Abstract
Differences in life-history traits between tropical and temperate lineages are often attributed to differences in their climatic niche dynamics. For example, the more frequent appearance of migratory behaviour in temperate-breeding species than in species originally breeding in the tropics is believed to have resulted partly from tropical climatic stability and niche conservatism constraining tropical species from shifting their ranges. However, little is known about the patterns and processes underlying climatic niche evolution in migrant and resident animals. We evaluated the evolution of overlap in climatic niches between seasons and its relationship to migratory behaviour in the Parulidae, a family of New World passerine birds. We used ordination methods to measure seasonal niche overlap and niche breadth of 54 resident and 49 migrant species and used phylogenetic comparative methods to assess patterns of climatic niche evolution. We found that despite travelling thousands of kilometres, migrants tracked climatic conditions across the year to a greater extent than tropical residents. Migrant species had wider niches than resident species, although residents as a group occupied a wider climatic space and niches of migrants and residents overlapped extensively. Neither breeding latitude nor migratory distance explained variation among species in climatic niche overlap between seasons. Our findings support the notion that tropical species have narrower niches than temperate-breeders, but does not necessarily constrain their ability to shift or expand their geographical ranges and become migratory. Overall, the tropics may have been historically less likely to experience the suite of components that generate strong selection pressures for the evolution of migratory behaviour.
Keywords: evolution, migration, niche breadth, niche overlap
1. Introduction
Although the evolution of migration in birds has been the focus of multiple studies, much remains to be learned about the selective pressures and evolutionary processes underlying the origin of migratory behaviour [1,2]. A widely accepted ‘southern home’ hypothesis posits that migration originated primarily through shifts in breeding ranges of tropical organisms to take advantage of resource booms and reduced predation risk in the temperate zone [1,3,4]. Although some evidence supports a southern-home origin of migration [4,5], shifts in breeding ranges out of the tropics cannot account for the repeated evolution of migration in the largest radiation of New World migrants, the Emberizoid passerines [6]. In this clade, migration evolved predominantly via shifts in winter ranges from temperate to tropical regions, supporting a ‘northern-home’ hypothesis [7,8]; in turn, shifts in breeding ranges have been rare [6].
Shifts in winter ranges in species evolving migratory behaviour presumably occurred as populations moved in search of favourable environmental conditions escaping the increased harshness of temperate winters associated with global cooling since the Late Oligocene [6,8]. Individuals may escape harsh winter conditions either by following a set of fixed climatic conditions throughout the year (niche tracking) or by changing their climatic niche between periods of the annual cycle (niche switching; [9–12]). Winger et al. [6] suggested that shifts in winter range from temperate to tropical latitudes in Emberizoids probably involved niche tracking; however, these authors did not examine the extent to which species track or switch between climatic conditions throughout the year. Whether species track or switch climatic niches between seasons is probably influenced by their niche breadth [13,14], but we are unaware of studies relating niche breadth and seasonal niche overlap to the evolution of migration.
The rarity of shifts in breeding ranges out of the tropics in Emberizoid passerines [6] despite their remarkable diversification in the tropics and the relative ease with which migration has been gained and lost in this clade and others [1,15,16] suggests that pressures to evolve migratory behaviour via shifts in the breeding range may not be as strong in lineages breeding in the tropics. This makes intuitive sense assuming that temperature is a main dimension of the climatic niche because tropical temperatures are more stable and less extreme than temperate ones, which presumably minimizes the need to move [17]. However, many tropical environments do exhibit marked climatic seasonality (especially in precipitation; [13]), which suggests that some tropical species, despite being non-migratory, may effectively experience seasonally variable climates to a greater extent than migrants breeding in the temperate zone and which track climatic conditions during their seasonal movements [12]. If this were the case, then the origin of migration via niche tracking may have resulted in a reversal of the geographical pattern of seasonal variation in climatic conditions hypothesized to have driven the more frequent evolution of migratory behaviour in temperate-zone lineages (i.e. tropical species would now experience greater annual climatic variation than their temperate counterparts).
The study of climatic niches in resident and migratory birds has only recently been subject to research in a comparative phylogenetic framework [12]. However, patterns of niche evolution remain poorly studied and are important to determine whether the evolution of migration involves niche tracking or niche switching. This is because the tendency for species to track or switch between climatic niches will partly depend on how conserved niches are, on the rate at which niches evolve, and on whether niches are affected by similar or different selection pressures across groups and regions [12,18,19]. If the degree to which species track their climatic niches between seasons were independent of migratory behaviour, then closely related migratory and resident species should not differ consistently in values of seasonal niche overlap. Alternatively, if migratory and resident species were under different selection pressures to track climatic conditions across the year, then one would expect lineages differing in migratory status to exhibit different patterns of seasonal niche occupation and evolution regardless of their phylogenetic relationships. Other factors such as niche breadth, breeding latitude and migratory distance could also influence the tendency of species to track or switch between climatic niches on a seasonal basis [12]. For example, one may expect species with wide niches and short-distance migrants to be better able to track climatic conditions than species with narrow niches or those migrating over long distances [11,12].
Insights about the relationship between climatic niche dynamics and migratory behaviour can be gained by evaluating the overlap in climatic niches between seasons within lineages containing both migrant and resident species [12]. A few studies have attempted this, finding examples of both seasonal niche-trackers and niche-switchers among migrants [11,12]. Another study concluded that migrants are niche-switchers which evolved such a derived condition from ancestral, niche-tracking residents [10]. However, this study only involved a small group of species (the Passerina buntings, Cardinalidae), which may be exceptional because they represent one of the rare cases in the Emberizoids in which migration was inferred to have evolved through shifts in the breeding range from the tropics to the temperate zone [6]. More generally, studies examining overlap in climatic niches between breeding and non-breeding periods may have suffered from methodological limitations. First, estimates of niche overlap were based on geographical projections of species distribution models during different periods of the annual cycle [10,11]. This may be problematic when comparisons are made between non-equivalent geographical areas in which combinations of climatic conditions are unequally represented [20,21], as would be the case when projecting a model based on the climate of North America (breeding period) onto the climate of Central or South America (non-breeding period) for a Nearctic–Neotropical migratory bird. Furthermore, several earlier studies did not employ robust phylogenies to correct for phylogenetic effects and to test evolutionary hypotheses using comparative methods (but see [12]).
