Abstract
Insects are a hyper-diverse group, comprising nearly three-quarters of all named animal species on the Earth, but the environmental drivers of their richness and the roles of ecological interactions and evolutionary innovations remain unclear. Previous studies have argued that family-level insect richness increased continuously over the evolutionary history of the group, but inclusion of extant family records artificially inflated the relative richness of younger time intervals. Here we apply sampling-standardization methods to a species-level database of fossil insect occurrences, removing biases present in previous richness curves. We show that insect family-richness peaked 125 Ma and that Recent values are only 1.5–3 times as high as the Late Palaeozoic. Rarefied species-richness data also tentatively suggest little or no net increase in richness over the past 125 Myr. The Cretaceous peak in family richness was coincident with major radiations within extant groups but occurred prior to extinctions within more basal groups. Those extinctions may in part be linked to mid-Cretaceous floral turnover following the evolution of flowering plants. Negligible net richness change over the past 125 Myr implies that major radiations within extant groups were offset by reduced richness within groups that are now relict or extinct.
Keywords: insecta, biodiversity, evolutionary radiation
1. Introduction
Nearly three-quarters of all named living animal species are insects, yet the factors enabling this immense richness remain unclear [1]. Evolutionary innovations such as the evolution of flight and the origin of complete metamorphosis may be among the key drivers [2–4]. Ecological characteristics of insect clades probably also played an important role in their rapid diversification. The evolution of herbivory [5] and interactions between insects and their host plants, most notably flowering plants [6], have been hypothesized to be important drivers of diversification. Despite the importance of insects across ecosystems, the history of their taxonomic richness remains as uncertain as the causes of rapid diversification.
In the fossil record, insect family richness has traditionally been quantified on the basis of first and last appearances of families (‘range-through diversity’), extending the range of extant families beyond the youngest fossil occurrence to the Recent [7–10]. These results have suggested that insects diversified steadily over their evolutionary history to reach unparalled family-level richness in the Recent. The range-through curves further imply that modern family richness may be twice as great as the Early Cretaceous, 125–150 Ma. However, such range-through measures are known to be biased, particularly at their edges, whereas extending fossil ranges of extant families will inflate diversity estimates in younger time intervals (the ‘Pull of the Recent’) because the living insect fauna is substantially better sampled than fossil faunas [11,12]. We compiled a species-level, occurrence-based dataset, which records more than 37 000 individual insect fossil occurrences (representing more than 24 500 valid species) rather than only the oldest and youngest known records. This database enables us to apply less-biased methods of counting diversity, which can account for variable sampling intensity and eliminate the Pull of the Recent bias [12]. Our dataset closely approximates previously published diversity curves when we use the same range-through counting methods and extant records, but reveals new insights when we apply less-biased methods of reconstructing diversity.
2. Material and methods
We compiled a species-level database of insect occurrences from 6334 primary references, recording more than 39 000 insect fossil occurrences of more than 24 500 valid species, available through the Paleobiology Database (www.paleobiodb.org). Each occurrence is a record of the presence of a taxon in a collection, which is a group of fossils from a single stratigraphic interval at a particular geographical locality. We constructed diversity curves using the ‘10 million year bin’ timescale available in the Paleobiology Database (bins composed of adjacent geological stages totalling approx. 10 Myr in length). Although the ages of many insect collections are resolved more precisely, there are too few occurrences in many finer time bins for subsampling calculations. We used the diversity curve tools at Fossilworks (www.fossilworks.org) to perform sampling standardization on sampled-in-bin richness, downloading occurrences identified to the genus level (without ? or “” qualifiers) and excluding trace fossils and form taxa.
We focus our analyses on the family-level record of insect richness. Despite potentially inconsistent definitions of higher taxa [13], the family level has traditionally been used to reconstruct changes in fossil insect richness [7–9], although some studies have also analysed genus-level data [8]. At the family level, we used shareholder quorum subsampling (SQS) [12] to account for large variations in the number of insect occurrences over time. SQS is designed to subsample a constant fraction of the occurrence-frequency distribution, selecting taxa until the sum of their occurrence frequencies reaches the chosen quorum. Family-level data were subsampled at a quorum of 0.64 for all records, the highest value that allowed us to evaluate all time bins other than the extremely sparse Early Triassic (28 occurrences) and Maastrichtian (15 occurrences), although the relative richness pattern is unaffected by the choice of sampling quorum (electronic supplementary material, figure S1). We also downloaded all insects using the same criteria as previously outlined but excluding records with lithology marked as amber (which occur in the Cretaceous-Recent only). Those occurrences were subsampled with the SQS method at the same quorum (0.64) to reconstruct richness trends within a consistent taphonomic category (compression/impression fossils) through time. For both analyses, Good's u (a measure of data coverage) was calculated from single-reference taxa, ignoring the most common taxon and the largest collection, following Alroy [12], although these choices have only negligible effects on richness trends (electronic supplementary material, figure S2). We did not downweight collections coming from large references because most collections contain occurrences derived from multiple references (therefore, the identity of the primary reference is less meaningful). This choice also had no effect on the results (electronic supplementary material, figure S2). Although classical-rarefaction (CR), which subsamples a uniform number of occurrences, tends to flatten relative richness differences [12], we also analysed family-level richness at a quota of 170 occurrences. We used both SQS and CR subsampling to assess the robustness of richness trends because the two methods respond differently to changes in the occurrence-frequency distribution of the underlying taxon pool by sampling either a uniform frequency (SQS) or absolute quota (CR) of occurrences.
