in red abalone because modern diagnostic tools can determine infection without harming landed abalone and the diagnostic cost is minor relative to the catch value. The non-selective abalone fishing required to eradicate parasites exceeded thresholds for abalone sustainability, but targeting infected abalone allowed the fishery to generate yield and reduce parasite prevalence while maintaining stock densities at or above the densities attainable if the population was closed to fishing. The effect was strong enough that stock and yield increased even when the catch was one-third uninfected abalone. These results could apply to other fisheries as the diagnostic costs decline relative to catch value.
Keywords: abalone, fisheries management, host–parasite models, presumptive diagnosis, withering syndrome
1. Introduction
Worth a hundred oysters, the red abalone is the biggest and most prized abalone species. Beginning in the 1980s, a new abalone disease called withering syndrome (WS) devastated southern and central California's valuable abalone (Haliotis spp.) fisheries. By autumn 1997, the California Fish and Game Commission closed the fishery, an understandable action, given the crisis [1]. Despite WS persisting for decades, some red abalone (H. rufescens) populations have maintained high densities in southern California. The remaining abalones' high market value has created interest in reopening a limited fishery.
Some fishery models suggest that harvesting an infected population might be more sustainable than closure. If the host threshold density for transmission is higher than the maximum sustainable yield, reducing population abundance can eliminate the disease from the system while maintaining sustainable harvest [2–4]. Indeed, some parasites are less abundant where fishing is intense [5]. Work in terrestrial ecosystems supports the fishing-out-parasites hypothesis [6–8], but with dispersive planktonic transmission stages [9], the fishing required to eradicate a marine disease is likely to exceed levels needed in terrestrial systems, and thus can surpass sustainable thresholds [2,4]. In other words, you might be able to fish out a parasite, but at the risk of also overfishing the stock.
An intermediate strategy is to target and cull infected animals before they infect additional hosts. Many marine diseases remain asymptomatic until late in infection, and before those late stages such infections do not degrade landing value. In these cases, culled hosts are included in the fishery harvest (e.g. [10]). Culling infected hosts is a standard yet often contentious way to manage terrestrial wildlife diseases [11–13]. In marine systems, this strategy faces added challenges, such as difficulties and costs associated with diagnosing cryptic infections. Recently, presumptive diagnoses of many marine diseases have been streamlined with rapid, inexpensive and non-invasive methods (table 1), including observing external parasites [14,16,23,26], morphological and behavioural changes in hosts [20,27,30], and non-lethal immunological and molecular assays [29,31]. For instance, polymerase chain reaction (PCR) methods can detect genes specific to the WS bacterium in abalone faeces [29]. Detecting infection in harvested stocks makes it easier for targeted fishing to reduce parasite transmission.
Table 1.
Presumptive diagnoses for selected parasites impacting marine and freshwater fisheries.
| condition | parasite | fishery | region | presumptive diagnosis | reference | remarks |
|---|---|---|---|---|---|---|
| bacterial cold water disease (BCWD) | Flavobacterium psychrophilum | Atlantic salmon (Salmo salar) | global | lesions and dark, torn, split or frayed fins. Heavily infected fish are often lethargic and stop feeding | [14] | |
| chinook salmon (Oncorlynchus tshawytscha) | ||||||
| sockeye salmon (Oncorlynchus nerka) | ||||||
| chum salmon (Oncorlynchus keta) | ||||||
| cutthroat trout (Oncorlynchus clarki) | ||||||
| brook trout (Salvelinus fontinalis) | ||||||
| brown trout (Salvelinus trutta) | ||||||
| columnaris disease | Cytophage columnaris | channel catfish (Ictaluris punctatus) | global | greyish-white lesions | [15] | |
| common carp (Cyprinus carpio) | ||||||
| goldfish (Cyprinus auratus) | ||||||
| American eel (Anguilla rostrata) | ||||||
| Japanese eel (Anguilla japonica) | ||||||
| European eel (Anguilla anguilla) | ||||||
| rainbow trout (Oncorhynchus mykiss) | ||||||
| brown trout (Salvelinus trutta) | ||||||
| brook trout (Salvelinus fontinalis) | ||||||
| epizootic shell disease | multiple parasites | American lobster (Homarus americanus) | Nebraska | external shell lesions | [16–19] | not readily transmissible |
| Hematodinium/bitter crab disease | Hematodinium perezi | blue crab (Callinectes sapidus) | global | hyperpigmentation of the carapace and arthrodial membranes | [16,20,21] | presumptive signs only observed in late stage infections |
| Norway lobster (Nephrops norvegicus) | ||||||
| snow crab (Chionoecetes opilio) | ||||||
| tanner crab (Chinocecetes bairdi) | ||||||
| velvet swimming crab (Necora puber) | ||||||
| limp lobster disease | Vibrio fluvialis-like organisms | American lobster (Homarus americanus) | Nebraska | weakness, lethargy, slow or ineffectual responses to sensory stimuli | [22] | |
| parasitic nematodes | Anguillicota crassus | American eel (Anguilla rostrata) | Patuxent River, USA | direct observation of external parasites | [23] | |
| PaV1 | Panulirus argus virus 1 | spiny lobster (Panulirus argus) | Caribbean Sea | lethargy and restricted movement | [16,24] | |
| red tail disease | Aerococcus viridans (var.) homari | American lobster (Homarus americanus) | Nebraska | pink coloration of the ventral abdomen | [25] | |
| salmon lice | Lepeophtheirus salmonis and Caligus spp. | Atlantic salmon (Salmo salar) | global | removal of skin over the head and presence of female lice bearing egg strings on the back | [26] | |
| coho salmon (Oncorhynchus kisutch) | ||||||
| rainbow trout (Oncorhynchus mykiss) | ||||||
| striped bass (Morone saxatilis) | ||||||
| Atlantic cod (Gadus morhua) | ||||||
| Arctic charr (Salvelinus alpinus) | ||||||
| scoliosis | Myxobolus acanthoobii | Japanese mackerel (Scomber japonicus) | Japan | kyphosis (dorso-ventral curvature) | [27] | |