Here, we use climatic data, species occurrences and a comprehensive molecular phylogeny to address questions seeking to understand how seasonal dynamics in climatic niches are related to, and may have influenced, the evolution of migratory behaviour in a large avian radiation, the New World warblers (Parulidae, hereafter warblers). Warblers are an appropriate study system because they are one of the largest clades of the Emberizoid passerines, which have both tropical residents and long-distance migrants, including species with some of the longest migrations among landbirds in the Americas [6,15,22]. Most warblers breeding in the tropics are permanent residents not known to engage in seasonal movements such as altitudinal migration ([22], but see [23]). Long-distance migration in warblers is thought to have evolved through shifts in non-breeding ranges from temperate North America to the tropics, and it has been lost and gained repeatedly over time [6,15]. We quantified overlap in climatic niches between periods of the annual cycle for 103 species of warblers (54 resident, 49 migratory) using ordination techniques accounting for spatial autocorrelation in climatic conditions between regions. Additionally, we used comparative methods to examine the evolution of climatic niche overlap between seasons. Based on these approaches, we: (i) examined whether migrant warblers are climatic niche-trackers or niche-switchers across seasons; (ii) compared seasonal niche overlap and niche breadth between resident and migrant species to examine whether migratory behaviour is related to these dimensions of ecological niches; and (iii) explored whether seasonal niche overlap and niche breadth vary as a function of migratory distance or breeding latitude.
2. Material and methods
(a). Data preparation
We obtained geographical coordinates of verified observations and museum specimens (from 1980 to 2013) of warblers from eBird and the Global Biodiversity Information Facility ([24], www.gbif.org). Additional coordinates for a rare species (Setophaga kirtlandii) were obtained from the literature [25]. We divided records into those corresponding to the breeding period (June–August) and the non-breeding period (December–February) of migratory (i.e. temperate-breeding) species [10,11]. These three-month periods do not necessarily coincide with the breeding and non-breeding periods of resident species, which are often poorly known [22]. However, because most resident warblers remain on their breeding ranges year-round, this lack of information does not affect our assessment of seasonality in climatic niches; dividing data temporally using the same criteria used for temperate-breeding species facilitates comparisons between migrant and resident species.
Records from migration periods (March to May and September to November) and those of species with less than five unique localities in either the breeding or non-breeding period (i.e. Basileuterus hypoleucus, Setophaga subita, Myioborus cardonai and Geothlypis semperi) were excluded from analyses. In total, we analysed data for 103 species of which 49 are migrants and 54 are resident [15]. We discarded any records not strictly within the known breeding and non-breeding ranges of species (e.g. records of early and late migrants, or of vagrants) [22,26]. Some warblers begin migrating in August, but most juveniles are still moulting at this time and, like many adults, they do not migrate until September [22,27]. Thus, to minimize the inclusion of migrating birds within our breeding-period sample, we eliminated all August records outside of known breeding ranges. To reduce biases related to uneven distribution of occurrence records due to the activity of observers and collectors, we used a kernel density function to remove spatially clustered localities and produce a smoothed density of occurrence for every cell in the map [21,28]. We did not apply the kernel correction to 20 species having less than 20 spatially unique records (electronic supplementary material, table S1) to avoid reducing record numbers further. The median number of records for migrants after data preparation was 195 for June–August and 98 for December–February; for residents, the medians were 167 and 93 records, respectively.
(b). Characterization of climatic niches
Following earlier studies describing the seasonal climatic niches of birds [10–12], we selected seven environmental variables to describe species' climatic niches and to quantify niche breadth and overlap in climatic niches between seasons: maximum temperature, minimum temperature, temperature seasonality, mean precipitation, precipitation seasonality, slope and aspect [29]. These variables describe ecologically relevant components of tropical and temperate climates, including average conditions, seasonality and sources of microclimatic variation (i.e. topography; [13]). See the electronic supplementary material for details on the procedures used to prepare climatic variables for analyses.
(c). Seasonal niche overlap and niche breadth
The environmental variables we selected appropriately predicted the distributions of our study species during breeding and non-breeding periods (see the electronic supplementary material for methods and results of distribution modelling); this justified our use of these variables to estimate overlap in climatic niches between breeding and non-breeding periods. We conducted separate analyses using all environmental variables together and for temperature (minimum, maximum and seasonality), precipitation (mean and seasonality) and topographic variables (slope and aspect) separately; this allowed us to examine the contribution of each group of variables to patterns of seasonal niche overlap. Because topography probably influences thermal microclimate, we also analysed temperature and topographic variables together.
Niche overlap between breeding and non-breeding seasons was quantified for each species using an approach derived from multivariate ordination theory [21]. Because this method accounts for the differential availability of climates between regions and accounts for spatial bias in occurrence records [12,21,30], it avoids pitfalls derived from the geographical projection of niche models [20,31]. The procedure involves a principal components analysis (PCA) to associate climatic values with species occurrence densities in each period (breeding or non-breeding; [21]). A grid of climatic conditions reflecting the first two PCA axes is created and species occurrence densities from each period are mapped onto it [21]. Densities are rescaled to take into account the availability of climatic conditions in the background [21] and are then used to estimate the D statistic to measure seasonal niche overlap [32]. D ranges from 0 (niches completely different, i.e. seasonal niche-switching) to 1 (niches identical, i.e. seasonal niche-tracking; [21,32]). To examine the extent to which climatic niches occupied by species in either period differ from each other relative to what would be expected by chance given background variation in climate, we used a similarity test involving 100 iterations where the overlap between the observed niche in one period is compared to simulations of a null niche based on the available climates of the other period [21,32]. If the observed overlap falls outside the 95% confidence interval of the simulated values, then the niches from the two periods are considered more similar or different than expected by chance.
To estimate seasonal and total niche breadth, random points were sampled from the complete environmental space available to all species in both seasons. Sample points were selected in proportion to the density of occurrence of each species in the climatic space. The median and variance of these points along the first two environmental axes of the PCA was then measured using the ‘ecospat’ package in R [33]. We multiplied the variances from PC1 and PC2 in each season to obtain a measure of niche breadth (area) and then calculated the sum of these values as an estimate of total niche breadth of each study species [33,34].