We also downloaded species-level insect occurrences from the Paleobiology Database API (www.paleobiodb.org/data1.2), again removing occurrences that did not fall within a ‘10 million year’ bin. We kept all species-level occurrences regardless of the qualifiers applied to the genus identification. Because palaeoentomologists rarely mention additional occurrences of a species after its initial description, Good's u cannot be estimated reliably for SQS subsampling. Therefore, we used CR subsampling of species-level data at 500, 1000 and 2000 occurrences, despite its problems [12]. All code is available at https://github.com/mclapham/insect_div.
3. Results
(a). Family-richness trends
The underlying data within Paleobiology Database captures the information contained in range-based compilations (figure 1a; electronic supplementary material, figure S3), but the family-richness curve after SQS differs considerably from previous range-through curves and does not show a continuous increase in family-level richness to the present (figure 1b). Much of the post-Jurassic increase in previous range-through curves arises from Pull of the Recent bias. All fossil ranges are incomplete relative to the true duration of the taxon and the unidirectional range extension enabled by extremely well-known modern data artificially inflates the magnitude of richness increase (figure 1a). Range-through curves also suffer from edge effects introduced by artificial range truncations; this effect is most noticeable in the youngest time interval of the fossil-only range-through richness curve (figure 1a). These and other biases can be reduced by assessing richness only from taxa recorded within each time interval and by applying subsampling methods [12]. In the subsampled curve, insect family richness increased by about 50% from the mid-Carboniferous to the Middle Jurassic and then more rapidly to a peak in the Early Cretaceous. Subsampled richness in most Cenozoic intervals was lower than the Early Cretaceous peak; only the Cenozoic 3 interval, which contains the exceptionally well-sampled Baltic amber, is equal or higher. However, extreme volatility within the Cretaceous and Cenozoic makes it difficult to determine typical richness levels for the interval, so the presence and magnitude of any decrease are uncertain. Nevertheless, the SQS record strongly argues against a large increase in family richness since the Early Cretaceous.
Figure 1.
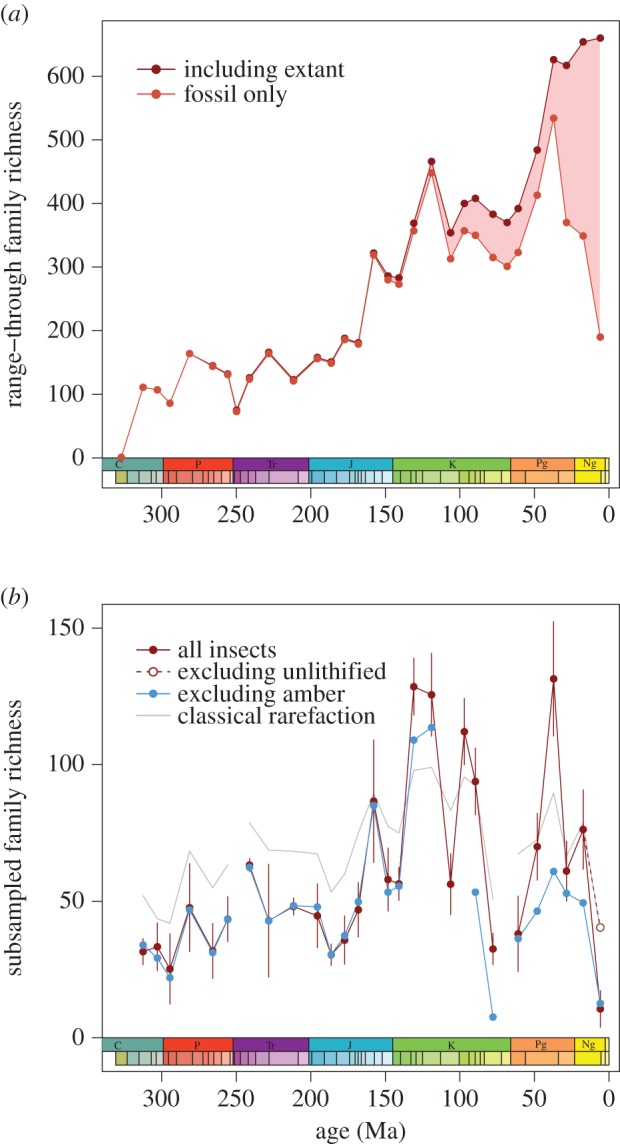
(a) Range-through family richness trends generated by extending ranges of extant families to the Recent (dark red) and only considering fossil data (light red). Including extant data imposes the Pull of the Recent bias (shaded area), while the fossil-only curve suffers from edge effects (notably the decrease in the youngest interval). As a result, these curves are not accurate records of insect richness, particularly when including extant data. (b) Sampling-standardized trends in insect family richness. Curves show shareholder quorum subsampling of all insects (red) and excluding amber fossils (blue), as well as classical rarefaction subsampling (grey line). Error bars (shown for all insects only) are 1 s.d. The open circle shows richness after excluding unlithified and poorly lithified occurrences. Differences between the pre-Cretaceous curves for all insects and when excluding amber arise because sampling-standardization methods take random subsamples of the dataset. The Early Triassic and Maastrichtian intervals are poorly sampled and cannot be analysed with these methods. (Online version in colour.)
