Abstract
During the Pleistocene, Australia and New Guinea supported a rich assemblage of large vertebrates. Why these animals disappeared has been debated for more than a century and remains controversial. Previous synthetic reviews of this problem have typically focused heavily on particular types of evidence, such as the dating of extinction and human arrival, and have frequently ignored uncertainties and biases that can lead to misinterpretation of this evidence. Here, we review diverse evidence bearing on this issue and conclude that, although many knowledge gaps remain, multiple independent lines of evidence point to direct human impact as the most likely cause of extinction.
Keywords: quaternary, prehistory, palaeoecology, archeology, human impacts, climate change
1. Introduction
Alfred Russel Wallace (1876) identified the extinction of the ‘hugest, fiercest and strangest’ animals from most land environments as one of the most significant biological changes in recent Earth history [1]. The ‘marvellous fact’ of megafaunal extinction is now far better described than it was in Wallace's lifetime, but its cause is controversial. The two most widely accepted agents of extinction are human impact and climate change, but whether one or the other was dominant and how their importance varied globally is unclear [2–6].
Sahul—mainland Australia, New Guinea and Tasmania, as connected by dry land through much of the Pleistocene [7,8]—is crucial to this debate. This is because people reached Sahul by an ocean crossing mid-way through the last glacial cycle [9–14]. Later continental migrations through Eurasia and into the Americas were governed by changing climates in the approach to the Pleistocene/Holocene transition. These controlled the extent of ice sheets, the availability of migration routes and the distribution of environments suitable for people. The same changes also drove shifts in habitat for megafaunal species, making it difficult to separate the human and climatic contributions to megafaunal extinction [3]. The decoupling of migration from these global shifts should allow a clearer test of the impacts of newly arrived humans on ecosystems in Sahul.
2. Hypotheses
Today, Sahul has no native terrestrial animal larger than about 40 kg, but for much of the Pleistocene it supported diverse large vertebrates up to almost three tonnes [6,15,16]. The overkill hypothesis proposes that human hunting drove these animals extinct. Conceivably, this resulted from selective killing of big animals [17,18]. It is also possible that non-selective hunting differentially removed large species because of their low population growth rates and consequent sensitivity to small increases in mortality [15,19,20]. The main alternative to overkill is the idea that the megafauna disappeared because of climate change. Several authors argue that over the last 450 ka (thousands of years) the climate of Sahul became more variable and arid. This is thought to have placed increasing environmental stress on large vertebrates, reducing their distribution and abundance and causing a staggered series of extinctions over several glacial cycles [6,21,22]. A third hypothesis envisages anthropogenic fire as a cause of extinction of at least some megafauna. Many of Australia's extinct megaherbivores appear to have been browsers, and so presumably benefitted from a high diversity of shrubs and small trees; perhaps burning removed or degraded habitat for these species [23,24]. Although these causal mechanisms can be evaluated independently, they might also have combined in various ways.
Several types of evidence can be used to test these hypotheses. Most obviously, evidence on the timing of extinction and human arrival is essential to show if the extinctions were synchronized and closely followed human arrival, rather than being spread over some long interval unrelated to human impact. Data on past climates are needed to test for trends that might have driven megafaunal decline. The pattern of change in population size preceding extinction is also crucial: if climate trends caused gradual attrition of megafauna, populations of large vertebrates should have been in long-term decline under the stress of worsening environmental conditions before finally disappearing; on the other hand, human impact ought to have precipitated abrupt decline to extinction of species that need not have been declining beforehand. Palaeoecological reconstructions can test whether extinctions were associated with specific environmental changes, particularly shifts in fire regime (possibly caused by people) or alterations of vegetation state that might have been caused by anthropogenic fire or climate change. Also relevant is archaeological evidence on interactions between humans and extinct megafauna.
Here, we synthesize current understanding of this problem. Our review has two main aims. First, we aim to encompass the broadest possible range of evidence. This is important because the use of multiple independent lines of evidence is the most promising avenue to resolve this problem, in Sahul and globally. Second, we address uncertainties and biases that are inevitable features of data on events from the distant past, and deal with these explicitly in interpreting evidence.
3. Human arrival
It is still uncertain when people first set foot on Sahul: it might have been around 50 ka, or as much as 10 ka earlier [10,25,26]. However, it is generally accepted that people were widespread over the continent by 45 ka or a few millennia earlier [13,14,27,28]. There is as yet no obvious geographical pattern in first-appearance dates to indicate the progress of a wave of colonization across Sahul. This is not surprising, because dates older than 40 ka typically have uncertainty ranges of several thousand years. If people dispersed over Sahul within a few millennia, we would be unable to resolve that process. In addition, we still have few dated sites from the earliest phase of the prehistory of Sahul. Only 20 archaeological sites have been dated to 40 ka or older [14]; for comparison, the archaeological record in Australia consists of 1748 dated sites [29]. Most of the arid centre lacks evidence of human occupation until just after 40 ka [30], but whether this truly indicates late settlement rather than poor preservation and limited sampling is unclear. Occupation of Tasmania had to await the emergence of a land bridge at 43 ka [8].
This picture suggests that declines and extinctions of species owing to human impact should have been concentrated in the period 50–40 ka. Quantitative population models suggest that if hunting was the primary driver of decline, demographic lags might have caused delays of several hundred to several thousand years between first contact and extinction in any given region [20,31]. Late occupation of some regions might plausibly have delayed continent-wide extinction several thousand years more. Taking these factors into account, a concentration of extinctions between about 50 and 35 ka might be attributable to the impact of human arrival. Later extinctions are less likely to have been owing to direct human impact, although they could conceivably have resulted from the slow emergence of interactions between human and climate impacts or other delayed effects of people on ecosystems [32]. Extinctions earlier than 60 ka can be attributed to non-human factors.
4. Chronology of megafaunal extinction
There are two contending views on the timing of extinction. A series of recent studies restricted to specimens and sites dated with high confidence suggest that the extinctions were concentrated between 50 and 40 ka on mainland Australia [24,26,33–36], and slightly later in Tasmania [37]. On the other hand, more extensive compilations of occurrences in the fossil record, lacking controls on date quality, suggest staggered extinction through the period from 400 to about 20 ka [6].