| Japanese bluefish (Scombrops boops) | ||||||
| red gurnard (Chelidonichthys spinosus) | ||||||
| brown-lined puffer (Canthigaster rivulata) | ||||||
| white spot syndrome | Whispovirus | kuruma shrimp (Penaeus japonicus) | global | white spots on carapace and appendages | [28] | |
| tiger prawn (Penaeus monodon) | ||||||
| redtail prawn (Penaeus penicillatus) | ||||||
| withering foot syndrome | Candidatus Xenohaliotis californiensis | red abalone (Haliotis rufescens) | California | withered appearance, lethargy, faecal PCR | [29] | |
| white abalone (Haliotis sorenseni) | ||||||
| black abalone (Haliotis cracherodii) | ||||||
| green abalone (Haliotis fulgens) | ||||||
| pink abalone (Haliotis corrugata) |
We parametrized a general fisheries model for the California red abalone (Haliotis rufescens) infected by the WS rickettsial bacterium Candidatus Xenohaliotis californiensis (WS-RLO) [32]. We used this model to contrast how fishery closure, culling infected hosts, and harvesting uninfected hosts affect parasite prevalence, fishery yield and abalone density, finding that a successful management strategy could sustain a modest harvest while protecting the stock from disease mortality. We conclude by considering strategies to maximize yield while conserving disease-affected fisheries.
2. A general fisheries model
We used a simple population model that tracked stock density over time in two states, susceptible S and infected I. This general model was not specific to abalone, but was sufficiently flexible to accommodate the various life histories of marine hosts and their parasites. Parasite transmission to susceptible hosts occurred through direct density-dependent contact with free-living, water-borne parasites P at the disease transmission rate β. Parasite stages in the water column were produced by the infected population at a per capita rate (σ) and either died at rate δ if they failed to contact a susceptible host or were removed from the water column after host contacts. We assumed that infection did not reduce fecundity so that host recruitment came from all abalone (N = S + I) at the per capita rate r. Natural mortality acted on both the susceptible and infected classes with per capita base rate μ. Intraspecific competition led to a density-dependent loss term c, so that carrying capacity without disease and fishing was set to (r − μ)/c. Disease increased host mortality by the additive rate α, and therefore, without harvest and density-dependent mortality, the infected host's expected lifespan was (μ + α)−1. The fishery harvested susceptible and infected hosts at the respective per capita rates fS, and fI, with the following coupled differential equations:
| 2.1 |
| 2.2 |
| 2.3 |
It was convenient to rewrite the system as total population density N and disease prevalence i, where S = (1 − i)N and I = iN. Therefore,
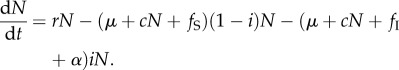 |
2.4 |
After some transformations (see the electronic supplementary material), we calculated the stock density at the endemic disease equilibrium N* as:
| 2.5 |
This solution gives two important insights into harvesting uninfected hosts (fS) and targeted culling (fI): (i) harvesting uninfected hosts, as expected, will drive down population density, because the endemic equilibrium infection prevalence (i*) must always be less than 1; and (ii) targeted culling's effect on N* will be scaled by its effect on i*. The solution for i* was not interpretable, so we used the next-generation matrix solution [33] to define the parasite's basic reproduction number (R0) at the disease-free equilibrium S = N, I = 0, P = 0 (see the electronic supplementary material), finding the condition for disease persistence:
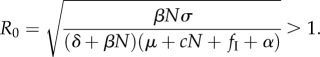 |
2.6 |
We interpreted this as the probability that an infectious parasite stage contacts a host before it dies (a function that saturates with host density, 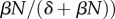 times the number of infectious stages produced over an infected host's life (
times the number of infectious stages produced over an infected host's life (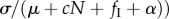 . Harvesting uninfected hosts did not directly reduce infection rate because fS did not enter into equation (2.6). Rather, harvesting uninfected hosts reduced R0 only indirectly, by its effect on host population density (N). This agrees with past models [4], demonstrating that fishing can remove parasites by driving the fished population below the density threshold for transmission, so long as this density threshold exceeds thresholds defining maximum sustainable yield. However, there are two ways that limit the extent that increased fishing interferes with transmission. First, if infective stages are lived long in the environment, infection follows a saturating functional response, making transmission less sensitive to reductions from high host density. Second, as Potapov et al. [34] observed, when crowding limits host abundance through adult mortality, fishing increases an infected host's lifespan along with the infectious stages it produces. On the other hand, when we added targeted culling (fI) into equation (2.6), infection rate decreased with fishing.
. Harvesting uninfected hosts did not directly reduce infection rate because fS did not enter into equation (2.6). Rather, harvesting uninfected hosts reduced R0 only indirectly, by its effect on host population density (N). This agrees with past models [4], demonstrating that fishing can remove parasites by driving the fished population below the density threshold for transmission, so long as this density threshold exceeds thresholds defining maximum sustainable yield. However, there are two ways that limit the extent that increased fishing interferes with transmission. First, if infective stages are lived long in the environment, infection follows a saturating functional response, making transmission less sensitive to reductions from high host density. Second, as Potapov et al. [34] observed, when crowding limits host abundance through adult mortality, fishing increases an infected host's lifespan along with the infectious stages it produces. On the other hand, when we added targeted culling (fI) into equation (2.6), infection rate decreased with fishing.