When excluding amber fossils to allow direct comparison with pre-Cretaceous intervals (prior to common amber fossilization), Cenozoic richness of roughly 50 subsampled families per interval is comparable with the Triassic and Jurassic and only 50% higher than the Carboniferous. The youngest time interval (Late Miocene–Pleistocene) has unusually low subsampled richness because most localities represent a fundamentally different preservation mode (unlithified sediments) and are overwhelmingly dominated by a few families of ground-dwelling beetles. After excluding unlithified sediments, Late Miocene–Pleistocene subsampled richness is comparable with the compression fossil record from other Cenozoic time intervals.
(b). Species-richness trends
Although there are many extant insect families, the notion of insects as a hyper-diverse group arises primarily from the immense number of insect species. Extant species richness is disproportionately driven by a few clades with extremely high diversity (e.g. ichneumonid wasps, staphylinid beetles) [4]. Those clades are also diverse and abundant in the fossil record; however, the true ancient richness of such hyper-diverse groups is probably underestimated for two reasons. First, in many cases their small body size may reduce the likelihood of collection and identification. Second, fossil specimens, especially those preserved as rock compressions, typically do not preserve all of the subtle anatomical features used for species discrimination of living specimens. Those biases, however, should be consistently present in deposits of similar preservation type throughout the geological record and therefore are unlikely to produce spurious trends in richness estimates when comparing fossil faunas of different ages. Fragile clades (such as the hyper-diverse Lepidoptera) will also be under-represented relative to more robust taxa, but this also should be true throughout the insect record.
It is possible, therefore, that increases in the number of species per family led to large increases in insect species richness, despite the lack of trend in family-level richness since the Early Cretaceous. However, the nature of the published insect fossil record, unusually dominated by species with occurrences reported only from a single reference (91% of species) and from a single database collection (82% of species), poses a challenge for construction of sampling-standardized, particularly SQS, species-level richness curves. CR subsampling can be applied instead, even though only a few intervals have a sufficient number of occurrences for robust subsampling. The resulting species-richness curves (figure 2) exhibit a mid-Jurassic increase (visible at 1000 occurrences) and suggest no or a small net increase in species richness since the Early Cretaceous (at 1000 and 2000 occurrences, respectively), both broadly similar to the family-level richness curve.
Figure 2.
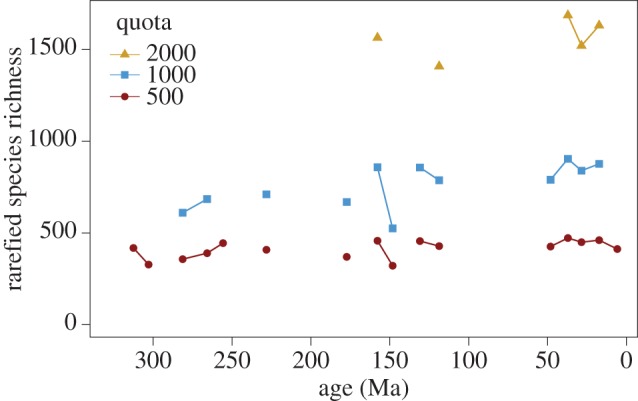
Species richness estimates from classical rarefaction at quotas of 500, 1000 and 2000 occurrences. The analyses were performed after removing occurrences from unlithified and poorly lithified sediments (only affecting the youngest interval). One standard deviation error bars are smaller than the symbols. Rarefied species richness increases in the mid-Jurassic, similar to the family-level curve, and has a small net increase from the Early Cretaceous to the Neogene. (Online version in colour.)
