Abstract
Infectious marine diseases can decimate populations and are increasing among some taxa due to global change and our increasing reliance on marine environments. Marine diseases become emergencies when significant ecological, economic or social impacts occur. We can prepare for and manage these emergencies through improved surveillance, and the development and iterative refinement of approaches to mitigate disease and its impacts. Improving surveillance requires fast, accurate diagnoses, forecasting disease risk and real-time monitoring of disease-promoting environmental conditions. Diversifying impact mitigation involves increasing host resilience to disease, reducing pathogen abundance and managing environmental factors that facilitate disease. Disease surveillance and mitigation can be adaptive if informed by research advances and catalysed by communication among observers, researchers and decision-makers using information-sharing platforms. Recent increases in the awareness of the threats posed by marine diseases may lead to policy frameworks that facilitate the responses and management that marine disease emergencies require.
Keywords: adaptive management, marine disease, response plan, surveillance, impact mitigation, environmental law
1. Introduction
Frequent and severe disease outbreaks are hypothesized to be a consequence of cumulative natural and anthropogenic stressors and could affect many marine species [1–3]. Most recently, a wasting disease outbreak decimated populations of sea stars (Asteroidea) in intertidal and sub-tidal regions of eastern and western North America [4]. In addition to uncounted deaths in the east, millions of individuals died in California, Oregon and Washington in 2013 and 2014 and more are dying now in Alaska [5]. The high mortality rate, unprecedented geographical scope and multi-species scale of impacts caught the scientific and resource management community by surprise, emphasizing that we lack an effective framework for the detection and management of marine diseases.
The recent experience with the sea star wasting disease (SSWD) outbreak suggests early detection and diagnosis is the key to response and management. Fortunately, as reports of marine disease impacts are increasing, so is interest in creating policy to manage marine diseases. Such policy needs to address several basic questions including: (i) which marine disease outbreaks warrant management responses? And, (ii) how can we prepare for and manage marine disease emergencies? To answer these questions, we describe how coordinated research and management of marine diseases can reduce disease and its impacts for a variety of marine organisms. We provide examples of ongoing marine disease surveillance and response and discuss some of the inconsistencies in the coordination of these efforts. In the USA and Canada, governmental, non-profit and academic agencies often coordinate management responses. However, many recent marine disease outbreaks constitute emergencies not covered under current policies. Managing emerging outbreaks is essential and viable with strategic investment in research and response.
2. Which marine disease outbreaks are emergencies that warrant management responses?
Every disease is not an emergency. Parasites and pathogens are common in seaweeds and fishes. The typical fish caught by any marine angler often contains several parasite species, yet may still appear healthy. Most infectious agents do not cause noticeable disease and, even when they do, the resulting population impacts may be beneficial to the marine community by returning host abundances to carrying capacity [6]. Even mass mortalities may not constitute an emergency if the die-off is localized, the outbreak is self-limiting, the system is resilient to the loss of the host, the species' existence is not under other threats, or the infectious agent does not put human communities at risk. However, when a disease causes large declines in the host population resulting in endangerment of that taxa or precipitating lasting ecological, economic or social impacts (figure 1), it becomes an emergency [2,3,7].
Figure 1.
Marine diseases classifiable as emergencies due to the scope and scale of ecological, economic and social impacts. (a) Sea star wasting disease, (b) eelgrass wasting disease, (c) shrimp white spot disease, (d) white plague disease in the Caribbean coral Dendrogyra cylindrus, (e) Vibrio parahaemolyticus and V. vulnificus infections in oysters and (f) epizootic shell disease in lobsters. Most of these as well as many other marine disease emergencies cause significant impacts in more than one category. (Online version in colour.)
We define marine diseases as emergencies for their disruption of ecosystem functioning if they remove keystone predators or foundational species. For instance, the recent SSWD outbreak described in the introduction remains an emergency (figure 1a). Resultant ecological impacts are still unfolding and include a reduction in long-term ecological integrity through shifts in populations of foundation species such as mussels and ecosystem engineers like sea urchins. Seagrass wasting disease is a historical example (figure 1b) [8]. In the 1930s, a seagrass wasting disease epidemic in the North Atlantic extirpated an entire coastal ecosystem that had provided food for migratory birds and valuable habitat for commercially important fish and shellfish [9]. A range of ecosystem services deteriorated as a result, including sediment retention, filtration of waste nutrients and carbon sequestration [8,10]. While some ecological effects may be expected from most marine diseases, they are of higher concern when they reduce ecosystem services, biodiversity or ecosystem-level resilience to additional stresses.
The most costly epidemics are those affecting commercial species. The annual global value of wild and farmed fisheries is estimated in the 100s of billions of US dollars [11]. At least 67 infectious diseases have been identified as negatively impacting the economy of marine-based industries [12]. For example, billions of dollars were lost in the early 1990s as a result of a global pandemic of white spot syndrome in penaeid shrimp [12] (figure 1c). The pandemic was exacerbated by the high susceptibility of hosts to this viral pathogen and the movement of infected product among farms [12]. Subsequent management and mitigation of white spot has resulted in a return to profitable shrimp farming [13]. Economic losses associated with reduced ecotourism following disease outbreaks can also be substantial. Coral diseases have led to widespread mass mortality of acroporid corals throughout their geographical distribution [14]. In the early 1980s, white band disease changed the structure and composition of Caribbean coral reefs and affected reef-dependent fisheries and tourism industries (figure 1d) [14]. These examples demonstrate that marine diseases can cause emergencies if the economic costs are substantial.