3. Case study: abalone fisheries impacted by withering syndrome
Given the general results above, we turn to how culling affects WS-RLO infection in California red abalone and interacts with general harvest to shape abalone yields, density and sustainability. Since WS emerged in the 1980s, it spread throughout southern California [35,36]. Diver surveys over 3 years (2006–2008) showed that red abalone density on south San Miguel Island, California, USA, was among the highest anywhere [37], motivating a proposal by former abalone divers to open a limited-entry fishery in this region. However, WS-RLO infects half the abalone at San Miguel Island [35–37], and concerns over fishing populations already affected by disease led managers to deny the request. We used published red abalone density and WS-RLO prevalence from San Miguel Island to ask whether this denial was warranted and to also consider how to manage harvest to moderate disease impacts.
Without disease (β = 0), natural, density-dependent mortality regulates unfished abalone populations (fS = fI = 0 yr−1) [38]. Red abalone densities at San Miguel Island often exceeded 6000 abalone ha−1 before WS, and, from the maximum densities reported in the California Abalone Recovery and Management Plan, we estimated the disease-free carrying capacity (KH) as 6800 abalone ha−1 [39]. By simplifying equation (2.4), we estimated the abalone fishery's disease-free maximum sustainable yield as:
| 3.1 |
Using red abalone population growth (0.32 yr−1) and natural mortality (0.15 yr−1) from Tegner et al. [40], and estimating c = 0.025 m2 abalone−1 from KH (equation 3.1), the WS-free maximum sustainable yield (MSYH) was 288 abalone ha−1 yr−1, leaving 3365 abalone ha−1 in the wild (figure 1).
Figure 1.
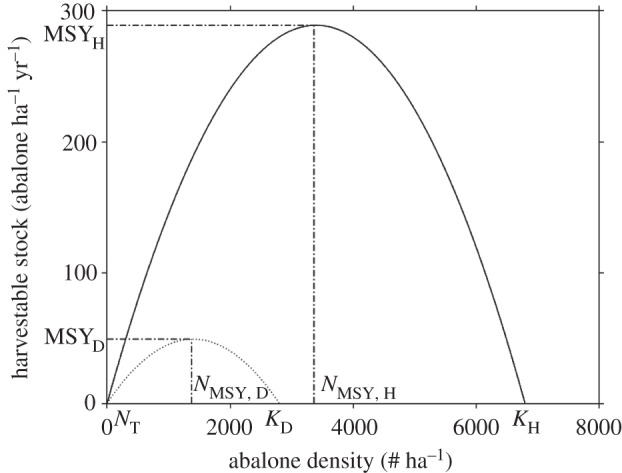
Predicted annual population growth and harvestable stock of San Miguel Island red abalone before WS (solid line) and when disease is present (dotted line). The maximum sustainable yield occurs when the population is maintained at the maximum growth rate. For the population before the emergence of WS, MSYH = 288 abalone ha−1 yr−1 when the population is maintained at 3365 abalone ha−1. When disease is present MSYD = 49 abalone ha−1 yr−1 when the population is maintained at 1374 abalone ha−1. The threshold density for disease invasion (NT) is 120 abalone ha−1.
We used the additive WS mortality rate (α = 0.20 yr−1) at the northern Channel Islands, California estimated by Ben-Horin [41] from data in Moore et al. [35] to obtain the WS-impacted abalone carrying capacity without fishing (KD). The WS mortality rate increases with temperature and varies across the California coast [36,42,43]. The early and advancing infections detectable by PCR remain asymptomatic in cold water without decreasing the sale price. Advanced infections impact the host digestive gland, leading to the withered host foot muscle that characterizes WS, and which is soon followed by death [36,43]. We assumed WS is in near equilibrium at San Miguel Island and used the value i* = 0.50 [35,37] to rewrite equation (2.4) to consider abalone population growth at San Miguel Island when impacted by disease, but without fishing:
| 3.2 |
We estimated the abalone carrying capacity impacted by disease as KD = 2889 abalone ha−1. Maximum population growth without fishing (fS = fI = 0 yr−1), and the maximum sustainable yield for harvest that is non-selective with respect to infection (MSYD), was 49 abalone ha−1 yr−1 at a population maintained at 1374 abalone ha−1. As should be expected, WS reduced the expected equilibrium abundance and maximum sustainable yield.