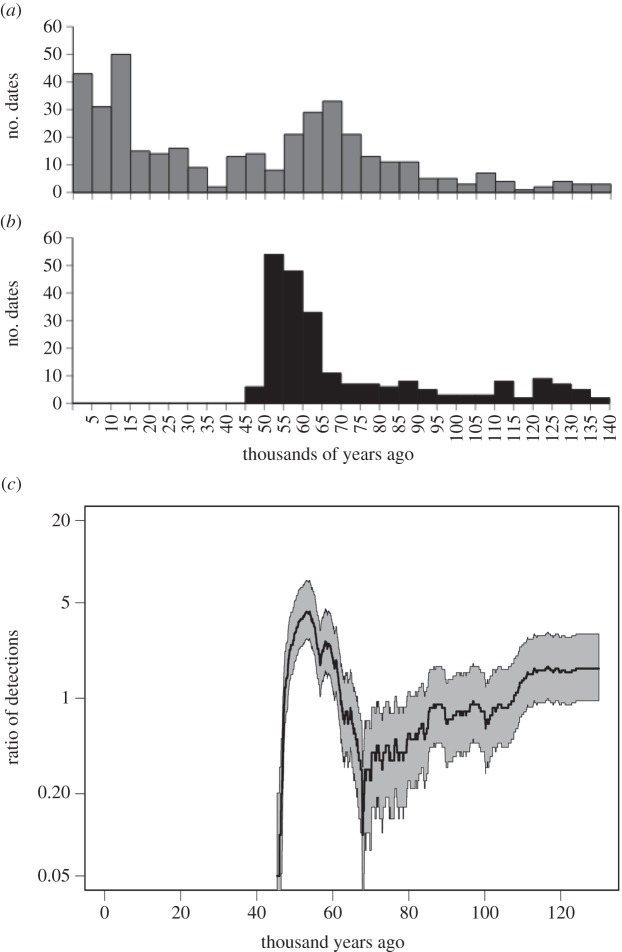
Two methodological problems affect these inferences. First, dates on fossil remains are subjected to many technical limitations and potential biases. Therefore, it is necessary to screen date-lists for reliability. Because in Sahul, the period of potential human–megafauna overlap is close to or beyond the limit of 14C dating, a wide range of techniques in addition to 14C has been applied to the problem, making it difficult to standardize the reliability of age determinations. In response, we developed a set of criteria for assessing reliability of age determinations across the full range of methods applied to Quaternary palaeontology and archaeology, and used these to assess reliability of all published age estimates on Sahul's extinct megafauna [38]. Figure 1 illustrates the impact of screening of dates using the example of Diprotodon sp., the largest marsupial. There are approximately 100 ages on Diprotodon from more than 1 Myr to 2 ka. After filtering for reliability, only 23 reliable dates remained, none younger than 44 ka.
Figure 1.
Time-series of dated specimens of Diprotodon sp., arranged in sequence from youngest to oldest, with ±1 s.d. High-reliability dates [38] are black and low-quality dates grey; youngest reliable date is arrowed. Diprotodon sketch by Peter Murray.
The second problem is sparse sampling in the fossil record. Many megafaunal taxa from Sahul are represented by few specimens, of which even fewer have reliable dates. The date of extinction of a species is inferred from the absence of fossils, but when we have few dates this inference is highly uncertain. This uncertainty can create the appearance of staggered extinction even if all species disappeared at the same time [39]. To draw statistically robust inferences on the pattern of extinction of assemblages of species, we need quantitative approaches that infer probability intervals for extinction from the incomplete presence data furnished by the fossil record [40–42].
A recent study addresses both problems. Saltré et al. [26] compiled all available dates on Australia's Pleistocene megafauna and screened them for reliability [38], then derived statistically robust estimates of extinction timing for the 14 genera with sufficient reliable dates for analysis. They concluded that these genera went extinct between approximately 61 and 35 ka, with a peak in extinction probability at 42.1 ka. The picture remains incomplete because we cannot infer extinction chronologies for many poorly dated taxa, representing as many as 15 [6] genera.
5. Climate trends and variability
The Quaternary record of terrestrial climate change in Sahul is sparse, so climate trends are mainly inferred from ice cores in Antarctica and syntheses of marine sequences, which indicate broad trends in temperature. Unusually warm interglacials and cool glacials are irregularly distributed through almost the entire 800 ka of the EPICA Dome C ice-core from Antarctica [43]. Several syntheses have suggested a cooling trend over the last million years, with variable expression of the Mid-Brunhes event at approximately 430 ka, but this is not universally recognized [44–46]. Records of sea-surface temperatures near Sahul in the Coral Sea [47,48] do not show cooling over the last million years, nor increased variability across the Mid-Brunhes event or thereafter [46]. There is no clear trend through the last million years in the rate of change in oxygen-isotope composition in the global benthic oxygen-isotope record (the LR04 stack) [49].
Some authors have suggested [6] that the EPICA Dome C ice core reveals increasing variability in temperature proxies over the last 450 ka. We tested for this at millennial time scales by calculating the mean deviation (sum of absolute differences from the mean divided by the number of measurements) in δD (deuterium; this is a proxy for temperature, in which more negative values indicate lower temperatures) from 450 ka to the present in this core. A challenge for this analysis is that the time resolution of the core increases towards the present. For example, the mean interval between successive temperature estimates for the last 12.2 ka is 12.2 years, increasing to 418.6 years from 500 to 550 ka. Fluctuations on short time-scales are therefore more likely to be visible in recent parts of the record. To control for this uneven sampling, we resampled 1000 times (with a random uniform start date from within the first interval) increasing temporal window lengths from 3 ka to 12 ka across the series to 450 ka. For each resampled interval width, we calculated the mean deviation and tested for a linear trend of increasing mean deviation towards the present. We used the range of evidence ratios (ER) to compare the slope model (trend in increasing mean deviation towards the present) to the intercept-only (null) model with no trend. An 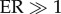 would support a linear change over the null ‘no trend’ model, and thus the claim that variability increased. The linear trend model was rarely favoured, the slope of the trend being near zero (supporting the null model) for most sampling intervals (figure 2a). To visualize the absence of trend, we resampled at an interval of 3 ka (to ensure at least five temperature values were available to calculate mean deviations) over 1000 iterations, splitting the 450 ka-to-present series into four periods (24–156 ka, 156–271 ka, 271–342 ka and 342–437 ka; figure 2b), and calculated the temperature mean deviations for each interval and iteration. The temperature record actually became less variable from 450 to 156 ka; variability then increased, but only slightly, from 156 to 24 ka (figure 2c).
would support a linear change over the null ‘no trend’ model, and thus the claim that variability increased. The linear trend model was rarely favoured, the slope of the trend being near zero (supporting the null model) for most sampling intervals (figure 2a). To visualize the absence of trend, we resampled at an interval of 3 ka (to ensure at least five temperature values were available to calculate mean deviations) over 1000 iterations, splitting the 450 ka-to-present series into four periods (24–156 ka, 156–271 ka, 271–342 ka and 342–437 ka; figure 2b), and calculated the temperature mean deviations for each interval and iteration. The temperature record actually became less variable from 450 to 156 ka; variability then increased, but only slightly, from 156 to 24 ka (figure 2c).
