Abstract
Body size exerts multiple effects on plankton food-web interactions. However, the influence of size structure on trophic transfer remains poorly quantified in the field. Here, we examine how the size diversity of prey (nano-microplankton) and predators (mesozooplankton) influence trophic transfer efficiency (using biomass ratio as a proxy) in natural marine ecosystems. Our results support previous studies on single trophic levels: transfer efficiency decreases with increasing prey size diversity and is enhanced with greater predator size diversity. We further show that communities with low nano-microplankton size diversity and high mesozooplankton size diversity tend to occur in warmer environments with low nutrient concentrations, thus promoting trophic transfer to higher trophic levels in those conditions. Moreover, we reveal an interactive effect of predator and prey size diversities: the positive effect of predator size diversity becomes influential when prey size diversity is high. Mechanistically, the negative effect of prey size diversity on trophic transfer may be explained by unicellular size-based metabolic constraints as well as trade-offs between growth and predation avoidance with size, whereas increasing predator size diversity may enhance diet niche partitioning and thus promote trophic transfer. These findings provide insights into size-based theories of ecosystem functioning, with implications for ecosystem predictive models.
Keywords: size diversity, biodiversity–ecosystem functioning, trophic transfer efficiency, functional diversity, body size, predator–prey dynamics
1. Introduction
A major challenge in contemporary ecology is the development of a more mechanistic understanding of the relationships between biodiversity and ecosystem functioning (BEF) [1]. Although biodiversity has been demonstrated to promote efficiency of resource use and productivity within a trophic level, no consensus has been reached concerning the mechanism, with some advocating for sampling effects (i.e. likelihood of finding a species of high productivity) and others arguing for complementarity (i.e. niche partitioning and/or facilitation) [2]. Moreover, the issue of BEF becomes much more complicated when considering more than one trophic level. For example, experimental manipulation of insect and plant diversity has shown that resource diversity counteracts the positive effect of consumer diversity on the efficiency of resource use [3]. These studies point out apparently contrasting effects of predator and prey diversity on trophic transfer; nevertheless, studies on how the interaction of diversity across trophic levels influences measures of ecosystem function, such as trophic transfer efficiency (TTE) remain scarce [4]. Another knowledge gap is that most BEF research consists of manipulation studies in controlled environments. As Hillebrand & Cardinale [5] stress, manipulation studies are important for their control capacity but are still unrealistic (i.e. simplified interactions and too few species) and therefore, any conclusions are prone to bias. Thus, in order to complement and clarify our understanding of BEF, it is necessary to address these questions for natural communities.
A significant step towards understanding the role of biodiversity on ecosystem functioning has been made with the growing research on functional diversity, which focuses on functional traits rather than on species [6]. Recent studies on aquatic systems have found that functional-trait diversity tends to perform better than taxonomic diversity as a metric for linking community structure to aspects of ecosystem function such as carbon export and productivity [7]. In particular, individual size of plankton has been designated as the ‘meta-trait’ integrating several functional traits into one measurement [8,9]. The structuring role of body size in aquatic ecosystems arises because of physiological constraints as well as predator–prey mechanical and energetic constraints [10,11]. Physiological constraints emerge because metabolism scales with body size, and thus population traits such as abundance, secondary production and nutrient turnover rates, also scale with size [10]. With respect to predator–prey dynamics, predators tend to be larger than their prey [11], the size of predators generally conditions the size of their prey [12] and larger predators usually take advantage of a larger size range of prey [13].
Consequently, the size diversity of a community is expected to influence biomass transfer between trophic levels. This is a very old idea originally conceptualized in the seminal works of Elton [14] and Odum [15]. Unfortunately, this idea has not been fully explored, because most models have relied on Lindeman's [16] principles of trophic dynamics and assumed an average transfer efficiency of 10% (but see [17] for a derivation of TTE based on size-related predator–prey interactions). However, resurgent interest in size structure has recently revived the ideas of Elton [14] and Odum [15]. For example, Yvon-Durocher et al. [18] reported that the reduced cell sizes of prey (phytoplankton) accompanying warming in their mesocosm experiment increased trophic transfer (using the predator/prey biomass ratio as proxy). The authors hypothesized that enhanced prey turnover rates owing to metabolic acceleration and smaller cell size explained the increased energy flux. In another experiment, Steiner [19] explained that reduced predation effects with increasing phytoplankton cell size and diversity were related to reduced edibility, but only under conditions of nutrient enrichment. These findings demonstrate that prey (phytoplankton) size represents a pivotal trade-off between population growth rates and susceptibility to grazing by predators (zooplankton), meaning that larger cells experience slower growth, but also reduced mortality because their size exceeds handling capacity of predators and/or because they invest more energy in predation avoidance. From the perspective of predators, Ye et al. [20] analysed the size structure of mesozooplankton communities in the East China Sea (ECS), and found that zooplankton size diversity explained a significant proportion of the variation of the predator/prey biomass ratio. The authors proposed diet niche partitioning as the mechanism behind an observed positive effect of predator size diversity on trophic transfer.