We then asked if fishing could drive abalone populations down below the threshold density for transmission (NT) and eliminate the WS-RLO at San Miguel Island. In part, this depends on how far infective stages can travel from outside the stock. For WS-RLO, transmission occurs when abalone ingest water-borne stages derived from contaminated faeces [44]. Infectious stages are short-lived and dilute in the water column once released by hosts. This short lifespan is supported by observations that the highest WS-RLO densities in seawater occur only near effluent from abalone farms effluent [45], which often contain many WS-RLO infected abalone [42]. San Miguel Island is located almost 50 km from farms on the California mainland and, at its closest point, is 5 km from abalone on neighbouring Santa Rosa Island. Small remnant black abalone populations persist on both San Miguel and Santa Rosa Islands [46], but these alternate host populations seem to contribute little to WS-RLO transmission in red abalone. For this reason, we suspect that San Miguel Island can be modelled as a closed system for the WS-RLO pathogen. The short infective stage lifespan also suggests that parasite stages lost to host contacts are negligible compared with parasite mortality in the water column (i.e. 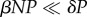 ), allowing us to simplify equation (2.3) to
), allowing us to simplify equation (2.3) to
| 3.3 |
which simplifies the condition for disease persistence (see the electronic supplementary material) to
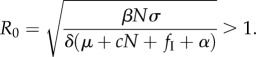 |
3.4 |
We used the transmission coefficient β = 0.03 m2 abalone−1 estimated from red abalone at San Miguel Island [41] to obtain the threshold population density for transmission as NT = 120 abalone ha−1. This is well below the population density at maximum sustainable yield when disease is present (figure 1). Removing the WS-RLO by fishing abalone populations down below NT is, therefore, not a reasonable option for management, because the estimated exploitation required to eradicate this parasite is unsustainable. Furthermore, sustainable yield leads to a stock density or biomass that is 50–75% lower than unfished stocks, but a more conservative yield is often mandated for fisheries impacted by disease, including California abalone [39]. For this reason, the decision to not reopen the red abalone to general fishing seems warranted.
We then considered conservative scenarios where targeted culling reduced transmission and thus death from disease, allowing harvested stocks to maintain population densities at or above KD (i.e. a fishery strategy that ironically increased abalone abundance). We numerically simulated equations (2.1), (2.2) and (3.2) for fS = 0 : 0.5 yr−1 and fI = 0 : 0.5 yr−1 for T = 1000 years, using initial population densities of S0 = 3 abalone m−2, I0 = 1 abalone m−2 and P0 = 1 parasite m−2. For each fS and fI combination, we obtained abalone population density, WS-RLO prevalence and fishery yield. Mathematical details and Matlab (Mathworks, Natick, MA, USA) code reproducing the simulations are available in the electronic supplementary material.
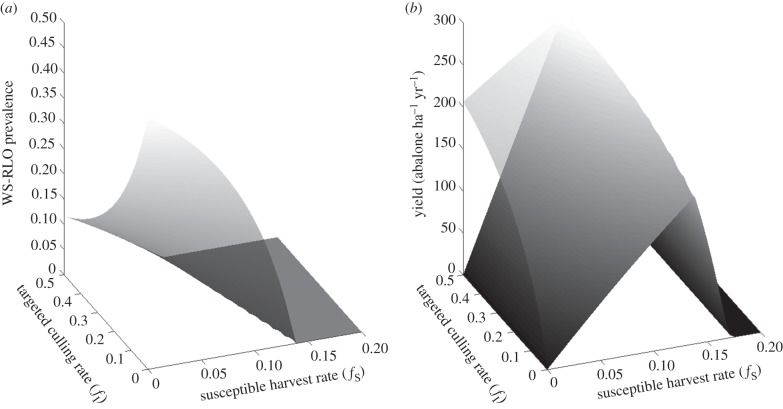
Culling infected abalone allowed abalone density (NF) to exceed the carrying capacity (KD) when disease was present (figure 2). However, this occurred only with targeted culling > 0.22 yr−1 when fS = 0 yr−1. This introduced an important result relevant for managing abalone fisheries impacted by WS: minor efforts to cull abalone infected with the WS-RLO (fI < 0.22 yr−1) were ineffective because the compensatory decreases in disease transmission from culling were not enough to balance the direct decline in population density from removing infected abalone. Moreover, adding a susceptible abalone harvest increased the culling rate required for NF ≥ KD in a near linear fashion, leading to a second key result: the fished population was maintained at or above KD when the infected culling rate was no less than twice the susceptible harvest rate, plus the initial culling rate required for NF ≥ KD
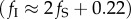 . Equivalence in population density between fished and unfished populations was therefore maintained even when uninfected abalone were harvested, so long as fI increased with fS. This equivalence was no longer possible when fS > 0.1, because here, WS-RLO prevalence was near zero (figure 3a) and increasing the uninfected abalone harvest led to overfishing (figure 3b). Increasing fI decreased the uninfected abalone harvest rate that maximized yield. In other words, a fishery that also culled diseased abalone attained higher yields at lower harvest rates. These harvests exceeded the maximum sustainable yield attainable when fishing was non-selective with respect to infection (MSYD; figure 1) and approached the maximum sustainable yield attainable before WS (MSYH). This highlights our third result relevant to management: disease-impacted abalone populations were managed conservatively, at densities equal to or greater than those if the fishery were closed (NF ≥ KD), while generating modest yields and maintaining an intermediate WS-RLO prevalence. Unfortunately, removing the WS-RLO altogether required harvesting beyond sustainable thresholds, even when culling was included as a harvest strategy. In other words, the fishery could live with the disease but should not expect to eradicate it.
. Equivalence in population density between fished and unfished populations was therefore maintained even when uninfected abalone were harvested, so long as fI increased with fS. This equivalence was no longer possible when fS > 0.1, because here, WS-RLO prevalence was near zero (figure 3a) and increasing the uninfected abalone harvest led to overfishing (figure 3b). Increasing fI decreased the uninfected abalone harvest rate that maximized yield. In other words, a fishery that also culled diseased abalone attained higher yields at lower harvest rates. These harvests exceeded the maximum sustainable yield attainable when fishing was non-selective with respect to infection (MSYD; figure 1) and approached the maximum sustainable yield attainable before WS (MSYH). This highlights our third result relevant to management: disease-impacted abalone populations were managed conservatively, at densities equal to or greater than those if the fishery were closed (NF ≥ KD), while generating modest yields and maintaining an intermediate WS-RLO prevalence. Unfortunately, removing the WS-RLO altogether required harvesting beyond sustainable thresholds, even when culling was included as a harvest strategy. In other words, the fishery could live with the disease but should not expect to eradicate it.
