Abstract
Interactions between multiple ecosystem stressors are expected to jeopardize biological processes, functions and biodiversity. The scientific community has declared stressor interactions—notably synergies—a key issue for conservation and management. Here, we review ecological literature over the past four decades to evaluate trends in the reporting of ecological interactions (synergies, antagonisms and additive effects) and highlight the implications and importance to conservation. Despite increasing popularity, and ever-finer terminologies, we find that synergies are (still) not the most prevalent type of interaction, and that conservation practitioners need to appreciate and manage for all interaction outcomes, including antagonistic and additive effects. However, it will not be possible to identify the effect of every interaction on every organism's physiology and every ecosystem function because the number of stressors, and their potential interactions, are growing rapidly. Predicting the type of interactions may be possible in the near-future, using meta-analyses, conservation-oriented experiments and adaptive monitoring. Pending a general framework for predicting interactions, conservation management should enact interventions that are robust to uncertainty in interaction type and that continue to bolster biological resilience in a stressful world.
Keywords: ecological surprises, non-additive effects, global change, ecological experiments
1. Introduction
The human footprint on natural ecosystems is now almost inescapable [1]. The pressures of habitat loss, pollution, overexploitation, invasive species and climate change are increasing in reach and intensity around the globe [2,3]. Their cumulative effects are eroding biodiversity [4], triggering concerns that we are in the midst of a sixth mass extinction [5] and approaching a planetary-scale tipping point [6,7].
Integral to the idea of thresholds and critical transitions is the notion that the cumulative effects of multiple stressors are magnified by synergistic interactions [6,8]. A synergy is conventionally defined as a combined effect of multiple stressors that exceeds the sum of individual stressor effects [8]. Synergies are caused by amplifying feedbacks and can provoke unpredictable ‘ecological surprises' that can accelerate biodiversity loss [8] and impair the functioning of ecosystems [9,10]. The scientific community has been nearly unanimous in declaring such synergies a major problem for conservation and management in the twenty-first century [9,11]. The conservation implications of synergies are that insidious, cascading impacts of co-occurring stressors will severely degrade ecosystems.
In this review, we cast a critical eye on the pervasiveness of ecological synergies. We first remind readers that synergies are one of several types of interactions that can occur between ecosystem stressors and highlight the difficulty in defining these interactions. By stressor, we mean any natural or anthropogenic pressure that causes a quantifiable change, whether positive or negative, in biological response (akin to ‘driver’, sensu [12]). We then examine whether synergies, as conventionally defined, are over-emphasized in ecological literature by examining trends of publications on synergies and other interactions in the ecological and conservation literature over the past four decades. If synergy has become a buzzword that encompasses all instances of multiple stressor effects, there is a real risk of unwarranted pessimism that may diminish the credibility of the scientific community and discourage policymakers from attempts to tackle environmental problems. Finally, we explore the consequences of misidentifying the type of stressor interaction at play, evaluate our current ability to predict interaction type, and provide a series of recommendations to increase and better incorporate knowledge about stressor interactions into applied outcomes.
2. Interaction types
At first glance, it seems simple to identify and label the different ways in which multiple ecosystem stressors can interact. As stated above, a synergy is conventionally defined as an interaction where the combined impact of two or more stressors on an ecological response (e.g. diversity, productivity, abundance, survival, growth, reproduction) is greater than a threshold calculated from the impacts of the individual stressors [13] (figure 1). By contrast, when the combined effect of multiple stressors generates a response that is smaller than this threshold, the interaction is conventionally deemed to be antagonistic [13] (figure 1). However, the devil of this simplicity is in the details.
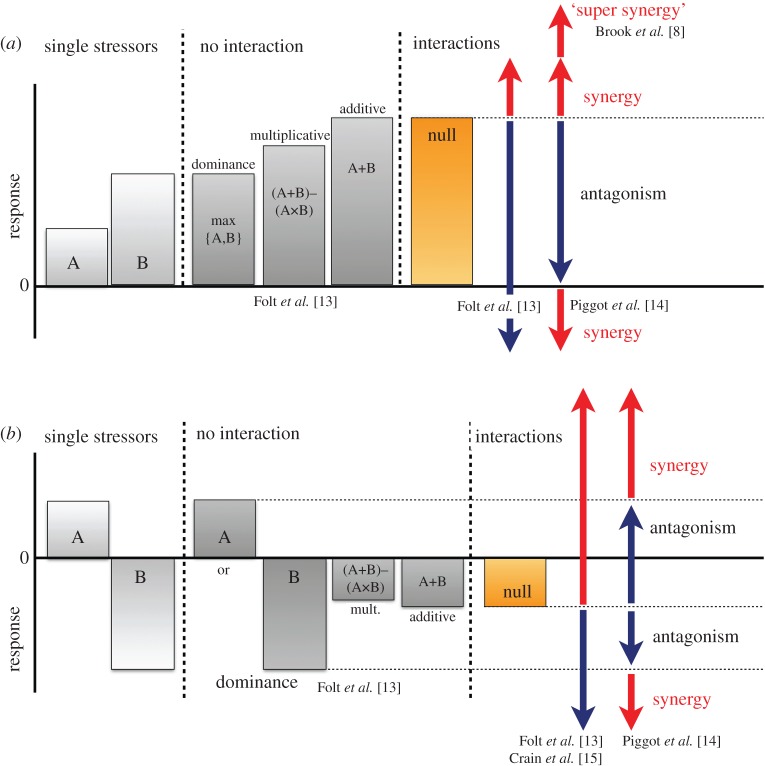
Figure 1.