To the best of our knowledge, no empirical study has focused on the concomitant effects of size diversity of predators and their potential prey on biomass trophic transfer. Thus, in this work, we explore the effect of the size diversity of predators (mesozooplankton excluding carnivores) and the size diversity of their potential prey (nano-microplankton) on biomass trophic transfer in natural planktonic communities. Here, size diversity is calculated as the analogue of the Shannon diversity index adapted to individual size distribution [21]. The ratio of mesozooplankton (predator) to nano-microplankton (prey) biomass in log-scale (log10(PPBR)) was used as a proxy for TTE from prey to predators [18,20]. TTE refers to the ecological efficiency of transferring biomass between adjacent trophic levels, thus the net result of integrating physiological and predator–prey dynamics. The choice of biomass ratio as a proxy is mainly due to the impossibility of routinely measuring turnover rates to estimate the ecological efficiency, which is the production rate (turnover rate × biomass). Based on the studies discussed above, we hypothesize that (i) a greater predator size diversity promotes trophic transfer and (ii) a greater prey size diversity hinders trophic transfer. We anticipate that the interactive effect of predator and prey size diversities has a stronger impact on trophic transfer than the effect of size diversity at a single trophic level. We also explored the effect of nutrients and temperature on size diversity and trophic transfer in order to detect environmental conditions favourable to trophic transfer, as well as to propose mechanisms underlying those relationships.
2. Material and methods
(a). Sampling and sample processing
We collected 106 sets of samples from 11 cruises covering the ECS and waters east of Taiwan between May and October from 2009 to 2013 (appendix A in electronic supplementary material). Nano-microplankton were sampled with Go-Flo bottles at every 10-m depth interval from 10 m below the chlorophyll maximum depth to the surface, and cell size was measured with the FlowCAM [22]. Mesozooplankton were sampled with oblique tows of an Ocean Research Institute (ORI) net from 10 m above the sea floor (or from 200 m for deeper stations) to the surface, and organisms were analysed with the ZooSCAN [23]. Carnivores were removed from the mesozooplankton counts prior to data analyses. Mesozooplankton were sampled through the entire water column, because some of these organisms carry out a daily vertical migration but still feed in the photic zone. On average, 2000 and 3500 individuals were measured for mesozooplankton and nano-microplankton, respectively, in each sample. Sampling, sample preservation and sample digitizing procedures with the ZooSCAN and FlowCAM are detailed in appendix A, electronic supplementary material.
Sea surface temperature (SST), sea surface salinity (SSS), nitrate (NO3), phosphate (PO4) and silicate (SiO3) concentrations were determined according to standard methods [24]. Depth-integrated nutrient concentrations were calculated using multiple depth-specific measurements from above the mixed layer depth (MLD; see details in appendix A, electronic supplementary material).
(b). Total biomass and plankton size diversity
Total biomass was estimated from individual biovolume using literature-based conversion factors for mesozooplankton and nano-microplankton (appendix B, electronic supplementary material). Size diversity was estimated from individual biovolume rather than biomass in order to retain the greatest possible accuracy of the two-dimensional size measurements. The major (M) and minor (m) axes of an ellipse containing the area of each individual were converted to ellipsoidal biovolume. We used the ellipsoidal volume (EllipVol) over the more popular equivalent spherical diameter (ESD), because most of the mesozooplankters in our samples were elongated. Nano-microplankton cell volumes were corrected for shrinkage owing to preservation. Size diversity (μ) corresponds to the analogue of the Shannon diversity index computed on the probability density function of individual biovolumes estimated with non-parametric kernel [21] (see appendix B, electronic supplementary material, for further details):
where px(x) is the probability density function of size x, and x represents log(EllipVol). Here, individual biovolume was log-transformed to adjust the apparent variance of organisms of diverse size [25]. The calculation of size diversity is based on relative contribution and is therefore mathematically independent from total biomass; a statistical relationship between size diversity and total biomass would therefore be due to biological processes and not to mathematical artefact [20]. In our dataset, predator size diversity exhibits a significant negative correlation with prey biomass (r = −0.32, p < 0.0001), whereas prey size diversity exhibits a significant positive correlation with prey biomass (r = 0.29, p < 0.0001).
(c). Trophic transfer efficiency
The ratio of mesozooplankton (predator) to nano-microplankton (prey) biomass in log-scale (log10(PPBR)) was used as a proxy for TTE from prey to predators [18,20]. In order to assess the suitability of the biomass ratio as a proxy for TTE calculated as the ratio of production rates (PPPR), we compared a parallel but reduced dataset (see appendix C, electronic supplementary material for detailed methodology). For the comparison, zooplankton biomasses and production rates were estimated only for copepods (representing 70–90% of total zooplankton biomass in our samples), and their growth rates were calculated using the ‘artificial cohort method’. Our comparison indicates that the proxy, log10(PPBR) and the direct TTE estimate, log10(PPPR), were strongly correlated (r = 0.84; p < 0.0001; n = 29; electronic supplementary material, figure C1). Furthermore, we tested how the potential uncertainty associated with using log10(PPBR) as a proxy for TTE could be propagated throughout our results. The uncertainty (e.g. standard errors of the log10(PPBR) : log10(PPPR)) was propagated via bootstrap, and the conclusions drawn from this study remained after accounting for this uncertainty (electronic supplementary material, figure C3).