Figure 2.
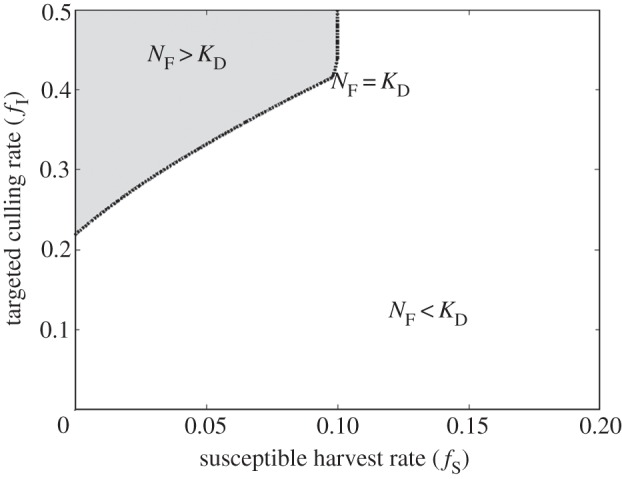
Isocline showing combinations of susceptible host harvest rate (fS) and targeted culling rate (fI) when the disease-impacted fished population density (NF) is equal to the unfished disease-impacted population (KD).
Figure 3.
(a) WS-RLO infection prevalence as a function of susceptible host harvest rate (fS) and targeted culling rate (fI). (b) Fishery yield including culled hosts in the catch (transparent grey surface) and excluding culled hosts from the catch (dark grey surface) as a function of the susceptible host harvest rate (fS) and targeted culling rate (fI).
4. Discussion and conclusion
For red abalone, and perhaps for other fishery species, targeting infected animals means that fishing can enhance stock abundance and sustainable yield, whereas non-selective fishing can further imperil a disease-impacted stock. Culling infected hosts leads to a compensatory decrease in disease-induced mortality, in turn producing harvestable stock that is unavailable in unfished or closed fisheries. With targeted harvesting, the effective decrease in natural mortality can allow fisheries to operate while maintaining stock densities at or above the maximum densities attainable were the populations closed to fishing. However, this result depends on details. First, culling must be intensive enough for the compensatory decrease in disease-induced mortality to balance direct losses to the stock due to removing infected individuals from the population. More importantly, although culling decreases the fishing effort that maximizes fishery yield, exploited populations are more sensitive to overfishing when they are also culled. Managing disease-impacted fisheries therefore sustains a modest harvest and protects the population at large from disease by (i) reducing parasite prevalence and (ii) maintaining stock density near, at or above the densities achieved by fishery closure.
Culling is a standard but often contentious way to control terrestrial wildlife diseases [11,13], bringing animal welfare, economic and conservation considerations into conflict [12]. Wild capture fisheries resolve this conflict in part because fish are treated more as a commodity than as wildlife [17,47]. The public perception of wild capture fisheries, seafood's popularity and the management infrastructure already in place suggest that the costs and animal welfare concerns do not prohibit this strategy in managing fisheries impacted by disease [48].
Our results do not apply to all fisheries. First, not all infectious diseases impact fisheries. Also, in practice, culling infected animals requires fisheries or fishery managers to non-destructively identify an infection at harvest or soon after. This can be time-consuming and costly apart from high-value fisheries such as abalone where the diagnostic cost (approx. $5 USD) is minor relative to the landing value. For abalone and the WS-RLO, presumptive diagnoses can be achieved by a PCR assay [31] applied to faeces collected from landed abalone or by swabbing a wild abalone's first open respiratory pore, where discharged faeces accumulate (T. Ben-Horin and D. Witting 2013, unpublished data). Although such molecular assays suggest parasite presence, PCR assays indicate only target DNA rather than established and viable infections [49,50], and therefore include inherent though quantifiable uncertainty. Regardless, the substantial additive mortality due to WS-RLO, coupled with the ability to diagnose wild abalone and abalone's high market value, makes our proposed strategy tractable.
How could managers implement this strategy? In practical terms, separate quotas could be set for uninfected and infected abalone, and all harvested abalone swabbed for WS-RLO once landed. The fishery might then operate until reaching the uninfected abalone quota. Beyond separate quotas, fishery-independent divers could use numbered tags to identify abalone within designated fishing areas and swab them for WS-RLO. After the PCR results were entered into a database, commercial divers could record these numbers and then either harvest abalone with numbers corresponding to a positive infection or harvest from high-prevalence fishing areas. Although this process sounds onerous, a single abalone can sell for $100 (USD) or more, and the alternative is a fishery that remains closed to harvest.
Our results might apply to some other fisheries for which infections can be diagnosed with non-lethal methods. We considered perfect and cost-free infection diagnosis in our model, but most diagnoses have inherent uncertainty and take effort [49,51]. Our simple deterministic model is extendable to stochastic frameworks, and including uncertainty in the infection status would allow one to determine how much information about infection status one needs to target infected hosts. Furthermore, in addition to individual diagnoses such as PCR for WS-RLO, factors such as punctuated mortality events, environmental factors such as water temperature and salinity, and for chronic diseases, the stock size or age structure, can help predict and forecast the infection status at a site [14,26,52–54], leading to an analogous fishing strategy based on targeting sites rather than individuals. Regardless, assessing other fisheries would require specific models, including subtracting diagnostic cost from the yield function.
