Abstract
Ecological traps, which occur when animals mistakenly prefer habitats where their fitness is lower than in other available habitats following rapid environmental change, have important conservation and management implications. Empirical research has focused largely on assessing the behavioural effects of traps, by studying a small number of geographically close habitat patches. Traps, however, have also been defined in terms of their population-level effects (i.e. as preferred habitats of sufficiently low quality to cause population declines), and this is the scale most relevant for management. We systematically review the ecological traps literature to (i) describe the geographical and taxonomic distribution of efforts to study traps, (ii) examine how different traps vary in the strength of their effects on preference and fitness, (iii) evaluate the robustness of methods being used to identify traps, and (iv) determine whether the information required to assess the population-level consequences of traps has been considered. We use our results to discuss key knowledge gaps, propose improved methods to study traps, and highlight fruitful avenues for future research.
Keywords: effect size, fitness, habitat selection, human-induced rapid environmental change, maladaptive, preference
1. Introduction
Human activities have dramatically changed environments worldwide [1,2]. These changes are occurring at significantly faster rates (i.e. human-induced rapid environmental change, HIREC [3]) than natural processes, and mean that animals increasingly encounter conditions they have not experienced in their evolutionary history. Initial responses by animals to HIREC are often behavioural, and can help them adjust to new conditions [4]. Worryingly though, the rate at which HIREC occurs can mean that animals fail to adapt and their fitness is compromised when previously adaptive behaviours become maladaptive [5].
Many animals use environmental cues to select habitats that maximize their fitness. HIREC can cause these cues to become uninformative of habitat quality; one consequence is that ecological traps form when animals prefer habitats where their fitness is lower than in other available options [6,7]. Ecological traps are likely to increase local extinction risk [8], so understanding how they can arise, how animals respond to them and how they can be mitigated are important questions for conservation biology [9]. Despite significant efforts to develop the theory underpinning ecological traps and to describe their potential effects (e.g. [5,7,10,11]), there has been limited exploration of strategies for preventing their formation or mitigating their impacts (but see [8,12,13]).
Three criteria must be met to demonstrate an ecological trap [7]: (i) individuals prefer one habitat over another (a ‘severe’ trap) or equally prefer multiple habitats (an ‘equal preference’ trap); (ii) fitness (or a reasonable surrogate) differs between habitats; and (iii) fitness is lower when animals exploit the (equally) preferred habitat. In two comprehensive reviews, Robertson and co-workers [5,7] identified studies that meet these criteria and assessed many of the characteristics of traps. This work has provided a solid framework for assessing maladaptive responses to HIREC and important insights including: (i) a broad array of anthropogenic activities can cause traps; (ii) most studies are of ‘severe’ traps, potentially reflecting a reporting bias against ‘equal preference’ traps; and (iii) changes in both cue sets and resource values cause severe traps in most cases [5].
To build on the findings of Robertson and co-workers [5,7], we present the results of a systematic and quantitative review of the ecological traps literature. A solid foundation of relevant evidence is required to ensure conservation decisions are science-based [14], and an improved library of empirical studies is vital to inform our understanding of the underlying mechanisms behind maladaptive responses to HIREC, and the conditions under which ecological traps might occur [7]. Our goal was to use this review to identify key knowledge gaps and suggest best-practice methods for future research.
Our first aim was to assess where and with which taxa ecological traps have been studied. If research efforts have been restricted geographically or taxonomically, scientific inference can be biased, and there may be uncertainty about the degree to which findings from a particular study system apply more generally. Conservation efforts can be hindered when knowledge gaps from taxonomic or geographical biases exist; for example, if the most threatened ecosystems/species are those that are most poorly understood [15]. Furthermore, the ‘ecological trap’ concept was originally formulated on studies with birds, and while a wide range of taxa are affected [5], is this diversity studied commonly? Animals may differ considerably in terms of the likelihood of maladaptive responses to HIREC [3], and taxonomic biases in research effort may mean we understand only a subset of this variability. Neither the taxonomic nor geographical distribution of ecological traps research has been examined, but doing so will help identify if a re-distribution of future research efforts should be encouraged.
Our second aim was to describe how the strength of the effects of traps on preference and fitness vary. Traps can have a variety of causes [5], and their fitness costs range from 100% mortality [16] through to more subtle effects such as reduced reproductive output [17]. We present the first evaluation of how the magnitude of changes in preference and fitness might vary among different causes of traps and affected taxa. Both the relative attractiveness and fitness costs of traps will influence how they affect animals [5], and we also examined if traps that are most attractive to animals are those with the greatest fitness costs. Examining the relationship between fitness and preference could shed light on the mechanisms causing traps that have the most severe consequences for animals and provide opportunities for better targeting of conservation actions.