Finally, we explored whether seasonal climatic niche overlap and total niche breadth varied as a function of each other and with migratory distance or breeding latitude. We estimated the geographical centroids of the breeding and non-breeding polygons of each species [26] and calculated the geographical distances between these points using the R packages ‘rgeos’ and ‘gmt’ [35,36]. To determine whether differences between resident and migrant species were significant, we used phylogenetic generalized linear models (PGLS) in the R package ‘caper’ [37] using a lambda model to describe the correlation of the residuals. These analyses were based on a comprehensive molecular phylogeny of warblers [38].
(d). Climatic niche evolution
To examine whether climatic niche has evolved in association with or independently from migratory behaviour and species relatedness, we tested various models of character evolution using the R package ‘OUwie’ [39]. We fitted four different models of evolution to test whether there were differences in character (i.e. seasonal niche overlap) evolution between resident and migrant species. The models considered were: (i) single-rate Brownian Motion (BM), where traits evolve gradually through a random-walk process at a fixed rate (σ2) [40]; (ii) BM with different rates (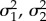 ), where the rate of evolution of a trait (i.e. seasonal niche overlap) can vary according to another trait on the tree (i.e. migratory status [41]; (iii) Ornstein–Uhlenbeck (OU) with a single mean, where traits evolve gradually at a constant rate (σ2) but are pulled towards a mean value (θ) with a strength (α) depending on how far values stray from the mean [42,43]; and (iv) OU with different state means (
), where the rate of evolution of a trait (i.e. seasonal niche overlap) can vary according to another trait on the tree (i.e. migratory status [41]; (iii) Ornstein–Uhlenbeck (OU) with a single mean, where traits evolve gradually at a constant rate (σ2) but are pulled towards a mean value (θ) with a strength (α) depending on how far values stray from the mean [42,43]; and (iv) OU with different state means (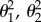 ) but with single rate (σ2) and pull (α) of evolution [44]. Evaluating the above models requires estimates of character states along the tree, which we obtained using stochastic mapping under an Mk1 model [45] (see the electronic supplementary material for details). Models were compared based on their corrected Akaike information criteria (AICc) weights.
) but with single rate (σ2) and pull (α) of evolution [44]. Evaluating the above models requires estimates of character states along the tree, which we obtained using stochastic mapping under an Mk1 model [45] (see the electronic supplementary material for details). Models were compared based on their corrected Akaike information criteria (AICc) weights.
3. Results
(a). Seasonal niche overlap and niche breadth
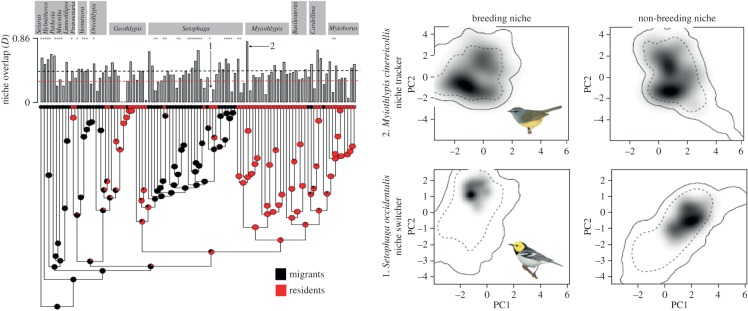
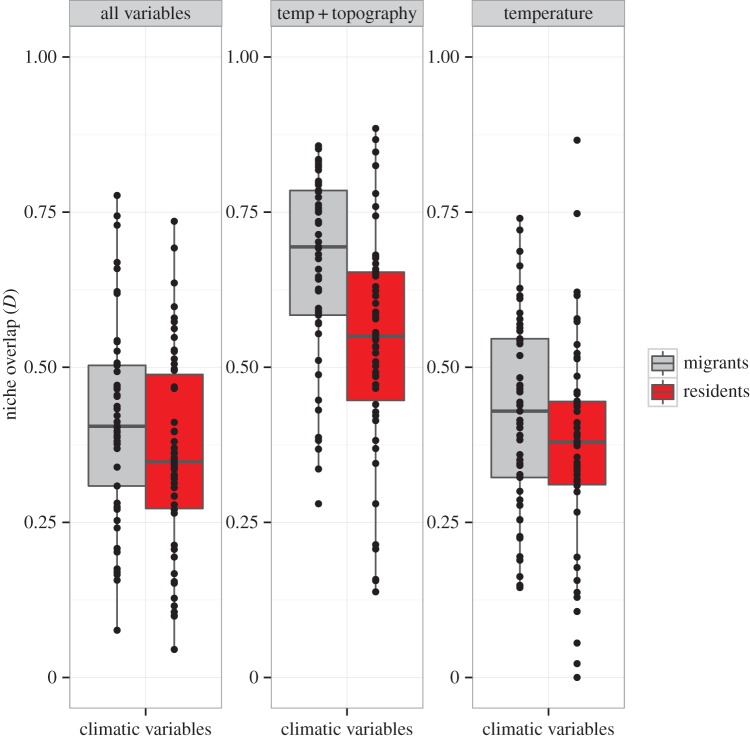
Our estimates of overlap in climatic niches between seasons were based on the comparison of the climatic spaces described by the first two components of the PCA for each period (breeding and non-breeding). On average, PC1 and PC2 (±s.d.) explained 64 ± 9% of the variation in species occurrence within the climatic space of each period. Values of niche overlap between seasons (D) ranged from 0.004 to 0.77 when including all climatic variables, and from 0.00 to 0.86 for temperature variables only (figure 1; electronic supplementary material, table S1). Migrant species showed consistently higher mean niche overlap between seasons than resident species based on all variables (PGLS, β ± s.e. = −0.05 ± 0.03, p = 0.05), only temperature variables (PGLS, β ± s.e. = −0.06 ± 0.03, p = 0.03), and for temperature and topography together (PGLS, β ± s.e. = −0.12 ± 0.03, p < 0.001; figure 2). Seasonal niche overlap estimated using variables describing precipitation and topography alone did not differ between migrant and resident species (table 1). Niche similarity tests based on all climatic variables indicated that seasonal niches were more similar than expected by chance in 73% of migrants (i.e. significant niche tracking), whereas only 50% of resident species showed this pattern (figure 1; electronic supplementary material, table S1).