Ecosystem-based fisheries management has gained traction as an alternative to single-species fisheries management [55,56], casting a wider focus on ecosystems and how fisheries affect them. Although parasites are in all ecosystems, modern fisheries management does not often consider marine diseases. When it does, the default responses are to ignore disease or shut fisheries down. Our model informs fisheries management to consider the interactions between fishing and marine disease, showing that considering disease in fisheries management can benefit both fisheries yield and sustainability.
Supplementary Material
Acknowledgement
We thank J. Marshall, C. Voss, A. Woodcock, J. Moore and C. Friedman for constructive discussions and help formulating ideas for the analyses. This manuscript benefitted from input from K. Markey-Lundgren, E. Aalto and two anonymous reviews.
Data accessibility
Matlab routines reproducing the models used here are in the electronic supplementary material.
Authors' contributions
T.B.H., K.D.L. and H.S.L. developed the conceptual model. T.B.H. assembled the input data. T.B.H. and G.B. worked out solutions to the models. T.B.H. wrote code and ran numeric solutions of the models. All authors analysed model outputs. T.B.H., K.D.L. and H.S.L. wrote the main paper. T.B.H. and G.B. wrote the electronic supplementary material. All authors discussed the results and implications and commented on the manuscript at all stages.
Funding
T.B.H. was supported by a grant from the National Science Foundation program in Ecology and Evolution of Infectious Diseases (OCE-1216220) and a standard cooperative agreement (SCA no. 58-1915-1-156) between the US Department of Agriculture Agricultural Research Service and the University of Rhode Island. Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
Competing interests
We have no competing interests
References
- 1.Drennan LT, McConnell A, Stark A. 2014. Risk and crisis management in the public sector. London, UK: Routledge. [Google Scholar]
- 2.Dobson AP, May RM. 1987. The effects of parasites on fish populations—theoretical aspects. Int. J. Parasitol. 17, 363–370. ( 10.1016/0020-7519(87)90111-1) [DOI] [PubMed] [Google Scholar]
- 3.Kuris AM, Lafferty KD. 1992. Modelling crustacean fisheries: effects of parasites on management strategies. Can. J. Fish. Aquat. Sci. 49, 327–336. ( 10.1139/f92-037) [DOI] [Google Scholar]
- 4.McCallum H, Gerber L, Jani A. 2005. Does infectious disease influence the efficacy of marine protected areas? A theoretical framework. J. Appl. Ecol. 42, 688–698. ( 10.1111/j.1365-2664.2005.01043.x) [DOI] [Google Scholar]
- 5.Wood CL, Lafferty KD, Micheli F. 2010. Fishing out marine parasites? Impacts of fishing on rates of parasitism in the ocean. Ecol. Lett. 13, 761–775. ( 10.1111/j.1461-0248.2010.01467.x) [DOI] [PubMed] [Google Scholar]
- 6.Arneberg P, Skorping A, Grenfell B, Read AF. 1998. Host densities as determinants of abundance in parasite communities. Proc. R. Soc. Lond. B 265, 1283–1289. ( 10.1098/rspb.1998.0431) [DOI] [Google Scholar]
- 7.Morand S, Poulin R. 1998. Density, body mass and parasite species richness of terrestrial mammals. Evol. Ecol. 12, 717–727. ( 10.1023/A:1006537600093) [DOI] [Google Scholar]
- 8.Arneberg P. 2002. Host population density and body mass as determinants of species richness in parasite communities: comparative analysis of directly transmitted nematodes of mammals. Ecography 25, 88–94. ( 10.1034/j.1600-0587.2002.250110.x) [DOI] [Google Scholar]
- 9.McCallum H, Harvell D, Dobson A. 2003. Rates of spread of marine pathogens. Ecol. Lett. 6, 1062–1067. ( 10.1046/j.1461-0248-2003.00545.x) [DOI] [Google Scholar]
- 10.Lenihan HS, Micheli F, Shelton SW, Peterson CH. 1999. How multiple environmental stresses influence parasitic infection of oysters. Limnol. Oceanogr. 44, 910–924. ( 10.4319/lo.1999.44.3_part_2.0910) [DOI] [Google Scholar]
- 11.Donnelly CA, et al. 2006. Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature 439, 843–846. ( 10.1038/nature04454) [DOI] [PubMed] [Google Scholar]
- 12.McCallum H, Hocking BA. 2005. Reflecting on ethical and legal issues in wildlife disease. Bioethics 19, 336–347. ( 10.1111/j.1467-8519.2005.00447.x) [DOI] [PubMed] [Google Scholar]
- 13.Joseph MB, Mihaljevic JR, Arellano AL, Kueneman JG, Preston DL, Cross PC, Johnson PT. 2013. Taming wildlife disease: bridging the gap between science and management. J. Appl. Ecol. 50, 702–712. ( 10.1111/1365-2664.12084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starliper CE. 2011. Bacterial coldwater disease of fishes caused by Flavobacterium psychrophilum. J. Adv. Res. 2, 97–108. ( 10.1016/j.jare.2010.04.001) [DOI] [Google Scholar]
- 15.Anderson JIW, Conroy DA. 1969. The pathogenic myxobacteria with special reference to fish diseases. J. Appl. Bacteriol. 32, 30–39. ( 10.1111/j.1365-2672.1969.tb02186.x) [DOI] [PubMed] [Google Scholar]
- 16.Shields JD. 2012. The impact of pathogens on exploited populations of decapod crustaceans. J. Invert. Pathol. 110, 211–224. ( 10.1016/j.jip.2012.03.011) [DOI] [PubMed] [Google Scholar]
- 17.Brashares JS, Arcese P, San MK, Coppolillo PB, Sinclair ARE, Balmford A. 2004. Bushmeat hunting, wildlife declines, and fish supply in West Africa. Science 306, 1180–1183. ( 10.1126/science.1102425) [DOI] [PubMed] [Google Scholar]