Marine disease emergencies can also have significant social impacts capable of disrupting public safety, threatening human health or decreasing the resilience of local human communities. Along with our reliance on ocean resources, the probability of humans acquiring infections from marine organisms is also increasing [15]. These include brucellosis, leptospirosis and trichinellosis from marine mammals, avian influenza from marine birds and cryptosporidiosis and vibriosis from shellfish [16]. The most common infection route is ingestion through seafood, such as oysters. For example, transmission of pathogenic Vibrio parahaemolyticus and V. vulnificus through shellfish or other means can cause human gastrointestinal illness, septicaemia, cellulitis and, in some cases, death (figure 1e) [17]. Both V. parahaemolyticus and the more lethal V. vulnificus have increased recently as a direct response to ocean warming [18]. The emergence of epizootic shell disease in lobsters also demonstrates how marine diseases can impact human communities. Shell disease has severely damaged the historic southern New England stock, reducing an important resource for lobster fishermen in Long Island Sound and Cape Cod [19,20]. In turn, these losses have downstream impacts on the livelihood and economic vitality of these fishing communities. Collectively, these examples demonstrate how disease outbreaks that constitute emergencies can have ecological, economic and social impacts.
3. How can we systematically prepare for and manage marine disease emergencies?
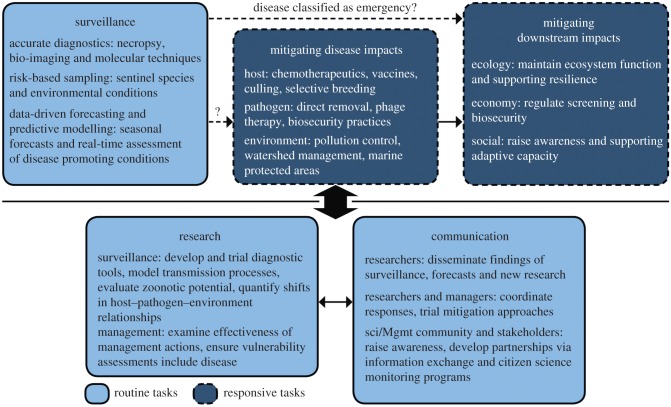
Preparing for and managing marine diseases requires surveillance and responsive mitigation (figure 2). Filling key information and capacity gaps through research, outreach and education informs management programmes for disease surveillance and mitigation. Consequently, the process used to prepare for and manage a disease emergency is adaptive; one refines diagnostic methods, initiates surveillance programmes and tailors impact mitigation as research advances and capacity among the community builds (figure 2). The response framework we recommend below highlights how effective disease surveillance creates opportunities to proactively mitigate disease and its impacts.
Figure 2.
A framework for adaptively managing marine disease emergencies. Routine disease surveillance enables early detection of more diseases. A working group then determines whether the disease is an emergency, triggering responsive efforts to mitigate disease and downstream impacts. Surveillance tools and mitigation approaches are informed by research and catalysed by effective communication among researchers, managers and stakeholders. (Online version in colour.)
(a). Pathogen and disease surveillance: the key to proactive impact mitigation
Disease surveillance requires the use of fast and accurate diagnostic tools to identify causative agents within clinically unhealthy individuals or to determine the presence of certain pathogens in a host or environment. Some governmental, non-profit and academic organizations do on-going marine disease surveillance for numerous diseases (electronic supplementary material, table S1). Federal agencies such as the National Oceanic and Atmospheric Administration and the National Institutes of Health monitor diseases of marine mammals in the USA, universities and state agencies monitor diseases in many crustaceans and shellfish in the USA and Canada, and a variety of organizations including academic institutions, international non-governmental organizations and federal and state agencies monitor diseases of corals around the globe. Diagnostic tools are critical for early detection in the absence of visible or clinical signs or when the causative agent is unknown or multifactorial (e.g. dual infections or a combination of infection and temperature). Such tools have to be paired with strategic spatio-temporal sampling designed to detect new or emerging pathogens close to their onset. This risk-based surveillance is important because knowledge gaps in how infectious agents propagate and disperse in the ocean constrain predictive modelling. Nonetheless, when possible, data-driven forecasting of disease-promoting conditions or modelling of disease dynamics can inform sampling programmes. This can result in the application of mitigation strategies before, or at the onset of an outbreak. Delaying diagnoses will be likely to make the problem more difficult to manage.
Fortunately, new diagnostic techniques are under development [21]. Recent advances in diagnostic tools such as quantitative PCR, flow cytometry and immunocapture techniques improve the ability to quantify specific pathogens at low cost. For example, in a single run with the commercially available platform Fluidigm BioMark®, it is possible to simultaneously perform 96 unique diagnostic tests on 96 samples while maintaining high analytical sensitivity [22]. The Department of Fisheries and Oceans Canada is using this platform to monitor wild and farmed salmonids for a multitude of infections [23]. In total, 47 assays for 46 microbes suspected or known to cause disease worldwide, including four viruses that are listed by the World Organisation for Animal Health (OIE), are in development for simultaneous assessment using this platform [23,24]. The utility of such diagnostic tools will continue to improve as we focus on their application to marine disease.
Although powerful, diagnostic methods have their limitations [25]. For example, qPCR recognizes and quantifies DNA of the target species (assuming high specificity of the assay), but does not imply viability or infection [26]. These assays, when fully validated, can be used as proxies for parasite or disease presence, especially in locations where the disease (agent) has been confirmed. Validation of diagnostic tools is a critical, non-trivial step. The OIE Manual of Diagnostic Tests for Aquatic Animals describes assay validation as a four-stage pathway to assess a test's ‘fitness-for-purpose’ (e.g. screening versus confirmatory assay) in a designated target population/species: stage 1, analytical characteristics; stage 2, diagnostic sensitivity and specificity; stage 3, reproducibility among laboratories; and stage 4, programme implementation [24]. Completion of stages 1, 2 and 3 is interpreted as being fit for the originally intended purpose, usually at a national level, and would be expected for tests used in a diagnostic laboratory setting. Without validation, interpretation of diagnostic test results becomes challenging with unknown false positive and negative rates. Development of validated diagnostic tools for pathogens in non-commercial species, such as seastars, sea urchins and many species of crabs or lobsters, is needed and may shed light on the ecological role of the pathogen, whether it is obligate, facultative or opportunist, a generalist or a specialist, and if it is newly introduced to an area.