4. Discussion
(a). Biases and reliability of the record
Our findings contrast markedly with previous studies that suggested a nearly-continuous, sixfold net increase in insect family richness from the Late Palaeozoic to Cenozoic [7,8]. We also argue that there was no net increase (perhaps even a decrease) in family-level richness over the past 125 Myr, rather than the near-doubling previously proposed [7]. Furthermore, species-level results suggest little net change over the past 125 Myr despite molecular evidence for major radiations among extant groups. Even though the previous range-through curves contain known artefacts, most notably from the Pull of the Recent bias, it is important to explore the reliability of the subsampled results.
The SQS curve (figure 1b) exhibits abrupt peaks and troughs with substantial volatility even between successive time intervals, which seems implausible over such short timescales. The episodic nature of exceptional preservation in amber provides one explanation for the volatility because amber fossilization enhances recorded richness in certain time intervals relative to others that lack rich amber deposits. Enhanced richness does not result simply from additional insect occurrences provided by amber fossilization; instead, amber and compression fossils tend to sample overlapping but distinct subsets of the insect fauna, largely because of different size-selectivity in the two preservation modes [14]. When combined, amber and compression fossils capture a broader taxon pool that is reflected as higher subsampled richness by the SQS method.
Although amber fossilization produces sharp peaks in sampled family richness (figure 1), the compression fossil record also exhibits considerable and probably also artificial volatility, particularly in the Cretaceous. That volatility is best explained by tectonically driven variations in the nature of depositional environments in which insects were fossilized. We assessed the role of tectonic setting by assigning each collection to ‘higher-subsidence’ (extensional, pull-apart and volcanic caldera basins) or ‘lower-subsidence’ basins (forearc or foreland basins, passive margins and cratonic basins). We then tested the role of tectonic setting with linear regression, first with changes in higher-subsidence occurrences as the independent variable and, in a separate analysis, with changes in lower-subsidence occurrences as the independent variable. All time-series data were differenced and we excluded unlithified or poorly lithified occurrences. Richness changes between successive time intervals are significantly associated (r2 = 0.225, p = 0.025) with shifts in the number of insect occurrences deriving from higher-subsidence basin types (figure 3a). By contrast, there is no relationship (r2 = 0.0004, p = 0.93) with changes in the number of occurrences from basin types with lower-subsidence rates on average (figure 3b). Basins with high-subsidence rates generate greater accommodation space for the formation of larger or deeper lakes, where finer grain size, lower energy and potentially anoxic bottom waters promote high-quality preservation of a broad range of insect families [15–17].
Figure 3.
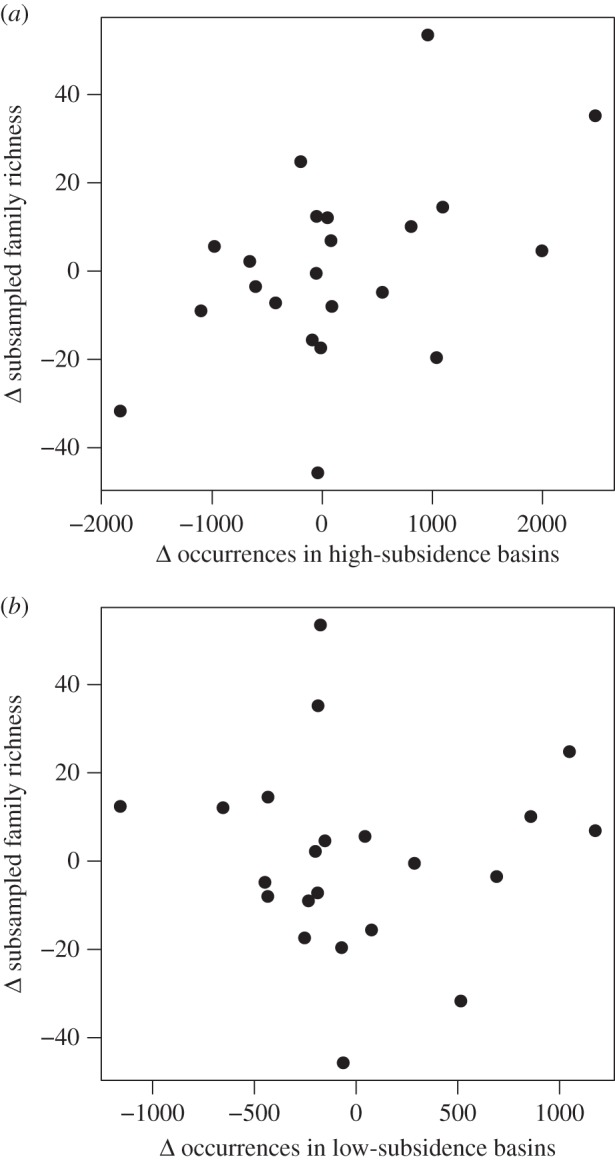
Relationship between changes in the tectonic setting of insect occurrences and interval-to-interval changes in subsampled richness. (a) Changes in the number of occurrences in typically high-subsidence basin types (extensional, pull-apart and volcanic caldera basins) are closely linked with changes in subsampled family richness (r2 = 0.225, p = 0.025). (b) Changes in the number of occurrences in other basins have no effect on subsampled richness (r2 = 0.0004, p = 0.93).
