Abstract
Compared with laboratory environments, complex natural environments promote brain cell proliferation and neurogenesis. Predators are one important feature of many natural environments, but, in the laboratory, predatory stimuli tend to inhibit brain cell proliferation. Often, laboratory predatory stimuli also elevate plasma glucocorticoids, which can then reduce brain cell proliferation. However, it is unknown how natural predators affect cell proliferation or whether glucocorticoids mediate the neurogenic response to natural predators. We examined brain cell proliferation in six populations of the electric fish, Brachyhypopomus occidentalis, exposed to three forms of predator stimuli: (i) natural variation in the density of predatory catfish; (ii) tail injury, presumably from predation attempts; and (iii) the acute stress of capture. Populations with higher predation pressure had lower density of proliferating (PCNA+) cells, and fish with injured tails had lower proliferating cell density than those with intact tails. However, plasma cortisol did not vary at the population level according to predation pressure or at the individual level according to tail injury. Capture stress significantly increased cortisol, but only marginally decreased cell proliferation. Thus, it appears that the presence of natural predators inhibits brain cell proliferation, but not via mechanisms that depend on changes in basal cortisol levels. This study is the first demonstration of predator-induced alteration of brain cell proliferation in a free-living vertebrate.
Keywords: predators, brain cell proliferation, neurogenesis, cortisol, fish
1. Introduction
One way the environment influences the structure of the brain is by changing the rate at which new cells are generated [1,2]. Animals living in more complex environments, such as in natural conditions in the wild or enriched conditions in the laboratory, tend to have higher rates of brain cell proliferation and neurogenesis than those living in simplified laboratory environments [3–6]. However, animals exposed to threats in the environment produce fewer cells and neurons than those in benign environments [7,8].
One such threat—exposure to predators—is known to decrease brain cell proliferation in the laboratory [9,10]. However, this effect has not been examined in the wild, where animals may experience predator threats differently because they can execute a broader range of behaviours to evade predators than they can in captivity. The presence of predators in the wild might provide a source of complex stimuli or provoke behaviours that activate cell proliferation. Subsequent neuronal differentiation and integration of new neurons might then contribute to enhanced cognitive abilities (e.g. improved spatial orientation or vigilance) that enable animals to avoid predation. Alternatively, predators might constitute a threat that directly suppresses cell proliferation or inhibits behaviours (e.g. exploration) that would otherwise promote cell proliferation.
In the laboratory, environmental stressors often inhibit brain cell proliferation by elevating glucocorticoid secretion [2,7]. Experimental exposure to predators or their odours can increase the production of glucocorticoids [9,11–13], and exogenous glucocorticoids can inhibit the production of proliferating cells [7,14]. However, the observed relationships between predators, glucocorticoids and brain cell proliferation are not consistent or simple: in some cases, predator exposure increases glucocorticoids without affecting brain cell proliferation [15], while in other cases environmental manipulations that increase glucocorticoids can enhance brain cell proliferation [7,16]. This inconsistency is paralleled by results from natural populations: in some cases, populations exposed to high predation pressure have higher basal glucocorticoid levels or glucocorticoid responses to acute stress [17,18], while in other cases they do not [19,20]. To the best of our knowledge, no one has examined the relationship between glucocorticoid concentrations and brain cell proliferation in animals living in their natural habitat.
In this study, we assessed the influence of naturally occurring predators on brain cell production and glucocorticoids by examining free-living electric fish, Brachyhypopomus occidentalis, in populations in Panama that vary in their exposure to electroreceptive, predatory catfish, Rhamdia quelen. Concurrent studies in Panama indicated that the behaviour and morphology of B. occidentalis are significantly affected by interactions with R. quelen [21]. Like all weakly electric fish, B. occidentalis emit electric discharges used for locating objects in the environment and for communication. Tran [21] showed that B. occidentalis living in streams with a high density of Rhamdia produce electric signals that should make them less detectable by the predator. Brachyhypopomus occidentalis in streams with high Rhamdia density also suffer a high prevalence of tail injury, reaching as high as 45% of the sampled population (electronic supplementary material, table S1). The positive correlation between Rhamdia density and tail injury in the field along with the lack of tail injury in captive B. occidentalis populations suggest that predation rather than intraspecific aggression is the source of tail damage [21].
The occurrence of differential predation pressure on B. occidentalis in Panamian populations offers an excellent opportunity to examine how exposure to predators and naturally occurring injury affect forebrain cell proliferation. We focused our study on the forebrain because it contains regions that probably help coordinate the behavioural response to predators [22,23]. In teleost fish, the dorsolateral telencephalon (DL) participates in spatial orientation and learning, and it is probably homologous to the mammalian hippocampus [23,24], a cell proliferation zone in mammals that is influenced by predator stimuli [9,25]. The dorsomedial telencephalon (DM), a likely homologue of the mammalian amygdala, is involved in conditioned avoidance [24,26], and the ventral telencephalon (V), a likely homologue of basal ganglia, is involved in selecting motor actions and evaluating their outcome [27].
Several studies in other teleosts have shown that forebrain cell proliferation can be enhanced or inhibited by environmental stimuli [28–32]. Moreover, in the congener B. gauderio, brain cell proliferation is greatly enhanced in free-living fish compared with those living in captivity, even those in enriched, semi-natural laboratory conditions [6]. The greater complexity of social interactions in the wild probably contributes to this enhanced cell proliferation, but we do not know whether interaction with natural predators also influences cell proliferation.
We examined variation in predator stimuli, glucocorticoids and forebrain cell proliferation in three ways. First, we compared populations that differ in predation pressure, allowing us to detect differences resulting from evolutionary changes among the populations or from plastic changes in individuals' lifetime due to predator exposure. Second, we compared injured and intact individuals to detect the consequence of a recent (in the previous days to months) sub-lethal encounter with a predator. Finally, we measured the short-term response (within hours) to threatening stimuli by comparing fish euthanized immediately after capture with those first held captive for 3 h. We found that forebrain cell proliferation correlated negatively with predator exposure and tail injury, suggesting a significant role of predators on brain plasticity in free-living animals. However, brain cell proliferation was not correlated with any measure of plasma cortisol levels, indicating that another mechanism probably links predation and reduced brain plasticity.