Models for defining ecological synergies and other interactions between multiple stressors. (a) Two stressors (A and B) impact a biological response in the same direction when acting separately. Their combined effect could simply be equal to the effect of one of the two stressor, i.e. a dominance effect, or be additive, i.e. the sum of the two stressor effects with or without a multiplicative-risk correction. Alternatively, stressors can interact either antagonistically or synergistically. Null models (from an additive or multiplicative risk expectation; we use the additive expectation as the null model here) provide the threshold for distinguishing between these interactions. Interaction type becomes difficult to label when the combined response is in a direction opposite that of the individual stressors (antagonism by Folt et al. [13] versus synergy by Piggot et al. [14]). Some authors [8] also identify an area of ‘super-synergy’ in relation to minimum population size. (b) Two stressors have opposing effects on a biological response. Here, some authors use a null model to define interaction types [13,15], while others advocate using the effects of each stressor to classify interactions [14]. (Online version in colour.)
One complication is that different null models can underpin interaction types, resulting in different outcomes. Conventional synergies and antagonisms are typically defined in relation to an additive expectation (i.e. the sum of the stressor effects when acting in isolation). While this null model might be appropriate for some metrics of impacts (e.g. growth), it is not for others. For example, mortality is a common response (e.g. [16]) that represents probabilistic events that are best combined with a multiplicative (or risk) model, which corrects for the fact that individuals killed by one stressor cannot be killed by another and which bounds the combined mortality estimates to a maximum of 100% (i.e. (A + B − (A × B)), [17,18]). Importantly, the cumulative mortality predicted by a multiplicative (risk) null model is smaller than an additive model, hence some interactions deemed to be antagonistic under an additive null model might be labelled synergistic under the multiplicative risk null model (figure 1).
Another complication occurs when one stressor is dominant and accounts for most or all of the biological response of interest [13]. For example, pollutants A and B might both be lethal to a vulnerable fraction of a focal population, but A acts more swiftly and removes all susceptible individuals such that B has no additional effect when both stressors act in concert [19]. Under the assumption of an additive or multiplicative null model, this situation would appear to be an antagonism (figure 1)—even though it is actually driven by a single stressor—which can have implications for conservation and management (see below).
A final complication is when stressors have opposite effects on the biological response of interest. The conventional approach is to sum (adjusting or not for multiplicative risk) the effects of individual stressors and use the resultant value as the threshold between synergy and antagonism (e.g. [15]). However, the threshold is invariably smaller in absolute terms than the responses to any of the independent stressors, hence the label of synergy might become attached to combined effects that are smaller than the effect of a single stressor (figure 1), i.e. what should logically be deemed an antagonism. For this reason, some authors recently advocated using the effects of independent stressors to delineate the boundaries between antagonisms (i.e. combined effect smaller than any independent effect) and synergies (combined effect exceeding independent effects), with additional clarifications regarding the direction of responses (positive or negative) [14] (figure 1). In addition, others have argued for the existence of a threshold in synergistic interactions [8], which identifies when populations are pushed beyond a minimum viable size and drawn into an extinction vortex [20]—essentially an area of ‘super-synergy’ (figure 1). These extreme synergies can also be of concern if they abruptly push a system beyond a threshold or tipping point into an alternative (and undesirable) state that may be difficult to reverse due to hysteresis (e.g. [10]).
While clarity is always desirable in science, we question the extent to which this proliferation of labels, with ever-finer distinctions between types and directions of synergies and antagonisms, is helpful. For conservation, labels will be of greatest relevance if they describe ecological responses to stressors that require different management interventions. To this end, it might seem only necessary to distinguish between interactions resulting in dominant, additive or non-additive effects, as well as tipping points beyond which the long-term security of populations, communities or ecosystems is compromised. Finally, knowing whether interactions among stressors result in ecologically beneficial or detrimental responses is also essential, since both synergies and antagonisms can do either, depending on the type of driver and the directions of its impact, and the underlying ecological mechanisms (e.g. [21]). However, to stay consistent with current practice, the rest of this review continues to focus on the three conventional types of interactions: synergies (which we will largely view as detrimental, nonlinear interactions), antagonisms (which we will largely view as beneficial, nonlinear interactions) and additive effects.
3. Are synergies over-emphasized in the ecological literature?
Despite the pitfalls in defining what a synergy is, the term has been enthusiastically adopted in the ecological literature. Over the past four decades (1986–2014), there has been a rapid increase in the annual number of papers deeming synergies to be important enough to appear in either their title or abstract (figure 2a; see the electronic supplementary material for methods). Mention of other interaction types (antagonisms and additive effects) is also growing, but not nearly as quickly (figure 2a). In total, we identified 616 papers, of which 69% mentioned the term ‘synergy’, while 35% mentioned additive effects and only 15% mentioned antagonisms (some papers mentioned more than one type of interaction). Perhaps because of the definitional pitfalls outlined above, a high proportion of stressor interactions (approx. 45%) are stated but not formally tested, either by examining statistical interactions, by comparison to an appropriate null model or by other statistical means (e.g. survival curves or modelling approaches; electronic supplementary material, figure S1). Notably, synergies are more likely to be claimed without being tested (40.2% of 393 papers) than additive effects (19.5% of 133 papers) and antagonisms (18.5% of 92 papers; see the electronic supplementary material, table S1).
Figure 2.