- 18.Smolowitz R, Chistoserdov AY, Hsu A. 2005. A description of the pathology of epizootic shell disease in the American lobster, Homarus americanus H. Milne Edwards 1837. J. Shellfish Res. 24, 749–756. ( 10.2983/0730-8000(2005)24%5B749:ADOTPO%5D2.0.CO;2) [DOI] [Google Scholar]
- 19.Cawthorne RJ. 2011. Diseases of American lobsters (Homarus americanus): a review. J. Invert. Pathol. 106, 71–79. ( 10.1016/j.jip.2010.09.010) [DOI] [PubMed] [Google Scholar]
- 20.Butler MJ, Tiggelaar JM, Shields JD, Butler MJ. 2014. Effect of the parasitic dinoflagellate Hematodinium perezi on blue crab (Callinectes sapidus) behavior and predation. J. Exp. Mar. Biol. Ecol. 461, 381–388. ( 10.1016/j.jembe.2014.09.008) [DOI] [Google Scholar]
- 21.Chualáin CN, Robinson M. 2011. Comparison of assessment methods used to diagnose Hematodinium sp. infections in Cancer pagurus. ICES J. Mar. Sci. 68, 454–462 ( 10.1093/icesjms/fsq197) [DOI] [Google Scholar]
- 22.Tall BD, et al. 2003. Characterization of Vibrio fluvialis-like strains implicated in limp lobster disease. Appl. Environ. Microbiol. 69, 7435–7446. ( 10.1128/AEM.69.12.7435-7446.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barse AM, Secor DH. 1999. An exotic nematode parasite of the American eel. Fisheries 24, 6–10. ( 10.1577/1548-8446(1999)024-0006) [DOI] [Google Scholar]
- 24.Shields JD. 2011. Diseases in spiny lobsters: a review. J. Invertebr. Pathol. 106, 79–91. ( 10.1016/j.jip.2010.09.015) [DOI] [PubMed] [Google Scholar]
- 25.Stewart JE. 1975. Gaffkemia, the fatal infection of lobsters (genus Homarus) caused by Aerococcus viridans (var.) homari: a review. Mar. Fish. Rev. 37, 20–24. [Google Scholar]
- 26.Costello MJ. 2006. Ecology of sea lice parasitic on farmed and wild fish. Trends Parasitol. 22, 475–483. ( 10.1016/j.pt.2006.08.006) [DOI] [PubMed] [Google Scholar]
- 27.Yokoyama H, Freeman MA, Itoh N, Fukuda Y. 2005. Spinal curvature of cultured Japanese mackerel Scomber japonicas associated with a brain myxosporean, Myxobolus acanthogobii. Dis. Aquat. Org. 66, 1–7. ( 10.3354/dao066001) [DOI] [PubMed] [Google Scholar]
- 28.Chou H-Y, Huang C-Y, Wang C-H, Chiang H-C, Lo C-F. 1995. Pathogenicity of a baculovirus infection causing white spot syndrome in cultured penaeid shrimp in Taiwan. Dis. Aquat. Org. 23, 165–173. ( 10.3354/dao023165) [DOI] [Google Scholar]
- 29.Friedman CS, Wright N, Crosson LM, White SJ, Strenge RM. 2014. Validation of a quantitative PCR assay for detection and quantification of ‘Candidatus Xenohaliotis californiensis’. Dis. Aquat. Org. 108, 251–259. ( 10.3354/dao02720) [DOI] [PubMed] [Google Scholar]
- 30.Stentiford GD, Shields JD. 2005. A review of the parasitic dinoflagellates Hematodinium species and Hematodinium-like infections in marine crustaceans. Dis. Aquat. Org. 66, 47–70. ( 10.3354/dao066047) [DOI] [PubMed] [Google Scholar]
- 31.Park K-I, Yang H-S, Kang H-S, Cho M, Park K-J, Choi K-S. 2010. Isolation and identification of Perkinsus olseni from feces and marine sediment using immunological and molecular techniques. J. Invert. Pathol. 105, 261–269. ( 10.1016/j.jip.2010.07.006) [DOI] [PubMed] [Google Scholar]
- 32.Friedman CS, Andree KB, Beauchamp KA, Moore JD, Robbins TT, Shields JD, Hedrick RP. 2000. ‘Candidatus Xenohaliotis californiensis’, a newly described pathogen of abalone, Haliotis spp., along the west coast of North America. Int. J. Syst. Evol. Microbiol. 50, 847–855. ( 10.1099/00207713-50-2-847) [DOI] [PubMed] [Google Scholar]
- 33.Diekmann O, Heesterbeek JAP, Roberts MG. 2010. The construction of next-generation matrices for compartmental epidemic models. J. R. Soc. Interface 7, 873–885. ( 10.1098/rsif.2009.0386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potapov A, Merrill E, Lewis MA. 2012. Wildlife disease elimination and density dependence. Proc. R. Soc. B 279, 3139–3145. ( 10.1098/rspb.2012.0520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore JD, Marshman BC, Chun CS. 2011. Health and survival of red abalone Haliotis rufescens from San Miguel Island, California, USA, in a laboratory simulation of La Niña and El Niño conditions. J. Aquat. Anim. Health 23, 78–84. ( 10.1080/08997659.2011.568860) [DOI] [PubMed] [Google Scholar]
- 36.Crosson LM, Wight N, VanBlaricom GR, Kiryu I, Moore JD, Freidman CS. 2014. Abalone withering syndrome: distribution, impacts, current diagnostic methods and new findings. Dis. Aquat. Org. 108, 261–270. ( 10.3354/dao02713) [DOI] [PubMed] [Google Scholar]
- 37.California Department of Fish and Game. 2012. San Miguel Island red abalone fishery considerations: a report to the Marine Resources Committee. Monterey, CA: Marine Region. [Google Scholar]
- 38.Shepherd SA. 1990. Studies on southern abalone (genus Haliotis) XII*. Long-term recruitment and mortality dynamics of an unfished population. Aust. J. Mar. Freshwater Res. 41, 475–492. ( 10.1071/MF9900475) [DOI] [Google Scholar]
- 39.California Department of Fish and Game. 2005. Abalone recovery and management plan. Monterey, CA: Marine Region. [Google Scholar]
- 40.Tenger MJ, Breen PA, Lennert CE. 1989. Population biology of red abalones, Haliotis rufescens, in southern California and management of the red and pink H. corrugata, abalone fisheries. Fish. Bull. 87, 313–339. [Google Scholar]
- 41.Ben-Horin T. 2013. Withering syndrome and the management of California abalone fisheries. PhD Thesis, University of California Santa Barbara.