Our third aim was to assess if suitable methods are being used to identify traps. Difficulty in adequately demonstrating preference was highlighted as a major impediment to identifying traps nearly a decade ago [7]. We assess if correlative approaches, which can provide ambiguous information about whether observed patterns are due to preference, or experimental methods, which provide the most definitive evidence of preference, are more commonly used to assess traps. We also assess if before–after control-impact (BACI) principles are being considered in ecological trap studies. These designs are widely used in environmental impact assessment monitoring [18], and eliminate the possibility that observed differences between sites simply reflect underlying patterns of spatial variability rather than a disturbance. While it may be impossible to predict the formation of some traps, others, such as those arising as unintended consequences of management activities, may be more predictable [13], making these designs possible (e.g. [19]), and in fact the most unambiguous way to determine if a trap has formed.
(a). The landscape-scale consequences of ecological traps
Traps were originally defined as behavioural phenomena (i.e. as habitats that animals prefer where their fitness is compromised) [6,7]. Reflecting this, most studies evaluate traps at only a small number of geographically close habitat patches. Traps, however, have also been defined as population-level phenomena (i.e. as low-quality but preferred habitats that cannot sustain a population; e.g. [20]) and act as ‘attractive sinks' [21]. Given that effective habitat management depends on considering how local patches fit within the mosaics of habitat present across the landscape [22], examining the effects of traps for metapopulations will be important [10]. This will require behavioural studies to determine if a trap has formed and, if so, further studies to understand the potential consequences (e.g. in terms of population/metapopulation growth rate and persistence) for animals across the landscape [9,10].
Traps that are highly attractive and severely reduce fitness are likely to compromise metapopulations, especially when they represent a large proportion of available habitat [10,23,24], but there has been little consideration of what is required to assess such landscape-level consequences. Recently, we proposed that these landscape consequences are likely to be related to: (i) the probability that dispersing animals encounter traps; (ii) the likelihood that they select them; (iii) how and by how much traps reduce fitness; and (iv) the susceptibility of animals to these fitness costs [10] (table 1, columns a–d). However, each of these criteria encapsulates the effects of a range of factors acting at both local and landscape scales (table 1, columns a–d). Our fourth aim was to determine which of these factors are being considered in current attempts to study ecological traps, in order to identify where data are likely to be deficient.
Table 1.
Summary of the information required to assess the landscape consequences of traps. This table outlines (a and b) the variables that are likely to determine the landscapes consequences of traps (following [10]); (c) the conditions under which animals are most likely to encounter/select traps, when traps are likely to most reduce fitness, and when animals are most susceptible to these effects; (d) the scale (patch or landscape) at which these variables should be considered; (e) the nine variables evaluated in this study, in terms of the degree to which they have been considered in studies of ecological traps (see main text and electronic supplementary material for further information); and (f) the information required to consider the variables outlined in (b). The top row identifies the section that each column is most relevant to.
| introduction |
methods |
results and discussion |
|||
|---|---|---|---|---|---|
| (a) category | (b) variable | (c) worst case scenario/examples | (d) scale (P: patch, L: landscape) | (e) variables evaluated | (f) required information |
| probability of encounter | proportion of traps in the landscape | large % of habitats are traps [8,10,20,21] | P: is a habitat a trap? L: % of traps in the landscape |
T. pro: proportion of habitats that are traps H. avail: habitat availability—distribution of trap/non-trap habitat in study region |
—GIS analysis—proportion of traps, distribution of habitats |
| landscape topology | likely when traps are large and central | P: Size of traps L: landscape topology |
Size: size of trap and non-trap patches | —importance of different patches for connectivity [25] | |
| dispersal capacity | vagility increases trap encounters [10] | L | n.a. | —parametrized dispersal kernel | |
| perceptual range | larger perceptual range attracts animals to traps from further away | L | n.a. | —characterize perceptual range (e.g. [26]) | |
| likelihood of selection | attractiveness of traps | traps highly attractive [10,11,27] | P | T. att: strength of preference for traps | —tests of habitat preference |
| habitat selection | animals use simple/single cues [8] | P | H. sel: do studies consider habitat selection behaviour? | —knowledge of habitat selection behaviour, e.g. which/how many cues used? | |
| influence of natal experience on habitat selection | natal experience increases probability of selecting poor quality habitats [28,29] | P | Np: does natal experience influence habitat selection? | —test if natal experience influences habitat selection | |
| disperser physiology | animals become less choosy and more likely to select traps as dispersal progresses | P | Phys: potential influence of disperser condition on preference | —assess if preferences change in relation to condition or dispersal time | |
| fitness costs of selection | fitness costs of traps | traps greatly reduce fitness, especially mortality [16] | P | T. fit: fitness comparison of traps/non-traps | —fitness in traps versus non-traps |
| susceptibility to costs of selection | intrinsic fitness/life-history traits | animals have ‘slow’ life histories, no capacity for learning/adaptation [8,24] | P | Traits: are traits/intrinsic fitness considered? | —knowledge of life history |
2. Material and methods
(a). Basic search protocols
We searched the Web of Science database using the same search terms (‘ecological trap*’ OR ‘evolutionary trap*’ OR ‘maladaptive’ OR ‘behavioural/behavioral mismatch’) and methods (electronic supplementary material) as per the two reviews by Robertson and co-workers [5,7]. We identified 29 studies that met the criteria for demonstrating a trap (hereafter ‘demonstrated trap’ studies; see the electronic supplementary material, table S2 for full details of these studies). We identified an additional 98 studies that tested for but did not find a trap, or speculated that a trap had been found but failed to meet the required criteria. We classified these as studies where a trap had not been demonstrated (hereafter ‘undemonstrated trap’ studies).