Figure 2.
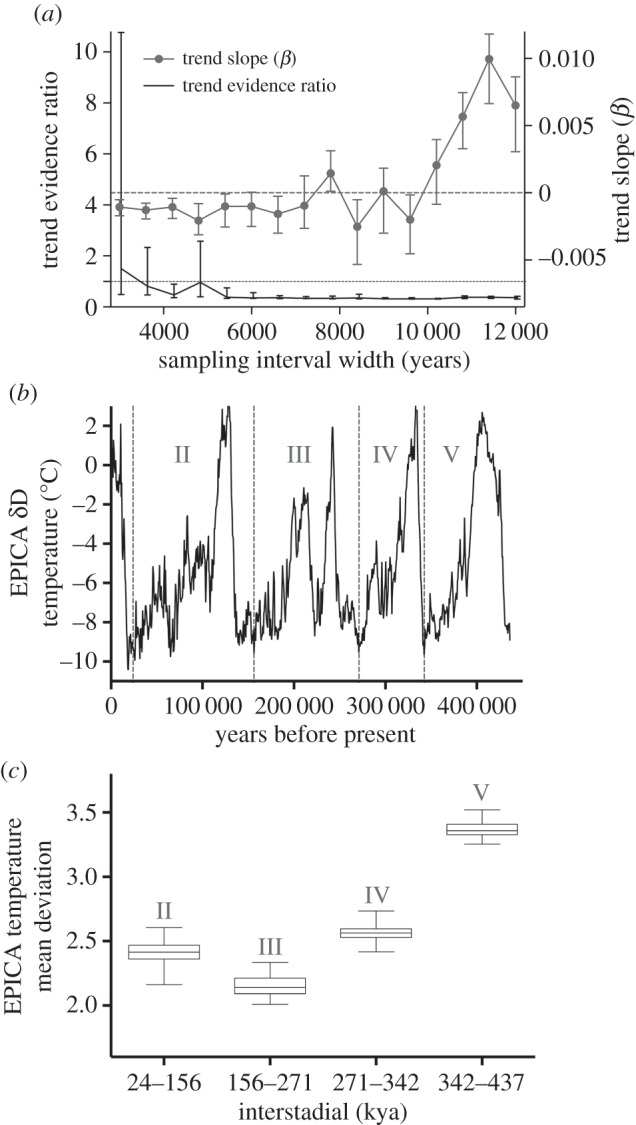
Trends in variability of temperature in the EPICA Dome C core over the last 450 000 years. (a) ER for linear trend in mean deviation in EPICA δD (δ deuterium = a proxy for temperature: more negative values indicate lower temperatures) from 450 to 24 ka (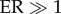 indicates evidence for linear trend) across sampling intervals of increasing width; also shown is the mean trend slope (β) per sampling interval width; (b) example EPICA temperature series resampled at a constant window of 600 years from present back to 450 ka; and (c) average and 95 percentile mean deviation of temperature within the four interstadials since 450 ka.
indicates evidence for linear trend) across sampling intervals of increasing width; also shown is the mean trend slope (β) per sampling interval width; (b) example EPICA temperature series resampled at a constant window of 600 years from present back to 450 ka; and (c) average and 95 percentile mean deviation of temperature within the four interstadials since 450 ka.
Much of the discussion of environmental stress on megafaunal populations has focused on moisture availability rather than temperature [50]. The last few glacial–interglacial cycles (excluding the current interglacial) have been characterized by wetter conditions during interglacial stages and comparatively arid conditions during glacials [11,51–54]. However, available moisture records not support the existence of a strong trend to increasing aridity over the last few glacial cycles.
There is little evidence for exceptional climate change around the time of human arrival. During Marine Isotope Stage (MIS) 3 (57–29 ka), dust flux into the Tasman Sea from south-eastern Australia, and into the Indian Ocean from north-western Australia, remained approximately constant [52], and there was no substantial variation in summer rainfall and dry season length over the Arafura Sea [55], or in discharge from the Murrumbidgee River in south-eastern Australia [56]. Australian palaeo-lake levels were high in early MIS 3, generally decreasing after 48–42 ka over a period of 10–15 ka [11,50,54]. Millennial-scale Asian monsoon variability, which is probably coupled with Australian monsoon variability, is similar in amplitude throughout the interval 60–30 ka [57–59]. While grass pollen is anomalously high off northwest Australia during the last interglacial [60,61], this was evidently not part of a longer trend to increased aridity in northern Australia. Water levels in the Lake Eyre and Lake Frome mega-lakes, in the southeast of the arid zone, fell between 50 and 40 ka, after which those lakes filled only intermittently [50]. Possibly, this drying provides an explanation for the extinction of the giant bird Genyornis newtoni in that region [50], although it is unknown if these changes were exceptional or typical of a pattern that recurred through successive glacial cycles.
6. Trends in megafaunal abundance
Trends in abundance of species cannot easily be inferred from the fossil record, because the abundance of fossils varies for many reasons unrelated to abundance in the source populations, such as age-dependent preservation bias [62,63] or stochastic variation in conditions affecting the likelihood of preservation. Three datasets attempt to overcome these problems in different ways.
First, cave deposits from subterranean galleries with openings to the surface that act as passive pitfall traps should accumulate remains at rates roughly proportional to population abundance. If conditions for preservation are excellent there may be little loss of fossil material over the period of accumulation. Two such cases from southern Australia, spanning periods of 500–150 ka, revealed long-term stability in the mammalian assemblage, despite climate-related variation in the relative abundance of small and large species. Large species declined relative to smaller ones during dry periods, probably owing to local range contractions, but rebounded subsequently [64,65].
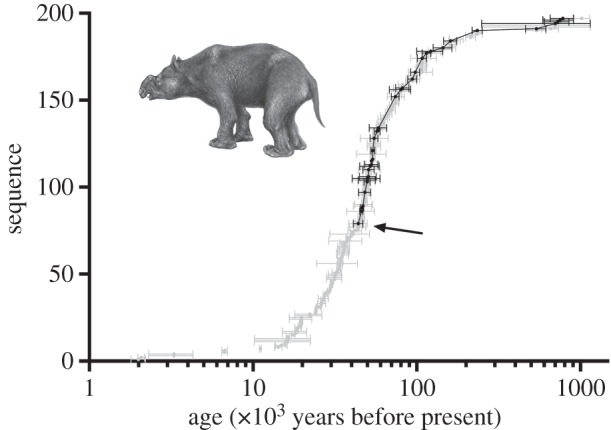
Second, comparison of the frequencies through time of remains of species that are subject to similar preservation biases may reveal shifts in their relative abundance [63]. Genyornis newtoni was a flightless, ground-nesting bird with a distribution overlapping the emu Dromaius novaehollandiae, another flightless ground-nester. Eggshells of both birds are abundant in the same sedimentary contexts and so are subject to the same processes of deposition and preservation, and are dateable by the same methods [24,66]. If abundances of fossil eggshells of both species are affected by the same biases, the ratio of their abundances should be free of bias. Changes in that ratio through time depict trends in the abundance of a species that went extinct—Genyornis—relative to a species that survived (emu). Figure 3 collates the relative abundance of Genyornis and emu eggshells through time, and shows that Genyornis tended to decline relative to the emu from the last interglacial to about 70 ka, then increased from about 65 to 50 ka, before crashing to extinction just after 50 ka.