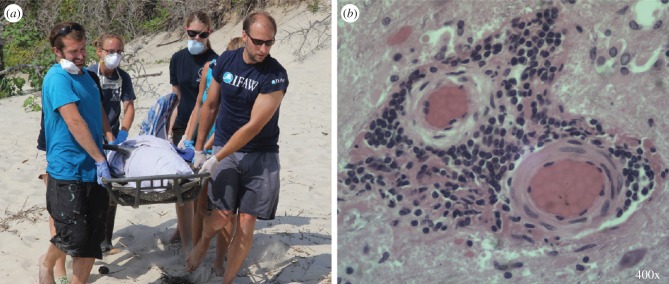
The advantages of rapid diagnostic tools are only realized with strategic spatio-temporal sampling, which can be informed through identification of risk factors using epidemiological models, risk analysis and disease simulations. Risk factors can relate to the host (e.g. species, demographic stage and sex), pathogen (e.g. range expansions or changes in virulence) or environment (e.g. temperature or salinity). Filter-feeding shellfish that act as bioaggregators of microbes in the water column can help monitor for the presence of human pathogens. For instance, mussels in the northeast coast of the USA have the highest disease prevalence and parasite burdens in the USA mussel watch programme, suggesting that this may be an area to target future surveillance [27]. Similarly, marine mammals are monitored as sentinel species for zoonotic pathogens, due to their phylogenetic and dietary similarity to humans, long-life and high-level of exposure to pathogens [16]. In both cases, diagnostic tools and strategic sampling have been paired to detect and respond to diseases as well as understand their ultimate causes (figure 3).
Figure 3.
Cetacean morbillivirus (CeMV) causes dolphin stranding and mortality. Identifying CeMV as the cause of a mortality event depends on: fresh tissues, trained responders (a), and available, equipped diagnostic laboratories. In 2013/2014 CeMV was detected by PCR, virus isolation and histology, which stains intensely brown where the virus is present (b). This rapid response effort was made possible under the US Marine Mammal Health and Stranding Program. Under future legislation, similar coordinated responses could be possible for diseases in other marine taxa. Photos courtesy of Virginia Aquarium & Marine Science Center (both) and David Rotstein (b). (Online version in colour.)
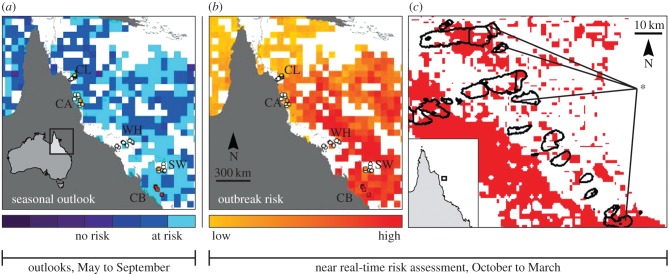
For some diseases affecting tropical corals, web-accessible seasonal forecasts and near real-time assessments of outbreak likelihoods exist. White syndrome outbreaks in Australia's Great Barrier Reef occurred with greater severity following mild winters and when summer conditions were warm [28,29]. These empirical findings were made possible by strategic monitoring programmes that assessed disease presence and severity for 10 years. The forecasting tools developed from such observations can visualize outbreak likelihoods as high, medium or low based on data-driven mathematical algorithms that query remotely sensed sea surface temperature datasets (figure 4). Assessments showing high outbreak likelihoods trigger managers to target monitoring efforts and, if disease is severe, implement actions to reduce impacts or support recovery. Essentially, forecasting disease-promoting conditions increases support for and the vigilance of those engaged in disease surveillance, which can result in earlier and more robust disease detection.
Figure 4.
Predictive tools developed for the coral disease white syndromes (WS) in Australia's Great Barrier Reef. Statistical analyses were used to relate sea surface temperature patterns in the winter (seasonal forecast (a)) and summer (near real-time risk assessment (b,c)) to WS prevalence during outbreak and non-outbreak years [28,29]. These web-accessible tools are monitored by managers and scientists and used to target response efforts. Forecasting and near real-time monitoring of disease-promoting conditions can increase vigilance and support for surveillance efforts, resulting in earlier disease detection. (Online version in colour.)
Forecasting tools can be developed for other diseases if two criteria are met: (i) the major environmental risk factors for disease are known; and (ii) the relevant environmental data are available regularly and at a sufficiently high quality and spatial and temporal resolutions to represent conditions the organisms experience [30]. Candidate diseases for the development of forecasting tools include seagrass wasting disease and abalone withering syndrome because field and experimental data exist to calibrate and validate models. Recent advances allow such forecasts and real-time assessments of environmental conditions to be paired with models of disease transmission and spread. For example, three-dimensional oceanographic models that enable hydrodynamic modelling of environmentally sensitive pathogens has proved useful for predicting transmission probabilities of salmon pathogens, thereby influencing aquaculture strategies in Norway [31]. Monitoring of environmental conditions conducive to marine disease outbreaks will be increasingly important as the climate changes. As with diagnostics and sampling, developing data-driven forecasting tools and predictive modelling for marine diseases will require new investments in research that could be facilitated by policy changes.
(b). Shifting host–pathogen–environment relationships to mitigate disease
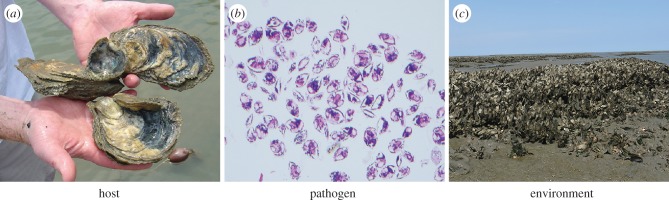
Management interventions can reduce the extent or severity of the outbreak itself, dispelling the notion among some that nothing can be done to reduce marine diseases [12]. Mitigation options include targeting the host or the infectious agent, ameliorating disease-promoting environmental conditions, or some combination (figure 5). The majority of mitigation efforts undertaken to date have been for diseases related to aquaculture and for marine mammals in wildlife hospitals. Nonetheless, there are some examples of disease mitigation in wild populations (table 1). These programmes often target the host by reducing the pool of infectious or susceptible individuals. This includes vaccination (e.g. on fish farms [39] or, for marine mammals [40]), application of chemical treatments to lower pathogen intensity [39], culling of diseased individuals or even whole populations (e.g. sabellid worms in abalone and various pathogens in farmed salmon [12]), and proper disposal of sick individuals that incorporates biosecurity measurements (e.g. crabs with bitter crab syndrome, bycatch or offal from processing plants) [41,42]. Host populations can also be manipulated by promoting increased resistance to disease. Frequencies of resistant genotypes may be increased in wild populations through the designation of marine protected areas (MPAs) or sanctuaries, or in aquaculture populations through selective breeding. Both of these methods are being used to increase resistance in oysters affected by MSX and dermo diseases [33]. Managers are not forced to watch in despair as a marine disease emergency unfolds.