2. Methods
(a). Field sites and fish capture
Adult B. occidentalis (body length > 120 mm) were captured from six streams in the Republic of Panama in February–May 2014 (figure 1). Fish were detected by probing underwater vegetation with electrodes that transduce their electric signal into an audio signal and were captured by dip net within minutes of detection. The populations are located in three independent river drainages: Guarumo in western Panama (Camarón and Teribe), Chagres in central Panama (Puente and Frijolito) and Bayano in eastern Panama (Tapagrilla and Tumagantí; figure 1). These populations are exposed to different degrees of predation pressure as indicated by a direct measure of the density of predatory catfish (Rhamdia quelen) and indirectly by the prevalence of tail injury, presumably from predation attempts (electronic supplementary material, table S1) [21]. By comparing populations within a drainage, we minimized the effect of genetic and environmental variation among populations [33].
Figure 1.
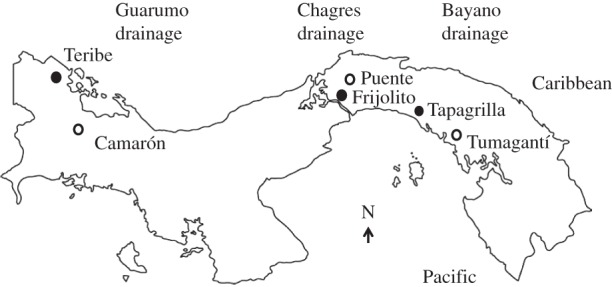
Map of the Republic of Panama showing the six field sites within three drainages. Filled circles indicate the populations with relatively high predation pressure within a drainage; open circles indicate the populations with relatively low predation pressure within a drainage. For data on predation pressure, see the electronic supplementary material, table S1.
(b). Tissue collection in the field
Fish in each population were euthanized at one of two time points: (i) 5–10 min after capture for basal values (n = 7) and (ii) 3–3.25 h after capture for stressed values (n = 6–7). During the 3 h stress period, fish were held in buckets containing approximately 8 l of stream water. After overdose with anaesthetic (0.075% 2-phenoxyethanol), fish were immediately bled from the caudal vein. The blood was held on ice in the field for 3–6 h and then centrifuged. Immediately after blood sampling, brains were dissected and placed in a series of solutions on ice: formaldehyde (4%, 2 h) for fixation, PBS (0.1 M, 2 × 1 h) for rinse and sucrose (25%, overnight) for cryoprotection. On the following morning, brains were frozen with pulverized dry ice. Plasma and brains were stored on dry ice in the field and afterwards at −80°C in the laboratory.
(c). Immunohistochemistry
To label proliferating cells, we used immunohistochemistry for the mitotic marker proliferating cell nuclear antigen (PCNA). Brain sections were treated sequentially with HCl (2N, 37°C, 30 min), borate buffer (0.1 M, pH 8.5, 2 × 10 min), PBS (0.1 M, 1 h), blocking solution (5% donkey serum, 0.3% Triton X in PBS), rabbit anti-PCNA (1 : 50 in blocking solution, Santa Cruz Biotechnology, FL-261), PBS (3 × 20 min) and Cy-3 conjugated donkey anti-rabbit secondary antibody (1 : 300, Jackson Immunoresearch). All solutions (except HCl) were at room temperature.
In all fish (n = 79), we quantified the abundance of proliferating cells by counting unilaterally the PCNA+ cells in three forebrain regions—the DL, DM and V (electronic supplementary material, figure S1)—at axial levels corresponding to sections 30–36 in the brain atlas of another electric fish, Apteronotus leptorhynchus [34]. For the midbrain, we examined the periventricular zone (sections 17–19 of Apteronotus atlas) in a subset of fish (n = 22): uninjured fish euthanized immediately after capture. In each region, we confined counting to a 100 µm band at the periphery of the brain or ventricle, and estimated the area of these regions using NIH ImageJ v. 4.0. We then calculated the density (PCNA+ cells mm−3) by dividing cell counts by the area of each region and section thickness (30 µm).
(d). Cortisol immunoassays
Plasma cortisol levels were quantified using enzyme immunoassays (Cortisol EIA kit; Cayman Chemical Co.) as described and validated previously for B. gauderio [35]. Intra-assay coefficients of variation were 8.9, 9.3, 6.5, 7 and 7.3%; inter-assay coefficient of variation was 5.2%.
(e). Statistics
To assess the effect of predation on forebrain cell proliferation, we performed repeated-measures ANCOVA with stepwise removal of non-significant covariates. We used PCNA+ cell density as the dependent variable, population, injury and capture stress as independent variables, brain region as the repeated measure, and body size (mass and length), body condition (residuals of body mass/body length regression) and sex as covariates. Because there were no interactions between forebrain region and treatments (population, injury or capture stress), we used total PCNA+ cell density across all forebrain regions in subsequent analyses.
To assess the effect of predation on cortisol levels, we performed ANCOVA with plasma cortisol concentration as the dependent variable, and the same independent variables and covariates as above. When necessary, variables were log transformed to give them normality. Post hoc analyses included pairwise comparisons using Bonferroni confidence intervals to adjust for multiple comparisons (family-wise alpha = 0.05). Relationships among population pressure, plasma cortisol and proliferating cell density were determined through Pearson product–moment correlations.
3. Results
(a). Forebrain cell proliferation is negatively correlated with predation pressure
Across all forebrain regions, cell proliferation varied by population and injury state, but was only marginally affected by capture stress (tables 1 and 2; figures 2–4). Comparisons within a drainage, which allow us to control for many genetic and environmental differences among populations, show that, among all fish (both injured and intact), those living in high-predation streams had lower density of proliferating cells than those living in low-predation streams (table 1). This same relationship holds true even when examining intact fish alone (figure 2a). Furthermore, in intact fish across all populations in all drainages, mean density of proliferating cells correlated negatively with predation pressure (as measured by the fraction of fish with injured tails; figure 3a). By contrast, cell proliferation in the midbrain did not vary by population among intact fish (F = 0.3, d.f. = 3, p > 0.05), and within drainages, populations with differing predation pressure showed similar midbrain cell proliferation (figure 2b).
Table 1.