There is a strong association between richness changes and the abundance of occurrences in extensional or other high-subsidence basin types, yet not all extensional basins yield exceptional insect localities [18]. Although each basin has a unique subsidence history, initial fault-controlled subsidence rates typically are higher in rift basins in regions of greater lithospheric thickness [19], consistent with the occurrence of exceptional insect localities in extensional basins associated with collapse of orogenically thickened continental crust (e.g. [20]). A bimodal palaeolatitudinal distribution of insect occurrences (electronic supplementary material, figure S4), with peaks near the equator and at temperate mid-latitudes, also suggests that precipitation exceeding evaporation is an important additional control on the formation of exceptional localities. This bimodal pattern is probably not driven by the location of well-sampled regions like North America or Europe because it independently occurs within each region (electronic supplementary material, figure S4) and within a single time interval, when sufficient sampling breadth is available (electronic supplementary material, figure S5). However, it is difficult to distinguish our preservation hypothesis from true variations in the abundance of insects, which may be less abundant in arid climate zones. Large-scale insect abundance patterns are not well described, and the contributing factors are probably complex [21], but water availability is one important control on richness in insects and other terrestrial groups [22,23].
Only a small fraction of all discovered fossil insect specimens have been formally described. The number of described fossil insects varies considerably among time intervals, in part due to the area of suitable basins, and also probably owing to worker effort concentrated on richer time intervals [24,25]. We use sampling standardization to account for variations in the number of published occurrences, but non-random researcher practices, such as the logical preference for describing well-preserved, rare or unusual specimens, may also influence the occurrence-frequency distribution in a particular interval. This effect is likely to introduce noise and perhaps amplifies volatility in the record, because extremely rich intervals also yield a greater number of rare and better-preserved specimens, enabling palaeoentomologists to focus on describing rare or unique taxa. It is unlikely, however, to vary systematically or predictably with the geological age of the deposit (excluding differences between amber and compression fossils). The insect fossil record is also dominated by only a small number of localities in most time periods, but there is no evidence that geographical extent of sampling, as measured by the length of the minimum spanning tree between all localities [26], biases subsampled richness overall or with compression fossils (electronic supplementary material, figure S6).
Short-term fluctuations in the SQS family richness record are therefore best explained as artefacts from changes in the underlying taxon pool driven by tectonic setting and climate, as well as by episodic amber preservation. These biases are particularly likely explanations of the volatility in the Late Cretaceous record and the abrupt decrease in the youngest interval (Late Miocene–Pleistocene). Given that, can any conclusions be drawn about the overall trajectory of insect family richness? The CR method of subsampling provides the opposite end-member case for richness estimation by drawing a uniform number of occurrences from each time interval-independent of variations in the taxon pool [12]. The overall shape of the CR family-level richness curve closely matches SQS data (figure 1), even if potentially less reliable older literature is excluded from the analyses (electronic supplementary material, figure S7). Furthermore, net family-level richness change since the beginning of the Cretaceous is negligible even in range-through data after accounting for the Pull of the Recent (figure 1a). Because CR and SQS make different assumptions about subsampling from the broader taxon pool, yet result in consistent overall richness trends through time, the broad shape of the sampling-standardized family-richness curve is probably a robust result. Short-term fluctuations are difficult to resolve with confidence, but all methods yield a Late Jurassic–Early Cretaceous increase followed by little net change or perhaps decreasing family-level richness.