Figure 3.
Relative abundance of eggshells of Genyornis and emu Dromaius novaehollandiae through the last glacial cycle: (a) and (b) numbers of dated samples from Genyornis and emu, respectively, from [24]. (c) Ratio of frequencies of Genyornis to emu samples, with 95% confidence intervals, calculated using a moving window (scaled to density of samples) to generate a smoothed curve.
Third, spores of fungi (Sporormiella spp. and others) that sporulate on the dung of large herbivores indicate the presence of those animals in past environments [67,68]. The spores are abundant and so provide a continuous measure of activity of large herbivores that can be quantified as spore-influx rates or indexed relative to pollen counts. A dung-fungus record from north-eastern Australia showed no trend from 130 ka until a steep decline at about 41 ka [69]. This decline cannot be explained by climate, which was evidently stable at the time [70]. Analysis of potential deposition biases suggest that the drop in dung fungi was a genuine indicator of an abrupt decline of the biomass of large herbivores [67].
Studies of ancient DNA in other regions have revealed long-term trends in population size [3], and local extinctions [71–73]. Unfortunately, we have little genetic information on Sahul's extinct megafauna because of poor DNA preservation in this region. Recent advances in molecular techniques have resulted in the first complete mitochondrial genome sequences of extinct marsupial megafauna [74]. These methods hold promise for phylogenetic and demographic studies, but population genetic analyses are currently out of reach.
7. Palaeoecological reconstructions
Prideaux et al. [75] reconstructed the ecology of Procoptodon goliah, a large kangaroo that once occurred through semi-arid southern and eastern Australia [76]. Dental morphology and microwear showed that P. goliah had a tough browse diet, and stable isotopes confirmed that a major component was C4 chenopods (saltbush, Chenopodiaceae). Chenopod shrublands remain widespread through the southern semi-arid and arid zones. Because chenopods are poorly flammable, it seems unlikely that anthropogenic fire had a large impact on P. goliah's habitat, but the species could have been highly exposed to hunters in its shrubland habitat.
At the Lynch's Crater site, Rule et al. [69] used counts of spores of dung fungi, pollen grains and charcoal particles to reconstruct environmental changes associated with megafaunal extinction. Before the decline of dung fungi at 41 ka, the vegetation around the site was a mixture of angiosperm and gymnosperm rainforests and dry sclerophyll forest with little or no fire. Decline of dung fungi was closely followed by a sharp increase in the influx of charcoal and a more gradual change in vegetation composition leading to replacement of the original mixed forest by uniform sclerophyll forest of higher density. Possibly, increased fire was caused by a build-up of fine fuel following the relaxation of herbivory, while vegetation changes resulted from some combination of release from herbivore pressure and impacts of fire. A parallel study at a cool alpine site in south-eastern Australia [77] also revealed a steep and unprecedented drop in dung fungi in the middle of the last glacial cycle, but this was not accompanied by any change in fire activity or vegetation, which remained a grass/shrub steppe.
Stable isotope analysis of eggshells showed that extinction of Genyornis coincided with a sustained change in diet of sympatric emus, from mixed feeding on C3 and C4 plants to predominantly C3 plants [24]. The change was unprecedented in a record reaching back to 140 ka and cannot be attributed to climate, but its cause remains unclear. Possibly, an altered fire regime induced a shift in the composition of vegetation, but there are no suitable charcoal records to verify this. Alternatively, the change in emu diet could reflect vegetation change resulting from megaherbivore extinction. This also cannot be tested owing to the lack of pollen records for the arid zone. A marine core with a source area overlapping part of the same region shows a transient increase in biomass burning from 43 to 40 ka [78] and an excursion to C3-dominated vegetation; a low-resolution terrestrial record to the southwest, in the same climate zone, reveals no such increase in charcoal [79].
A synthesis of charcoal records from the Australasian region found some indication of increased charcoal input between 50 and 40 ka but the deviation during that period was small compared with variation before 50 and after 40 ka [80]. Some sites do show charcoal peaks around the time of human arrival and megafaunal extinction, but others do not. Some caution in the interpretation of charcoal records is warranted, because human and natural fire regimes might differ in their ecological effects while producing similar influxes of charcoal, especially when these are averaged over long intervals. In addition, many charcoal records have only loose chronological control through the crucial period between 50 and 35 ka, so sharp changes could be obscured by imprecision when different records are combined. Bearing these reservations in mind, it is unlikely that human firing of Sahul landscapes produced continent-wide impacts, although some environments may have been sensitive to changes in the frequency and timing of ignition with human colonization.
8. Human–megafauna interaction
Archaeological evidence of hunting is rare and questionable for most species of Sahul's megafauna [15]. The one clear exception is the giant bird Genyornis [81]. Some eggshells of Genyornis show distinct charring patterns indicating they were heated over campfires, but only from 54 to 47 ka, during the interval when Genyornis declined to extinction. Similar charring patterns first appear on emu eggshells at the same time, and continue thereafter. Simultaneous onset of charring at widespread locations provides a signal of the early arrival and rapid spread of human populations through the arid regions of southern Australia. It also shows that these early populations exerted hunting pressure that could have contributed to the extinction of Genyornis.
Otherwise, does the lack of evidence for hunting of other species mean that hunting must have been negligible, as several authors have argued [6,82,83]? Surovell & Grund [84] argue that for Sahul especially, archaeological evidence of hunting of species that went extinct soon after human arrival ought to be rare even if that hunting was ecologically important. The main reason is that, given the early date of human arrival, the period of interaction between humans and extinct megafauna is only a small proportion of the total archaeological record of Sahul. Further, the quantity of evidence should be limited by the fact that hunting rates would have been highest early in the interaction when hunted populations were abundant but human populations were still small and of low archaeological visibility, and the effects of time-dependent loss of evidence would increase the rarity of signs of human–megafauna interaction. The predicted rarity of this evidence means that a very large archaeological and palaeontological sample would be needed to detect it, and it would be dangerous to use the failure to detect such evidence in a small sample to conclude that no such interaction occurred. Given these considerations, it is not surprising that the strongest evidence for hunting comes from Genyornis, whose remains are outstandingly abundant (1327 eggshell collections [81]).