(b). Extraction of information
We extracted basic information about each study (e.g. location, methods used to assess preference, if the study spanned the period before and after traps), supplementing information in [5,7]. The process causing the trap was classified into the categories used in [5]: agriculture and forestry, exotic species, human structures (e.g. buildings), hunting and fishing, and restoration. We used the log response ratio [30] as an effect size to quantify the attractiveness and fitness costs of traps, calculated as ln(response variable in traps/response variable for non-traps) where the response variable reflects the measure of preference or fitness being used. Effect sizes were calculated based on the measures used by authors to identify traps, and from the one with the largest effect size if multiple measures were used. Studies that did not provide data that could be used to calculate effect sizes were excluded when these values were required. Nine studies did not provide data but either explicitly stated or implied that mortality was 100% in traps (eight examining egg laying by insects on artificial structures, plus [16]). Because mortality in non-trap habitat is unknown for these studies, we calculated three potential effect sizes assuming a 5-, 10- or 20-fold reduction in survival, which are likely to be conservative estimates based on effect sizes observed in other studies. All quantitative analyses were performed with each of these effect sizes in turn and excluding these studies to ensure they did not bias our findings.
It is unreasonable to assess studies undertaken at the patch scale against all of the patch- and landscape-scale criteria outlined in table 1, given that studies were not implemented to assess effects at the latter scale. We therefore selected nine parameters (table 1, column e) that patch-scale studies could reasonably be expected to consider, and determined if any of them were considered in the 127 studies. This assessment was done across both ‘demonstrated’ and ‘undemonstrated’ categories so we could compare if studies meeting the criteria to identify traps generally collected more of the required information that those that did not. The required levels of evidence for our qualitative scores are outlined in the electronic supplementary material, table S1.
3. Results and discussion
(a). Where and with which taxa have traps been studied?
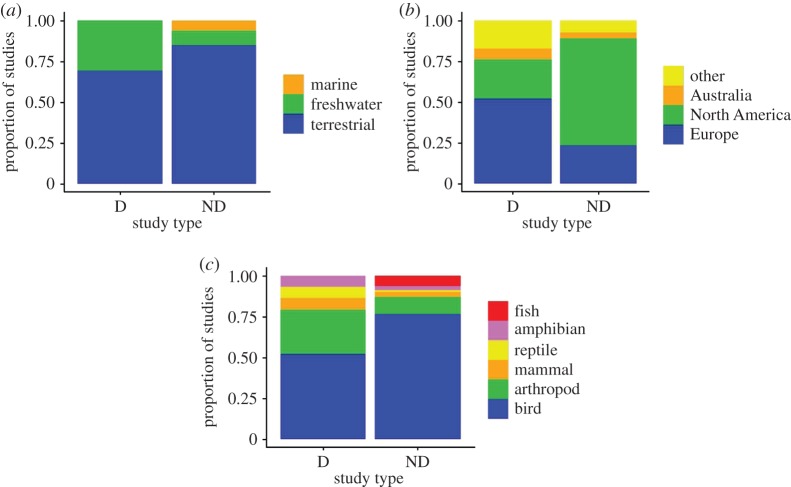
Traps affect a variety of taxa [5] and studies are published in a range of journals (electronic supplementary material, table S2), but traps have mainly been identified for birds (reflecting the initial development of the concept), especially in Europe and North America (figure 1). The geographical and ecosystem distributions we observed are consistent with other similar assessments [31,32]. Other than the compelling evidence of the attraction of insects to artificial structures, only three studies have demonstrated a trap in freshwater ecosystems, and none in marine environments. However, rates and extent of habitat transformation have been considerable in aquatic environments (e.g. [2,33]), making it likely that traps remain undetected due to, at least in part, less research being undertaken in these ecosystems (e.g. [31]).
Figure 1.
The (a) ecosystems (b) continents and (c) focal taxa where ecological traps have been demonstrated (D) and studied but not demonstrated (ND).
(b). Do the effects of traps on preference and fitness vary?