The classical-rarefaction species-richness curve appears to record the same major features as the family-richness curves, despite the limited number of intervals meeting a quota of 1000 or 2000 occurrences. However, there are additional caveats to its interpretation. First, richness differences between intervals will be muted by the rarefaction method, especially at smaller quotas [12]. Second, subsampling assumes that the rank-order distribution of published occurrences is an accurate representation of the underlying rank-order distribution of fossil occurrences among collections. The dominance of single-collection or single-reference fossil insect species suggests that the rank-order distribution may be artificially flattened by non-random publication practices in which new species, rather than additional records of existing taxa, are preferentially described. If that effect varies systematically among time periods, which seems unlikely but cannot be ruled out, it may obscure true richness changes. Taken at face value, species-level richness trends (figure 2) appear to broadly follow our family-level curve (figure 1b): a more pronounced increase from the mid-Jurassic to Early Cretaceous followed by little change over the past 125 Myr. In contrast with the family-level curve, which implies decreasing richness from an Early Cretaceous peak, the species-level curve provides more support for little net change or a small net increase in richness since the Early Cretaceous (figure 2).
(b). Ecological and environmental controls on insect richness
Our analyses suggest that the net increase in insect richness from the Late Palaeozoic to Recent was much less than previously estimated, primarily due to bias from the Pull of the Recent in previous range-through curves. This implies that insects had evolved high richness early in their evolutionary history; family-level richness may have reached one-third (on the basis of range-through data excluding the Pull of the Recent) to three-quarters (on the basis of SQS richness of compression fossils) of modern levels by the Permian. Rapid early diversification is consistent with the hypothesis that the evolution of flight was among the key innovations enabling high richness in insects [1,2]. All methods show a more rapid increase in richness from the mid-Jurassic to the Early Cretaceous. The Late Jurassic also marked a fundamental shift in the preservation quality of insect compression fossils [17], so we cannot rule out taphonomic biases artificially reducing richness in older intervals. However, a pronounced richness increase in the Late Jurassic and Early Cretaceous is supported by phylogenomic evidence for rapid diversification within extant lineages, particularly Holometabola [4,27].
We find no evidence for a net increase in family-level insect richness, and perhaps also in species richness, since the Early Cretaceous, despite major biotic changes such as the mid-Cretaceous diversification of flowering plants. Negligible effects of the angiosperm radiation are consistent with previous findings [7] and suggest that positive ecological interactions in some groups may have been offset by negative effects in others, for example, from disruption of existing habitats and resources during the floral turnover [28]. Limited net richness change over the last 125 Myr agrees with the pattern exhibited by beetles, the richest extant insect order, which also have not increased in richness since the Early Cretaceous when the Pull of the Recent bias is excluded (supplementary figures in [29]). Although sampling-related volatility in the data preclude interpretation of shorter term or more subtle richness changes, a post-Early Cretaceous decrease in subsampled family richness is supported by both SQS and CR methods. This is consistent with beta diversity data (the difference in taxonomic composition among sites), which also argues for Palaeogene global insect richness higher than present-day levels [30]. Archibald et al. [31] also found that local species richness within a Palaeogene mid-latitude community was comparable with a modern tropical example and greater than a modern mid-latitude community, potentially consistent with a broader-scale reduction in richness since the Palaeogene. Our subsampled species-level richness data tentatively imply a small net richness increase since the Early Cretaceous at that taxonomic level, although we lack resolution to assess finer patterns. Regardless, these multiple lines of evidence argue against large increases in richness over the past 50–125 Myr.
The Early Cretaceous richness peak may therefore reflect a transitional period in insect evolution where radiating extant families coexisted with basal taxa that are rare today or that became extinct [32–35], consistent with phylogenetic evidence for downshifted diversification rates during the Cretaceous in some basal members of groups [4]. Minimal net richness change since the Early Cretaceous implies that the spectacular evolutionary radiations within extant clades were offset by decreasing diversity in extinct or relict groups. Biotic changes, like the transition to angiosperm-dominated plant communities, and climatic shifts, probably provided evolutionary opportunities for some groups but negatively influenced others. Thus, the evolution of insects, rather than representing a continuous accumulation of taxa to the present day, has been shaped by ecological and environmental forces and the waxing and waning of clades over their long history.
Supplementary Material
Acknowledgements
We thank S. Finnegan and J. Payne for discussion and reading of the manuscript, and two anonymous reviewers for helpful feedback. This is Paleobiology Database publication 249.