Population-level data (mean ± s.e.) on all fish (both intact and injured) according to drainage and relative predation pressure; n = 12–14 for forebrain, body size and condition measurements; n = 6–7 for cortisol measurements.
| population | relative predation pressurea | forebrain cell proliferation (PCNA+ cells mm−1) | basal cortisol (ng ml−1) | stress cortisol (ng ml−1) | body length (mm) | body conditionb |
|---|---|---|---|---|---|---|
| western drainage (Guarumo) | ||||||
| Camarón | low | 8321 ± 763 | 21.6 ± 5.1 | 58.5 ± 10.2 | 150 ± 5 | 0.003 ± 0.020 |
| Teribe | high | 6190 ± 511c | 36.0 ± 9.4 | 41.9 ± 6.7 | 146 ± 9 | −0.026 ± 0.041 |
| central drainage (Chagres) | ||||||
| Puente | low | 7698 ± 537 | 33.5 ± 8.1 | 45.1 ± 2.4 | 148 ± 6 | 0.034 ± 0.019 |
| Frijolito | high | 6456 ± 505c | 37.1 ± 6.5 | 53.7 ± 8.9 | 160 ± 6 | 0.061 ± 0.025 |
| eastern drainage (Bayano) | ||||||
| Tumagantí | low | 14 027 ± 719 | 37.4 ± 7.4 | 41.6 ± 10.1 | 155 ± 6 | −0.068 ± 0.023 |
| Tapagrilla | high | 10 603 ± 564b | 30.5 ± 6.1 | 61.9 ± 13.7 | 163 ± 9 | 0.034 ± 0.019c |
aBased on incidence of tail injury and density of predators. See the electronic supplementary material, table S1 for these data.
bBody condition is defined as residuals of the regression of log body mass and log body length across in all sampled fish. Positive value indicates fish that were heavier than predicted based on their body length.
cSignificantly different from the low-predation population within the drainage.
Table 2.
ANOVA results showing the effect of predator stimuli on forebrain cell proliferation and plasma cortisol in all fish.
| forebrain cell proliferation (PCNA+ cells mm−3) |
plasma cortisol (ng ml−1) |
||||
|---|---|---|---|---|---|
| effect | d.f. | F | P | F | p |
| population | 5 | 4.0 | <0.001 | 1.1 | >0.05 |
| injury | 1 | 4.2 | <0.05 | 1.2 | >0.05 |
| capture stress | 1 | 3.5 | =0.06 | 11.1 | <0.005 |
| population × injury | 5 | 1.6 | >0.05 | 0.7 | >0.05 |
| population × capture stress | 5 | 3.1 | >0.05 | 2.1 | >0.05 |
| injury × capture stress | 1 | 0.02 | >0.05 | 0.3 | >0.05 |
| population × injury × capture stress | 5 | 0.5 | >0.05 | 0.2 | >0.05 |
Figure 2.
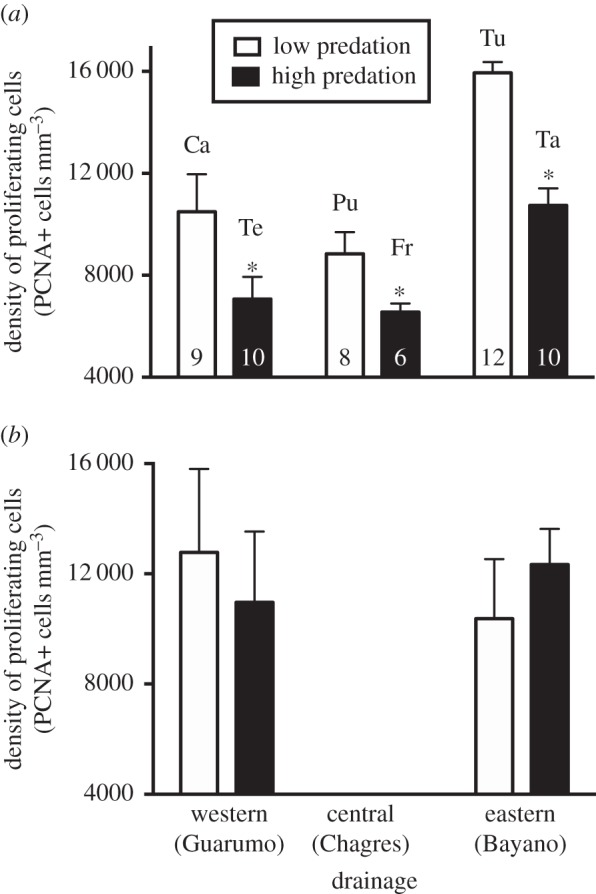
Brain cell proliferation in the (a) forebrain and (b) midbrain in high- (black bars) and low-predation (white bars) populations, comparing populations within three drainages. Data (mean ± s.e.) are from intact fish; no data were available from the midbrain in the Chagres drainage. For the forebrain, the high-predation population had significantly lower density of proliferating cells than the low-predation population in all three drainages; there were no population differences in the midbrain. Asterisks indicate significant differences between populations within a drainage. Sample sizes are at the base of each bar. Tu, Tumagantí; Ca, Camarón; Ta, Tapagrilla; Pu, Puente; Fr, Frijolito; Te, Teribe.
Figure 3.
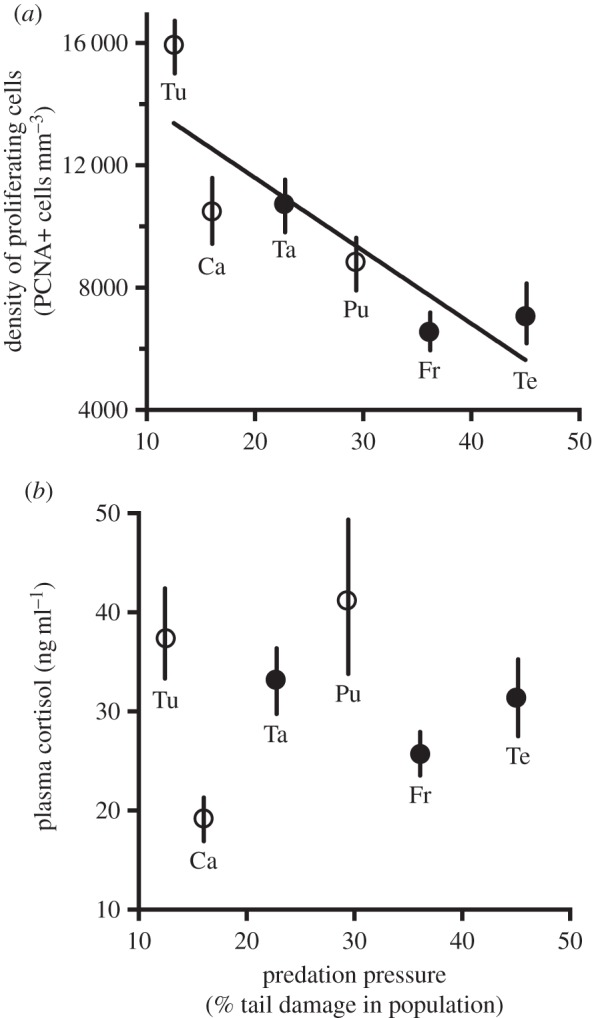
(a) Forebrain cell proliferation and (b) basal concentration of cortisol in populations differing in predation pressure. Data (mean ± s.e.) are from intact fish. As in figure 1, filled circles indicate the population with relatively high predation pressure within a drainage; open circles indicate the population with relatively low predation pressure within a drainage. Sample sizes and population abbreviations are identical to those in figure 2. For cell proliferation, mean density of proliferating cells correlated significantly and positively with frequency of tail damage in the population (r2 = 0.73, p < 0.01).