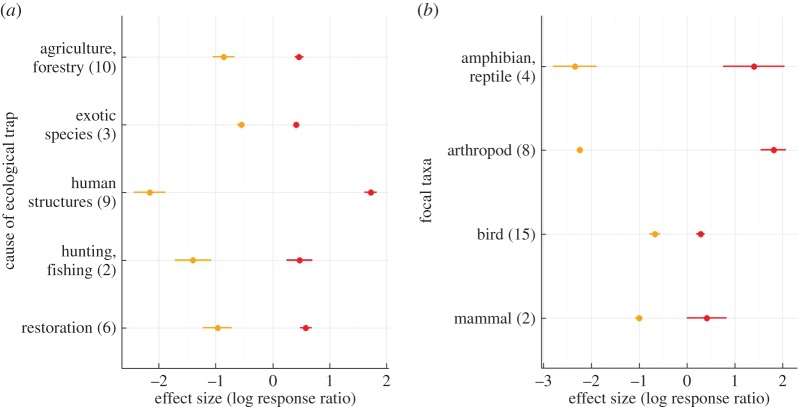
The likelihood that animals respond maladaptively to environmental changes [3], and the magnitude of the effects of traps on fitness and preference, clearly vary depending on the cause of the trap and which taxa are affected (figure 2; electronic supplementary material, figure S1). Discussing the mechanisms underlying this variability is difficult given the small number of available studies, and the fact that particular causes of traps have been studied with only a subset of taxa (e.g. arthropods and human structures, birds and agriculture, forestry and restoration). However, maladaptive behaviours are most likely when the environment changes dramatically and animals are exposed to conditions that are fundamentally different from those that shaped their traits [3]. Whether animals prefer ecological traps is likely related to their sensory ability and behaviour. For example, animals with larger brains might be more flexible or innovative, facilitating more effective adjustments to HIREC (e.g. [34]). Furthermore, animals capable of more complex behaviours (e.g. use of multiple habitat selection cues, multiple sensory modalities) may be less susceptible to making mistakes when selecting habitats [10]. Traps that greatly reduce fitness, especially those that cause death, are likely to have worse consequences for animals, especially when they have life-history traits that increase their susceptibility to these fitness costs [8]. One of the most common, and compelling, cases of ecological traps is insects tricked into laying eggs on artificial structures on the basis of polarized light [7]. This form of HIREC results in insects experiencing habitats that are very different to those under which their evolutionary traits have developed (i.e. buildings versus wetlands/streams), and have amplified cues (polarized light) that make them supernormally attractive. In comparison, the apparent decreased susceptibility of birds and mammals to traps might be due to their greater cognitive ability, enabling more complex habitat selection behaviours.
Figure 2.
Effect sizes (log response ratio) of differences in fitness (orange) and preference (red) between ecological traps and non-trap habitats (a) caused by different processes and (b) affecting different taxa. Mean (and standard error) effect sizes shown. The number in brackets on the y-axis indicates sample size.
(i). The benefits of widening the focus of ecological traps research
Ecological traps have been studied in an ad hoc, species- and system-specific manner, reflecting the relative infancy of this subfield. We suggest that a wider taxonomic, geographical and ecosystem focus could have three important benefits.
First, studying a wider range of ecosystems and taxonomic groups may provide new insights to complement the knowledge collected largely with European and North American birds. For example, birds commonly experience traps caused by predation (e.g. [35]), but aquatic taxa may be susceptible to traps that arise in other ways (e.g. pollution [36]). A wider focus could allow the degree to which findings from particular studies might transfer to other sites and systems to be assessed, which is a key component of a general, predictive understanding of ecology [37]. In addition, understanding the potential intricacies of traps in different systems could inform the development of management options tailored to address the underlying cause—for example, should management efforts be focused on reducing habitat attractiveness (e.g. by removing tree species that birds prefer [12]), improving habitat quality (e.g. treating polluted sediment in urban stormwater wetlands [13]) or excluding animals from potential traps (e.g. using fencing)?
Second, a wider geographical focus may mean that more research is conducted in areas where landscape changes are most rapid, and traps most likely to form [8]. While conservation research in human modified landscapes is likely to be most important in areas where human pressure is greatest, these areas are also the least studied [32]. There has been much interest in understanding the biodiversity impacts of land-use change in the tropics (e.g. [38]), but little consideration of the potential for ecological traps in tropical ecosystems. Many biodiversity studies use species richness as an indicator of responses to land use (e.g. [38]), but this will not allow traps to be detected until they cause decreases in the number of species at a site. By working in systems that are human-dominated (e.g. in urban areas) or in geographical areas where habitat transformation is greatest, we may find that traps are more common, or their effects significantly stronger, than previously documented.
Third, more replication of trap studies with different causes and affected taxa will improve current understanding of variability in maladaptive responses to HIREC, and provide information that could help prioritize when managing traps is important. Interspecific differences in life-history traits and behaviours will affect the likelihood and fitness consequences of selecting traps (e.g. [8,10]). By studying animals with differing traits/behaviours and examining how they respond to traps that vary from those that cause only minor changes to the environment through to completely novel conditions will allow ‘high-risk’ activities and highly susceptible taxa to be identified in the future. An improved library of empirical studies could also help inform attempts to examine the likely impacts of traps, for example, through simulation models that are parametrized with demographic data informed from field studies. Ultimately, such studies will hopefully enable the effects of traps on animals to be compared with other conservation threats, and to identify when the management of traps should be prioritized.