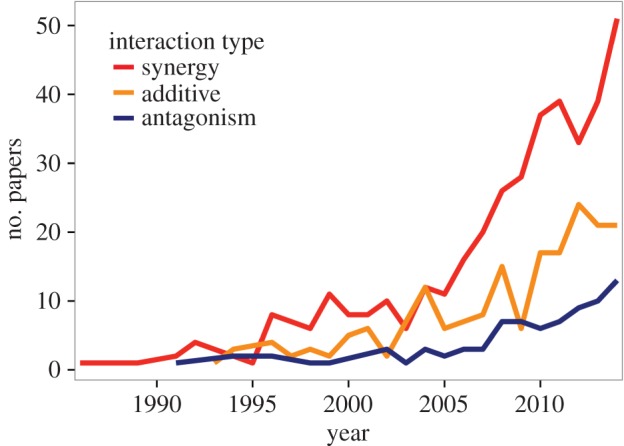
Trends in annual mentions of each of the three conventional types of interactions among multiple stressors in the ecological literature between 1986 and 2014 (for methodological details, see the electronic supplementary material). (Online version in colour.)
This apparent preponderance of ecological synergies in the ecological literature can be compared to their prevalence in quantitative meta-analyses that sought to measure empirically the effects of multiple stressors on a variety of physiological and ecological responses (table 1). Meta-analysts usually control the quality of the contributing studies, for example by selecting only fully factorial experiments. Such reviews might therefore provide a more objective base rate for tested synergies, although differences among meta-analyses in null models (i.e. additive or multiplicative), and hence thresholds for identification of interaction types, will still bias our perspective to some extent. The only meta-analysis to date to compare the effect of using different null models on the same pool of studies found that 4% of experiments were synergistic under an additive model versus 9% under a multiplicative model [29]. Notwithstanding these problems, the proportion of contributing studies that reported synergies varied from 4 to 68% (table 1) in meta-analyses of multiple stressor effects. In terms of overall cumulative effects, the majority of meta-analyses report additivity of multiple stressor effects across pooled studies; overall synergies are uncommon (table 1).
Table 1.
Prevalence of synergies and overall interaction types in meta-analyses of experimental studies testing stressor interactions. Full references to sources are given in the electronic supplementary material.
| source | focal group/ecosystem | stressors | responses | tests | % tests showing synergy | overall interaction type |
|---|---|---|---|---|---|---|
| Przeslawski et al. [22] | early life stages, marine | various abiotic | variable | 131 | 65 | additive for temperature × salinity; additive for temperature × pH |
| Ban et al. [23] | corals | many | variable | 111 | 54 | additive |
| Harvey et al. [24] | marine | temperature and pH | variable | 623 | — | synergistic for rates of calcification, photosynthesis, reproduction and survival; additive for growth |
| Holmstrup et al. [25] | all | abiotic and chemical | variable | 151 | 68 | not reported |
| Darling & Côté [16] | all | many | mortality | 57 | 35 | additive |
| Crain et al. [15] | marine | many | variable | 171 | 36 | synergistic; synergistic for population-level responses, antagonistic for community-level responses |
| Gruner et al. [26] | all | nutrients and herbivory | producer biomass | 191 | — | additive |
| Jackson [27] | all | multiple invasions | abundance, survival, growth, diet, behaviour of native species | 45 | 4 | antagonistic; antagonistic for all responses |
| Strain et al. [28] | canopy- or mat-forming marine algae | many | growth, survival | 118 | 38 | not reported |
| Stephens et al. [29] | terrestrial plants | multiple enemies | performance metrics | 74 | 9 | additive |
| Vadeboncoeur [30] | N American forests | multiple nutrients | above-ground 98 tree production | — | dominance effect | |
| Wu et al. [31] | terrestrial | temperature and precipitation | plant growth, carbon balance | 338 | — | antagonistic for biomass, net production and respiration; additive for net ecosystem exchange and photosynthesis |
| Dieleman et al. [32] | terrestrial | CO2 and temperature | plant biomass, soil processes | 73 | 34 | antagonistic for biomass; possibly additive for soil respiration |
| Wahl et al. [33] | Fucus seaweeds | various biotic and abiotic | various performance traits | 41 | 32 | additive |
| Burkepile & Hay [34] | marine primary producers | nutrients and herbivory | abundance | 54 | — | synergistic |
Synergies thus appear to be over-emphasized in the ecological literature. Why? It may be that researchers are confused about what synergies are, and they inadvertently label as synergies interactions that are simply additive or even antagonistic, depending on the definitions used (figure 1). However, synergies do offer a more compelling narrative than other interaction types. Antagonisms in particular are counterintuitive: how can two stressors that each cause negative impacts have a less detrimental effect together than on their own? Concluding that a synergy exists, even when multiple stressors have a simple dominance or additive effect, bolsters the notion that natural ecosystems are on the verge of irreparable deterioration and collapse. This is a perception that makes popular news headlines [35] and also one that results in a higher rate of citations of papers referring to synergies than to other interaction types, especially for review articles (electronic supplementary material, figure S2).
In short, synergies do occur and are a popular and powerful narrative, but the evidence to date suggests that they are not rampant. Their exact prevalence would be clearer with a more transparent use of appropriate null models and consideration of the direction of individual effects. This is not just an academic concern about terminology, but also has important implications for conservation and ecosystem management.
4. Are there conservation implications to getting it wrong?
Identifying stressor interactions is important for prioritizing conservation actions because it can inform which stressor to act on, and when and where to intervene [36–38]. Because management capacity is constrained by time and resources, priority actions should be those that achieve the greatest benefits to biodiversity, as measured against the counterfactual, i.e. what would happen if resources were spent on an alternative action [39,40]. Actions that disrupt true synergies between stressors can have the greatest benefit to ecosystems because the counterfactual situation would be immensely worse [36,41]. However, when addressing synergies is very expensive (compared to other interactions), a greater net conservation efficiency (i.e. benefit per unit cost) might be achieved by addressing other interactions instead.