Figure 4.
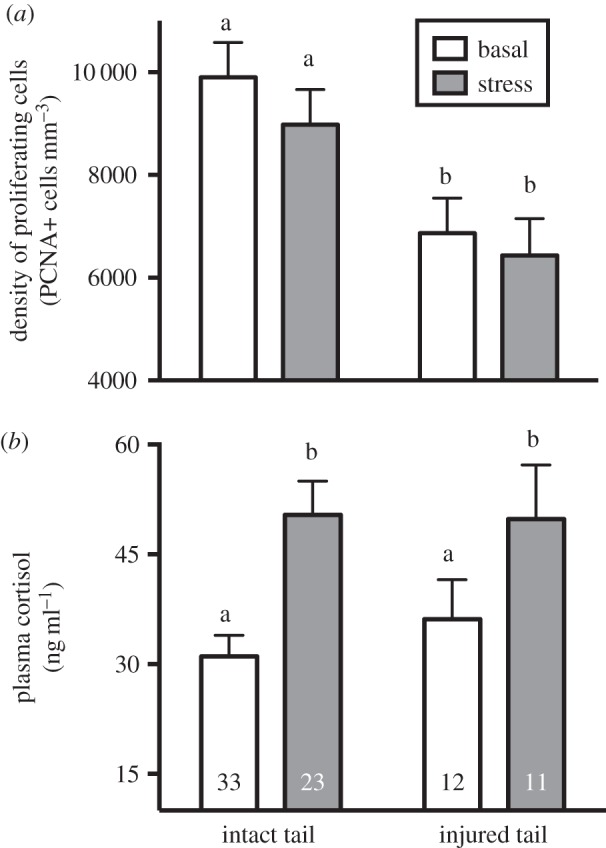
(a) Forebrain cell proliferation and (b) plasma cortisol concentration according to tail injury and capture stress across all six populations. Injured fish had lower density of proliferating cells than intact fish, but there was only a marginally significant (p = 0.06) effect of capture stress on proliferating cell density. Plasma cortisol was significantly elevated by capture stress, but was not significantly associated with tail injury. Bars with different letters above them are significantly different. Numbers at the base of bars are the sample size for each group.
Across populations, fish with injured tails had lower densities of proliferating cells in the forebrain than those with intact tails (table 2; figure 4a). Capture stress also appeared to inhibit cell proliferation; however, the effect was only marginally significant (p = 0.064; table 2; figure 4a). There were no significant interactive effects between independent variables, indicating that the presence of one kind of predator stimulus did not influence the response to other forms of predator stimuli.
Across all individuals, cell proliferation rate was not correlated with either basal or stress levels of cortisol. However, among intact fish, there was a weak but statistically significant positive correlation between proliferating cell density and basal cortisol (r2 = 0.122, F = 4.3, p < 0.05). Cell proliferation rates did not covary significantly with body size (length or mass), body condition or sex.
(b). Plasma cortisol levels are not correlated with predation pressure or forebrain cell proliferation
Basal cortisol concentrations in our field sample were similar to those in captive fish (Brachyhypopomus gauderio) that were bled 2–3 min after capture [35], suggesting that our values were close to those found in undisturbed fish. Plasma cortisol increased significantly in response to capture (tables 1 and 2; figure 4b). However, there was no significant variation in cortisol by population (table 2; figure 3b) or by tail injury, and no significant interaction between capture stress and population or tail injury (table 2; figures 3b and 4b). Plasma cortisol did not covary significantly with density of proliferating cells, body size or body condition.
(c). Response of cell proliferation to predators is consistent across all three forebrain regions
Adult B. occidentalis showed a spatial distribution of proliferating cells in the telencephalon consistent with the adult telencephalon of other teleost species, including two other gymnotiform electric fish: Gymnotus omarorum [36] and Apteronotus leptorhynchus [37]. Within individuals, the density was highly correlated among the three forebrain regions. We found no significant interaction between forebrain region and population, injury or capture stress (table 3), indicating that all three forebrain regions respond similarly to predator stimuli. Density of proliferating cells in the midbrain periventricular zone was statistically indistinguishable from that of pooled forebrain regions (F = 0.51, p > 0.05), and forebrain and midbrain rates of cell proliferation were not correlated (r2 = 0.18, p > 0.05). Thus, it appears that cell proliferation in forebrain regions is uniformly responsive to predator stimuli, coordinated across forebrain regions but at different proliferative rates between forebrain regions, and independent from midbrain cell proliferation.
Table 3.
ANOVA results on the effect of forebrain region and the interaction with predator stimuli on forebrain cell proliferation.
| effect | d.f. | F | p-value |
|---|---|---|---|
| brain region | 2 | 4.7 | <0.001 |
| brain region × population | 10 | 1.5 | >0.05 |
| brain region × injury | 2 | 2.1 | >0.05 |
| brain region × capture stress | 2 | 1.9 | >0.05 |
(d). Population variation in body condition and size
Populations did not differ in mean body size (length and mass) but differed significantly in body condition (table 1; F = 3.51, p < 0.01). When analysed within drainages, the only significant population difference in body condition was that, in the western drainage, fish from Tapagrilla had significantly higher mean body condition than those from Tumagantí (table 1). However, body condition did not correlate with predator pressure, brain cell proliferation or cortisol levels.