(d). Data analyses
We used linear mixed-effects modelling (LMM) to investigate which factors affect biomass TTE, with the trophic transfer proxy, log10(PPBR), as the response variable and predator and prey size diversities as well as environmental factors as explanatory variables. Pseudo-replication was accounted for by allowing the intercept to vary with sampling station as a random effect (i.e. 40 stations and thus 65 instead of 105 degrees of freedom in models with one explanatory variable). We then, investigated the extent of top-down and bottom-up control by exploring factors explaining predator and prey size diversity; that is, we considered the size diversity of prey or predators as the response variable, and the size diversity of predators or prey respectively, nutrient and temperature conditions as explanatory variables. For all models, we also report model fits with sea surface salinity as a way to dissociate temperature and nutrient effects from solely coastal–offshore differences. Furthermore, spatial autocorrelation of response variables was thoroughly explored and did not affect the conclusions of our study (electronic supplementary material, appendix D).
In each case, we first-ranked single explanatory variables using univariate models, and then tested all possible combinations of explanatory variables to determine the most parsimonious model according to the Akaike's information criterion corrected for sample size (AICc). In order to gain greater insights into potential mechanisms, the most parsimonious model explaining the predator (or prey) size diversity was identified through inclusion and exclusion of size diversity of prey (or predators) as one of the explanatory variables. Nutrient concentrations were log-transformed to approach normality prior to analyses. The basic statistics and pairwise relationships for all of the variables used in this study are presented in appendix E, electronic supplementary material.
Size diversity calculations and data analyses were carried out with Matlab® v. 7.9 (The Mathworks, Inc., Natick, MA). Models were implemented with the ‘lme’ function in the nlme package [26] of R (R Development Core Team, 2010), and the most parsimonious models were selected with the ‘dredge’ function of the MuMIn package in R [27].
3. Results
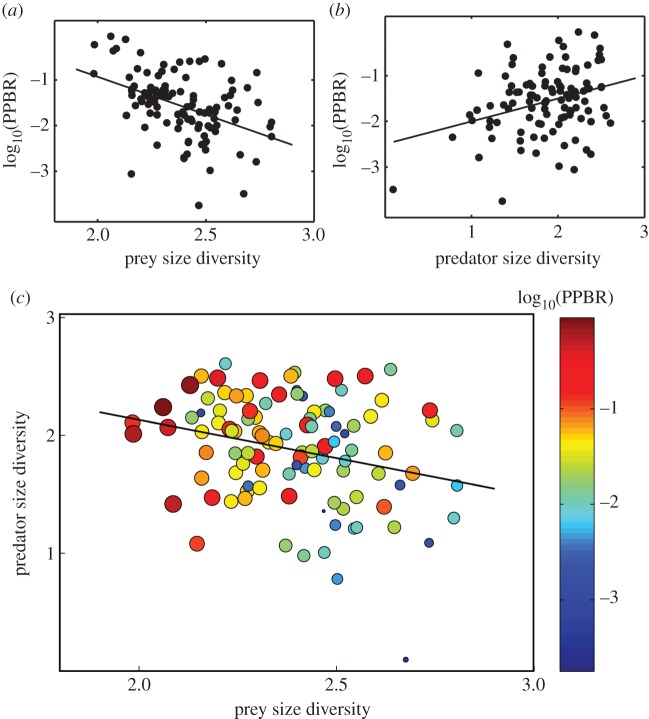
Mesozooplankton biomass was correlated with the biomass of potential prey, nano-microplankton, even though the relationship is not especially strong (r = 0.23; p = 0.02; electronic supplementary material, figure B2). The best single factor explaining prey–predator biomass transfer efficiency (log10(PPBR) as proxy) was the size diversity of prey (table 1). The most parsimonious model explaining biomass transfer was the interaction of predator and prey size diversity (table 1 and figure 1; electronic supplementary material, figure F1a and figure F2a). We also note that predator and prey size diversities exhibit a significant, albeit weak, negative correlation (table 2; r = −0.27; p = 0.006; n = 106).
Table 1.
Results of LMM explaining the biomass transfer efficiency (log10(PPBR)). The best univariate explanatory variable is prey size diversity according to the Akaike information criterion corrected for sample size (AICc) and highlighted in italics. The most parsimonious model (with the lowest AICc) includes the interaction of predator and prey size diversities. Intrinsic variables (plankton) and extrinsic (environment) are separated by a division line. (*p < 0.05, **p < 0.005, ***p < 0.0005.)
| response variable: log10(PPBR) | ||||||
|---|---|---|---|---|---|---|
| slope |
||||||
| one explanatory variable | AICc | estimate | s.e. | t-value | p-value | d.f. |
| predator size diversity | 221.03 | 0.497 | 0.145 | 3.423 | 0.001** | 65 |
| prey size diversity | 209.99 | −1.655 | 0.332 | −4.980 | <0.0001*** | 65 |
| water salinity (SSS) | 232.17 | 0.009 | 0.025 | 0.376 | 0.71 | 65 |
| water temperature (SST) | 225.68 | 0.046 | 0.018 | 2.614 | 0.011* | 65 |
| NO3 | 228.00 | −0.147 | 0.071 | −2.087 | 0.041* | 65 |
| PO4 | 227.95 | −0.755 | 0.359 | −2.098 | 0.040* | 65 |
| SiO3 | 223.86 | −0.278 | 0.094 | −2.955 | 0.004** | 65 |
| most parsimonious model: log10(PPBR) ∼ prey size diversity × predator size diversity AICc: 205.63. | ||||||
Figure 1.