In reality, conservation practitioners must often act without knowing the types of stressor interactions at play, or the long-term costs of addressing them. In such cases, the bias towards reporting synergies may lead many to a false presumption that most interactions are synergistic. This assumption will affect prioritization of conservation actions [36], and can also affect significantly the outcome of management. For example, the conservation of threatened seagrass populations may depend on the nature of the interaction between two important stressors: turbid water caused by land run-off, which can be managed locally, and heat waves, a global stressor that cannot be managed at a local scale [36]. A theoretical analysis found that when turbidity interacted additively with heat waves, improving water clarity resulted in lower seagrass mortality and longer population persistence, compared with a no-management baseline [36]. These benefits of water quality management were magnified when the interaction was synergistic, but were minimal when the climate stressor was dominant. Importantly, local management became detrimental if the interaction was antagonistic, increasing seagrass mortality rate and hastening the timing of population collapse [36]. Mitigative antagonisms have proved difficult to document, but in this case it can occur because turbid waters reduce light stress on photosynthesis centres already stressed by increased temperature [42]. Thus, for some primary producers, improving water clarity, and light transfer, can increase mortality induced by heat waves. In conservation science, the management of local stressors is commonly advocated, partly for pragmatic reasons since global stressors are nearly impossible to control [38,43] but also because of the implicit assumption of additive or, more often, synergistic interactions. As the example for seagrass shows [36], and as others have argued [44], managing an antagonistic stressor interaction as if it were synergistic might actually worsen an ailing ecosystem and result in wasted resources.
There are currently too few exercises that attempt to understand the conservation consequences of getting the stressor interaction wrong. As a result, we do not know whether the conclusions of the seagrass study [36] are specific to the ecosystem, stressors and response metric they used, and whether inadvertent mismanagement might be more or less detrimental under different circumstances. This is an urgent research need that, when fulfilled, will clarify how essential it is to elucidate interaction types before they are managed.
5. Can we predict stressor interaction types?
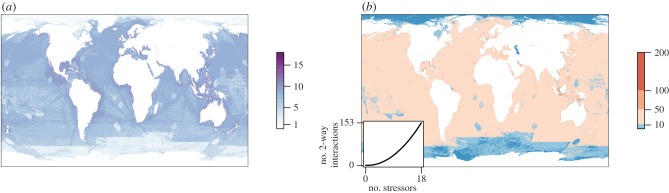
Whether it proves essential or not, identifying interactions occurring among stressors before managing them will be challenging at any scale. There are simply too many stressors acting on natural ecosystems to be able to measure all potential interactions between every combination of stressors. As an example, the number of stressors currently affecting the global ocean [3] translates into an accelerating number of potential two-way interactions (figure 3), let alone higher order interactions. Much of the world's oceans has more than 10 two-way interactions (which would require at least 16 experimental treatments to quantify) and coastal areas typically have upwards of 100 two-way interactions! This sheer number in fact underestimates the complexity of the situation because for a given set of stressors, interaction type will differ across organisms (e.g. [45]) and response metrics (e.g. physiological, population level, ecosystem level; [46]), and might even change over time or with varying severity of the stressors. In some places, there may be so many possible interactions that the system will be strongly altered by the time researchers document them and identify appropriate management actions. Is there a solution to this problem?
Figure 3.
The number of potential interactions increases nonlinearly with the number of stressors. (a) The number of different ocean stressors globally (data from [3]); (b) the number of potential two-way interactions based on the number of stressors in each grid cell. Inset shows how the number of two-way interactions accelerates with the number of stressors. See the electronic supplementary material for methods. (Online version in colour.)
Instead of identifying the outcome of every possible stressor combination—an impossible task—identifying generalities about ecosystems, stressors and/or responses that can reliably predict interaction types is a more realistic goal and could provide real guidance to conservation scientists and managers. This would help inform managers and decision-makers about the likelihood of any given interaction type for their specific system and the stressors that generate this interaction, and whether the interaction can be addressed through management.
Meta-analyses offer a potential avenue to achieve this goal. Many meta-analyses have examined the effect of various moderators in an effort to explain heterogeneity in the combined effect of multiple stressors. However, the list of significant covariates is long. Depending on the meta-analysis, interaction type has been found to covary with stressor combination, biome or ecosystem, latitude, taxon, trophic group, life-history traits, ontogenetic stage, experimental method and response metric. Generalities are not obvious. Life stage is sometimes important [22], sometimes not [16,47]. Laboratory, field and mesocosm studies sometimes reliably lead to certain interaction types [15], but not always [27,29]. The prevalence of different interactions is often taxonomically biased (e.g. [16,26,29,34,47]), but taxonomic correlates are not useful predictors unless we can understand the general life-history, behavioural or ecological traits underpinning these associations.
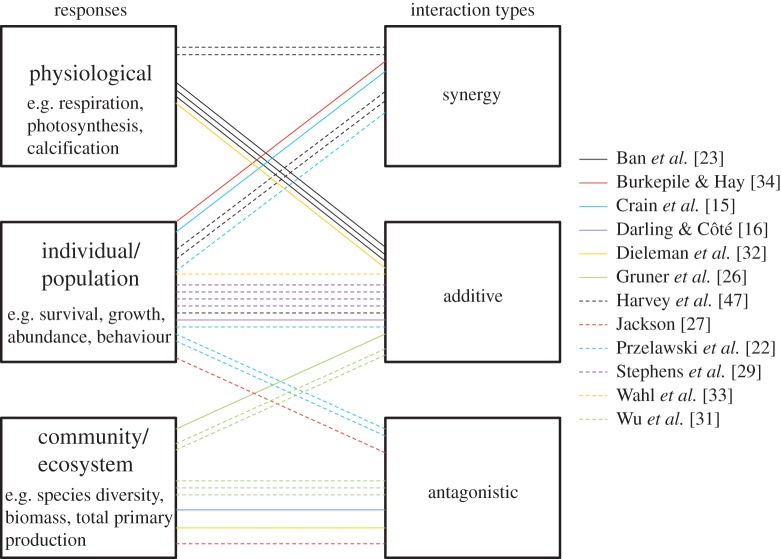
There is perhaps more hope in identifying linkages between general categories of responses and specific interaction types. For example, community-level metrics might be expected to be antagonistic more often than population-level responses if interactions among species act to dampen or diffuse impacts on individual species (e.g. [15]). Unfortunately, a qualitative meta-analysis of the meta-analyses in table 1 that report interaction type for individual response metrics suggests that broad classes of responses, corresponding roughly to levels of ecological organization, can be associated with more than one type of interaction (figure 4). However, it is interesting that physiological response variables have so far not yielded evidence of antagonisms, while community- or ecosystem-level responses are not consistent with synergistic interactions, at least in the meta-analyses considered in figure 4. This might be good news for managers interested in protecting assemblages rather than individual species from the effects of multiple stressors. A robust pattern of associations might emerge with more data.