Data accessibility
All data are deposited and available in the Paleobiology Database (www.paleobiodb.org).
Author contributions
M.E.C. and J.A.K. conceived the study and collected the data. M.E.C. analysed the data and wrote the first draft of the manuscript. All authors contributed to interpretation of the data and revisions of the manuscript.
Competing interests
We have no competing interests.
Funding
We received no funding for this study.
References
- 1.Mayhew PJ. 2007. Why are there so many insect species? Perspectives from fossils and phylogenies. Biol. Rev. 82, 425–454. ( 10.1111/j.1469-185X.2007.00018.x) [DOI] [PubMed] [Google Scholar]
- 2.Nicholson DB, Ross AJ, Mayhew PJ. 2014. Fossil evidence for key innovations in the evolution of insect diversity. Proc. R. Soc. B 281, 20141823 ( 10.1098/rspb.2014.1823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nel A, et al. 2013. The earliest known holometabolous insects. Nature 503, 257–261. ( 10.1038/nature12629) [DOI] [PubMed] [Google Scholar]
- 4.Rainford JL, Hofreiter M, Nicholson DB, Mayhew PJ. 2014. Phylogenetic distribution of extant richness suggests metamorphosis is a key innovation driving diversification in insects. PLoS ONE 9, e109085 ( 10.1371/journal.pone.0109085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiens JJ, Lapoint RT, Whiteman NK. 2015. Herbivory increases diversification across insect clades. Nat. Commun. 6, 8370 ( 10.1038/ncomms9370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wahlberg N, Wheat CW, Peña C. 2013. Timing and patterns in the taxonomic diversification of Lepidoptera (butterflies and moths). PLoS ONE 8, e80875 ( 10.1371/journal.pone.0080875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labandeira CC, Sepkoski JJ. 1993. Insect diversity in the fossil record. Science 261, 310–315. ( 10.1126/science.11536548) [DOI] [PubMed] [Google Scholar]
- 8.Jarzembowski EA, Ross AJ. 1996. Insect origination and extinction in the Phanerozoic. Geol. Soc. Spec. Publ. 102, 65–78. ( 10.1144/GSL.SP.1996.001.01.05) [DOI] [Google Scholar]
- 9.Nicholson DB, Mayhew PJ, Ross AJ. 2015. Changes to the fossil record of insects through fifteen years of discovery. PLoS ONE 10, e0128554 ( 10.1371/journal.pone.0128554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dmitriev VY, Zherikhin VV. 1988. Changes in the familial diversity of insects and demonstration of a method of data analysis. In The Mesozoic-Cenozoic crisis in the evolution of insects (ed. Ponomarenko AG.), pp. 208–215. Moscow, Russia: Akademii Nauk. [Google Scholar]
- 11.Foote M. 2000. Origination and extinction components of taxonomic diversity: general problems. Paleobiology 26, 74–102. ( 10.1666/0094-8373(2000)26%5B74:OAECOT%5D2.0.CO;2) [DOI] [Google Scholar]
- 12.Alroy J. 2010. Fair sampling of taxonomic richness and unbiased estimation of origination and extinction rates. In Quantitative methods in paleobiology, vol. 16 (eds Alroy J, Hunt G), pp. 55–80. New Haven, CT: The Paleontological Society. [Google Scholar]
- 13.Stadler T, Rabosky DL, Ricklefs RE, Bokma F. 2014. On age and species richness of higher taxa. Am. Nat. 184, 447–455. ( 10.1086/677676) [DOI] [PubMed] [Google Scholar]
- 14.Martínez-Delclòs X, Briggs DEG, Peñalver E. 2004. Taphonomy of insects in carbonates and amber. Palaeogeogr. Palaeoclimatol. Palaeoecol. 203, 19–64. ( 10.1016/S0031-0182(03)00643-6) [DOI] [Google Scholar]
- 15.Wilson MVH. 1980. Eocene lake environments: depth and distance-from-shore variation in fish, insect, and plant assemblages. Palaeogeogr. Palaeoclimatol. Palaeoecol. 32, 21–44. ( 10.1016/0031-0182(80)90029-2) [DOI] [Google Scholar]
- 16.Smith DM. 2012. Exceptional preservation of insects in lacustrine environments. Palaios 27, 346–353. ( 10.2110/palo.2011.p11-107r) [DOI] [Google Scholar]
- 17.Karr JA, Clapham ME. 2015. Taphonomic biases in the insect fossil record: shifts in articulation over geologic time. Paleobiology 41, 16–32. ( 10.1017/pab.2014.3) [DOI] [Google Scholar]