Figure 5.
Management of oysters in the eastern US to reduce the impacts of Perkinsus marinus (dermo) and Haplosporidium nelsoni (MSX) can target the host, the pathogen or the environment. Disease-resistant hosts are protected in sanctuaries from harvest to promote an increase in the frequency of resistant genotypes (a). Biosecurity management focuses on pathogen screening in aquacultured seed (viewed histologically here) to prevent disease introduction into new areas and exacerbation of disease where it occurs (b). Conserving and restoring three-dimensional reef habitat enhances growth, reproduction and recruitment of healthy oyster metapopulations (c). (Online version in colour.)
Table 1.
Marine disease mitigation in wild North American populations. Included are the disease mitigated, host and pathogen species, the agencies that have conducted the mitigation, the strategies implemented and the project goal.
| disease | host | pathogen | mitigation approach | agencya | goal |
|---|---|---|---|---|---|
| bitter crab disease | snow crabs | Hematodinium sp. | dispose of infected animals in landfills; no culling of diseased animals at sea [19] | DFO | control |
| bitter crab disease | Tanner crab | Hematodinium sp. | include disease in fisheries models [32] | NOAA | control |
| epizootic shell disease | American lobster | bacterial dysbiosis | moratorium on being considered on mid-Atlantic fishery [20] | ASMFC | recovery |
| MSX, dermo | oysters | Haplosporidium nelsoni, Perkinsus marinus | promotion of resistant populations through sanctuaries from harvest, rotational harvest programs [33,34] | VMRC, MDNR | recovery |
| sabellid infestation | abalone, black turban snails | sabellid polychaete Terebrassabella heterouncinata | culling of highly susceptible and preferred hosts (black turban snails), installing screens at abalone mariculture facility [35] | CDFG | eradication |
| black band disease | 12 coral species | bacterial colonies | vacuuming bacterial mat from affected area and then sealing with underwater epoxy [36] | NOAA, universities | control disease |
| disease outbreak | corals | various | close the reef to any human activities [36] | NOAA | prevent transmission |
| viral haemorrhagic septicaemia (VHS) | salmonids | viral haemorrhagic septicaemia virus (VHSv) | quarantine and cull hatchery salmon testing positive [37] | WDFW | control |
| icthyophoniasis, VHS | Pacific herring | Ichthophonus hoferi, VHSv | include disease in fisheries models [38] | NOAA | control |
aWDFW, Washington Department of Fish and Game; CDFG, California Department of Fish and Game; DFO, Department of Fisheries and Oceans Canada; ASMFC, Atlantic States Marine Fisheries Commission; VMRC, Virginia Marine Resources Commission; MDNR, Maryland Department of Natural Resources.
Interventions that directly target marine pathogens are effective by either direct removal of pathogens or manipulation of microbial communities to reduce pathogen virulence. Methods for direct removal of sea lice on salmon farms include biological control by co-stocking with endemic cleaner fish that are sea louse predators [43]. In another approach, mussels (Mytilus edilus) are placed near salmon farms to filter larval sea lice and pathogenic Vibrio bacteria species from the water column [44,45]. These multi-trophic approaches are promising for sustainable management of various types of infections in aquaculture and warrant continued development. Phage therapy also has potential. In this approach, pathogenic bacteria are targeted with specific viruses that lyse the cells [46] or, in the case of the rickettsia-like organisms that cause withering syndrome in abalone, reduce the virulence of the infected cells [47]. Interventions like these require creative problem-solving based on research.
Altering the environmental conditions where marine diseases occur can also be effective at reducing impacts. Many natural marine habitats provide ecosystem services in the form of disease reduction. For example, shrimp aquaculture benefits from nearby mangrove forests, through filtering of water-borne pollutants and supply of larval broodstock [48]. Mangroves restoration could improve water quality and reduce nutrients that trigger disease and reduce the need to import shrimp larvae (which might introduce disease). Similarly, because seagrasses have been shown to filter and detoxify human pathogens and other pollutants [8,49], protecting or restoring seagrasses that harbour valuable bacteria with algicidal properties against harmful algal blooms could have beneficial effects [50]. While environmental manipulations are most feasible on smaller scales, such as those relevant to aquaculture, they can also occur at larger scales. For example, Vibrio sp. are known to increase with nutrient-driven increases in estuarine plankton [51]. Watershed-based reductions of nutrient loading to estuarine systems can retain the natural abundance and diversity of bacterial and phytoplankton species in the receiving waters, thereby reducing risks to human health [52]. Another effective strategy is to reduce the impacts of other stressors on diseased species. For example, designation of MPAs can decrease the amount of damage resulting from boats, abandoned fishing gear and human impacts [53]. Such areas have lower coral disease prevalence than adjacent areas that are frequently visited and fished [54].
(c). Mitigating downstream impacts of marine diseases
Mitigating marine disease itself reduces downstream ecological, economic and social impacts; however, in many cases the impacts themselves require mitigation. The local context and constraints, including whether the affected populations and communities can benefit from changes in human activities or habitat restoration, will dictate what types of restoration are possible. A recent meta-analysis suggests that, while often slow, restoration of impacted ecosystems can be successful [55]. These actions may help to mitigate economic and social impacts, though there are also more direct options.
Direct mitigation of economic impacts of diseases can include revising stock-recruitment fishery models to explicitly account for disease [56] and ensuring biosecurity practices reduce or eliminate transport of infected individuals and product. Both the Pacific herring and Tanner crab industries have used stock assessment models that include disease and adjust allowable catch to account for disease-induced mortality [32,38]. Changes to those fisheries models were implemented after disease outbreaks occurred, but such model adjustments can be made more proactively in the future for other marine diseases through increased monitoring of risk factors.