Figure 4.
Links between various categories of responses to multiple stressors and the types of interactions occurring between these stressors. Coloured lines represent response-specific interaction types reported in published meta-analyses of the ecological effects of multiple stressors on a variety of ecosystems (table 1). Multiple lines of the same colour represent multiple responses reported in a given study. Note that responses were sometimes reported as individual metrics (e.g. survival, growth) or as aggregated responses (e.g. survival and growth combined into a single population-level response).)
Even if meta-analyses have not so far revealed universal covariates, they still play an important role in setting expectations for interaction types. Given the preponderance of studies that assume synergistic interactions (figure 1), meta-analyses offer an important baseline—in general, we should not expect more than one-third of stressor interactions to be synergistic (table 1; [15,16]). Without detailed case-specific information, conservation managers should therefore work under the assumption that synergisms, antagonisms and additive effects are equally likely.
In the end, the ability to predict the nature of interactions between stressors might boil down to understanding the underlying processes and mechanisms. The conceptual framework of co-tolerance [19] is a useful starting point. When tolerance to one stressor confers tolerance to another stressor, for example because both stressors act on the same physiological or ecological processes, then antagonisms might be expected when the stressors act in concert [19]. Vulnerable individuals or species are thus removed from a community, and replaced by more resistant individuals or species, which diminishes the combined impact of the stressors (e.g. [48]). Conversely, when different stressors act on different mechanisms, individuals or species are equipped to resist one or a few but not all stressors, which can increase the susceptibility of the community to multiple stressors (e.g. [49]). Here, the impacts of different stressors become mismatched rather than overlapping, which can lead to additive or synergistic cumulative effects. This simple and intuitive framework recasts the problem as one of understanding what underpins the effects of single stressors rather than their interaction—a slightly more tractable endeavour. Of course, ecological complexity is never far. In a comparison of co-tolerance patterns on coral reefs, reef fish displayed a negative co-tolerance relationship, compared to the positive co-tolerance relationship of reef corals [50], complicating the conservation decisions for different parts of the same ecosystem.
6. Conclusion: how to move from theory to real-world application
Multiple stressors and ecological interactions have largely remained within the realm of academic discussions because jargon and uncertainty have limited applications to the real world, where interactions continue to multiply and impact ecological systems. We have cast a critical eye on the uncertainties that remain in defining and predicting ecological interactions, despite four decades of research. We believe that the urgency of the problem requires a drastic change in how the science of multiple stressors is carried out. We now offer three priorities to move this enterprise from theory to real-world application for conservation and management.
(a). Designing realistic experiments for conservation
A critical difference exists between current research on multiple stressors and conservation practice. The first typically focuses on adding stressors to a system, while the latter is often about removing stressors. Yet, the trajectory of population or community change following stress alleviation is unlikely to be a mirror image of what happens with the intensification of stress. We therefore need to conduct experiments to understand the effects of stressor removal, and design them to reflect realistically the constraints of management actions. For instance, it may often be more feasible for managers to reduce each of the key stressors acting on a system by a small amount than to totally eliminate a single stressor. Furthermore, a large proportion of experiments consider only interactions between drivers of global change, such as acidification and temperature [51]. Because managers cannot act directly to alleviate global stressors, experiments that include interactions with manageable local stressors (e.g. [43]) will be more directly useful for management.
Experimental designs can also be more relevant to management if they use realistic baseline conditions, stressor levels and rates of change in stressor intensity. For instance, it would be more realistic to use impacted sites (or current stressor levels), rather than un-impacted state as the control conditions, and then remove stressors [41,43], effectively simulating the processes of management or restoration in the experimental design. In fact, restoration experiments that document ecological recovery following the removal of causes of degradation [52] are directly relevant to experimental manipulations of multiple stressors to inform management. Stressor removal experiments should also simulate realistic rates of stressor change. For example, nutrient change will not occur instantaneously but gradually over longer timescales. In addition, local observations can help set levels of stressors that are locally relevant (e.g. the local water quality at a site or local temperature profiles).
Finally, the unwieldy number of potential interactions conservation managers must consider (figure 3) can be simplified by using experimental treatments that reflect natural combinations of stressors, rather than designs that separate the effects of individual stressors. This suggestion will be anathema to experimental purists who insist on fully factorial designs, but in real life some stressors are inextricably linked. For instance, global change stressors are correlated in space, so that areas of high temperature change will often experience large changes in salinity [53]. Aggregating stressors reduces the number of factorial combinations required to quantify the benefits of managing stressors involved in multiple interactions. A down-side is that aggregated experiments cannot isolate the effects of individual stressors, so they may less successfully elucidate the mechanisms of organism responses to stress. However, trading-off mechanistic detail in experiments with practicality may provide a quicker means of evaluating how ecosystems stressed by numerous pressures respond to management. For instance, aggregated experimental designs could reveal dominant drivers that, if acted on, would have the largest benefit for the ecosystem [54].