4. Discussion
(a). Predation pressure correlates negatively with forebrain cell proliferation in natural populations of electric fish
Fish in natural populations of B. occidentalis differ by over twofold in rate of cell proliferation across the forebrain. Although several studies have demonstrated that population variation in brain size is correlated with predation [38,39], ours is the first to document the relationship between predators and brain cell proliferation in free-living vertebrates.
We found that variation in forebrain cell proliferation in B. occidentalis correlated closely with predation pressure when examined both across all streams and in comparisons within drainages. Moreover, the relationship between predators and the brain cell proliferation is found in both intact and injured fish. When intact fish are compared across populations, those that live in high-predation streams have lower cell proliferation (table 1; figures 2 and 3), indicating that simply detecting predators appears to affect brain plasticity, even when fish are not directly predated. This may result from the so-called ‘non-consumptive effects’ or ‘intimidation’ by predators [40,41]. In addition, direct, injurious encounters with predators are associated with reduction in brain cell proliferation (table 2). Compared with intact fish, fish with tail injuries had approximately 30% lower density of proliferating cells in the forebrain (figure 4).
Several lines of evidence indicate that the presence of predators and tail injury contribute causally to this correlation. First, the comparison within drainages controls for many variables that might otherwise drive population variation in cell proliferation. Fish from streams within the same drainage are genetically similar [33] and probably face similar environmental conditions (water composition, climate, etc.). Yet even when we compare populations within drainages, those with greater predator pressure have fish that, on average, have lower forebrain cell proliferation. Second, in the congener B. gauderio, fish exposed experimentally to a simulated predator or with experimentally amputated tails have fewer proliferating forebrain cells than undisturbed fish and intact controls [42]. The reduction in cell proliferation resulting from these experimental treatments was quantitatively similar to that of fish faced with predation in the wild. This finding suggests that predator presence and tail injury can indeed cause a reduction in cell proliferation and that reduced brain plasticity might be a naturally occurring cost of sub-lethal interactions with predators in the field.
In the presence of abundant predators, prey probably move more cautiously and in a more limited range [13,23,43] (however, see [44]). Thus, predators could influence prey by directly influencing the brain through the ‘physiology of fear’ (e.g. neurochemical changes in response to aversive stimuli) or indirectly through their effects on restricting behaviour [18]. In other animals, predator stimuli quickly reduce cell proliferation separate from any influence on behaviour [9], and, in B. occidentalis, our data on capture stress indicate that they also may lower brain cell proliferation directly in response to fear (although this effect is only marginally significant). However, given that locomotion and exploration in novel environments increase neurogenesis in many animals [45], it is also conceivable that B. occidentalis in predator-abundant environments have lower cell proliferation rates because they engage less frequently in behaviours that would otherwise promote cell proliferation. Assessing this possibility will require a more detailed analysis of the antipredator behaviours of fish across populations and the influence of these behaviours on brain cell proliferation.
The lower brain cell proliferation in fish with tail injuries may arise from the process of tail regeneration, rather than the injury from predation itself. Regenerative processes in other animals are energy-intensive [41]. It is possible that, in B. occidentalis, the production of brain cells slows as a consequence of the fish redirecting energy to the production of tail cells. If so, this would indicate that injured fish give priority to tail regeneration over brain plasticity. Tail injury immediately compromises the structure of the electrocommunication signal, making fish potentially more detectable by electroreceptive predators [21] and thus placing a premium on rapid tail repair. It would be interesting to determine whether there is a trade-off between brain cell proliferation and injury recovery by tracking the temporal relationship between cell proliferation rates in the brain and regenerating tail.
(b). Predation-related reduction in forebrain cell proliferation is not mediated by cortisol in natural populations of electric fish
Naturally occurring conditions (predator exposure and tail injury) that appear to inhibit forebrain cell proliferation had no effect on basal cortisol levels (figures 3 and 4). Capture stress significantly increased cortisol, but caused only a marginally significant decrease in brain cell proliferation, and among individuals, capture-induced levels of cortisol did not correlate with concurrent density of proliferating cells. These observations indicate that changes in brain cell proliferation and cortisol are uncoupled in both acute and long-term response to predators.
However, a more detailed parsing of the data indicates a possible positive role of glucocorticoids in regulating the brain cell proliferation rates when fish are in a relatively undisturbed state: in uninjured fish, basal cortisol correlated positively with density of proliferating cells. This effect is relatively small, with only 12% of the variation in proliferating cell density attributable to basal cortisol levels. Nonetheless, it offers support from natural populations that small glucocorticoid increases in unstressed conditions promote cell proliferation [2,14]. This finding is also consistent with laboratory studies of another electric fish, A. leptorhynchus, in which small elevations in basal cortisol from exogenous treatment or social interaction both promote brain cell addition [16,46], and of zebrafish, in which elevated cortisol from environmental enrichment correlates positively with brain cell proliferation [29].
While our data indicate that the predator-induced reduction in brain cell proliferation is not mediated by elevated basal cortisol levels or amplified adrenocortical response to acute stress, it is nonetheless possible that more frequent adrenocortical surges associated with living among abundant predators might contribute to population differences in brain cell proliferation. To evaluate this possibility, we will need to examine the time course of cortisol responses to predator interaction and determine whether repeated pulses of cortisol can inhibit brain cell production.
(c). Response to predation is specific to the forebrain and coordinated across forebrain regions
Forebrain cell proliferation appears inhibited by the presence of predators, but midbrain cell proliferation is unaffected (figure 2). Given that midbrain cell proliferation does not vary by population and that the forebrain probably plays a particularly large role in responding to predators [23], it appears that the effect of predators is specific to the forebrain. However, we cannot rule out the possibility that predators suppress cell division rates all over the body by reducing growth rate, but that the midbrain is resistant to this effect. To determine whether the effect of predators is specific to the forebrain, it will be necessary to assay proliferation rates in a larger range of brain regions and non-brain tissues, and to measure growth rates across populations that differ in predator exposure.
Although forebrain and midbrain were differentially responsive to predation pressure, regions within the forebrain (DL, DM and V) responded uniformly (table 3). This pattern indicates either that predator stimuli affect a single set of internal signals (e.g. growth factors or hormones) that act globally across all forebrain regions, or that predator stimuli act similarly but independently on each of these three brain regions. In other animals, brain cell proliferation and neurogenesis depend on neural activity within the brain region, allowing for region-specific, experience-dependent responses to environmental change [1,2,45]. In another electric fish, A. leptorhynchus, an enhanced social environment increases cell addition in midbrain regions associated with electrocommunication, but not in midbrain regions less closely tied to social interaction [16,46]. However, in B. occidentalis, we did not observe such differential proliferative response by region, indicating that all three brain regions show similar neural activity or that the forebrain cell proliferation of electric fish is not activity-dependent.