The billions of dollars lost due to white spot disease among panaeid shrimp in the 1990s demonstrates the role that biosecurity practices play in reducing economic impacts (figure 1c). Biosecurity is also critically important for reducing the most alarming of social impacts: human illness and death. Concerns about spillover of marine diseases into humans usually involve food or water-borne contact. Such contact can often be reduced through public health messaging. For example, when a V. parahaemolyticus outbreak occurs in a particular area, the Interstate Shellfish Sanitation Conference requires the Shellfish Control Authority to immediately close the affected area, issue an advisory and initiate a recall of oyster and other shellfish products [57]. These examples can be tailored for use with other marine diseases.
Disease management must be adaptive and involve information exchange. Importantly, timely implementation and trial of mitigation actions hinges entirely on disease surveillance. For some types of marine disease management decisions, competing interests will have to be balanced and many groups will need to be involved. Resolving contentious issues that may involve public health or industry viability will require clear policy and coordinated efforts. This attests to the value of future policy for bringing increased attention and resource mobilization to marine disease responses.
4. Future directions and conclusion
Some marine disease outbreaks that qualify as emergencies are ongoing (e.g. sea star wasting in Alaska and morbillivirus in dolphins along the eastern seaboard of the USA) and new outbreaks are certain to occur in the coming years. The framework we used to summarize marine disease management can maximize opportunities to mitigate the impacts of future disease emergencies. For the framework we recommend to be adaptive, both surveillance and responsive impact mitigation need to be informed by research and catalysed by effective communication among research, management and stakeholder groups. Key areas for investment of research effort include as follows.
(a). Surveillance
— Developing and evaluating more diagnostic tools and increasing capacity among the science and management communities to use these tools.
— Developing hydrodynamic models of pathogen propagation and dispersal.
— Quantifying the nature of shifts in host, pathogen and environment relationships under climate and anthropogenic change and using these data to develop monitoring and forecasting tools.
— Developing data-sharing and disease-mapping tools to transfer information among scientists, managers and the public.
(b). Impact mitigation
— Trialling and evaluating management actions implemented to mitigate disease and its downstream impacts.
— Supporting the adaptive capacity of human communities dependent on fisheries.
— Ensuring that vulnerability assessments of fisheries include disease.
— Developing models and monitoring programmes to assess disease impacts and mitigation plans.
(c). Development of marine disease adaptive management policies
How might changes to policy help us diagnose and manage marine diseases? One limitation is the lack of a coordinated response or timely funding. For instance, investigation of the sea star wasting disease depended on interested parties donating time and resources. The need for policy that supports marine disease emergency responses and management is starting to be recognized by some governing bodies. In response to the ongoing SSWD outbreak, Washington state representative Dennis Heck of the US Congress introduced a bill that, if passed, will become the Marine Disease Emergency Act (MDE Act, HR936). This bill would increase capacity for timely and coordinated responses to future marine disease outbreaks and would: (i) ensure marine disease outbreaks are considered for classification as ‘emergencies’; (ii) appoint a working group in the US National Oceanic and Atmospheric Administration to advise on assessing, declaring and responding to emergencies; (iii) form a data repository to disseminate and facilitate research to manage disease impacts; and (iv) designate financial resources for research and response coordination. A similar programme, the US Unusual Mortality Event (UME) programme, coordinates responses to marine mammal mortality events. After unprecedented mortalities of dolphins along the eastern seaboard of the USA in 1987 and 1988 [58], this programme was established under the Marine Mammal Protection Act (MMPA) amendments in 1992. The value of the MMPA for marine mammals was demonstrated during the rapid response to increasing dolphin mortalities following the Deepwater Horizon oil spill in the Gulf of Mexico [59]. However, the MMPA does not cover either fish or invertebrates, hence the need for the proposed MDE Act.
Disseminating surveillance data and research findings through open access data repositories will facilitate research and uptake of findings among decision-makers. The recent proliferation of information-sharing platforms is a positive part of this era of rapid change within which we have to manage marine disease. Indeed, linked open data that enables sophisticated data queries is already revolutionizing human disease diagnosis and care management (e.g. [60]). Such communication platforms can connect marine disease researchers with decision-makers. Consequently, management and responses coordinated under the MDE Act or future similar legislation can be adaptive and effective (figure 6). Research advances can be incorporated into management strategy and responses can be coordinated when disease is detected or anticipated, warranting these marine emergencies the attention they require.
Figure 6.
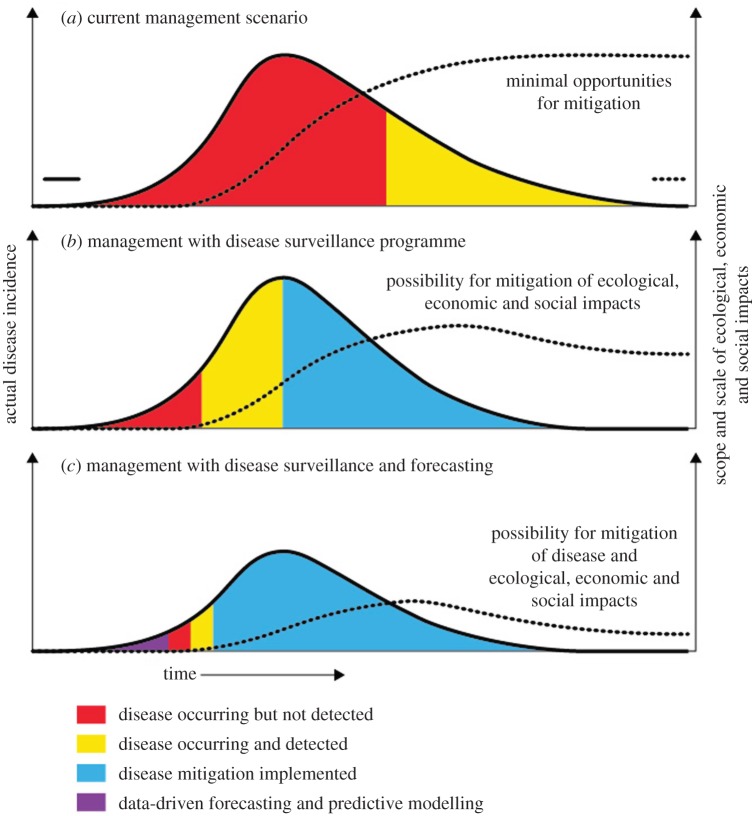
How the proposed disease management framework improves the timing of disease detection and extent of impact mitigation. Currently, marine diseases are detected near or after their epidemic peak (a) and there is limited management of the disease outbreak or downstream ecological, economic or social and cultural impact. Diseases could be detected earlier with greater disease surveillance, which increases management opportunities, especially for mitigating downstream impacts (b). Diseases are best managed when surveillance programmes can include data-driven forecasting and predictive modelling, ensuring mitigation starts before the epidemic peak (c) (see also figure 4). (Online version in colour.)