Figure 1.
Climatic niche overlap between breeding and non-breeding seasons, as measured for temperature variables only, shows high lability when mapped over the phylogeny of the Parulidae [38]. Seventy-three per cent of the migrant species (in black) showed more similar niches between seasons than would be expected by chance (marked with an asterisk (*)). Dashed horizontal lines show the mean niche overlap between seasons for migrants (black D = 0.43) and for residents (light D = 0.36). The panels on the right show examples of climatic spaces occupied by (1) a niche switcher and (2) a niche tracker; the position of these species in the phylogeny is indicated with arrows. Shaded areas represent the breeding and non-breeding climatic niches described by the first two components of the PCA. Darker shades of grey represent higher densities of occurrence for each species and season. The solid and dashed contour lines surround 100% and 50% of the available background climates, respectively. Bird illustrations reproduced with permission from the Handbook of birds of the world alive [46,47]. (Online version in colour; residents in red.)
Figure 2.
Despite wide variation within groups, mean climatic niche overlap between seasons was consistently higher in migrant than in resident warblers when including all climatic variables (p = 0.05), temperature and topographic variables (p < 0.001), and temperature variables only (p = 0.03). p-values were obtained from PGLS models of seasonal niche overlap as a function of migratory status. Precipitation and topographic variables alone did not show significant differences between groups (not shown). (Online version in colour.)
Table 1.
An OUM model describing the evolution of niche overlap in the Parulidae consistently received the greatest support estimated using AICc, except for precipitation variables. (Parameter estimates and their variance are given for each of the models and the different combinations of climatic variables. OUM, multiple-mean Ornstein–Uhlenbeck; OU, single mean Ornstein–Uhlenbeck; BMS, multiple-rate Brownian motion; BM, single-rate Brownian motion. α = evolutionary rate, σ2 = variance, θ = phenotypic mean.)
| model parameters (±variance) |
|||||||
|---|---|---|---|---|---|---|---|
| Δ AICc | Wi | α | σ2 | θ migrants | θ residents | stationary variance | |
| all variables | |||||||
| OUM | 0 | 0.7 | 4.80 ± 0.15 | 0.24 ± 3.9 × 10−3 | 0.41 ± 3.3 × 10−6 | 0.35 ± 3.4 × 10−6 | 0.03 |
| OU | 1.65 | 0.3 | 4.89 ± 0.10 | 0.25 ± 0.001 | 0.38 ± 0.02 | 0.03 | |
| BMS | 107.09 | 0 | 0.003 ± 0.001 | 0.36 ± 0.20 | |||
| BM | 115.02 | 0 | 0.003 ± 0.001 | 0.37 ± 1.6 × 10−5 | |||
| temperature | |||||||
| OUM | 0 | 0.75 | 4.57 ± 1.09 | 0.25 ± 0.003 | 0.43 ± 1.1 × 10−6 | 0.36 ± 1.0 × 10−6 | 0.03 |
| OU | 2.17 | 0.25 | 0.28 ± 0.01 | 0.01 ± 0.001 | 0.39 ± 0.02 | 0.02 | |
| BM | 70 | 0 | 0.002 ± 4.8 × 10−7 | 0.46 ± 0.17 | |||
| BMS | 70.46 | 0 | 0.002 ± 3.5 × 10−8 | 0.46 ± 8.8 × 10−6 | |||
| topography | |||||||
| OUM | 0 | 0.57 | 0.79 ± 0.09 | 0.029 ± 0.001 | 0.62 ± 4.9 × 10−6 | 0.67 ± 3.9 × 10−6 | 0.02 |
| OU | 0.52 | 0.43 | 0.44 ± 0.01 | 0.02 ± 3.9 × 10−6 | 0.65 ± 0.01 | 0.02 | |
| BM | 58.89 | 0 | 0.001 3.9 × 10−4 | 0.64 ± 0.13 | |||
| BMS | 60.27 | 0 | 0.001 ± 7.3 × 10−9 | 0.65 ± 3.9 × 10−6 | |||
| temp + topo | |||||||
| OUM | 0 | 0.99 | 4.66 ± 0.68 | 0.25 ± 0.002 | 0.67 ± 1.9 × 10−6 | 0.53 ± 1.7 × 10−6 | 0.03 |
| OU | 13.42 | 0.01 | 4.86 ± 0.59 | 0.31 ± 0.002 | 0.59 ± 0.01 | 0.03 | |
| BMS | 112.61 | 0 | 0.002 ± 9.1 × 10−7 | 0.68 ± 1.2 × 10−4 | |||
| BM | 116.25 | 0 | 0.003 ± 7.5 × 10−7 | 0.67 ± 0.21 | |||
| precipitation | |||||||
| OU | 0 | 0.74 | 0.26 ± 0.01 | 0.018 ± 0.001 | 0.29 ± 0.02 | 0.03 | |
| OUM | 2.07 | 0.26 | 0.29 ± 1.6 × 10−2 | 0.02 ± 7.6 × 10−5 | 0.29 ± 1.9 × 10−5 | 0.32 ± 1.9 × 10−5 | 0.56 |
| BM | 72.65 | 0 | 0.003 ± 0.001 | 0.23 ± 0.19 | |||
| BMS | 74.19 | 0 | 0.003 ± 0.001 | 0.23 ± 4.1 × 10−6 | |||
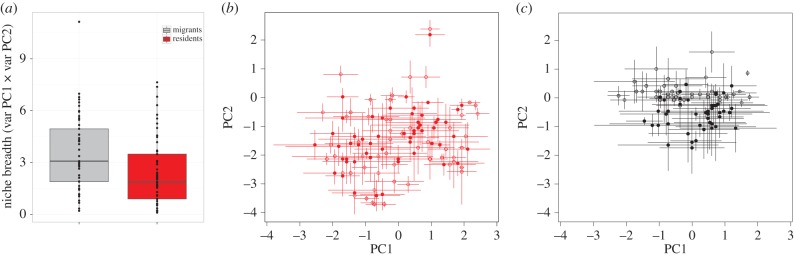
On average, migrant species had significantly wider niches than resident species (figure 3a; PGLS β ± s.e. = −1.2 ± 0.4, p = 0.009) although the correlation between niche breadth and breeding latitude was only marginally significant (PGLS β ± s.e. = 0.02 ± 0.01, p = 0.06). Also, resident species as a group occupied a wider area of climatic space than migrants, and climatic niches of migrant and resident species overlapped extensively (figure 3b,c). We found a significant and positive relationship between seasonal niche overlap and niche breadth (figure 4; PGLS β ± s.e. = 0.02 ± 0.007 p < 0.001), but no significant relationships between seasonal niche overlap and breeding latitude (PGLS, β ± s.e. = 0.7 × 10−3 ± 0.7 × 10−3, p = 0.30) or migratory distance (PGLS, β ± s.e. = 0.7 × 10−5 ± 0.8 × 10−5, p = 0.37).