9. Conclusion
Evidence on causes of megafaunal extinction in Sahul is still patchy: we have less information on the changing climate of Sahul through the Middle and Late Pleistocene than for other parts of the world; many species that went extinct during this period are poorly dated; we have few archaeological sites attesting to the timing and pattern of early human occupation; and we lack detailed ecological information for most extinct megafauna. As a result, we still lack a detailed picture of the processes leading to megafaunal extinction in Sahul. Nonetheless, the weight of the evidence that we do have points clearly to direct human impact as the main cause of extinction. Although it is likely that there was a general cooling trend over Sahul through much of the Pleistocene, the evidence that megafaunal extinction was related to an increased rate of drying and amplified climate variability is weak at best. There were periods of aridity in the last glacial cycle, but they appear not to have been exceptional in comparison with previous cycles. There is no rigorously tested evidence for a staggered series of extinctions, either within the last glacial cycle or over several glacial cycles. Instead, high-quality dates indicate synchronous extinction within a few thousand years of human arrival. Where it is possible to interpret dynamics of megafaunal populations, populations appear to have crashed to extinction shortly after human occupation of Sahul rather than declining gradually over long periods beforehand. For species with well-described habitat preferences, it is clear that the animals disappeared despite their habitat remaining widespread. Reconstruction of the environmental context of extinction suggests that extinction preceded vegetation change, and that increased fire (where it occurred) was a consequence rather than a cause of decline of large herbivores.
Extinction of megafauna in Sahul presaged comparable losses on other continents and large islands over the last 50 000 years. Because the arrival of people in Sahul in the middle of the last glacial cycle was the first time in Earth history that modern humans reached a large landmass not already occupied by other hominids, Sahul provides an exceptionally valuable case for our understanding of the impacts of early humans on naive ecosystems. The evidence summarized here for a dominant role of direct human impact is therefore globally significant. Several recent analyses of megafaunal extinction using global databases agree in finding a dominant role for humans in most of the world [2,5,85]. The evidence from Sahul in these analyses is less clear, probably because the data on Sahul's megafauna were relatively sparse and of variable quality. The more comprehensive approach to data sources taken in this review is therefore valuable in clarifying evidence on the relative contributions of humans and climate to megafaunal extinction in Sahul, and strengthening support for a consistently large global impact of early humans on the diversity of large vertebrates.
Authors' contributions
All authors contributed to planning and drafting of the manuscript; C.N.J. led the writing; C.J.A.B. conducted the analysis of climate variability; N.J.B. conducted the analysis of Genyornis and emu abundance; M.R.R. and C.J.A.B. analysed the Diprotodon date list; all authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
The Australian Research Council provided funding for this work through research fellowships to C.N.J., J.A., M.I.B., A.C., R.G.R., C.S.M.T., G.J.P. and C.J.A.B. The Environment Institute of the University of Adelaide funded research workshops.
References
- 1.Wallace AR. 1876. The geographical distribution of animals, with a study of the relations of living and extinct faunas as elucidating past changes of the Earth's surface, vol. 1. New York, NY: Harper and Brothers. [Google Scholar]
- 2.Sandom C, Faurby S, Sandel B, Svenning J-C. 2014. Global late Quaternary megafauna extinctions linked to humans, not climate change. Proc. R. Soc. B 281, 20133254 ( 10.1098/rspb.2013.3254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorenzen ED, et al. 2011. Species-specific responses of Late Quaternary megafauna to climate and humans. Nature 479, 359–364. ( 10.1038/nature10574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lima-Ribeiro MS, Hortal J, Varela S, Diniz-Filho JAF. 2014. Constraint envelope analyses of macroecological patterns reveal climatic effects on Pleistocene mammal extinctions. Quat. Res. 82, 260–269. ( 10.1016/j.yqres.2014.02.003) [DOI] [Google Scholar]
- 5.Bartlett LJ, Williams DR, Prescott GW, Balmford A, Green RE, Eriksson A, Valdes PJ, Singarayer JS, Manica A. 2015. Robustness despite uncertainty: regional climate data reveal the dominant role of humans in explaining global extinctions of Late Quaternary megafauna. Ecography 38, 1–10. ( 10.1111/ecog.01566) [DOI] [Google Scholar]
- 6.Wroe S, et al. 2013. Climate change frames debate over the extinction of megafauna in Sahul (Pleistocene Australia-New Guinea). Proc. Natl Acad. Sci. USA 110, 8777–8781. ( 10.1073/pnas.1302698110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokoyama Y, Purcell A, Lambeck K, Johnston P. 2001. Shore-line reconstruction around Australia during the Last Glacial Maximum and Late Glacial Stage. Quat. Int. 83–85, 9–18. ( 10.1016/S1040-6182(01)00028-3) [DOI] [Google Scholar]
- 8.Lambeck K, Chappell J. 2001. Sea level change through the last glacial cycle. Science 292, 679–686. ( 10.1126/science.1059549) [DOI] [PubMed] [Google Scholar]
- 9.Roberts RG, Jones R, Smith MA. 1990. Thermoluminescence dating of a 50,000-year-old human occupation site in northern Australia. Nature 345, 153–156. ( 10.1038/345153a0) [DOI] [Google Scholar]