Supplementary Material
Acknowledgements
Figures were developed in collaboration with D. Tracey. The manuscript benefited from input by K. Lafferty, J. Lamb, P. Hershberger and anonymous reviewers.
Authors' contributions
All authors contributed to the conception of the article and figure content and design. M.L.G., J.M. and C.D.H. led drafting of the manuscript with substantial input from all other authors.
Competing interests
We have no competing interests.
Funding
This paper was conceived during a meeting of the NSF-supported Research Coordination Network (RCN) on the Ecology and Evolution of Infectious Disease (grant OCE-1215977 awarded to C.D.H.) made possible by logistical support from Friday Harbor Laboratories of the University of Washington. Financial support was also provided by an NOAA Climate Program Office grant to the supervising author (NA13OAR4310127) and to the first author by the Canadian Excellence Research Chair in Aquatic Epidemiology at the University of Prince Edward Island.
References
- 1.Doney SC, et al. 2012. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 4, 11–37. (doi:10.1146/annurev-marine-041911-111611) [DOI] [PubMed] [Google Scholar]
- 2.Harvell D, et al. 2004. The rising tide of ocean diseases: unsolved problems and research priorities. Front. Ecol. Environ. 2, 375–382. (doi:10.1890/1540-9295(2004)002[0375:TRTOOD]2.0.CO;2) [Google Scholar]
- 3.Burge CA, et al. 2014. Climate change influences on marine infectious diseases: implications for management and society. Annu. Rev. Mar. Sci. 6, 249–277. (doi:10.1146/annurev-marine-010213-135029) [DOI] [PubMed] [Google Scholar]
- 4.Hewson I, et al. 2014. Densovirus associated with sea-star wasting disease and mass mortality. Proc. Natl Acad. Sci. USA 111, 17 278–17 283. (doi:10.1073/pnas.1416625111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenlord ME, et al. 2016. Ochre star mortality during the 2014 wasting disease epizootic: role of population size structure and temperature. Phil. Trans. R. Soc. B 371, 20150212 (doi:10.1089/rstb.2015.0212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrens MD, Lafferty KD. 2004. Effects of marine reserves and urchin disease on southern Californian rocky reef communities. Mar. Ecol. Prog. Ser. 279, 129–139. (doi:10.3354/meps279129) [Google Scholar]
- 7.Groner ML, et al. 2015. Emergency response for marine diseases. Science 347, 1210 (doi:10.1126/science.347.6227.1210-a) [DOI] [PubMed] [Google Scholar]
- 8.Short FT, Wyllie-Echeverria S. 1996. Natural and human-induced disturbance of seagrasses. Environ. Conserv. 23, 17–27. (doi:10.1017/s0376890003821) [Google Scholar]
- 9.Godet L, Fournier J, Van Katwijk M, Olivier F, Le Mao P, Retière C. 2008. Before and after wasting disease in common eelgrass Zostera marina along the French Atlantic coasts: a general overview and first accurate mapping. Dis. Aquat. Org. 79, 249–255. (doi:10.3354/dao01897) [DOI] [PubMed] [Google Scholar]
- 10.Barbier EB, Hacker SD, Kennedy C, Koch EW, Stier AC, Silliman BR. 2011. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 81, 169–193. (doi:10.1890/10-1510.1) [Google Scholar]
- 11.Food and Agricultural Organization of the United Nations. 2014. The state of world fisheries and aquaculture: opportunities and challenges. Rome, Italy: FAO. [Google Scholar]
- 12.Lafferty KD, Harvell CD, Conrad JM, Friedman CS, Kent ML, Kuris AM, Powell EN, Rondeau D, Saksida SM. 2015. Infectious diseases affect marine fisheries and aquaculture economics. Annu. Rev. Mar. Sci. 7, 471–496. (doi:10.1146/annurev-marine-010814-015646) [DOI] [PubMed] [Google Scholar]
- 13.Lightner DV. 2011. Status of shrimp diseases and advances in shrimp health management. Dis. Asian Aquac. 7, 121–133. [Google Scholar]
- 14.Aronson RB, Precht WF. 2001. White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460, 25–38. [Google Scholar]
- 15.Broglia A, Kapel C. 2011. Changing dietary habits in a changing world: emerging drivers for the transmission of foodborne parasitic zoonoses. Vet. Parasitol. 182, 2–13. (doi:10.1016/j.vetpar.2011.07.011) [DOI] [PubMed] [Google Scholar]
- 16.Bossart GD. 2011. Marine mammals as sentinel species for oceans and human health. Vet. Pathol. 48, 676–690. (doi:10.1177/0300985810388525) [DOI] [PubMed] [Google Scholar]
- 17.Drake SL, DePaola A, Jaykus LA. 2007. An overview of Vibrio vulnificus and Vibrio parahaemolyticus. Comp. Rev. Food Sci. Food Safety 6, 120–144. (doi:10.1111/j.1541-4337.2007.00022.x) [Google Scholar]
- 18.Baker-Austin C, Trinanes JA, Taylor NG, Hartnell R, Siitonen A, Martinez-Urtaza J. 2013. Emerging Vibrio risk at high latitudes in response to ocean warming. Nat. Clim. Change 3, 73–77. (doi:10.1038/nclimate1628) [Google Scholar]
- 19.Shields JD. 2012. The impact of pathogens on exploited populations of decapod crustaceans. J. Invertebr. Pathol. 110, 211–224. (doi:10.1016/j.jip.2012.03.01) [DOI] [PubMed] [Google Scholar]
- 20.Shields JD. 2013. Complex etiologies of emerging diseases in lobsters (Homarus americanus) from Long Island Sound. Can. J. Fish. Aquat. Sci. 70, 1576–1587. (doi:org/10.1139/Cjfas-2013-0050) [Google Scholar]
- 21.Burge CA, et al. 2016. Complementary approaches to diagnosing marine diseases: a union of the modern and the classic. Phil. Trans. R. Soc. B 371, 20150207 (doi:10.1098/rstb.2015.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weaver S, Dube S, Mir A, Qin J, Sun G, Ramakrishnan R, Jones RC, Livak KJ. 2010. Taking qPCR to a higher level: analysis of CNV reveals the power of high throughput qPCR to enhance quantitative resolution. Methods 50, 271–276. (doi:10.1016/j.ymeth.2010.01.003) [DOI] [PubMed] [Google Scholar]
- 23.Department of Fisheries and Oceans Canada. 2015. Proceedings of the national peer review of the Fluidigm® Biomark platform: evaluation to assess fitness for purpose in microbial monitoring, 2–4 December 2014. DFO Can. Sci. Advis. Sec. Proceed. Ser. 2015/039.