Effects of (a) prey size diversity on biomass transfer efficiency (log10(PPBR)) (r = −0.43, p < 0.0001), (b) predator size diversity on biomass transfer efficiency (log10(PPBR)) (r = 0.32, p < 0.0001) and (c) prey and predator size diversities on biomass transfer efficiency (log10(PPBR)). In panel (c), colour as well as symbol size indicate the biomass transfer efficiency. The solid line represents the relationship between prey and predator size diversity (r = −0.27, p = 0.006).
Table 2.
Results of LMM explaining (a) predator size diversity and (b) prey size diversity. The best model (i.e. predator size diversity explained by SiO3; prey size diversity explained by temperature) is selected according to the Akaike information criterion corrected for sample size (AICc) and highlighted in italics. The most parsimonious model explaining the predator (or prey) size diversity was computed through including and excluding the size diversity of prey (or predators) as one of the explanatory variables. (*p < 0.05, **p < 0.005, ***p < 0.0005.)
| slope |
||||||
|---|---|---|---|---|---|---|
| one explanatory variable | AICc | estimate | s.e. | t-value | p-value | d.f. |
| (a) response variable: predator size diversity | ||||||
| prey size diversity | 129.03 | −0.648 | 0.226 | −2.857 | 0.005** | 65 |
| water salinity (SSS) | 130.63 | 0.040 | 0.015 | 2.560 | 0.01* | 65 |
| water temperature (SST) | 128.44 | 0.033 | 0.011 | 2.962 | 0.004** | 65 |
| NO3 | 123.97 | −0.161 | 0.043 | −3.698 | <0.0001*** | 65 |
| PO4 | 128.36 | −0.670 | 0.225 | −2.979 | 0.004** | 65 |
| SiO3 | 107.75 | −0.313 | 0.054 | −5.760 | <0.0001*** | 65 |
| most parsimonious model: predator size diversity ∼ SiO3 + prey size diversity AICc: 107.44 excluding prey size diversity: predator size diversity ∼ SiO3 AICc: 107.75 | ||||||
| (b) response variable: prey size diversity | ||||||
| predator size diversity | −59.36 | −0.109 | 0.039 | −2.818 | 0.006** | 65 |
| water salinity (SSS) | −51.83 | −0.003 | 0.006 | −0.515 | 0.61 | 65 |
| water temperature (SST) | −70.35 | −0.020 | 0.004 | −4.491 | <0.0001*** | 65 |
| NO3 | −58.11 | 0.047 | 0.018 | 2.574 | 0.01* | 65 |
| PO4 | −52.57 | 0.095 | 0.096 | 0.997 | 0.32 | 65 |
| SiO3 | −60.73 | 0.075 | 0.024 | 3.066 | 0.003** | 65 |
| most parsimonious model: prey size diversity ∼ SST + pred. size diversity AICc: −71.45 excluding predator size diversity: prey size diversity ∼ SST + SiO3 + PO4 + SST × SiO3 AICc: −71.34 | ||||||
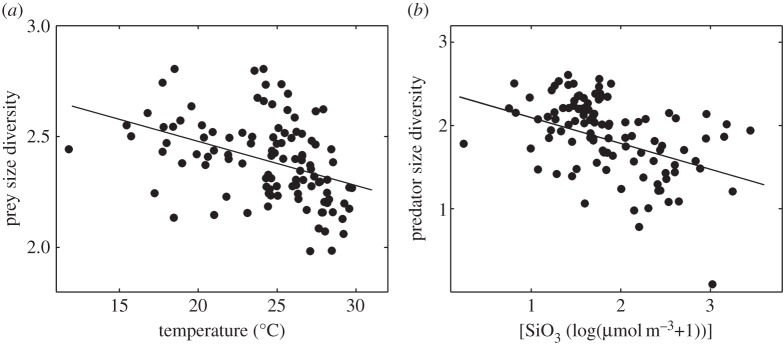
The concentration of SiO3 was the best single factor explaining predator size diversity (table 2a and figure 2b), whereas temperature was the best single factor explaining prey size diversity (table 2b and figure 2a). Predator size diversity tended to be lower in waters with high SiO3 concentrations, and prey size diversity tended to be lower in warm waters. The most parsimonious model explaining predator size diversity included prey size diversity as the second factor accompanying the best single predictor, as did the model for prey size diversity (table 2; see ranking of all possible models in electronic supplementary material, figure F1b and figure F1c). We also considered the most parsimonious models for predator size diversity in the absence of prey size diversity and for prey size diversity in the absence of predator size diversity as explanatory variables. Under these model constraints, the most parsimonious model explaining predator size diversity included only SiO3, and that explaining prey size diversity included the effects of PO4 and SiO3 in addition to that of temperature (table 2; electronic supplementary material: second rows of figure F1c and figure F1b, and first row of figure F2d).
Figure 2.
Effects of (a) temperature on prey size diversity (r = −0.40, p < 0.0001) and (b) silicate concentration on predator size diversity (r = −0.49, p < 0.0001).