Figure 3.
(a) Migrant species have wider climatic niches than residents (p = 0.01), but resident species as a whole (b) occupy a wider niche space than migrants (c) although niche overlap between migrants and residents is substantial. Niche breadth is measured as the variance along PC1 and PC2 (horizontal and vertical lines) of the full climatic space available to all species. Open symbols represent the breeding period (June–August) and closed symbols the non-breeding period (December–February). Dots are the median points in niche space of each species. (Online version in colour; residents in red.)
Figure 4.
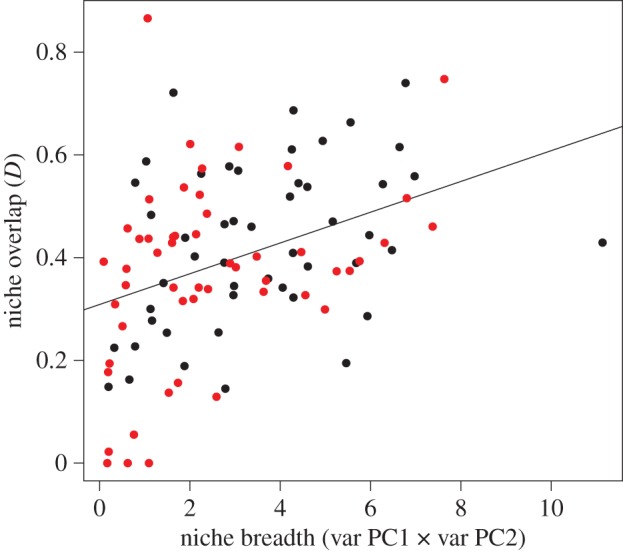
Seasonal niche overlap significantly increased as a function of niche breadth (PGLS β ± s.e. = 0.02 ± 0.007, p < 0.001), but there was no significant relationship between niche overlap and breeding latitude or migratory distance (not shown). Resident species are shown in a lighter colour and migrants in black. (Online version in colour; residents in red.)
(b). Climatic niche evolution
The model of trait evolution consistently receiving the highest support was a multiple-mean OU model where the phenotypic means (θ) of seasonal niche overlap differed significantly between migrant and resident species (table 1). This was the case for all climatic variables, only temperature variables, only topographic variables, and for temperature and topography together. The best-fit models estimated a higher mean theta parameter (θ) of seasonal niche overlap for migrants than for residents (table 1), supporting the notion that migrants tend to track their annual climatic niches to a greater extent than residents, and that presence or absence of migratory behaviour is associated with the evolution of seasonal niche overlap in different directions.
4. Discussion
Despite travelling hundreds to thousands of kilometres between breeding and wintering grounds, migrant species of warblers track climatic niches throughout the year to a greater extent than resident tropical species that remain in the same areas year-round. We obtained this striking result both through quantitative estimates of overlap in climatic niches between seasons (figure 2) and through analyses fitting evolutionary models in a phylogenetic framework (table 1). The greater tendency to track climatic niches across seasons in migrants may be partly explained by their wider climatic niches relative to resident species (figure 3a) as well as by their historical experience of more dramatic climatic changes forcing them to move in search of favourable conditions [1,8].
Our findings support the widely accepted notion that tropical species have narrower niches relative to temperate-breeders, a likely consequence of tropical temperatures being more stable and less extreme than those in the temperate zone [48,49]. Nevertheless, despite having individually narrower niches, resident species as a group occupy a wider climatic space, which encompasses a large part of that occupied by migrants during their breeding period (figure 3b,c). Given that resident species are more numerous than migrants, it is not surprising that as a group the former occupy a larger area of climatic space [48]. Also, if biotic interactions are stronger in the tropics as argued by some authors, then competition may cause niches to diverge into unused climatic space to a greater extent in resident, tropical species [50]. Independently of the reasons why resident species may occupy a broader overall climatic space than their migratory counterparts, our work shows that the narrower niches of resident species do not imply that such species experience more uniform climatic niches on an annual basis than temperate-breeding migrants, nor that residents are unable to occupy climatic spaces occupied by migrants [48]. Because residents switch climatic niches between seasons of a year to a greater extent than migrants and because there is substantial overlap in climatic conditions of warbler breeding areas in the temperate zone with the climatic space occupied by many resident species (figure 3), our work suggests that, in contrast to results of earlier studies [18,48], some tropical lineages may not be physiologically constrained to expand their geographical ranges into temperate latitudes owing to niche conservatism.
Migratory behaviour in Emberizoid passerines evolved more often through shifts of winter ranges from temperate to tropical areas than through shifts in breeding ranges from the tropics to the temperate zone [6,8]. Our findings suggest that this did not happen only because tropical species are somehow constrained to switch their climatic niches [6,12]. Instead, the tropics may have been historically less likely to experience the suite of components that generate strong selection pressures for the evolution of migratory behaviour [1,16,51–53]. Our study supports previous suggestions that the harshness of temperate winters forces birds to leave or experience a greatly reduced probability of survival, whereas the seasonality experienced by tropical residents need not importantly compromise survival and would therefore not be enough to trigger migratory behaviour [1,8]. Overall, then, climatic availability, and not only features of species' niches, probably explains why the evolution of migration from tropical to temperate regions has not been as common [1,6,8].