- 24.OIE (World Organisation for Animal Health). 2013. Principles and methods of validation of diagnostic assays for infectious diseases. In Manual of diagnostic tests for aquatic animals 2013, Chapter 1.1.2. See www.oie.int/international-standard-setting/aquatic-manual/access-online/ (accessed 18 August 2014).
- 25.Carnegie RB, Arzul I, Bushek D. 2016. Managing marine mollusc diseases in the context of regional and international commerce: policy issues and emerging concerns. Phil. Trans. R. Soc. B 371, 20150215 (doi:10.1098/rstb.2015.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burreson EM. 2008. Misuse of PCR assay for diagnosis of mollusc protistan infections. Dis. Aquat. Org. 80, 81–83. (doi:10.3354/dao01925) [DOI] [PubMed] [Google Scholar]
- 27.Kim Y, Powell EN. 2007. Distribution of parasites and pathologies in sentinel bivalves: NOAA status and trends ‘Mussel Watch’ program. J. Shell Res. 26, 1115–1151. (doi:10.2983/0730-8000(2007)26[1115:DOPAPI]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 28.Heron SF, Willis BL, Skirving WJ, Mark Eakin C, Page CA, Miller IR. 2010. Summer hot snaps and winter conditions: modelling white syndrome outbreaks on Great Barrier Reef corals. PLoS ONE 5, e12210 (doi:10.1371/journal.pone.0012210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maynard JA, Anthony KRN, Harvell CD, Burgman MA, Beeden R, Sweatman H, Heron SF, Lamb JB, Willis BL. 2011. Predicting outbreaks of a climate-driven coral disease in the Great Barrier Reef. Coral Reefs 30, 485–495. (doi:10.1007/s00338-010-0708-0) [Google Scholar]
- 30.Maynard J, et al. 2016. Improving marine disease surveillance through sea temperature monitoring, outlooks and projections. Phil. Trans. R. Soc. B 371, 20150208 (doi:10.1089/rstb.2015.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stene A, Viljugrein H, Yndestad H, Tavornpanich S, Skjerve E. 2014. Transmission dynamics of pancreas disease (PD) in a Norwegian fjord: aspects of water transport, contact networks and infection pressure among salmon farms. J. Fish Dis. 37, 123–134. (doi:10.1111/jfd.12090) [DOI] [PubMed] [Google Scholar]
- 32.Siddeek MSM, Zheng J, Morado JF, Kruse GH, Bechtol WR. 2010. Effect of bitter crab disease on rebuilding in Alaska Tanner crab stocks. ICES J. Mar. Sci. 67, 2027–2032. (doi:10.1093/icesjms/fsq061) [Google Scholar]
- 33.Ragone Calvo LM, Calvo GW, Burreson EM. 2003. Dual disease resistance in a selectively bred eastern oyster, Crassostrea virginica, strain tested in Chesapeake Bay. Aquaculture 220, 69–87. (doi:10.1016/S0044-8486(02)00399-X) [Google Scholar]
- 34.Carnegie RB, Burreson EM. 2011. Declining impact of an introduced pathogen: Haplosporidium nelsoni in the oyster Crassostrea virginica in Chesapeake Bay. Mar. Ecol. Progress Ser. 432, 1–15. (doi:10.3354/meps09221) [Google Scholar]
- 35.Culver CS, Kuris AM. 2000. The apparent eradication of a locally established introduced marine pest. Biol. Invasions 2, 245–253. (doi:10.1023/A:1010082407254) [Google Scholar]
- 36.Raymundo LJ, Couch CS, Harvell CD.2008. Coral disease handbook: guidelines for assessment, monitoring and management. St Lucia, Queensland, Australia: Coral Reef Targeted Research and Capacity Building for Management Program.