In summary, TTE (log10(PPBR)) exhibited a significant positive correlation with temperature and significant negative correlations with nutrient concentrations, associated with a tendency for prey size diversity to be low and predator size diversity to be high in those conditions (tables 1 and 2; figures 1 and 2). High TTE was primarily related to low prey size diversity. Yet, predator size diversity exerted additional secondary positive effects on TTE, especially when size diversity of prey was high (figure 1c). In accordance, the most parsimonious model to explain TTE is the interaction of prey and predator size diversities (table 1).
4. Discussion
(a). Predator and prey size diversity affect trophic transfer efficiency
We found that both predator and prey size diversity exhibit significant relationships with TTE in planktonic communities (table 1 and figure 1c). While this is solely based on correlation analysis, our findings support both hypotheses: (i) predator size diversity promotes biomass trophic transfer through enhanced diet niche partitioning [20] and (ii) prey size diversity hinders biomass transfer via an increasing incidence of predation defence [19] or a slowing of population turnover rates with increasing cell size [18]. To the best of our knowledge, this is the first field study showing the opposite effect of predator and prey size diversity on biomass transfer efficiency. In this study, the size diversity of prey has a greater effect on trophic transfer than the size diversity of predators. Yet, the interaction of predator and prey size diversities is significantly more important than the size diversity of either predator or prey alone (table 1 and electronic supplementary material, figure F1a). Specifically, when the size diversity of prey is low, trophic transfer tends to be high; when the size diversity of prey is high, trophic transfer tends to be higher with high predator size diversity (figure 1c). We hypothesize that enhanced diet niche partitioning related to high predator size diversity would mitigate the negative effects of prey diversity. Another alternative and non-exclusive hypothesis would be that under conditions of low prey size diversity, the mesozooplankton with a target size range of prey that does not overlap the size of available prey may relax their size-based selectivity, thus increasing the target size range of prey. Wider target size ranges among the mesozooplankton could on the one hand potentially enhance competition for the same prey, whereas, on the other hand, potentially decrease prey control via lower than expected ingestion rates from such a diverse mesozooplankton community. This idea has been developed through a mechanistic model in which size dependency of prey selection is divided into a fixed mechanical ingestion dependency, and a behavioural selection dependency driven by trade-offs related to food availability [28].
One might argue that the promotion of trophic transfer by predator size diversity may be solely attributed to an accompanying increase in the proportion of large (but rare) predators. This greater proportion of large predators would increase the predator–prey individual mass ratio (PPMR), enhance attack rates, decrease handling times and ultimately promote predation success [29]. Yet, according to our further analyses (electronic supplementary material, appendix G), the effect of PPMR on log10(PPBR) was weaker than that of the ratio of predator–prey size diversities. Likewise, the effect of average body size on log10(PPBR) was weaker than the effect of size diversity (electronic supplementary material, table G1) and supports our hypothesis.
The interaction of bitrophic diversities as ultimately determining predation performance has recently been reported in experimental studies [30,31]. Using mesocosm manipulations of prey and predator species richness, both Gamfeldt et al. [31] and Saleem et al. [30] highlighted the importance of the presence of multiple, functionally distinct species for determining the strength of predation performance. The role of resource partitioning (via specialization) as a mechanism for reducing total prey (resource) standing stock and for increasing total consumer biomass seems to be generalizable across natural communities, as reviewed by Duffy et al. [32] and reported in a meta-analysis [33]. The underlying mechanism proposed in these studies is similar to that reflected in our results, albeit we focus on size diversity rather than on taxonomic diversity. Neither the Saleem et al. [30] nor Gamfeldt et al. [31] experiments identified the negative effect of prey diversity on predation found in our study. This may be due to the low experimental diversity levels (max. three to five species) and because none of the manipulated prey species exhibited predation defences, a mechanism likely to be common in natural communities. In addition, these experiments used species richness as a measure of diversity, whereas we measured the entropy of individual sizes, which may be better suited for characterizing functional differentiation especially given the identification of size as a ‘meta-trait’ [7–9].
(b). Trophic transfer efficiency in the environmental context
We found that trophic transfer exhibits significant negative relationships with nutrient concentrations, and a significant positive relationship with temperature (table 1). This agrees with previous findings suggesting that trophic transfer increases with food scarcity [34–36]. Indeed, Calbet's [36] global comparative study on mesozooplankton ingestion rates concluded that the relative proportion of primary production removed by mesozooplankton decreased with nutrient availability. Moreover, recent estimates of mesopelagic fish biomass have indicated a 10-fold greater biomass than has been traditionally estimated by models, suggesting underestimation of trophic transfer from primary producers to fishes in the oligotrophic ocean [37]. Those high transfer efficiencies in oligotrophic conditions have been explained as owing to the fast turnover rates of the dominant small primary producers and therefore, an enhanced capacity to support more predator biomass as well as a more stable and clear water column that also aids greater predator–prey coupling [34–36]. Our results provide further insights, by suggesting that prey size diversity may tend to be low and predator size diversity to be high in the stable oligotrophic environments (table 2), and therefore facilitate a greater efficiency of trophic transfer (figure 1).