In contrast to our results showing that migrant warblers are largely niche trackers, a recent study found that both resident and migratory species of Old World warblers (Sylvia, Sylviidae) are climatic niche switchers [12]. Unlike us, that study compared the climate of the breeding period with the climate that species would experience during the winter if individuals did not migrate [12]. Furthermore, that study did not carry out a similarity test accounting for differences in the climatic conditions potentially available in breeding and wintering areas [21]. Hence, it is unclear whether migrants actively switch niches by selecting climatically distinct environments in different seasons or whether they are forced to do so owing to geographical variation in climate (i.e. climatic differences between regions where species breed and winter may not allow for niche tracking). Recent work indicates that migratory behaviour in Sylvia warblers may change rapidly as a consequence of changes in food availability [54], suggesting that species in this clade, perhaps unlike migratory parulids, may be more influenced by resources than by climate when selecting favourable sites in different moments of their annual cycle. If this were the case, then it would be a plausible explanation for the contrasting patterns in seasonal niche dynamics in Sylvia and New World warblers.
The study on Sylvia did not relate niche overlap to niche breadth directly, but like us, it found no relationship between niche breadth or seasonal niche overlap with migratory distance [12]. This is surprising because intuitively one would imagine that short-distance migrants might more easily locate sites with similar climate to that of their breeding grounds (i.e. be niche trackers) relative to long-distance migrants, which we expected to be more likely to be niche switchers. Likewise, we expected breeding latitude to be related to seasonal niche overlap, with species from temperate and subtropical areas showing lower overlap relative to species from the more stable tropics. Although not finding the expected patterns calls for an explanation, our results (see also [18]) are not free of caveats and should be interpreted cautiously. First, we estimated breeding latitude and migratory distance of species using geographical centroids of breeding and non-breeding ranges, which may not accurately reflect true breeding latitude or migratory distance of individuals, especially for species with large geographical ranges and for those having populations with different migratory routes and wintering destinations [12,55]. Lack of information precludes population-level analyses at this time, but the question of whether breeding latitude and migratory distance are related to the extent to which individuals track climatic niches should be revisited once data on migratory connectivity and migration routes are available at the population level. Future studies should also consider that widely distributed species of tropical warblers may consist of distinct evolutionary lineages [56] which probably experience dissimilar environmental conditions and may differ in the extent to which they engage in intratropical (i.e. altitudinal) migration [23]; it would be of interest to treat these lineages separately in analyses like those conducted in this study.
That migratory species have wider niches than resident species is consistent with the importance of climatic variability as a trigger for the evolution of migration [1,8]. Previous work has shown that niche breadth is strongly influenced by local climatic seasonality [34]. Therefore, a combination of narrower niches [6], less historical exposure to climatic changes [1,8] and potentially, greater importance of biotic interactions limiting species distributions and hence their ability to shift their ranges [50], have probably interacted to result in migration not evolving as frequently in species occurring in tropical latitudes.
We found that seasonal overlap in climatic niche evolves following a model of OU-type evolution, a result consistent with those of various studies of niche evolution on a wide range of organisms [30,31,52,57]. This makes sense because physiological constraints and stabilizing selection coupled with restrictions in the availability of climates impose limits on the occupation and evolution of climatic niches. However, our finding that migrants and residents are likely under distinct selective pressures to track climatic conditions across the year suggests that species differing in migratory status may respond in different ways to varying selective regimes. For example, although tropical resident species have traditionally been regarded as being highly sensitive to climatic change owing to their narrow and conserved niches [58,59], the more restricted temporal structure of seasonal climatic niches of temperate-breeding migrant species suggests that their sensitivity to climate change may be greater than currently thought.
Supplementary Material
Acknowledgements
We thank Irby Lovette for making the warbler phylogeny available for comparative analyses. Oscar Ramos, Jaime García-Márquez, Camila González, Dan L. Warren, A. Townsend Peterson, Liam Revell, Gustavo Bravo and Juan Ignacio Areta kindly gave suggestions that guided methodological aspects of this study. Members of the Laboratorio de Biología Evolutiva de Vertebrados, associate editor Anne Charmantier and two anonymous reviewers provided constructive feedback on the manuscript.
Data accessibility
All the data used for this study are publicly available in the eBird (www.ebird.org) and WorldClim databases (www.worldclim.org/). The phylogeny of the Parulidae has been published [38] and a tree file was provided by the authors.
Authors' contributions
C.G. and C.D.C. designed the study. C.G., E.A.T. and P.M. carried out data analyses. All authors contributed to interpreting and discussing results. C.G. drafted the manuscript and all authors reviewed and edited the text.
Competing interests
We declare we have no competing interests.
Funding
Colciencias provided funding for this study.