(d). Predator influence on brain cell proliferation: cost or adaptation?
Our study provides evidence that the presence of predators and direct injuries from predators decrease brain cell proliferation, but it is not clear whether this response is costly or adaptive. Several authors have proposed that regulating adult neurogenesis may enable animals to alter their behaviour in response to novel environments [22,47,48]. In some animals, brain cell proliferation and subsequent neurogenesis is causally linked to increased cognitive function (memory and learning) and behavioural performance [49]. If this is true in electric fish, individuals that have predator-induced inhibition of brain cell proliferation, particularly in brain regions likely to be involved in predator avoidance, may suffer decrements in behaviour that make them further vulnerable to predators or other environmental challenges. Alternatively, decreased neurogenesis also increases anxiety-related behaviours, including causing animals to retreat more quickly and stay hidden longer when exposed to a predator [50]. When confronted with predators, perhaps the most adaptive response is to retain stereotyped and cautious behaviour [8]. In this case, reduced cell proliferation may be adaptive if it causes fish in high-predation environments to limit exploration behaviour that would potentially expose them to more predators.
(e). Implications for laboratory studies of environmental regulation of brain cell proliferation
In many vertebrate taxa, animals in natural populations add brain cells at greater rates than those living in captivity [2,4–6]. One motivation of our study was to address whether the presence of predators, an important feature of most natural habitats, constitutes an environmental enrichment that contributes positively to greater brain cell proliferation in the wild or an environmental stressor that negatively affects brain cell production. In laboratory rodents, combinations of environmental influences on neurogenesis are generally additive, with positive and negative regulators (e.g. sexual experience and stress) tending to offset each other. We do not have data from captive B. occidentalis to directly compare with our field-captured animals. However, forebrain cell proliferation in free-living B. occidentalis, even in populations with the highest predator densities, is still higher than that of captive B. gauderio [42]. Thus, it appears that the positive effect of living in a complex natural environment outweighs the negative effect of natural predators.
Many laboratory studies have demonstrated that exposure to predator stimuli increases plasma glucocorticoids [9,12,13]. However, under laboratory circumstances, animals lack many of the behavioural options to respond to predators, and given that the perception of control is a prime determinant of the stress response [2], the relevance of these studies to naturally occurring glucocorticoid secretion is not clear. Indeed, in the few cases in which fish from field populations were examined, those living among abundant predators had lower cortisol production and reduced stress responses compared with fish in low-predation environments [20,44]. We found that basal and stress-induced cortisol levels were unrelated to predator exposure and tail injury, indicating that B. occidentalis regulate their glucocorticoid levels independently of predator stimuli. However, despite this adrenocortical ‘stress resistance’, they nonetheless experienced predator-related reductions in brain cell proliferation. Thus, the model developed primarily in laboratory rodents in which predator stimuli elevate glucocorticoid levels, which then inhibit brain cell proliferation, does not appear to apply to free-living fish.
5. Conclusion
Laboratory studies have been crucial for isolating specific features of the environment that influence neurogenesis and for identifying mechanisms by which these features alter neurogenic processes. However, the natural environments that shape animals' brains during both ontogeny and evolution are always dynamic combinations of influences [1]. Moreover, free-living animals determine in part their own environment through behavioural options (e.g. where, when and with whom to be active) that are usually prevented or restricted in the laboratory.
Here we show that fish exposed to naturally occurring predators and injuries have reduced forebrain cell proliferation even when they have a natural range of environmental complexity and antipredator behaviours. However, the enhancing properties of living in a natural (compared with a laboratory) environment still appear to exceed the inhibiting properties of predator pressure. While predation pressure appears to significantly affect cell proliferation, it does not alter basal cortisol, suggesting a dissociation of basal glucocorticoid secretion and cell proliferation in the wild.
Supplementary Material
Acknowledgements
We thank R. Gonzalez, L. F. De Leon, D. Sharpe, F. Alda, C. Martinez, J. Benda, H. Fotowat and J. Molina for assistance in the field, J. Dertien for housing in Panama, E. Mostoller for logistical support and T. Williams for editorial comments. Field research was conducted while K.D.D., A.T. and R.K. were visiting researchers at the Smithsonian Tropical Research Institute.
Ethics
This research was approved for ethical treatment of animals by the Institutional Animal Use and Care Committee of the Smithsonian Tropical Research Institute.
Data accessibility
The data supporting this article have been deposited in an external repository (Dryad) and can be accessed at: http://dx.doi.org/10.5061/dryad.3rd2v.
Authors' contributions
K.D.D. helped design the study, conducted fieldwork, quantified brain data, analysed all data and wrote the manuscript. A.T. helped design the study, conducted the fieldwork and revised the manuscript. M.A.R. conducted the immunolabelling and revised the manuscript. R.K. helped design the study, conducted the fieldwork and contributed to drafting the manuscript. V.L.S. quantified the hormones and contributed to drafting the manuscript.
Competing interests
We have no competing interests.
Funding
This study was supported by a grant from the Trinity Faculty Research Committee to K.D.D., by a graduate fellowship to A.T. from Le Fonds Québécois de la Recherche sur la Nature et les Technologies, from Discovery grants from the Natural Sciences and Engineering Council of Canada to V.L.S. and R.K., and CFI LOF and NSRIT to V.L.S.