(ii). Preferences for traps and their fitness consequences are strongly related
Traps that are more attractive also have the most deleterious fitness costs (figure 3). This suggests that severe traps pose the most serious demographic threats to animals, and that removing these is likely to be more important than removing equal preference traps. However, removing traps depends on understanding the mechanism(s) underlying the relationship between fitness and preference. The simplest explanation is that one HIREC activity simultaneously alters the quality and attractiveness of habitats (e.g. electronic supplementary material, figure S3a); for example, dragonflies die after being attracted to and landing on oil spills in lakes [27]. However, the underlying mechanisms could be more complicated. One HIREC activity could create two agents of change that independently act on fitness and habitat selection (e.g. electronic supplementary material, figure S3b). For example, fishing could cause an ecological trap for spiny lobsters (Panulirus argus) attracted to small artificial structures known as casitas, and killed once captured [39]. In this instance, the lobsters are attracted to the casita and another agent of change (fishing) reduces fitness. Animals could also encounter multiple HIREC activities that affect preference and fitness via independent pathways (e.g. electronic supplementary material, figure S3c). This will be most likely when covariance in HIREC activities occurs, with particular locations likely to be stressed in multiple ways. For example, catchment disturbance, water resource development and pollution are spatially well correlated in rivers [2]. Animals that encounter multiple forms of HIREC or agents of change are likely to face complex challenges [40,41]. Given these potential complexities, a more mechanistic approach is required to understand why more severe traps are worse for animals, in terms of identifying the cues used to select habitats, and examining how different HIREC activities/agents of change affect preference and fitness in isolation and in combination.
Figure 3.
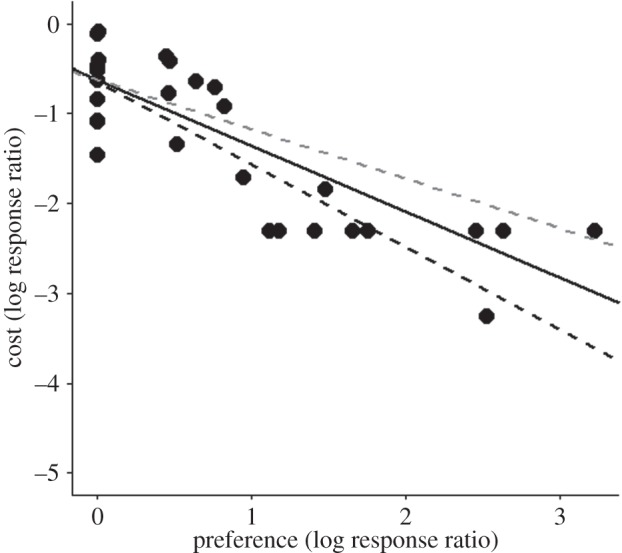
The relationship between the fitness costs of ecological traps and the preferences animals exhibit for them (both log response ratios). Zero values on the x-axis represent equal preference traps. Three potential effect sizes are shown for nine studies where no data on fitness reductions were present: a 10 times reduction in fitness in traps (solid black line), a 5 times reduction (dashed grey line) and a 20 times reduction (dashed black line)—see the methods section for further details. Linear regression models for each of these effect sizes and when these studies were removed all p < 0.01, R2 > 0.50.
(c). Are appropriate methods being used to study ecological traps?
(i). Preferences for traps should not be inferred from correlative data alone
Identifying preference as opposed to habitat association requires specific tests to demonstrate that the observed distributional patterns are due to behaviour [42], rather than other explanations (e.g. differential post-settlement motality [43]). Ambiguities with assessing preferences were identified as a major impediment to ecological trap studies nearly a decade ago [7,8], yet more than 70% of studies that demonstrated a trap used observational methods alone to infer preferences. This could result in uncertainty in accurately identifying traps, raising the possibility that some remain unidentified or alternatively that some non-trap habitats are mistakenly classified as traps.
Confidence in detecting ecological traps will be greatly enhanced if experiments are used to demonstrate preference whenever possible. There will undoubtedly be cases when this is impossible (e.g. when working with very large animals). In such instances, a number of methods exist to collect indirect evidence of habitat preference, which differ in terms of their likely suitability for different species/systems (e.g. [7]). Alternative explanations for observed patterns other than preference will need to be considered, however, when using these methods. For example, arrival time could be a useful proxy for migratory species that select breeding habitats each year, but could be a poor indicator of preference if alternative habitat selection strategies exist within populations (e.g. among age classes), or if variability in habitat use between years reflects changing physiological needs [7]. When preference cannot be demonstrated experimentally, several observational measures should be used to provide multiple lines of evidence indicating animals probably exhibit preference [7].
(ii). Traps are rarely studied both before and after they form
Monitoring before and after an environmental disturbance is a critical element of BACI designs. We found only one study that spanned the periods both before and after the formation of a trap. Rotem et al. [16] illustrate how knowledge of temporal changes in the relationship between preference and fitness can yield important insights, by demonstrating a temporal shift from ideal density-dependent habitat selection to an ecological trap in reptiles.