References
- 1.Salewski V, Bruderer B. 2007. The evolution of bird migration: a synthesis. Naturwissenschaften 94, 268–279. ( 10.1007/s00114-006-0186-y) [DOI] [PubMed] [Google Scholar]
- 2.Zink RM. 2011. The evolution of avian migration. Biol. J. Linn. Soc. 104, 237–250. ( 10.1111/j.1095-8312.2011.01752.x) [DOI] [Google Scholar]
- 3.Sekercioglu CH. 2010. Partial migration in tropical birds: the frontier of movement ecology. J. Anim. Ecol. 79, 933–936. ( 10.1111/j.1365-2656.2010.01739.x) [DOI] [PubMed] [Google Scholar]
- 4.Levey DJ, Stiles FG. 1992. Evolutionary precursors of long-distance migration: resource availability and movement patterns in Neotropical landbirds. Am. Nat. 140, 447–476. ( 10.1086/285421) [DOI] [Google Scholar]
- 5.Boyle WA, Conway CJ. 2007. Why migrate? A test of the evolutionary precursor hypothesis. Am. Nat. 169, 344–359. ( 10.1086/511335) [DOI] [PubMed] [Google Scholar]
- 6.Winger BM, Barker FK, Ree RH. 2014. Temperate origins of long-distance seasonal migration in New World songbirds. Proc. Natl Acad. Sci. USA 111, 12 115–12 120. ( 10.1073/pnas.1405000111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell CP. 2000. Process in the evolution of bird migration and pattern in avian ecography. J. Avian Biol. 31, 258–265. ( 10.1034/j.1600-048X.2000.310218.x) [DOI] [Google Scholar]
- 8.Louchart A. 2008. Emergence of long distance bird migrations: a new model integrating global climate changes. Naturwissenschaften 95, 1109–1119. ( 10.1007/s00114-008-0435-3) [DOI] [PubMed] [Google Scholar]
- 9.Joseph L, Stockwell D. 2000. Temperature-based models of the migration of Swainson's flycatcher (Myiarchus swainsoni) across South America. A new use for museum specimens of migratory birds. Proc. Acad. Natl Sci. Phila. 150, 293–300. [Google Scholar]
- 10.Martínez-Meyer E, Peterson AT, Navarro-Siguenza AG. 2004. Evolution of seasonal ecological niches in the Passerina buntings (Aves: Cardinalidae). Proc. R. Soc. Lond. B 271, 1151–1157. ( 10.1098/rspb.2003.2564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakazawa Y, Peterson AT, Martínez-Meyer E, Navarro-Siguenza AG. 2004. Seasonal niches of migratory birds: implications for the evolution of migration. Auk 121, 610–618. ( 10.1642/0004-8038(2004)121%5B0610:SNONMB%5D2.0.CO;2) [DOI] [Google Scholar]
- 12.Laube I, Graham CH, Böhning-Gaese K. 2015. Niche availability in space and time: migration in Sylvia warblers. J. Biogeogr. 42, 1896–1906. ( 10.1111/jbi.12565) [DOI] [Google Scholar]
- 13.Vázquez DP, Stevens RD. 2004. The latitudinal gradient in niche breadth: concepts and evidence. Am. Nat. 164, E1–E19. ( 10.1086/421445) [DOI] [PubMed] [Google Scholar]
- 14.Nakazawa Y. 2013. Niche breadth, environmental landscape, and physical barriers: their importance as determinants of species distributions. Biol. J. Linn. Soc. 108, 241–250. ( 10.1111/j.1095-8312.2012.02018.x) [DOI] [Google Scholar]
- 15.Winger BM, Lovette IJ, Winkler DW. 2012. Ancestry and evolution of seasonal migration in the Parulidae. Proc. R. Soc. B 279, 610–618. ( 10.1098/rspb.2011.1045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolland J, Jiguet F, Jønsson KA, Condamine FL, Morlon H. 2014. Settling down of seasonal migrants promotes bird diversification. Proc. R. Soc. B 281, 20140473 ( 10.1098/rspb.2014.0473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiens JJ, Graham CH. 2005. Niche conservatism: integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 36, 519–539. ( 10.1146/annurev.ecolsys.36.102803.095431) [DOI] [Google Scholar]
- 18.Wiens JJ, Donoghue MJ. 2004. Historical biogeography, ecology and species richness. Trends Ecol. Evol. 19, 639–644. ( 10.1016/j.tree.2004.09.011) [DOI] [PubMed] [Google Scholar]
- 19.Wiens JJ, et al. 2010. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 13, 1310–1324. ( 10.1111/j.1461-0248.2010.01515.x) [DOI] [PubMed] [Google Scholar]
- 20.Elith J, Kearney M, Phillips S. 2010. The art of modelling range-shifting species. Methods Ecol. Evol. 1, 330–342. ( 10.1111/j.2041-210X.2010.00036.x) [DOI] [Google Scholar]
- 21.Broennimann O, et al. 2012. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 21, 481–497. ( 10.1111/j.1466-8238.2011.00698.x) [DOI] [Google Scholar]
- 22.Curson J. 1994. New World warblers. London, UK: Christopher Helm (Publishers) Ltd. [Google Scholar]
- 23.Merkord CL. 2010. Seasonality and elevational bird migration in an Andean bird community. PhD dissertation, University of Missouri, Columbia, MO, USA.
- 24.eBird. 2014. eBird: an online database of bird distribution and abundance. Ithaca, NY. See www.ebird.org.
- 25.Jones TM, Akresh ME, King DI. 2013. Recent sightings of Kirtland's warblers on San Salvador Island, The Bahamas. Wilson J. Ornithol. 125, 637–642. ( 10.1676/13-007.1) [DOI] [Google Scholar]
- 26.Natureserve. 2013. NatureServe Web Service. Arlington, VA, USA. See http://services.natureserve.org.
- 27.Pyle P. 1997. Identification guide to North American birds, part I: Columbidae to Ploceidae. Bolinas, CA: Slate Creek Press. [Google Scholar]
- 28.García-Márquez JR, Dormann CF, Sommer JH, Schmidt M, Thiombiano A, Da SS, Chatelain C, Dressler S. 2012. A methodological framework to quantify the spatial quality of biological databases. Biodivers. Ecol. 4, 25–39. ( 10.7809/b-e.00057) [DOI] [Google Scholar]
- 29.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 30.Grandcolas P, Nattier R, Legendre F, Pellens R. 2011. Mapping extrinsic traits such as extinction risks or modelled bioclimatic niches on phylogenies: does it make sense at all? Cladistics 27, 181–185. ( 10.1111/j.1096-0031.2010.00324.x) [DOI] [PubMed] [Google Scholar]
- 31.Boucher FC, Thuiller W, Davies TJ, Lavergne S. 2014. Neutral biogeography and the evolution of climatic niches. Am. Nat. 183, 573–584. ( 10.1086/675506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warren DL, Glor RE, Turelli M. 2008. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62, 2868–2883. ( 10.1111/j.1558-5646.2008.00482.x) [DOI] [PubMed] [Google Scholar]
- 33.Broennimann O. et al. . 2015. ecospat: spatial ecology miscellaneous methods. R package v. 1.1. See https://cran.r-project.org/web/packages/ecospat/index.html.
- 34.Quintero I, Wiens JJ. 2013. What determines the climatic niche width of species? The role of spatial and temporal climatic variation in three vertebrate clades. Glob. Ecol. Biogeogr. 22, 422–432. ( 10.1111/geb.12001) [DOI] [Google Scholar]
- 35.Bivand R, Rundel C. 2014. rgeos: interface to geometry engine—open source (GEOS). R package v. 0.3-8. See https://cran.r-project.org/web/packages/rgeos/index.html.