References
- 1.Opendak M, Gould E. 2015. Adult neurogenesis: a substrate for experience-dependent change. Trends Cogn. Sci. 19, 151–161. ( 10.1016/j.tics.2015.01.001) [DOI] [PubMed] [Google Scholar]
- 2.LaDage LD. 2015. Environmental change, the stress response, and neurogenesis. Integr. Comp. Biol. 55, 372–383. ( 10.1093/icb/icv040) [DOI] [PubMed] [Google Scholar]
- 3.Kempermann G, Kuhn HG, Gage FH. 1997. More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495. ( 10.1038/386493a0) [DOI] [PubMed] [Google Scholar]
- 4.Amerin I, Lipp HP, Boonstra R, Wojtowicz J. 2008. Adult hippocampal neurogenesis in natural populations. In Adult neurogenesis (eds Gage FH, Kempermann G, Song H), pp. 645–660. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 5.LaDage LD, Roth TC, Fox RA, Pravosudov VV. 2010. Ecologically relevant spatial memory use modulates hippocampal neurogenesis. Proc. R. Soc. B 277, 1071–1079. ( 10.1098/rspb.2009.1769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunlap KD, Silva AC, Chung M. 2011. Environmental complexity, seasonality and brain cell proliferation in a weakly electric fish, Brachyhypopomus gauderio. J. Exp. Biol. 214, 794–805. ( 10.1242/jeb.051037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoenfeld TJ, Gould E. 2012. Stress, stress hormones, and adult neurogenesis. Exp. Neurol. 233, 12–21. ( 10.1016/j.expneurol.2011.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glasper ER, Schoenfeld TJ, Gould E. 2012. Adult neurogenesis: optimizing hippocampal function to suit the environment. Behav. Brain Res. 227, 380–383. ( 10.1016/j.bbr.2011.05.013) [DOI] [PubMed] [Google Scholar]
- 9.Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. 2001. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J. Comp. Neurol. 437, 496–504. ( 10.1002/cne.1297) [DOI] [PubMed] [Google Scholar]
- 10.Hill MN, Kambo JS, Sun JC, Gorzalka BB, Galea LA. 2006. Endocannabinoids modulate stress-induced suppression of hippocampal cell proliferation and activation of defensive behaviours. Eur. J. Neurosci. 24, 1845–1849. ( 10.1111/j.1460-9568.2006.05061.x) [DOI] [PubMed] [Google Scholar]
- 11.Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. 2005. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci. Biobehav. Rev. 29, 1123–1144. ( 10.1016/j.neubiorev.2005.05.005) [DOI] [PubMed] [Google Scholar]
- 12.Barcellos LJG, Ritter F, Kreutz LC, Quevedo RM, da Silva LB, Bedin AC, Finco J, Cericato L. 2007. Whole-body cortisol increases after direct and visual contact with a predator in zebrafish, Danio rerio. Aquaculture 272, 774–778. ( 10.1016/j.aquaculture.2007.09.002) [DOI] [Google Scholar]
- 13.Fürtbauer I, Pond A, Heistermann M, King AJ. 2015. Personality, plasticity and predation: linking endocrine and behavioural reaction norms in stickleback fish. Funct. Ecol. 29, 931–940. ( 10.1111/1365-2435.12400) [DOI] [Google Scholar]
- 14.Sorensen C, Bohlin LC, Overli O, Nilsson GE. 2011. Cortisol reduces cell proliferation in the telencephalon of rainbow trout (Oncorhynchus mykiss). Physiol. Behav. 102, 518–523. ( 10.1016/j.physbeh.2010.12.023) [DOI] [PubMed] [Google Scholar]
- 15.Thomas RM, Urban JH, Peterson DA. 2006. Acute exposure to predator odor elicits a robust increase in corticosterone and a decrease in activity without altering proliferation in the adult rat hippocampus. Exp. Neurol. 201, 308–315. ( 10.1016/j.expneurol.2006.04.010) [DOI] [PubMed] [Google Scholar]
- 16.Dunlap KD, Chung M, Castellano JF. 2013. Influence of long-term social interaction on chirping behaviour, steroid levels and neurogenesis in weakly electric fish. J. Exp. Biol. 216, 2434–2441. ( 10.1242/jeb.082875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monclús R, Palomares F, Tablado Z, Martínez-Fontúrbel A, Palme R. 2009. Testing the threat-sensitive predator avoidance hypothesis: physiological responses and predator pressure in wild rabbits. Oecologia 158, 615–623. ( 10.1007/s00442-008-1201-0) [DOI] [PubMed] [Google Scholar]
- 18.Clinchy M, Sheriff MJ, Zanette LY. 2013. Predator-induced stress and the ecology of fear. Funct. Ecol. 27, 56–65. ( 10.1111/1365-2435.12007) [DOI] [Google Scholar]
- 19.Creel S, Winnie JA Jr, Christianson D. 2009. Glucocorticoid stress hormones and the effect of predation risk on elk reproduction. Proc. Natl Acad. Sci. USA 106, 12 388–12 393. ( 10.1073/pnas.0902235106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer EK, Harris RM, Hofmann HA, Hoke KL. 2014. Predator exposure alters stress physiology in guppies across timescales. Horm. Behav. 65, 165–172. ( 10.1016/j.yhbeh.2013.12.010) [DOI] [PubMed] [Google Scholar]
- 21.Tran A. 2014. The effects of predation on electric fish signals. Master's thesis, McGill University, Canada.