- 10.Roberts RG, Jones R, Spooner NA, Head MJ, Murray AS, Smith MA. 1994. The human colonization of Australia: optical dates of 53,000 and 60,000 years bracket human arrival at Deaf Adder Gorge, Northern Territory. Quat. Sci. Rev. 13, 573–584. ( 10.1016/0277-3791(94)90080-9) [DOI] [Google Scholar]
- 11.Bowler JM, Johnston H, Olley JM, Prescott JR, Roberts RG, Shawcross W, Spooner NA. 2003. New ages for human occupation and climatic change at Lake Mungo, Australia. Nature 421, 837–840. ( 10.1038/nature01383) [DOI] [PubMed] [Google Scholar]
- 12.Summerhayes GR, Leavesley M, Fairbairn A, Mandui H, Field J, Ford A, Fullagar R. 2010. Human adaptation and plant use in highland New Guinea 49,000 to 44,000 years ago. Science 330, 78–81. ( 10.1126/science.1193130) [DOI] [PubMed] [Google Scholar]
- 13.O'Connell JF, Allen J. 2012. The restaurant at the end of the Universe: modelling the colonisation of Sahul. Aust. Archaeol. 74, 5–17. [Google Scholar]
- 14.Allen J, O'Connell JF. 2014. Both half right: updating the evidence for dating first human arrivals in Sahul. Aust. Archaeol. 79, 86–108. [Google Scholar]
- 15.Johnson C. 2006. Australia's mammal extinctions: a 50,000 year history. Melbourne, Australia: Cambridge University Press. [Google Scholar]
- 16.Long J, Archer M, Flannery T, Hand S. 2002. Prehistoric mammals of Australia and New Guinea. Sydney, Australia: University of New South Wales Press. [Google Scholar]
- 17.Martin PS. 1984. Prehistoric overkill: the global model. In Quaternary extinctions (eds Martin PS, Klein RG), pp. 354–403. Tucson, AZ: University of Arizona Press. [Google Scholar]
- 18.Flannery TF. 1994. The future eaters. Melbourne, Australia: Reed. [Google Scholar]
- 19.Johnson CN. 2002. Determinants of loss of mammal species during the Late Quaternary ‘megafauna’ extinctions: life history and ecology, but not body size. Proc. R. Soc. Lond. B 269, 2221–2227. ( 10.1098/rspb.2002.2130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brook BW, Johnson CN. 2006. Selective hunting of juveniles as a cause of the imperceptible overkill of the Australasian Pleistocene ‘megafauna’. Alcheringa 1, 39–48. [Google Scholar]
- 21.O'Connell JF, Allen J. 2015. The process, biotic impact, and global implications of the human colonization of Sahul about 47,000 years ago. J. Archaeol. Sci. 56, 73–84. ( 10.1016/j.jas.2015.02.020) [DOI] [Google Scholar]
- 22.Webb S. 2008. Megafauna demography and late Quaternary climate change in Australia: a predisposition to extinction. Boreas 37, 329–345. ( 10.1111/j.1502-3885.2008.00026.x) [DOI] [Google Scholar]
- 23.Bowman DMJS, Prior LD. 2004. Impact of Aboriginal landscape burning on woody vegetation in Eucalyptus tetrodonta savanna in Arnhem Land, northern Australia. J. Biogeogr. 31, 807–817. ( 10.1111/j.1365-2699.2004.01077.x) [DOI] [Google Scholar]
- 24.Miller GH, Fogel ML, Magee JW, Gagan MK, Clarke SJ, Johnson BJ. 2005. Ecosystem collapse in Pleistocene Australia and a human role in megafaunal extinction. Science 309, 287–290. ( 10.1126/science.1111288) [DOI] [PubMed] [Google Scholar]
- 25.Clarkson C, et al. 2015. The archaeology, chronology and stratigraphy of Madjedbebe (Malakunanja II): a site in northern Australia with early occupation. J. Hum. Evol. 83, 46–64. ( 10.1016/j.jhevol.2015.03.014) [DOI] [PubMed] [Google Scholar]
- 26.Saltre F, et al. 2016. Climate change not to blame for late Quaternary megafauna extinctions in Australia. Nat. Commun. 7, 10511 ( 10.1038/ncomms10511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bird MI, Hutley LB, Lawes MJ, Lloyd JON, Luly JG, Ridd PV, Roberts RG, Ulm S, Wurster CM. 2013. Humans, megafauna and environmental change in tropical Australia. J. Quat. Sci. 28, 439–452. ( 10.1002/jqs.2639) [DOI] [Google Scholar]
- 28.Hiscock P. 2008. Archaeology of ancient Australia. London, UK: Routeledge. [Google Scholar]
- 29.Williams AN, Ulm S, Smith M, Reid J. 2014. AustArch: a database of 14C and non-14C ages from archaeological sites in Australia: composition, compilation and review (data paper). Internet Archaeol. 36 See 10.11141/ia.36.6. [DOI] [Google Scholar]
- 30.Smith MA, Bird MI, Turney CSM, Fifield LK, Santos GM, Hausladen PA, Tada MLD. 2001. New ABOX AMS-14C ages remove dating anomalies at Puritjarra rock shelter. Aust. Archaeol. 53, 45–47. [Google Scholar]
- 31.Alroy J. 2001. A multispecies overkill simulation of the end-Pleistocene megafaunal mass extinction. Science 292, 1893–1896. ( 10.1126/science.1059342) [DOI] [PubMed] [Google Scholar]
- 32.Prowse TAA, Johnson CN, Bradshaw CJA, Brook BW. 2013. An ecological regime shift resulting from disrupted predator–prey interactions in Holocene Australia. Ecology 95, 693–702. ( 10.1890/13-0746.1) [DOI] [PubMed] [Google Scholar]
- 33.Roberts RG, et al. 2001. New ages for the last Australian megafauna: continent-wide extinction about 46,000 years ago. Science 292, 1888–1892. ( 10.1126/science.1060264) [DOI] [PubMed] [Google Scholar]
- 34.Grun R, Wells R, Eggins S, Spooner N, Aubert M, Brown L, Rhodes E. 2008. Electron spin resonance dating of South Australian megafauna sites. Aust. J. Earth Sci. 55, 917–935. ( 10.1080/08120090802097419) [DOI] [Google Scholar]
- 35.Grun R, Eggins S, Aubert M, Spooner N, Pike AWG, Muller W. 2010. ESR and U-series analyses of faunal material from Cuddie Springs, NSW, Australia: implications for the timing of the extinction of the Australian megafauna. Quat. Sci. Rev. 29, 596–610. ( 10.1016/j.quascirev.2009.11.004) [DOI] [Google Scholar]
- 36.Gillespie R, Brook BW, Baynes A. 2006. Short overlap of humans and megafauna in Pleistocene Australia. Alcheringa 1, 163–186. [Google Scholar]