(b). Adaptive monitoring: integrating long-term monitoring and adaptive management
Long-term monitoring of managed ecosystems is essential to allow managers to assess whether their intervention is achieving the desired outcome, but it can also provide an early warning signal of ecological surprises [55]. For example, if management is enacted but is not achieving the desired change, there may be a dominant or antagonistic interaction mitigating the effect of management. Similarly, the sudden success of a management activity may signal the interruption of an additive or synergistic interaction. Both are scenarios of adaptive monitoring that is driven by tractable questions (e.g. what type of stressor interaction is a manager dealing with?) and that can be used to target continued research and management [55].
Long-term monitoring can also act as fortuitous natural experiments to test interaction types when unexpected events and real-world variability occur. For example, Darling et al. [48,56] took advantage of a mass coral bleaching event that occurred 8 years into a long-term monitoring programme to identify an antagonism between fishing and thermal stress on reef corals, caused by the shifting composition of sensitive and tolerant coral species. Monitoring and evaluation are also desirable given an expectation of dynamic interactions, whereby interactive effects become stronger over time [57]. Ultimately, what matters for conservation is whether the management intervention is likely to change depending on the type of stressor interaction that is occurring.
(c). The most pressing need
Despite the fact that we can still not predict the ecological effects of interactions among stressors, ecosystems must still be managed to minimize losses within real-world political, social, economic and practical constraints. Perhaps the most important priority for conservation planning is to identify management actions that are robust to uncertainty in interaction types and that can build resilience (either resistance or recovery) to intensifying stressor regimes. For example, reducing nutrient impacts on seagrass in regions that are warming the fastest might yield the greatest benefits if eutrophication and warming interact synergistically. By contrast, if eutrophication and warming interact antagonistically, nutrient reductions in cool climate refuges will be more beneficial. If the interaction is unknown, then acting on nutrients in climate refuges may be the safest bet, because this will bring some benefits to seagrass regardless of the interaction [36].
Interactions among stressors will continue to generate surprises. When faced with this uncertainty, the bottom line remains the precautionary principle. Actions to maintain key ecological processes and that are as robust as possible to different interactions should continue to bolster biological resilience as science catches up—and scales up—to the global challenges of conservation and management in a rapidly changing world. We are optimistic that a better understanding of stressor interactions, keeping a firm eye on the needs of conservation and management, can provide key guidance into the future.
Supplementary Material
Data accessibility
Scripts and data to perform the analyses can be found at: https://github.com/esdarling/ProcB_Synergies/.
Authors' contributions
I.M.C., E.S.D. and C.J.B. wrote the manuscript. E.S.D. and C.J.B. prepared the figures.
Competing interests
We have no competing interests.
Funding
I.M.C. was supported by a Discovery grant of the Natural Sciences and Engineering Council of Canada (NSERC) and by the NSERC Canadian Healthy Oceans Network. E.S.D. was funded by a David H. Smith Conservation Research Fellowship from the Cedartree Foundation, and by the John D. and Catherine T. MacArthur Foundation. C.J.B. was funded by a Research Fellowship from Griffith University and an SFU/Griffith University Collaborative Travel Grant.
References
- 1.Millennium Ecosystem Assessment. 2005. Ecosystems and human well-being: synthesis. Washington, DC: Island Press. [Google Scholar]
- 2.Geldmann J, Joppa LN, Burgess ND. 2014. Mapping change in human pressure globally on land and within protected areas. Conserv. Biol. 28, 1604–1616. ( 10.1111/cobi.12332) [DOI] [PubMed] [Google Scholar]
- 3.Halpern BS, et al. 2015. Spatial and temporal changes in cumulative human impacts on the world's ocean. Nat. Comm. 6, 7615 ( 10.1038/ncomms8615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butchart SHM, et al. 2010. Global biodiversity: indicators of recent declines. Science, 328, 1164–1168. ( 10.1126/science.1187512) [DOI] [PubMed] [Google Scholar]
- 5.Barnosky AD, et al. 2011. Has the Earth's sixth mass extinction already arrived? Nature 471, 51–57. ( 10.1038/nature09678) [DOI] [PubMed] [Google Scholar]
- 6.Steffen W, et al. 2011. The Anthropocene: from global change to planetary stewardship. Ambio 40, 739–761. ( 10.1007/s13280-011-0185-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mace GM, et al. 2014. Approaches to defining a planetary boundary for biodiversity. Glob. Environ. Change 28, 289–297. ( 10.1016/j.gloenvcha.2014.07.009) [DOI] [Google Scholar]