- 37.Amos K, Thomas J, Hopper K. 1998. A case history of adaptive management strategies for viral hemorrhagic septicemia virus (VHSV) in Washington State. J. Aquat. Anim. Health 10, 152–159. (doi:10.1577/1548-8667(1998)010<0152:ACHOAM>2.0.CO;2) [Google Scholar]
- 38.Marty GD, Hulson PJF, Miller SE, Quinn TJ II, Moffitt SD, Merizon RA. 2010. Failure of population recovery in relation to disease in Pacific herring. Dis. Aquat. Org. 90, 1–14. (doi:10.3354/dao02210) [DOI] [PubMed] [Google Scholar]
- 39.Subasinghe R. 2009. Disease control in aquaculture and the responsible use of veterinary drugs and vaccines: the issues, prospects and challenges. Options Méditerranéennes 86, 5–11. [Google Scholar]
- 40.Jessup DA, Murray MJ, Casper DR, Brownstein D, kreuder-Johnson C. 2009. Canine distemper vaccination is a safe and useful preventative procedure for southern sea otters (Enhydra lutris nereis). J. Zoo Wildl. Med. 40, 705–710. (doi:10.1638/2008-0080.1) [DOI] [PubMed] [Google Scholar]
- 41.Stentiford GD, Shields JD. 2005. A review of the parasitic dinoflagellates Hematodinium species and Hematodinium-like infections in marine crustaceans. Dis. Aquat. Org. 66, 47–70. (doi:10.3354/dao066047) [DOI] [PubMed] [Google Scholar]
- 42.Karreman G, Klotins K, Bebak J, Gustafson L, Osborn A, Kebus MJ, Innes P, Tiwari A. 2015. Aquatic animal biosecurity: a case study of bioexclusion of viral hemorrhagic septicemia virus in an Atlantic salmon hatchery. J. Appl. Aquac. 27, 299–317. (doi:10.1080/10454438.2014.914996) [Google Scholar]
- 43.Groner ML, Cox R, Gettinby G, Revie CW. 2013. Use of agent-based modelling to predict benefits of cleaner fish in controlling sea lice, Lepeophtheirus salmonis, infestations on farmed Atlantic salmon, Salmo salar L. J. Fish. Dis. 36, 195–208. (doi:10.1111/jfd.12017) [DOI] [PubMed] [Google Scholar]
- 44.Molloy SD, Pietrak MR, Bouchard DA, Bricknell I. 2011. Ingestion of Lepeophtheirus salmonis by the blue mussel Mytilus edulis. Aquaculture 311, 61–64. (doi:10.1016/j.aquaculture.2010.11.038) [Google Scholar]
- 45.Pietrak MR, Molloy SD, Bouchard DA, Singer JT, Bricknell I. 2012. Potential role of Mytilus edulis in modulating the infectious pressure of Vibrio anguillarum 02β on an integrated multi-trophic aquaculture farm. Aquaculture 326–329, 36–39. (doi:10.1016/j.aquaculture.2011.11.024) [Google Scholar]
- 46.Oliveira J, Castilho F, Cunha A, Pereira MJ. 2012. Bacteriophage therapy as a bacterial control strategy in aquaculture. Aquacult. Intl. 20, 879–910. (doi:10.1007/s10499-012-9515-7) [Google Scholar]
- 47.Friedman CS, Wight N, Crosson LM, VanBlaricom GR, Lafferty KD. 2014. Reduced disease in black abalone following mass mortality: phage therapy and natural selection. Front. Microbiol. 5, 78 (doi:10.3389/fmicb.2014.00078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kautsky N, Rönnbäck P, Tedengren M, Troell M. 2000. Ecosystem perspectives on management of disease in shrimp pond farming. Aquaculture 191, 145–161. (doi:10.1016/S0044-8486(00)00424-5) [Google Scholar]
- 49.Shuval H. 2003. Estimating the global burden of thalassogenic diseases: human infectious diseases caused by wastewater pollution of the marine environment. J. Water Health 1, 53–64. [PubMed] [Google Scholar]
- 50.Onishi Y, Mohri Y, Tuji A, Ohgi K, Yamaguchi A, Imai I. 2014. The seagrass Zostera marina harbors growth-inhibiting bacteria against the toxic dinoflagellate Alexandrium tamarense. Fish. Sci. 80, 353–362. (doi:10.1007/s12562-013-0688-4) [Google Scholar]
- 51.Hsieh JL, Fries JS, Noble RT. 2007. Vibrio and phytoplankton dynamics during the summer of 2004 in a eutrophying estuary. Ecol. Appl. 17, S102–S109. (doi:10.1890/05-1274.1) [Google Scholar]
- 52.Suikkanen S, Pulina S, Engström-Öst J, Lehtiniemi M, Lehtinen S, Brutemark A. 2013. Climate change and eutrophication induced shifts in northern summer plankton communities. PLoS ONE 8, e66475 (doi:10.1371/journal.pone.0066475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamb JB, Wenger AS, Devlin MJ, Ceccarelli DM, Williamson DH, Willis BL. 2016. Reserves as tools for alleviating impacts of marine disease. Phil. Trans. R. Soc. B 371, 20150210 (doi:10.1098/rstb.2015.0210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamb JB, Williamson DH, Russ GR, Willis BL. 2015. Protected areas mitigate diseases of reef-building corals by reducing damage from fishing. Ecology 96, 2555–2567. (doi:10.1890/14-1952.1) [DOI] [PubMed] [Google Scholar]
- 55.Lotze HK, Coll M, Magera AM, Ward-Paige C, Airoldi L. 2011. Recovery of marine animal populations and ecosystems. Trends Ecol. Evol. 26, 595–605. (doi:10.1016/j.tree.2011.07.008) [DOI] [PubMed] [Google Scholar]
- 56.Ben-Horin T, Lafferty KD, Bidegain G, Lenihan HS. 2016. Fishing diseased abalone to promote yield and conservation. Phil. Trans. R. Soc. B 371, 20150211 (doi:10.1098/rstb.2015.0211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Interstate Shellfish Sanitation Conference (ISSC). 2015. V.p. illness response guidance document; 2015. See http://www.issc.org/client_resources/002015%20executive%20board%20utah/vp%20illness%20response%20interim%20guidance%2003-13-2015.pdf
- 58.Gulland FMD. 2006. Review of the Marine Mammal Unusual Mortality Event Response Program of the National Marine Fisheries Service. U.S. Department of Commerce, NOAA Technical Memorandum NMFS-OPR-33.
- 59.Venn-Watson S, et al. 2015. Demographic clusters identified within the northern Gulf of Mexico common bottlenose dolphin (Tursiops truncates) unusual mortality event: January 2010–June 2013. PLoS ONE 10, e0117248 (doi:10.1371/journal.pone.0117248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumarasamy KK, et al. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis. 10, 597–602. (doi:10.1016/S1473-3099(10)70143-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.