- 42.Moore JD, Robbins TT, Friedman CS. 2000. Withering syndrome in farmed red abalone Haliotis rufescens: thermal induction and association with a gastrointestinal Rickettsiales-like prokaryote. J. Aquat. Anim. Health 12, 26–34. () [DOI] [PubMed] [Google Scholar]
- 43.Braid BB, Moore JD, Robbins TT, Hedrick RP, Tjeerdema RS, Friedman CS. 2005. Health and survival of red abalone, Haliotis rufescens, under varying temperature, food supply, and exposure to the agent of withering syndrome. J. Invert. Pathol. 89, 219–231. ( 10.1016/j.jip.2005.06.004) [DOI] [PubMed] [Google Scholar]
- 44.Friedman CS, Biggs W, Shields JD, Hedrick RP. 2002. Transmission of withering syndrome in black abalone. J. Shellfish Res. 21, 817–824. [Google Scholar]
- 45.Lafferty KD, Ben-Horin T. 2013. Abalone farm discharges the withering syndrome pathogen into the wild. Front. Microbiol. 4, 373 ( 10.3389/fmicb.2013.00373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neuman M, Tissot B, VanBlaricom G. 2010. Overall status trends and threats assessment of black abalone (Haliotis cracherodii Leach, 1814) populations in California. J. Shellfish Res. 29, 577–586. ( 10.2983/035.029.0305) [DOI] [Google Scholar]
- 47.Naylor RL, et al. 2000. Effect of aquaculture on world fish supplies. Nature 405, 1017–1024. ( 10.1038/35016500) [DOI] [PubMed] [Google Scholar]
- 48.Diggles BK, Cooke SJ, Rose JD, Sawynok W. 2011. Ecology and welfare of aquatic animals in wild capture fisheries. Rev. Fish Biol. Fish. 21, 739–765. ( 10.1007/s11160-011-9206-x) [DOI] [Google Scholar]
- 49.Burreson EM. 2008. Misuse of PCR assay for diagnosis of molluscan protistan infections. Dis. Aquat. Org. 80, 81–83. ( 10.3354/dao01925) [DOI] [PubMed] [Google Scholar]
- 50.Burge CA, et al. 2016. Complementary approaches to diagnosing marine diseases: a union of the modern and the classic. Phil. Trans. R. Soc. B 371, 20150207 ( 10.1098/rstb.2015.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carnegie RB, Arzul I, Bushek D. 2016. Managing marine mollusc diseases in the context of regional and international commerce: policy issues and emerging concerns. Phil. Trans. R. Soc. B 371, 20150215 ( 10.1098/rstb.2015.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maynard J, et al. 2016. Improving marine disease surveillance through sea temperature monitoring, outlooks and projections. Phil. Trans. R. Soc. B 371, 20150208 ( 10.1098/rstb.2015.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Groner ML, et al. 2016. Managing marine disease emergencies in an era of rapid change. Phil. Trans. R. Soc. B 371, 20150364 ( 10.1098/rstb.2015.0364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chistoserdov AY, Smolowitz R, Mirasol F, Hsu A. 2005. Culture-dependent characterization of the microbial community associated with epizootic shell disease lesions in American lobster, Homarus americanus. J. Shellfish Res. 24, 741–747. ( 10.2983/0730-8000(2005)24%5B741:CCOTMC%5D2.0.CO;2) [DOI] [Google Scholar]
- 55.Leslie HM, McLeod KL. 2007. Confronting the challenges of implementing marine ecosystem-based management. Front. Ecol. Environ. 5, 540–548. ( 10.1890/060093) [DOI] [Google Scholar]
- 56.Smith ADM, Fulton EJ, Hobday AJ, Smith DC. 2007. Scientific tools to support the practical implementation of ecosystem-based fisheries management. ICES J. Mar. Sci. 64, 633–639. ( 10.1093/icesjms/fsm041) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Matlab routines reproducing the models used here are in the electronic supplementary material.