- 8.Brook BW, Sodhi NS, Bradshaw CJA. 2008. Synergies among extinction drivers under global change. Trends Ecol. Evol. 23, 453–460. ( 10.1016/j.tree.2008.03.011) [DOI] [PubMed] [Google Scholar]
- 9.Paine R, Tegner M, Johnson E. 1998. Compounded perturbations yield ecological surprises. Ecosystems 1, 535–545. ( 10.1007/s100219900049) [DOI] [Google Scholar]
- 10.Folke C, et al. 2004. Regime shifts, resilience and biodiversity in ecosystem management. Annu. Rev. Ecol. Evol. Syst. 35, 557–581. ( 10.1146/annurev.ecolsys.35.021103.105711) [DOI] [Google Scholar]
- 11.Laurance WF, Useche DC. 2009. Environmental synergisms and extinctions of tropical species. Conserv. Biol. 23, 1427–1437. ( 10.1111/j.1523-1739.2009.01336.x) [DOI] [PubMed] [Google Scholar]
- 12.Boyd PW, Hutchins DA. 2012. Understanding the responses of ocean biota to a complex matrix of cumulative anthropogenic change. Mar. Ecol. Prog. Ser. 470, 125–135. ( 10.3354/meps10121) [DOI] [Google Scholar]
- 13.Folt CL, Chen CY, Moore MV, Burnaford J. 1999. Synergism and antagonism among multiple stressors. Limnol. Oceanogr. 44, 864–877. ( 10.4319/lo.1999.44.3_part_2.0864) [DOI] [Google Scholar]
- 14.Piggott JJ, Townsend CR, Matthaei CD. 2015. Reconceptualizing synergism and antagonism among multiple stressors. Ecol. Evol. 5, 1538–1547. ( 10.1002/ece3.1465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crain CM, Kroeker K, Halpern BS. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304–1315. ( 10.1111/j.1461-0248.2008.01253.x) [DOI] [PubMed] [Google Scholar]
- 16.Darling ES, Côté IM. 2008. Quantifying the evidence for ecological synergies. Ecol. Lett. 11, 1278–1286. ( 10.1111/j.1461-0248.2008.01243.x) [DOI] [PubMed] [Google Scholar]
- 17.Soluk DA, Collins NC. 1988. Synergistic interactions between fish and stoneflies: facilitation and interference among stream predators. Oikos 52, 94–100. ( 10.2307/3565987) [DOI] [Google Scholar]
- 18.Sih A, Englund G, Wooster D. 1998. Emergent impacts of multiple predators on prey. Trends Ecol. Evol. 13, 350–355. ( 10.1016/S0169-5347(98)01437-2) [DOI] [PubMed] [Google Scholar]
- 19.Vinebrooke RD, et al. 2004. Impacts of multiple stressors on biodiversity and ecosystem functioning: the role of species co-tolerance. Oikos 104, 451–457. ( 10.1111/j.0030-1299.2004.13255.x) [DOI] [Google Scholar]
- 20.Fagan WF, Holmes EE. 2006. Quantifying the extinction vortex. Ecol. Lett. 9, 51–60. ( 10.1111/j.1461-0248.2005.00845.x) [DOI] [PubMed] [Google Scholar]
- 21.Didham RK, Tylianakis JM, Gemmell NJ, Rand TA, Ewers RM. 2007. Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol. Evol. 22, 489–496. ( 10.1016/j.tree.2007.07.001) [DOI] [PubMed] [Google Scholar]
- 22.Przeslawski R, Byrne M, Mellin C. 2015. A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Glob. Change Biol. 21, 2122–2140. ( 10.1111/gcb.12833) [DOI] [PubMed] [Google Scholar]
- 23.Ban SS, Graham NAJ, Connolly SR. 2015. Evidence for multiple stressor interactions and effects on coral reefs. Glob. Change Biol. 20, 681–697. ( 10.1111/gcb.12453) [DOI] [PubMed] [Google Scholar]
- 24.Harvey BP, Gwynn-Jones D, Moore PJ. 2013. Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol. Evol. 4, 1016–1030. ( 10.1002/ece3.516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmstrup M, et al. 2010. Interactions between effects of environmental chemicals and natural stressors: a review. Sci. Total Env. 408, 3746–3762. ( 10.1016/j.scitotenv.2009.10.067) [DOI] [PubMed] [Google Scholar]
- 26.Gruner DS, et al. 2008. A cross-system synthesis of consumer and nutrient resource control on producer biomass. Ecol. Lett. 11, 740–755. (doi:0.1111/j.1461-0248.2008.01192.x) [DOI] [PubMed] [Google Scholar]
- 27.Jackson MC. 2015. Interactions among multiple invasive animals. Ecology 96, 2035–2041. ( 10.1890/15-0171.1) [DOI] [PubMed] [Google Scholar]
- 28.Strain EMA, Thomson RJ, Micheli F, Mancuso FP, Airoldi L. 2014. Identifying the interacting roles of stressors in driving the global loss of canopy-forming to mat-forming algae in marine ecosystems. Global Change Biol. 20, 3300–3312. ( 10.1111/gcb.12619) [DOI] [PubMed] [Google Scholar]
- 29.Stephens AEA, Srivastava DS, Myers JH. 2013. Strength in numbers? Effects of multiple natural enemy species on plant performance. Proc. R. Soc. B 280, 20122756 ( 10.1098/rspb.2012.2756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vadeboncoeur MA. 2010. Meta-analysis of fertilization experiments indicates multiple limiting nutrients in northeastern deciduous forests. Can. J. Forest Res. 40, 1766–1780. ( 10.1139/X10-127) [DOI] [Google Scholar]
- 31.Wu Z, Dijkstra P, Koch GW, Peñuelas J, Hungate BA. 2011. Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Glob. Change Biol. 17, 927–942. ( 10.1111/j.1365-2486.2010.02302.x) [DOI] [Google Scholar]
- 32.Dieleman WIJ, et al. 2012. Simple additive effects are rare: a quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Glob. Change Biol. 18, 2681–2693. ( 10.1111/j.1365-2486.2012.02745.x) [DOI] [PubMed] [Google Scholar]
- 33.Wahl M, et al. 2011. Stress ecology in Fucus: abiotic, biotic and genetic interactions. Adv. Mar. Biol. 59, 37–106. ( 10.1016/B978-0-12-385536-7.00002-9) [DOI] [PubMed] [Google Scholar]