(c). Relationship between predator and prey size diversities
Another interesting finding is a significant, although weak, negative correlation between predator and prey size diversities (figure 1c). This finding contradicts a positive relationship of diversities (generally measured as species richness) between trophic groups reported by previous studies in terrestrial systems [38,39]. Yet, in the case of planktonic systems, the relationship between predator and prey diversities has already been reported to be weaker and with a variable sign [40,41]. This contrast may be partly explained by the knowledge that increasing terrestrial plant diversity is often accompanied by the creation of new spatial niches for herbivores, whereas in the water column, increased habitat complexity accompanying diversity of primary producers does not necessarily occur, because phytoplankton are unicellular. Besides, the positive effect of prey diversity on predator diversity via enhanced niche partitioning might be overcome in planktonic ecosystems because of a greater incidence of prey defence mechanisms accompanying increasing prey diversity [5]. Lastly, we cannot rule out the possibility that the negative correlation between predator and prey size diversities found in our study might be due to differences in the major forces driving them. While predator size diversity is mainly affected by food availability effects on secondary production in the ECS [42], prey size diversity is mainly driven by temperature via metabolic constraints [18] (table 2).
(d). Future research
Omnivory and intraguild predation have been reported to affect community size structure [43] and ecosystem function [44]. Although we did go to considerable effort to remove carnivores from the predators, we do acknowledge that our methodology limited our ability to account for these interactions. Calbet & Landry [45] demonstrated that the proportion of microzooplankton grazing on phytoplankton is relatively constant (60–75%) across environments; nevertheless, they also suggest that perhaps the length of protistan predatory chains may also play a role in TTE. Mesocosm manipulations further suggest that mesozooplankton may switch from phytoplankton to grazing on ciliates in warm nutrient-limited conditions [46]. Thus, we encourage future studies to explore and account for the effect of intraguild predation within mesozooplankton and microplankton groups when analysing TTE among these plankton groups.
5. Final remark
This study is, to the best of our knowledge, the first to report that the synergistic effect of size diversity at two contiguous trophic levels is stronger for biomass trophic transfer than the effect of size diversity at a single trophic level. While the size diversity of prey hinders transfer to consumers in planktonic communities, the size diversity of predators may promote the efficiency of resource utilization. Size-based top-down control seems to occur mainly via niche partitioning, whereas size-based bottom-up control takes place mainly via size-based metabolic constraints of unicellular growth and the trade-off between growth and predation avoidance. Furthermore, trophic transfer would be stronger in more oligotrophic, warm waters. Our findings rely on correlation analyses and cannot represent proof of causation; however, they do provide insights into size-based theories of ecosystem functioning and suggest mechanisms to test for modelling predictions of fisheries yields as well as the response of plankton size structure to global warming: a factor critical to the functioning of pelagic ecosystems.
Supplementary Material
Acknowledgements
We thank the crews of R/V ORI and R/V ORII for sampling and Yu-Ching Lee, Wei-Hsuan Teng and Elise Marquis for sample processing. Comments from Jyh-Min Chiang, Michio Kondoh, Yi-Ching Lin, Sheng-Feng Shen and Sami Souissi greatly improved this manuscript.
Data accessibility
Dryad data reference: http://dx.doi.org/10.5061/dryad.300k7.
Authors' contributions
C.-h.H. designed the study; C.G.-C., C.-h.H. and L.Y. developed the ideas and conceptual framework of the study; C.G.-C. performed the research; C.-Y.C., F.-S.L., M.-S.S. and G.-C.G. produced datasets; C.G.-C., A.S. and C.-h.H. wrote the manuscript. All authors made a substantial intellectual contribution to the manuscript.
Competing interests
Authors have no competing interests.
Funding
This study was supported by the Foundations for the Advancement of Outstanding Scholarship and Ministry of Science and Technology of Taiwan. C.G.-C. was supported by a project funded by CREST, JST (PI: S.L. Smith).