- 37.Turney CSM, et al. 2008. Late-surviving megafauna in Tasmania, Australia, implicate human involvement in their extinction. Proc. Natl Acad. Sci. USA 105, 12 150–12 153. ( 10.1073/pnas.0801360105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodríguez-Rey M, et al. 2015. Criteria for assessing the quality of Middle Pleistocene to Holocene vertebrate fossil ages. Quat. Geochronol. 30, 69–79. ( 10.1016/j.quageo.2015.08.002) [DOI] [Google Scholar]
- 39.Signor PW, Lipps JH. 1982. Sampling bias, gradual extinction patterns, and catastrophes in the fossil record. In Geological implications of impacts of large asteroids and comets on the Earth (eds Silver LT, Schultz PH), pp. 291–296. Washington, DC: Geological Society of America Special Publication. [Google Scholar]
- 40.Bradshaw CJA, Cooper A, Turney CSM, Brook BW. 2012. Robust estimates of extinction time in the geological record. Quat. Sci. Rev. 33, 14–19. ( 10.1016/j.quascirev.2011.11.021) [DOI] [Google Scholar]
- 41.Alroy J. 2014. A simple Bayesian method of inferring extinction. Paleobiology 40, 584–607. ( 10.1666/13074) [DOI] [Google Scholar]
- 42.Saltré F, Brook BW, Rodríguez-Rey M, Cooper A, Johnson CN, Turney CSM, Bradshaw CJA. 2015. Uncertainties in dating constrain model choice for inferring extinction time from fossil records. Quat. Sci. Rev. 112, 128–137. ( 10.1016/j.quascirev.2015.01.022) [DOI] [Google Scholar]
- 43.Masson-Delmotte V, et al. 2010. EPICA Dome C record of glacial and interglacial intensities. Quat. Sci. Rev. 29, 113–128. ( 10.1016/j.quascirev.2009.09.030) [DOI] [Google Scholar]
- 44.Candy I, Coope GR, Lee JR, Parfitt SA, Preece RC, Rose J, Schreve DC. 2010. Pronounced warmth during early Middle Pleistocene interglacials: investigating the Mid-Brunhes Event in the British terrestrial sequence. Earth-Sci. Rev. 103, 183–196. ( 10.1016/j.earscirev.2010.09.007) [DOI] [Google Scholar]
- 45.Lang N, Wolff EW. 2011. Interglacial and glacial variability from the last 800 ka in marine, ice and terrestrial archives. Clim. Past 7, 361–380. ( 10.5194/cp-7-361-2011) [DOI] [Google Scholar]
- 46.McClymont EL, Sosdian SM, Rosell-Melé A, Rosenthal Y. 2013. Pleistocene sea-surface temperature evolution: early cooling, delayed glacial intensification, and implications for the mid-Pleistocene climate transition. Earth-Sci. Rev. 123, 173–193. ( 10.1016/j.earscirev.2013.04.006) [DOI] [Google Scholar]
- 47.Lawrence KT, Herbert TD. 2005. Late Quaternary sea-surface temperatures in the western Coral Sea: implications for the growth of the Australian Great Barrier Reef. Geology 33, 677–680. ( 10.1130/g21595ar.1) [DOI] [Google Scholar]
- 48.Russon T, Elliot M, Sadekov A, Cabioch G, Corrège T, De Deckker P. 2010. Inter-hemispheric asymmetry in the early Pleistocene Pacific warm pool. Geophys. Res. Lett. 37, L11601 ( 10.1029/2010GL043191) [DOI] [Google Scholar]
- 49.Lisiecki LE, Raymo ME. 2005. A Pliocene-Pleistocene stack of 57 globally distributed benthic δ18O records. Paleoceanography 20, pPA1003. ( 10.1029/2004PA001071) [DOI] [Google Scholar]
- 50.Cohen TJ, Jansen JD, Gliganic LA, Larsen JR, Nanson GC, May J-H, Jones BG, Price DM. 2015. Hydrological transformation coincided with megafaunal extinction in central Australia. Geology 43, 195–198. ( 10.1130/g36346.1) [DOI] [Google Scholar]
- 51.Nanson GC, Price DM, Short SA. 1992. Wetting and drying of Australia over the past 300 ka. Geology 20, 791–794. () [DOI] [Google Scholar]
- 52.Hesse PP, Magee JW, van der Kaars S. 2004. Late Quaternary climates of the Australian arid zone: a review. Quat. Int. 118–119, 87–102. ( 10.1016/S1040-6182(03)00132-0) [DOI] [Google Scholar]
- 53.Bowler JM, Wyrwoll K-H, Lu Y. 2001. Variations of the northwest Australian summer monsoon over the last 300,000 years: the paleohydrological record of the Gregory (Mulan) Lakes System. Quat. Int. 83–85, 63–80. ( 10.1016/S1040-6182(01)00031-3) [DOI] [Google Scholar]
- 54.Cohen TJ, et al. 2011. Continental aridification and the vanishing of Australia's megalakes. Geology 39, 167–170. ( 10.1130/g31518.1) [DOI] [Google Scholar]
- 55.Beaufort L, van der Kaars S, Bassinot FC, Moron V. 2010. Past dynamics of the Australian monsoon: precession, phase and links to the global monsoon concept. Clim. Past 6, 695–706. ( 10.5194/cp-6-695-2010) [DOI] [Google Scholar]
- 56.Page K, Nanson G, Price D. 1996. Chronology of Murrumbidgee River palaeochannels on the Riverine Plain, southeastern Australia. J. Quat. Sci. 11, 311–326. () [DOI] [Google Scholar]
- 57.Carolin SA, Cobb KM, Adkins JF, Clark B, Conroy JL, Lejau S, Malang J, Tuen AA. 2013. Varied response of western Pacific hydrology to climate forcings over the Last Glacial Period. Science 340, 1564–1566. ( 10.1126/science.1233797) [DOI] [PubMed] [Google Scholar]
- 58.Wang YJ, Cheng H, Edwards RL, An ZS, Wu JY, Shen C-C, Dorale JA. 2001. A high-resolution absolute-dated Late Pleistocene monsoon record from Hulu Cave, China. Science 294, 2345–2348. ( 10.1126/science.1064618) [DOI] [PubMed] [Google Scholar]
- 59.Weninger B, Jöris O. 2008. A 14C age calibration curve for the last 60 ka: the Greenland-Hulu U/Th timescale and its impact on understanding the Middle to Upper Paleolithic transition in Western Eurasia. J. Hum. Evol. 55, 772–781. ( 10.1016/j.jhevol.2008.08.017) [DOI] [PubMed] [Google Scholar]
- 60.Kershaw AP, van der Kaars S, Moss PT. 2003. Late Quaternary Milankovitch-scale climatic change and variability and its impact on monsoonal Australasia. Mar. Geol. 201, 81–95. ( 10.1016/S0025-3227(03)00210-X) [DOI] [Google Scholar]
- 61.Kawamura H, Holbourn A, Kuhnt W. 2006. Climate variability and land–ocean interactions in the Indo Pacific Warm Pool: a 460-ka palynological and organic geochemical record from the Timor Sea. Mar. Micropaleontol. 59, 1–14. ( 10.1016/j.marmicro.2005.09.001) [DOI] [Google Scholar]