- 36.Magnusson A. 2014. gmt: interface between GMT map-making software and R. R package v. 1.2-0. See https://cran.r-project.org/web/packages/gmt/index.html.
- 37.Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W. 2014. caper: comparative analyses of phylogenetics and evolution in R. R package v. 0.5.2/r121. See https://cran.r-project.org/web/packages/caper/index.html.
- 38.Lovette IJ, et al. 2010. A comprehensive multilocus phylogeny for the wood-warblers and a revised classification of the Parulidae (Aves). Mol. Phylogenet. Evol. 57, 753–770. ( 10.1016/j.ympev.2010.07.018) [DOI] [PubMed] [Google Scholar]
- 39.Beaulieu JM, O'Meara BC. 2014. OUwie: analysis of evolutionary rates in an OU framework. R package v. 1.42. See https://cran.r-project.org/web/packages/OUwie/index.html.
- 40.Butler MA, King AA. 2004. Phylogenetic comparative analysis: a modeling approach for adaptive evolution. Am. Nat. 164, 683–695. ( 10.1086/426002) [DOI] [PubMed] [Google Scholar]
- 41.O'Meara BC, Ané C, Sanderson MJ, Wainwright PC. 2006. Testing for different rates of continuous trait evolution using likelihood. Evolution 60, 922–933. ( 10.1111/j.0014-3820.2006.tb01171.x) [DOI] [PubMed] [Google Scholar]
- 42.Revell LJ, Harmon LJ, Collar DC. 2008. Phylogenetic signal, evolutionary process, and rate. Syst. Biol. 57, 591–601. ( 10.1080/10635150802302427) [DOI] [PubMed] [Google Scholar]
- 43.Revell LJ, Harmon LJ. 2008. Testing quantitative genetic hypotheses about the evolutionary rate matrix for continuous characters. Evol. Ecol. Res. 10, 311–331. [Google Scholar]
- 44.Beaulieu JM, Jhwueng D-C, Boettiger C, O'Meara BC. 2012. Modeling stabilizing selection: expanding the Ornstein–Uhlenbeck model of adaptive evolution. Evolution 66, 2369–2383. ( 10.1111/j.1558-5646.2012.01619.x) [DOI] [PubMed] [Google Scholar]
- 45.Revell LJ. 2013. A comment on the use of stochastic character maps to estimate evolutionary rate variation in a continuously valued trait. Syst. Biol. 62, 339–345. ( 10.1093/sysbio/sys084) [DOI] [PubMed] [Google Scholar]
- 46.Curson J. 2015. Grey-throated warbler (Basileuterus cinereicollis). In Handbook of the birds of the world alive (eds del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E). Barcelona, Spain: Lynx Edicions; (accessed 28 January 2016; http://www.hbw.com/node/61547) [Google Scholar]
- 47.Curson J, Christie DA. 2015. Hermit warbler (Dendroica occidentalis). In Handbook of the birds of the world alive (eds del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E). Barcelona, Spain: Lynx Edicions; (accessed 28 January 2016; http://www.hbw.com/node/61476) [Google Scholar]
- 48.Smith BT, Bryson RW, Houston DD, Klicka J. 2012. An asymmetry in niche conservatism contributes to the latitudinal species diversity gradient in New-World vertebrates. Ecol. Lett. 15, 1318–1325. ( 10.1111/j.1461-0248.2012.01855.x) [DOI] [PubMed] [Google Scholar]
- 49.Janzen DH. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249. ( 10.1086/282487) [DOI] [Google Scholar]
- 50.Quintero I, Wiens JJ. 2013. Rates of projected climate change dramatically exceed past rates of climatic niche evolution among vertebrate species. Ecol. Lett. 16, 1095–1103. ( 10.1111/ele.12144) [DOI] [PubMed] [Google Scholar]
- 51.Shaw AK, Couzin ID. 2013. Migration or residency? The evolution of movement behavior and information usage in seasonal environments. Am. Nat. 181, 114–124. ( 10.1086/668600) [DOI] [PubMed] [Google Scholar]
- 52.Lawson AM, Weir JT. 2014. Latitudinal gradients in climatic-niche evolution accelerate trait evolution at high latitudes. Ecol. Lett. 17, 1427–1436. ( 10.1111/ele.12346) [DOI] [PubMed] [Google Scholar]
- 53.Bacon CD, Silvestro D, Jaramillo C, Tilston B, Chakrabarty P, Antonelli A. 2015. Biological evidence supports an early and complex emergence of the Isthmus of Panama. Proc. Natl Acad. Sci. USA 112, 6110–6115) ( 10.1073/pnas.1423853112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plummer KE, Siriwardena GM, Conway GJ, Risely K, Toms MP. 2015. Is supplementary feeding in gardens a driver of evolutionary change in a migratory bird species? Glob. Chang. Biol. 21, 4353–4363. ( 10.1111/gcb.13070) [DOI] [PubMed] [Google Scholar]
- 55.Rubenstein DR, Chamberlain CP, Holmes RT, Ayres MP, Waldbauer JR, Graves GR, Tuross NC. 2002. Linking breeding and wintering ranges of a migratory songbird using stable isotopes. Science 295, 1062–1065. ( 10.1126/science.1067124) [DOI] [PubMed] [Google Scholar]
- 56.Gutiérrez-Pinto N, Cuervo AM, Miranda J, Pérez-Emán JL, Brumfield RT, Cadena CD. 2012. Non-monophyly and deep genetic differentiation across low-elevation barriers in a Neotropical montane bird (Basileuterus tristriatus; Aves: Parulidae). Mol. Phylogenet. Evol. 64, 156–165. ( 10.1016/j.ympev.2012.03.011) [DOI] [PubMed] [Google Scholar]
- 57.Losos JB. 2008. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol. Lett. 11, 995–1003. ( 10.1111/j.1461-0248.2008.01229.x) [DOI] [PubMed] [Google Scholar]
- 58.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tewksbury JJ, Huey RB, Deutsch CA. 2008. Putting the heat on tropical animals. Science 320, 1296–1297. ( 10.1126/science.1159328) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data used for this study are publicly available in the eBird (www.ebird.org) and WorldClim databases (www.worldclim.org/). The phylogeny of the Parulidae has been published [38] and a tree file was provided by the authors.