References
- 1.Thompson RM, et al. 2012. Food webs: reconciling the structure and function of biodiversity. Trends Ecol. Evol. 27, 689–697. ( 10.1016/j.tree.2012.08.005). [DOI] [PubMed] [Google Scholar]
- 2.Hooper DU, et al. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35. ( 10.1890/04-0922) [DOI] [Google Scholar]
- 3.Wilby A, Orwin K. 2013. Herbivore species richness, composition and community structure mediate predator richness effects and top-down control of herbivore biomass. Oecologia 172, 1167–1177. ( 10.1007/s00442-012-2573-8) [DOI] [PubMed] [Google Scholar]
- 4.Duffy JE, Stachowicz JJ, Bruno JF. 2012. Multitrophic biodiversity and responses of marine ecosystems to global change. In Marine biodiversity and ecosystem fuctioning (eds Solan M, Aspden RJ, Paterson DM), pp. 164–184, 1st edn Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Hillebrand H, Cardinale BJ. 2004. Consumer effects decline with prey diversity. Ecol. Lett. 7, 192–201. ( 10.1111/j.1461-0248.2004.00570.x) [DOI] [Google Scholar]
- 6.Petchey OL, Gaston KJ. 2006. Functional diversity: back to basics and looking forward. Ecol. Lett. 9, 741–758. ( 10.1111/j.1461-0248.2006.00924.x) [DOI] [PubMed] [Google Scholar]
- 7.Barton AD, Pershing AJ, Litchman E, Record NR, Edwards KF, Finkel ZV, Kiørboe T, Ward BA. 2013. The biogeography of marine plankton traits. Ecol. Lett. 16, 522–534. ( 10.1111/ele.12063) [DOI] [PubMed] [Google Scholar]
- 8.Litchman E, Klausmeier CA. 2008. Trait-based community ecology of phytoplankton. Annu. Rev. Ecol. Evol. Syst. 39, 615–639. ( 10.1146/annurev.ecolsys.39.110707.173549) [DOI] [Google Scholar]
- 9.Litchman E, Ohman MD, Kiørboe T. 2013. Trait-based approaches to zooplankton communities. J. Plankton Res. 35, 473–484. ( 10.1093/plankt/fbt019) [DOI] [Google Scholar]
- 10.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789. ( 10.1890/03-9000) [DOI] [Google Scholar]
- 11.Cohen JE, Jonsson T, Carpenter SR. 2003. Ecological community description using the food web, species abundance, and body size. Proc. Natl Acad. Sci. USA 100, 1781–1786. ( 10.1073/pnas.232715699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes C, Maxwell D, Reuman DC, Jennings S. 2010. Global patterns in predator–prey size relationships reveal size dependency of trophic transfer efficiency. Ecology 91, 222–232. ( 10.1890/08-2061.1) [DOI] [PubMed] [Google Scholar]
- 13.Woodward G, Hildrew AG. 2002. Body-size determinants of niche overlap and intraguild predation within a complex food web. J. Anim. Ecol. 71, 1063–1074. ( 10.1046/j.1365-2656.2002.00669.x) [DOI] [Google Scholar]
- 14.Elton CS. 1927. Animal ecology. London, UK: Sidgwick and Jackson. [Google Scholar]
- 15.Odum HT. 1956. Efficiencies, size of organims, and community structure. Ecology 37, 592–597. ( 10.2307/1930184). [DOI] [Google Scholar]
- 16.Lindeman RL. 1942. Trophic–dynamic aspect of ecology. Ecology 23, 399–418. ( 10.2307/1930126) [DOI] [Google Scholar]
- 17.Andersen KH, Beyer JE, Lundberg P. 2009. Trophic and individual efficiencies of size-structured communities. Proc. R. Soc. B 276, 109–114. ( 10.1098/rspb.2008.0951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yvon-Durocher G, Montoya JM, Trimmer M, Woodward G. 2011. Warming alters the size spectrum and shifts the distribution of biomass in freshwater ecosystems. Glob. Change Biol. 17, 1681–1694. ( 10.1111/j.1365-2486.2010.02321.x) [DOI] [Google Scholar]
- 19.Steiner CF. 2003. Keystone predator effects and grazer control of planktonic primary production. Oikos 101, 569–577. ( 10.1034/j.1600-0706.2003.12309.x) [DOI] [Google Scholar]
- 20.Ye L, Chang C-Y, García-Comas C, Gong G-C, Hsieh C-H. 2013. Increasing zooplankton size diversity enhances the strength of top-down control on phytoplankton through diet niche partitioning. J. Anim. Ecol. 82, 1052–1061. ( 10.1111/1365-2656.12067) [DOI] [PubMed] [Google Scholar]
- 21.Quintana XD, Brucet S, Boix D, Lopez-Flores R, Gascon S, Badosa A, Sala J, Moreno-Amich R, Egozcue JJ. 2008. A nonparametric method for the measurement of size diversity with emphasis on data standardization. Limnol. Oceanogr. Methods 6, 75–86. ( 10.4319/lom.2008.6.75) [DOI] [Google Scholar]
- 22.Sieracki CK, Sieracki ME, Yentsch CS. 1998. An imaging-in-flow system for automated analysis of marine microplankton. Mar. Ecol. Prog. Ser. 168, 285–296. ( 10.3354/meps168285) [DOI] [Google Scholar]
- 23.Gorsky G, et al. 2010. Digital zooplankton image analysis using the ZooScan integrated system. J. Plankton Res. 32, 285–303. ( 10.1093/plankt/fbp124) [DOI] [Google Scholar]
- 24.Gong G-C, Shiah F-K, Liu K-K, Wen Y-H, Liang M-H. 2000. Spatial and temporal variation of chlorophyll a, primary productivity and chemical hydrography in the southern East China Sea. Cont. Shelf Res. 20, 411–436. ( 10.1016/S0278-4343(99)00079-5) [DOI] [Google Scholar]
- 25.Schartau M, Landry MR, Armstrong RA. 2010. Density estimation of plankton size spectra: a reanalysis of IronEx II data. J. Plankton Res. 32, 1167–1184. ( 10.1093/plankt/fbq072) [DOI] [Google Scholar]
- 26.Pinheiro J, Bates D, DebRoy S, Sarkar D, t.R.D.C.T 2011. nlme: linear and nonlinear mixed effects models. R package version 3.1-103. See http://CRAN.R-project.org/package=nlme.
- 27.Bartoń K.2015. MuMIn: multi-model inference. See http://CRAN.Rproject.org/package=MuMIn .