The vast majority of studies have been implemented with the ‘control-impact’ approach, which is not recommended for environmental monitoring since it is impossible to distinguish a treatment (i.e. disturbance) effect from inherent differences between sites [18]. Large numbers of control and impact sites can improve inferential strength using this approach, but the level of replication required is typically lacking in ecological trap studies. While some traps might arise unexpectedly, precluding any ‘before’ studies, the majority of traps are caused by human activities, which involve significant planning and lead-in times (e.g. restoration, building of structures—electronic supplementary material, table S2). Researchers may be able to work with managers to predict how management activities might alter habitats, and to monitor potential changes in fitness before and after the activity has commenced [13]. If fitness is decreased, studies could be undertaken to assess if poor quality habitats are preferred. Adopting principles of BACI designs, coupled with studies of habitat selection will result in greater confidence that differences between sites are caused by traps. Bro et al. [19] present a useful example of how monitoring fitness of grey partridges can be used to identify potential ecological traps resulting from replanting vegetation.
(d). Assessing the landscape-level consequences of ecological traps
(i). Are we collecting the right information?
All ‘demonstrated’ studies collected information about the attractiveness of traps and their fitness costs (figure 4: T. att and T. fit), which is required to meet the three criteria for documenting a trap [7]. By contrast, studies that did not demonstrate a trap only rarely assessed preference (less than 10% of studies; figure 4). The majority of both types of studies generally considered habitat selection behaviour (H. Sel), life-history traits/fitness (Traits), and the size of trap and non-trap habitats (Size), although slightly more so in ‘demonstrated’ studies.
Figure 4.
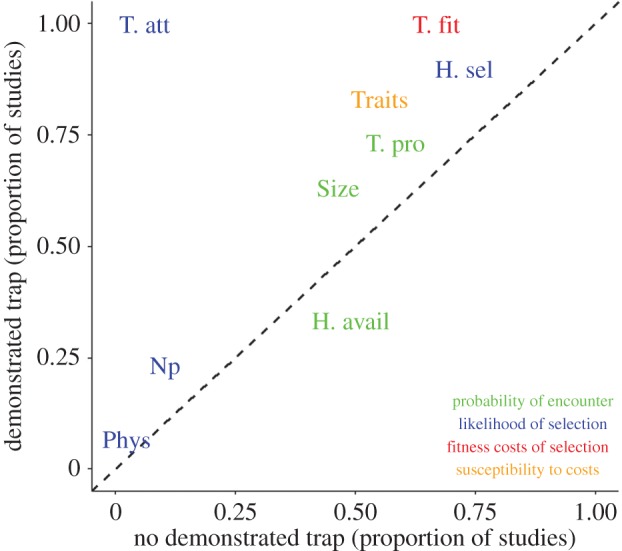
Assessing if studies consider the patch-level characteristics required to assess the landscape-scale consequences of traps. Nine variables were scored (table 1; electronic supplementary material, table S1) in 127 studies across the four categories (indicated by colouring) likely to determine the severity of traps [10]. The proportion of studies that considered a particular variable is denoted for studies that demonstrated a trap on the y-axis (n = 29), and studies that did not demonstrate a trap on the x-axis (n = 98). The dotted line shows the 1 : 1 linear relationship to indicate when the two types of studies provide comparable information. The area above this curve indicates when studies that demonstrated a trap have considered a variable more frequently than studies that did not demonstrate a trap.
Most (approx. 75%) of both types presented information about the proportion of observed traps (T. pro). These results were often not placed into context by considering the wider distribution of habitats within the study region (H. avail). This is an important knowledge gap, as effective management needs to consider how patches fit within the intrinsic variations in habitat quality likely to occur across the landscape [22]. Recent work mapping potential traps for Andean bears demonstrates the advantages of such a landscape approach [44].
How natal experiences or changes in disperser physiology might influence habitat selection and thus the probability of selecting a trap have rarely been considered (figure 4: Np, Phys), probably due to the limited research that has been undertaken in general about the effects of these factors on habitat selection. However, recent empirical work demonstrates that animals that select natal-like habitats can have compromised fitness [28,29], which could exacerbate the effects of traps [10]. Dispersers in poor physiological condition or under time constraints may become less choosy or more likely to choose sub-optimal habitats in general [45], but the degree to which this results in ecological traps has not been explored. More work is needed examining how experiences in early life history might cause maladaptive habitat selection later in life.
(ii). What additional information is required?
Many of the patch-level factors likely to determine the landscape-level consequences of traps are currently being considered at least in some studies. It will be important though to investigate the potential importance of the landscape-level variables not considered here. For example, landscape topology can affect the persistence of metapopulations (e.g. [46]), but the degree to which this is the case when traps are present remains unexplored. Similarly, little is known about how variation in dispersal ability or perceptual range might influence the probability that animals encounter traps.