- 62.Surovell TA, Brantingham PJ. 2007. A note on the use of temporal frequency distributions in studies of prehistoric demography. J. Archaeol. Sci. 34, 1868–1877. ( 10.1016/j.jas.2007.01.003) [DOI] [Google Scholar]
- 63.Surovell TA, Byrd Finley J, Smith GM, Brantingham PJ, Kelly R. 2009. Correcting temporal frequency distributions for taphonomic bias. J. Archaeol. Sci. 36, 1715–1724. ( 10.1016/j.jas.2009.03.029) [DOI] [Google Scholar]
- 64.Prideaux GJ, Roberts RG, Megirian D, Westaway KE, Hellstrom JC, Olley JM. 2007. Mammalian responses to Pleistocene climate change in southeastern Australia. Geology 35, 33–36. ( 10.1130/g23070a.1) [DOI] [Google Scholar]
- 65.Prideaux GJ, et al. 2010. Timing and dynamics of Late Pleistocene mammal extinctions in southwestern Australia. Proc. Natl Acad. Sci. USA 107, 22 157–22 162. ( 10.1073/pnas.1011073107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller GH, Magee JW, Johnson BJ, Fogel ML, Spooner NA, McCulloch MT, Ayliffe LK. 1999. Pleistocene extinction of Genyornis newtoni: human impact on Australian megafauna. Science 283, 205–208. ( 10.1126/science.283.5399.205) [DOI] [PubMed] [Google Scholar]
- 67.Johnson CN, Rule S, Haberle SG, Turney CSM, Kershaw AP, Brook BW. 2015. Using dung fungi to interpret decline and extinction of megaherbivores: problems and solutions. Quat. Sci. Rev. 110, 107–113. ( 10.1016/j.quascirev.2014.12.011) [DOI] [Google Scholar]
- 68.Baker AG, Bhagwat SA, Willis KJ. 2013. Do dung fungal spores make a good proxy for past distribution of large herbivores? Quat. Sci. Rev. 62, 21–31. ( 10.1016/j.quascirev.2012.11.018) [DOI] [Google Scholar]
- 69.Rule S, Brook BW, Haberle SG, Turney CSM, Kershaw AP, Johnson CN. 2012. The aftermath of megafaunal extinction: ecosystem transformation in Pleistocene Australia. Science 335, 1483–1486. ( 10.1126/science.1214261) [DOI] [PubMed] [Google Scholar]
- 70.Kaal J, Schellekens J, Nierop KGJ, Martínez Cortizas A, Muller J. 2014. Contribution of organic matter molecular proxies to interpretation of the last 55 ka of the Lynch's Crater record (NE Australia). Palaeogeogr. Palaeoclimatol. Palaeoecol. 414, 20–31. ( 10.1016/j.palaeo.2014.07.040) [DOI] [Google Scholar]
- 71.Hofreiter M, Muenzel S, Conard NJ, Pollack J, Slatkin M, Weiss G, Paabo S. 2007. Sudden replacement of cave bear mitochondrial DNA in the late Pleistocene. Curr. Biol. 17, R122–R123. ( 10.1016/j.cub.2007.01.026) [DOI] [PubMed] [Google Scholar]
- 72.Palkopoulou E, et al. 2013. Holarctic genetic structure and range dynamics in the woolly mammoth. Proc. R. Soc. B 280, 20131910 ( 10.1098/rspb.2013.1910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cooper A, Turney C, Hughen KA, Brook BW, McDonald HG, Bradshaw CJA. 2015. Abrupt warming events drove Late Pleistocene Holarctic megafaunal turnover. Science 349, 602–606. ( 10.1126/science.aac4315) [DOI] [PubMed] [Google Scholar]
- 74.Llamas B, et al. 2015. Upper Pleistocene Australian marsupial DNA clarifies the affinities of extinct megafaunal kangaroos and wallabies. Mol. Biol. Evol. 32, 574–584. ( 10.1093/molbev/msu338) [DOI] [PubMed] [Google Scholar]
- 75.Prideaux GJ, Ayliffe LK, DeSantis LRG, Schubert BW, Murray PF, Gagan MK, Cerling TE. 2009. Extinction implications of a chenopod browse diet for a giant Pleistocene kangaroo. Proc. Natl Acad. Sci. USA 106, 11 646–11 650. ( 10.1073/pnas.0900956106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prideaux GJ. 2004. Systematics and evolution of the sthenurine kangaroos. Berkeley, CA: University of California Publications in Geological Sciences. [Google Scholar]
- 77.Johnson CN, Rule S, Haberle SG, Kershaw AP, McKenzie GM, Brook BW. 2016. Geographic variation in the ecological effects of extinction of Australia's Pleistocene megafauna. Ecography 39, 109–116. ( 10.1111/ecog.01612) [DOI] [Google Scholar]
- 78.Lopes dos Santos RA, De Deckker P, Hopmans EC, Magee JW, Mets A, Sinninghe Damste JS, Schouten S. 2013. Abrupt vegetation change after the Late Quaternary megafaunal extinction in southeastern Australia. Nat. Geosci. 6, 627–631. ( 10.1038/ngeo1856). [DOI] [Google Scholar]
- 79.Turney CSM, Bird MI, Roberts RG. 2001. Elemental δ13C at Allen's Cave, Nullarbor Plain, Australia: assessing post-depositional disturbance and reconstructing past environments. J. Quat. Sci. 16, 779–784. ( 10.1002/jqs.633) [DOI] [Google Scholar]
- 80.Mooney SD, et al. 2010. Late Quaternary fire regimes of Australasia. Quat. Sci. Rev. 30, 28–46. ( 10.1016/j.quascirev.2010.10.010) [DOI] [Google Scholar]
- 81.Miller GH, et al. 2016. Human predation contributed to the extinction of the Australian megafaunal bird Genyornis newtoni ∼47 ka. Nat. Commun. 7, 10 496 ( 10.1038/ncomms10496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wroe S, Field J. 2006. A review of the evidence for a human role in the extinction of Australian megafauna and an alternative interpretation. Quat. Sci. Rev. 25, 2692–2703. ( 10.1016/j.quascirev.2006.03.005) [DOI] [Google Scholar]
- 83.Field J, Wroe S, Trueman CN, Garvey J, Wyatt-Spratt S. 2013. Looking for the archaeological signature in Australian megafaunal extinctions. Quat. Int. 285, 76–88. ( 10.1016/j.quaint.2011.04.013) [DOI] [Google Scholar]
- 84.Surovell T, Grund B. 2012. The Associational Critique of Quaternary overkill and why it is largely irrelevant to the extinction debate. Amer. Antiq. 77, 672–687. ( 10.7183/0002-7316.77.4.672) [DOI] [Google Scholar]
- 85.Araujo BBA, Oliveira-Santos LGR, Lima-Ribeiro MS, Diniz-Filho JAF, Fernandez FAS. In press. Bigger kill than chill: the uneven roles of humans and climate on late Quaternary megafaunal extinctions. Quat. Int. ( 10.1016/j.quaint.2015.10.045) [DOI] [Google Scholar]