- 28.Wirtz KW. 2014. A biomechanical and optimality-based derivation of prey-size dependencies in planktonic prey selection and ingestion rates. Mar. Ecol. Prog. Ser. 507, 81–94. ( 10.3354/meps10894) [DOI] [Google Scholar]
- 29.Brose U. 2010. Body-mass constraints on foraging behaviour determine population and food-web dynamics. Funct. Ecol. 24, 28–34. ( 10.1111/j.1365-2435.2009.01618.x) [DOI] [Google Scholar]
- 30.Saleem M, Fetzer I, Harms H, Chatzinotas A. 2013. Diversity of protists and bacteria determines predation performance and stability. ISME J. 7, 1912–1921. ( 10.1038/ismej.2013.95) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gamfeldt L, Hillebrand H, Jonsson PR. 2005. Species richness changes across two trophic levels simultaneously affect prey and consumer biomass. Ecol. Lett. 8, 696–703. ( 10.1111/j.1461-0248.2005.00765.x) [DOI] [Google Scholar]
- 32.Duffy JE, Cardinale BJ, France KE, McIntyre PB, Thébault E, Loreau M. 2007. The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecol. Lett. 10, 522–538. ( 10.1111/j.1461-0248.2007.01037.x) [DOI] [PubMed] [Google Scholar]
- 33.Griffin JN, Byrnes JEK, Cardinale BJ. 2013. Effects of predator richness on prey suppression: a meta-analysis. Ecology 94, 2180–2187. ( 10.1890/13-0179.1) [DOI] [PubMed] [Google Scholar]
- 34.San Martin E, Irigoien X, Harris RP, Lopez-Urrutia A, Zubkov MV, Heywood J. 2006. Variation in the transfer of energy in marine plankton along a productivity gradient in the Atlantic Ocean. Limnol. Oceanogr. 51, 2084–2091. ( 10.4319/lo.2006.51.5.2084) [DOI] [Google Scholar]
- 35.Gasol JM, del Giorgio PA, Duarte CM. 1997. Biomass distribution in marine planktonic communities. Limnol. Oceanogr. 42, 1353–1363. ( 10.4319/lo.1997.42.6.1353) [DOI] [Google Scholar]
- 36.Calbet A. 2001. Mesozooplankton grazing effect on primary production: a comparative analysis in marine ecosystems. Limnol. Oceanogr. 46, 1824–1830. ( 10.4319/lo.2001.46.7.1824) [DOI] [Google Scholar]
- 37.Irigoien X, et al. 2014. Large mesopelagic fishes biomass and trophic efficiency in the open ocean. Nat. Commun. 5, 3271. ( 10.1038/ncomms4271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haddad NM, Crutsinger GM, Gross K, Haarstad J, Knops JMH, Tilman D. 2009. Plant species loss decreases arthropod diversity and shifts trophic structure. Ecol. Lett. 12, 1029–1039. ( 10.1111/j.1461-0248.2009.01356.x) [DOI] [PubMed] [Google Scholar]
- 39.Castagneyrol B, Jactel H. 2012. Unraveling plant–animal diversity relationships: a meta-regression analysis. Ecology 93, 2115–2124. ( 10.1890/11-1300.1) [DOI] [PubMed] [Google Scholar]
- 40.Longmuir A, Shurin JB, Clasen JL. 2007. Independent gradients of producer, consumer, and microbial diversity in lake plankton. Ecology 88, 1663–1674. ( 10.1890/06-1448.1) [DOI] [PubMed] [Google Scholar]
- 41.Irigoien X, Huisman J, Harris RP. 2004. Global biodiversity patterns of marine phytoplankton and zooplankton. Nature 429, 863–867. ( 10.1038/nature02593) [DOI] [PubMed] [Google Scholar]
- 42.García-Comas C, Chang C-Y, Ye L, Sastri AR, Lee Y-C, Gong G-C, Hsieh C-H. 2014. Mesozooplankton size structure in response to environmental conditions in the East China Sea: how much does size spectra theory fit empirical data of a dynamic coastal area? Prog. Oceanogr. 121, 141–157. ( 10.1016/j.pocean.2013.10.010) [DOI] [Google Scholar]
- 43.Chang C-W, Miki T, Shiah F-K, Kao S-J, Wu J-T, Sastri AR, Hsieh C-H. 2013. Linking secondary structure of individual size distribution with nonlinear size–trophic level relationship in food webs. Ecology 95, 897–909. ( 10.1890/13-0742.1) [DOI] [PubMed] [Google Scholar]
- 44.Bruno JF, O'Connor MI. 2005. Cascading effects of predator diversity and omnivory in a marine food web. Ecol. Lett. 8, 1048–1056. ( 10.1111/j.1461-0n248.2005.00808.x) [DOI] [Google Scholar]
- 45.Calbet A, Landry MR. 2004. Phytoplankton growth, microzooplankton grazing, and carbon cycling in marine systems. Limnol. Oceanogr. 49, 51–57. ( 10.4319/lo.2004.49.1.0051) [DOI] [Google Scholar]
- 46.Lewandowska AM, Boyce DG, Hofmann M, Mathiessen B, Sommer U, Worm B. 2014. Effects of sea surface warming on marine plankton. Ecol. Lett. 17, 614–623. (doi/10.1111:ele.12265) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Dryad data reference: http://dx.doi.org/10.5061/dryad.300k7.