We only assessed here whether the variables likely to affect the landscape-level consequences of traps were being considered. Only a relatively small number of relevant studies was identified, and many of the variables were frequently not quantified (e.g. figure 4), preventing further quantitative comparisons. Collecting quantitative data on all of these would require a great deal of both desktop-based (e.g. GIS and network analyses) and field/laboratory-based (e.g. surveys and experiments) work (table 1, column f). Current knowledge has highlighted the importance of some variables (e.g. the proportion of habitat that are traps [10]), but further studies are required to assess the likely importance of others which are currently data limited. Another key consideration will be the degree to which the common tenets of metapopulation biology, landscape ecology and population demography are directly applicable to studying traps. For example, in some instances, traps that result in only minor fitness consequences could still be important stepping stones that facilitate connectivity [10], but in other cases, increasing landscape connectivity could have poor outcomes for animals if they are more likely to encounter traps [44]. Further work is needed to begin exploring some of these complexities.
4. Conclusion
Given the unprecedented rate at which humans are altering ecosystems, ecological traps are likely to become increasingly common. To improve the growing library of ecological trap studies, it will be important to: (i) study a wider variety of ecosystems and species to develop a better generalized understanding of ecological traps and their potential effects; (ii) examine in more detail the mechanisms underlying the relationships between habitat selection and fitness; and (iii) ensure that appropriate methods are used to accurately identify traps. Greater consideration also needs to be given to the information required to assess the consequences of traps at landscape scales, which are most relevant for management. We hope these recommendations will help identify and understand ecological traps and inform the development of improved methods to manage their effects on animals.
Supplementary Material
Acknowledgements
We thank Eric Treml, Luke Barrett, Michael Sievers and two anonymous reviewers for feedback.
Data accessibility
The data supporting this article have been uploaded as the electronic supplementary material.
Author contributions
R.H. and S.E.S. conceived the study and wrote the manuscript; R.H. undertook the systematic review.
Competing interests
We have no competing interests.
Funding
We acknowledge funding from Melbourne Water, the Centre for Aquatic Pollution Identification and Management, and the Australian Research Council under Project LP140100343.
References
- 1.Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. 1997. Human domination of Earth's ecosystems. Science 277, 494–499. ( 10.1126/science.277.5325.494) [DOI] [Google Scholar]
- 2.Vorosmarty CJ, et al. 2010. Global threats to human water security and river biodiversity. Nature 467, 555–561. ( 10.1038/nature09440) [DOI] [PubMed] [Google Scholar]
- 3.Sih A. 2013. Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim. Behav. 85, 1077–1088. ( 10.1016/j.anbehav.2013.02.017) [DOI] [Google Scholar]
- 4.Wong BBM, Candolin U. 2015. Behavioral responses to changing environments. Behav. Ecol. 26, 679–680. ( 10.1093/beheco/aru183) [DOI] [Google Scholar]
- 5.Robertson BA, Rehage JS, Sih A. 2013. Ecological novelty and the emergence of evolutionary traps. Trends Ecol. Evol. 28, 552–560. ( 10.1016/j.tree.2013.04.004) [DOI] [PubMed] [Google Scholar]
- 6.Schlaepfer MA, Runge MC, Sherman PW. 2002. Ecological and evolutionary traps. Trends Ecol. Evol. 17, 474–480. ( 10.1016/S0169-5347(02)02580-6)s [DOI] [Google Scholar]
- 7.Robertson BA, Hutto RL. 2006. A framework for understanding ecological traps and an evaluation of existing evidence. Ecology 87, 1075–1085. ( 10.1890/0012-9658(2006)871075:affuet%5D2.0.co;2) [DOI] [PubMed] [Google Scholar]
- 8.Battin J. 2004. When good animals love bad habitats: ecological traps and the conservation of animal populations. Conserv. Biol. 18, 1482–1491. ( 10.1111/j.1523-1739.2004.00417.x) [DOI] [Google Scholar]
- 9.Schlaepfer MA, Sherman PW, Runge MC. 2010. Decision making, environmental change, and population persistence. In Evolutionary behavioral ecology (eds Westneat DF, Fox CW), pp. 506–515. New York, NY: Oxford University Press. [Google Scholar]
- 10.Hale R, Treml EA, Swearer SE. 2015. Evaluating the metapopulation consequences of ecological traps. Proc. R. Soc. B 282, 20142930 ( 10.1098/rspb.2014.2930) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher RJ, Orrock JL, Robertson BA. 2012. How the type of anthropogenic change alters the consequences of ecological traps. Proc. R. Soc. B 279, 2546–2552. ( 10.1098/rspb.2012.0139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson BA. 2012. Investigating targets of avian habitat management to eliminate an ecological trap. Avian Conserv. Ecol. 7, 2 ( 10.5751/ace-00533-070202) [DOI] [Google Scholar]