- 34.Burkepile DE, Hay ME. 2006. Herbivore vs nutrient control of marine primary producers: context-dependent effects. Ecology 87, 3128–3139. ( 10.1890/0012-9658(2006)87%5B3128:HVNCOM%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 35.Duarte CM, et al. 2015. Reconsidering ocean calamities. Bioscience 65, 130–139. ( 10.1093/biosci/biu198) [DOI] [Google Scholar]
- 36.Brown CJ, Saunders MI, Possingham HP, Richardson AJ. 2013. Managing for interactions between local and global stressors of ecosystems. PLoS ONE 8, e65765 ( 10.1371/journal.pone.0065765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown CJ, Saunders MI, Possingham HP, Richardson AJ. 2014. Interactions between local and global stressors of ecosystems determine management effectiveness in cumulative impact mapping. Divers. Distrib. 20, 538–546. ( 10.1111/ddi.12159) [DOI] [Google Scholar]
- 38.Ghedini G, Russell BD, Connell SD. 2013. Managing local coastal stressors to reduce the ecological effects of ocean acidification and warming. Water 5, 1653–1661. ( 10.3390/w5041653) [DOI] [Google Scholar]
- 39.Wilson KA, McBride MF, Bode M, Possingham HP. 2006. Prioritizing global conservation efforts. Nature 440, 337–340. ( 10.1038/nature04366) [DOI] [PubMed] [Google Scholar]
- 40.Withey JC, et al. 2012. Maximising return on conservation investment in the conterminous USA. Ecol. Lett. 15, 1249–1256. ( 10.1111/j.1461-0248.2012.01847.x) [DOI] [PubMed] [Google Scholar]
- 41.Falkenberg LJ, Connell SD, Russell BD. 2013. Disrupting the effects of synergies between stressors: improved water quality dampens the effects of future CO2 on a marine habitat. J. Appl. Ecol. 50, 51–58. ( 10.1111/1365-2664.12019) [DOI] [Google Scholar]
- 42.Anthony KRN, Connolly SR, Hoegh-Guldberg O. 2007. Bleaching, energetics, and coral mortality risk: effects of temperature, light, and sediment regime. Limnol. Oceanogr. 52, 716–726. ( 10.4319/lo.2007.52.2.0716) [DOI] [Google Scholar]
- 43.Strain EMA, van Belzen J, van Dalen J, Bouma TJ, Airoldi L. 2015. Management of local stressors can improve the resilience of marine canopy algae to global stressors. PLoS ONE 10, e0120837 ( 10.1371/journal.pone.0120837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Côté IM, Darling ES. 2010. Rethinking resilience in the face of climate change. PLoS Biol. 8, e1000438 ( 10.1371/journal.pbio.1000438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muthukrishnan R, Fong P. 2014. Multiple anthropogenic stressors exert complex, interactive effects on a coral reef community. Coral Reefs 33, 911–921. ( 10.1007/s00338-014-1199-1) [DOI] [Google Scholar]
- 46.Boyd PW, Brown CJ. 2015. Modes of interactions between environmental drivers and marine biota. Front. Mar. Sci. 2, 9 ( 10.3389/fmars.2015.00009) [DOI] [Google Scholar]
- 47.Harvey BP, Gwynn-Jones D, Moore PJ. 2014. Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol. Evol. 3, 1016–1030. ( 10.1002/ece3.516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darling ES, McClanahan TR, Côté IM. 2013. Life histories predict coral community disassembly under multiple stressors. Glob. Change Biol. 19, 1930–1940. ( 10.1111/gcb.12191) [DOI] [PubMed] [Google Scholar]
- 49.Graham NAJ, et al. 2011. Extinction vulnerability of coral reef fishes. Ecol. Lett. 14, 341–348. ( 10.1111/j.1461-0248.2011.01592.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McClanahan TR, Graham NAJ, Darling ES. 2014. Coral reefs in a crystal ball: predicting the future from the vulnerability of corals and reef fishes to multiple stressors. Curr. Opin. Environ. Sustainability 7, 59–64. ( 10.1016/j.cosust.2013.11.028) [DOI] [Google Scholar]
- 51.Wernberg T, Smale DA, Thomsen MS. 2012. A decade of climate change experiments on marine organisms: procedures, patterns and problems. Glob. Change Biol. 18, 1491–1498. ( 10.1111/j.1365-2486.2012.02656.x) [DOI] [Google Scholar]
- 52.Anderson P. 2008. Ecological restoration and creation: a review. Biol. J. Linn. Soc. 56, S187–S211. ( 10.1111/j.1095-8312.1995.tb01133.x) [DOI] [Google Scholar]
- 53.Boyd PW, Lennartz ST, Glover DM, Doney SC. 2014. Biological ramifications of climate-change-mediated oceanic multi-stressors. Nat. Clim. Change 5, 71–79. ( 10.1038/NCLIMATE2441) [DOI] [Google Scholar]
- 54.Brennan G, Collins S. 2015. Growth responses of a green alga to multiple environmental drivers. Nat. Clim. Change 5, 892–899. ( 10.1038/NCLIMATE2682) [DOI] [Google Scholar]
- 55.Lindenmayer DB, Likens GE. 2009. Adaptive monitoring: a new paradigm for long-term research and monitoring. Trends Ecol. Evol. 24, 482–486. ( 10.1016/j.tree.2009.03.005) [DOI] [PubMed] [Google Scholar]
- 56.Darling ES, McClanahan TR, Côté IM. 2013. Combined effects of two stressors on Kenyan coral reefs are additive or antagonistic, not synergistic. Conserv. Lett. 3, 122–130. ( 10.1111/j.1755-263X.2009.00089.x) [DOI] [Google Scholar]
- 57.Bozec Y-M, Mumby PJ. 2015. Synergistic impacts of global warming on the resilience of coral reefs. Phil. Trans. R. Soc. B 370, 20130267 ( 10.1098/rstb.2013.0267) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Scripts and data to perform the analyses can be found at: https://github.com/esdarling/ProcB_Synergies/.