- 22.Ebbesson L, Braithwaite V. 2012. Environmental effects on fish neural plasticity and cognition. J. Fish Biol. 81, 2151–2174. ( 10.1111/j.1095-8649.2012.03486.x) [DOI] [PubMed] [Google Scholar]
- 23.Silva PI, Martins CI, Khan UW, Gjøen HM, Øverli Ø, Höglund E. 2015. Stress and fear responses in the teleost pallium. Physiol. Behav. 141, 17–22. ( 10.1016/j.physbeh.2014.12.020) [DOI] [PubMed] [Google Scholar]
- 24.Portavella M, Vargas JP. 2005. Emotional and spatial learning in goldfish is dependent on different telencephalic pallial systems. Eur. J. Neurosci. 21, 2800–2806. ( 10.1111/j.1460-9568.2005.04114.x) [DOI] [PubMed] [Google Scholar]
- 25.Falconer EM, Galea LA. 2003. Sex differences in cell proliferation, cell death and defensive behaviour following acute predator odor stress in adult rats. Brain Res. 975, 22–36. ( 10.1016/S0006-8993(03)02542-3) [DOI] [PubMed] [Google Scholar]
- 26.Portavella M, Torres B, Salas C. 2004. Avoidance response in goldfish: Emotional and temporal involvement of medial and lateral telencephalic pallium. J. Neurosci. 24, 2335–2342. ( 10.1523/JNEUROSCI.4930-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephenson-Jones M, Kardamakis AA, Robertson B, Grillner S. 2013. Independent circuits in the basal ganglia for the evaluation and selection of actions. Proc. Natl Acad. Sci. USA 110, E3670–E3679. ( 10.1073/pnas.1314815110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lema SC, Hodges MJ, Marchetti MP, Nevitt GA. 2005. Proliferation zones in the salmon telencephalon and evidence for environmental influence on proliferation rate. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 141, 327–335. ( 10.1016/j.cbpb.2005.06.003) [DOI] [PubMed] [Google Scholar]
- 29.von Krogh K, Sorensen C, Nilsson GE, Overli O. 2010. Forebrain cell proliferation, behaviour, and physiology of zebrafish, Danio rerio, kept in enriched or barren environments. Physiol. Behav. 101, 32–39. ( 10.1016/j.physbeh.2010.04.003) [DOI] [PubMed] [Google Scholar]
- 30.Maruska KP, Carpenter RE, Fernald RD. 2012. Characterization of cell proliferation throughout the brain of the African cichlid fish Astatotilapia burtoni and its regulation by social status. J. Comp. Neurol. 520, 3471–3491. ( 10.1002/cne.23100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salvanes AGV, Moberg O, Ebbesson LOE, Nilsen TO, Jensen KH, Braithwaite VA. 2013. Environmental enrichment promotes neural plasticity and cognitive ability in fish. Proc. R. Soc. B 280, 20131331 ( 10.1098/rspb.2013.1331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorensen C, Johansen IB, Overli O. 2013. Neural plasticity and stress coping in teleost fishes. Gen. Comp. Endocrinol. 181, 25–34. ( 10.1016/j.ygcen.2012.12.003) [DOI] [PubMed] [Google Scholar]
- 33.Picq S, Alda F, Krahe R, Bermingham E. 2014. Miocene and pliocene colonization of the Central American isthmus by the weakly electric fish Brachyhypopomus occidentalis. J Biogeogr. 41, 1520–1532. ( 10.1111/jbi.12309) [DOI] [Google Scholar]
- 34.Maler L, Sas E, Johnston S, Ellis W. 1991. An atlas of the brain of the electric fish Apteronotus leptorhynchus. J. Chem. Neuroanat. 4, 1–38. ( 10.1016/0891-0618(91)90030-G) [DOI] [PubMed] [Google Scholar]
- 35.Salazar VL, Stoddard PK. 2009. Social competition affects electric signal plasticity and steroid levels in the gymnotiform fish Brachyhypopomus gauderio. Horm. Behav. 56, 399–409. ( 10.1016/j.yhbeh.2009.07.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iribarne L, Castelló ME. 2014. Postnatal brain development of the pulse type, weakly electric gymnotid fish Gymnotus omarorum. J. Physiol. Paris 108, 47–60. ( 10.1016/j.jphysparis.2014.05.001) [DOI] [PubMed] [Google Scholar]
- 37.Zupanc GK, Horschke I. 1995. Proliferation zones in the brain of adult gymnotiform fish: a quantitative mapping study. J. Comp. Neurol. 353, 213–233. ( 10.1002/cne.903530205) [DOI] [PubMed] [Google Scholar]
- 38.Gonda A, Herczeg G, Merilä J. 2013. Evolutionary ecology of intraspecific brain size variation: a review. Ecol. Evol. 3, 2751–2764. ( 10.1002/ece3.627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonda A, Valimaki K, Herczeg G, Merila J. 2012. Brain development and predation: plastic responses depend on evolutionary history. Biol. Lett. 8, 249–252. ( 10.1098/rsbl.2011.0837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preisser EL, Bolnick DI, Benard MF. 2005. Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecol. 86, 501–509. ( 10.1890/04-0719) [DOI] [Google Scholar]
- 41.McCauley SJ, Rowe L, Fortin M. 2011. The deadly effects of ‘nonlethal’ predators. Ecology 92, 2043–2048. ( 10.1890/11-0455.1) [DOI] [PubMed] [Google Scholar]
- 42.Dunlap KD, Keane G, Ragazzi MA. In preparation. Experimental exposure to simulated predator and tail amputation reduces brain cell proliferation in an electric fish, Brachyhypopomus gauderio. [DOI] [PubMed]
- 43.Burns JG, Rodd FH. 2008. Hastiness, brain size and predation regime affect the performance of wild guppies in a spatial memory task. Anim. Behav. 76, 911–922. ( 10.1016/j.anbehav.2008.02.017) [DOI] [Google Scholar]
- 44.Archard GA, Earley RL, Hanninen AF, Braithwaite VA. 2012. Correlated behaviour and stress physiology in fish exposed to different levels of predation pressure. Funct. Ecol. 26, 637–645. ( 10.1111/j.1365-2435.2012.01968.x) [DOI] [Google Scholar]
- 45.Barker JM, Boonstra R, Wojtowicz JM. 2011. From pattern to purpose: How comparative studies contribute to understanding the function of adult neurogenesis. Eur. J. Neurosci. 34, 963–977. ( 10.1111/j.1460-9568.2011.07823.x) [DOI] [PubMed] [Google Scholar]
- 46.Dunlap KD, Castellano JF, Prendaj E. 2006. Social interaction and cortisol treatment increase cell addition and radial glia fiber density in the diencephalic periventricular zone of adult electric fish, Apteronotus leptorhynchus. Horm. Behav. 50, 10–17. ( 10.1016/j.yhbeh.2006.01.003) [DOI] [PubMed] [Google Scholar]
- 47.Kempermann G. 2008. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neurosci. 31, 163–169. ( 10.1016/j.tins.2008.01.002) [DOI] [PubMed] [Google Scholar]
- 48.Johansen IB, Sorensen C, Sandvik GK, Nilsson GE, Hoglund E, Bakken M, Overli O. 2012. Neural plasticity is affected by stress and heritable variation in stress coping style. Comp. Biochem. Physiol. D Genomics Proteomics 7, 161–171. ( 10.1016/j.cbd.2012.01.002) [DOI] [PubMed] [Google Scholar]
- 49.Castilla-Ortega E, Pedraza C, Estivill-Torrus G, Santin LJ. 2011. When is adult hippocampal neurogenesis necessary for learning? Evidence from animal research. Rev. Neurosci. 22, 267–283. ( 10.1515/rns.2011.027) [DOI] [PubMed] [Google Scholar]
- 50.Revest J, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza P, Abrous D. 2009. Adult hippocampal neurogenesis is involved in anxiety-related behaviours. Mol. Psychiatry 14, 959–967. ( 10.1038/mp.2009.15) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article have been deposited in an external repository (Dryad) and can be accessed at: http://dx.doi.org/10.5061/dryad.3rd2v.