- 18.Fraser NC, Grimaldi DA, Olsen PE, Axsmith B. 1996. A Triassic Lagerstätte from eastern North America. Nature 380, 615–619. ( 10.1038/380615a0) [DOI] [Google Scholar]
- 19.McKenzie DP. 1978. Some remarks on the development of sedimentary basins. Earth Planet. Sci. Lett. 40, 25–32. ( 10.1016/0012-821X(78)90071-7) [DOI] [Google Scholar]
- 20.Graham SA, Cope T, Johnson CL, Ritts B. 2012. Sedimentary basins of the late Mesozoic extensional domain of China and Mongolia. In Regional geology and tectonics: Phanerozoic rift systems and sedimentary basins (eds Roberts DG, Bally AW), pp. 442–461. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 21.Kocher SD, Williams EH. 2000. The diversity and abundance of North American butterflies vary with habitat disturbance and geography. J. Biogeogr. 27, 785–794. ( 10.1046/j.1365-2699.2000.00454.x) [DOI] [Google Scholar]
- 22.Keil P, Simova I, Hawkins BA. 2008. Water-energy and the geographical species richness pattern of European and North African dragonflies (Odonata). Insect Conserv. Divers. 1, 142–150. ( 10.1111/j.1752-4598.2008.00019.x) [DOI] [Google Scholar]
- 23.Hawkins BA, et al. 2003. Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117. ( 10.1890/03-8006) [DOI] [Google Scholar]
- 24.Raup DM. 1977. Systematists follow the fossils. Paleobiology 3, 328–329. [Google Scholar]
- 25.Dunhill AM, Benton MJ, Newell AJ, Twitchett RJ. 2013. Completeness of the fossil record and the validity of sampling proxies: a case study from the Triassic of England and Wales. J. Geol. Soc. Lond. 170, 291–300. ( 10.1144/jgs2012-025) [DOI] [Google Scholar]
- 26.Alroy J. 2010. Geographical, environmental and biotic controls on Phanerozoic marine diversification. Palaeontology 53, 1211–1235. ( 10.1111/j.1475-4983.2010.01011.x) [DOI] [Google Scholar]
- 27.Misof B, et al. 2014. Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767. ( 10.1126/science.1257570) [DOI] [PubMed] [Google Scholar]
- 28.Labandeira CC. 2005. The fossil record of insect extinction: new approaches and future directions. Am. Entomol. 51, 14–29. ( 10.1093/ae/51.1.14) [DOI] [Google Scholar]
- 29.Smith DM, Marcot JD. 2015. The fossil record and macroevolutionary history of the beetles. Proc. R. Soc. B 282, 20150060 ( 10.1098/rspb.2015.0060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Archibald SB, Greenwood DR, Mathewes RW. 2013. Seasonality, montane beta diversity, and Eocene insects: testing Janzen's dispersal hypothesis in an equable world. Palaeogeogr. Palaeoclimatol. Palaeoecol. 371, 1–8. ( 10.1016/j.palaeo.2012.10.043) [DOI] [Google Scholar]
- 31.Archibald SB, Bossert WH, Greenwood DR, Farrell BD. 2010. Seasonality, the latitudinal gradient of diversity, and Eocene insects. Paleobiology 36, 374–398. ( 10.1666/09021.1) [DOI] [Google Scholar]
- 32.Labandeira CC. 2006. The four phases of plant-arthropod associations in deep time. Geol. Acta 4, 409–438. [Google Scholar]
- 33.Nel A, Fleck G, Garcia G, Gomez B, Ferchaud P, Valentin X. 2015. New dragonflies from the lower Cenomanian of France enlighten the timing of the odonatan turnover at the Early-Late Cretaceous boundary. Cretaceous Res. 52, 108–117. ( 10.1016/j.cretres.2014.08.005) [DOI] [Google Scholar]
- 34.Peris D, Davis SR, Engel MS, Delclòs X. 2014. An evolutionary history embedded in amber: reflection of the Mesozoic shift in weevil-dominated (Coleoptera: Curculionoidea) faunas. Zool. J. Linn. Soc. 171, 534–553. ( 10.1111/zoj.12149) [DOI] [Google Scholar]
- 35.Rasnitsyn AP. 1988. Problema global'nogo krizisa nazemnykh biotsenozov v serdinie melovogo perioda. In Melovoi Biotsenoticheskii Krizis i Evolutsiya Nasekomykh (ed. Ponomarenko AG.), pp. 191–207. Moscow, Russia: Nauka. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are deposited and available in the Paleobiology Database (www.paleobiodb.org).