- 13.Hale R, Coleman R, Pettigrove V, Swearer SE. 2015. Identifying, preventing and mitigating ecological traps to improve the management of urban aquatic ecosystems. J. Appl. Ecol. 52, 928–939. ( 10.1111/1365-2664.12458) [DOI] [Google Scholar]
- 14.Sutherland WJ, Pullin AS, Dolman PM, Knight TM. 2004. The need for evidence-based conservation. Trends Ecol. Evol. 19, 305–308. ( 10.1016/j.tree.2004.03.018) [DOI] [PubMed] [Google Scholar]
- 15.Deikumah JP, McAlpine CA, Maron M. 2014. Biogeographical and taxonomic biases in tropical forest fragmentation research. Conserv. Biol. 28, 1522–1531. ( 10.1111/cobi.12348) [DOI] [PubMed] [Google Scholar]
- 16.Rotem G, Ziv Y, Giladi I, Bouskila A. 2013. Wheat fields as an ecological trap for reptiles in a semiarid agroecosystem. Biol. Conserv. 167, 349–353. ( 10.1016/j.biocon.2013.08.028) [DOI] [Google Scholar]
- 17.Hollander FA, Van Dyck H, San Martin G, Titeux N. 2011. Maladaptive habitat selection of a migratory passerine bird in a human-modified landscape. PLoS ONE 6, e25703 ( 10.1371/journal.pone.0025703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Downes BJ, Barmuta LA, Faith DP, Fairweather PG, Keough MJ, Lake PS, Mapstone BD, Quinn GP. 2002. Monitoring ecological impacts: concepts and practice in flowing waters. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 19.Bro E, Mayot P, Corda E, Reitz F. 2004. Impact of habitat management on grey partridge populations: assessing wildlife cover using a multisite BACI experiment. J. Appl. Ecol. 41, 846–857. ( 10.1111/j.0021-8901.2004.00939.x) [DOI] [Google Scholar]
- 20.Donovan TM, Thompson FR. 2001. Modelling the ecological trap hypothesis: a habitat and demographic analysis for migrant songbirds. Ecol. Appl. 11, 871–882. ( 10.1890/1051-0761(2001)0110871:mtetha%5D2.0.co;2) [DOI] [Google Scholar]
- 21.Delibes M, Gaona P, Ferreras P. 2001. Effects of an attractive sink leading into maladaptive habitat selection. Amer. Nat. 158, 277–285. ( 10.1086/321319) [DOI] [PubMed] [Google Scholar]
- 22.Lindenmayer D, et al. 2008. A checklist for ecological management of landscapes for conservation. Ecol. Lett. 11, 78–91. ( 10.1111/j.1461-0248.2007.01114.x) [DOI] [PubMed] [Google Scholar]
- 23.Kristan WB. 2003. The role of habitat selection behavior in population dynamics: source–sink systems and ecological traps. Oikos 103, 457–468. ( 10.1034/j.1600-0706.2003.12192.x) [DOI] [Google Scholar]
- 24.Kokko H, Sutherland WJ. 2001. Ecological traps in changing environments: ecological and evolutionary consequences of a behaviourally mediated Allee effect. Evol. Ecol. Res. 3, 537–551. [Google Scholar]
- 25.Urban D, Keitt T. 2001. Landscape connectivity: a graph-theoretic perspective. Ecology 82, 1205–1218. ( 10.1890/0012-9658(2001)082%5B1205:Lcagtp%5D2.0.Co;2) [DOI] [Google Scholar]
- 26.Zollner PA, Lima SL. 1997. Landscape-level perceptual abilities in white-footed mice: perceptual range and the detection of forested habitat. Oikos 80, 51–60. ( 10.2307/3546515) [DOI] [Google Scholar]
- 27.Horvath G, Bernath B, Molnar G. 1998. Dragonflies find crude oil visually more attractive than water: multiple-choice experiments on dragonfly polarotaxis. Naturwissenschaften 85, 292–297. ( 10.1007/s001140050503) [DOI] [Google Scholar]
- 28.Fletcher RJ, Robertson EP, Wilcox RC, Reichert BE, Austin JD, Kitchens WM. 2015. Affinity for natal environments by dispersers impacts reproduction and explains geographical structure of a highly mobile bird. Proc. R. Soc. B 282, 20151545 ( 10.1098/rspb.2015.1545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piper WH, Palmer MW, Banfield N, Meyer MW. 2013. Can settlement in natal-like habitat explain maladaptive habitat selection? Proc. R. Soc. B 280, 20130979 ( 10.1098/rspb.2013.0979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hedges LV, Gurevitch J, Curtis PS. 1999. The meta-analysis of response ratios in experimental ecology. Ecology 80, 1150–1156. ( 10.2307/177062) [DOI] [Google Scholar]
- 31.Lawler JJ, et al. 2006. Conservation science: a 20-year report card. Front. Ecol. Environ. 4, 473–480. ( 10.1890/1540-9295(2006)4473:csayrc%5D2.0.co;2) [DOI] [Google Scholar]
- 32.Trimble MJ, van Aarde RJ. 2012. Geographical and taxonomic biases in research on biodiversity in human-modified landscapes. Ecosphere 3, 119 ( 10.1890/es12-00299.1) [DOI] [Google Scholar]
- 33.Halpern BS, et al. 2012. An index to assess the health and benefits of the global ocean. Nature 488, 615–620. ( 10.1038/nature11397) [DOI] [PubMed] [Google Scholar]
- 34.Sol D, Lapiedra O, González-Lagos C. 2013. Behavioural adjustments for a life in the city. Anim. Behav. 85, 1101–1112. ( 10.1016/j.anbehav.2013.01.023) [DOI] [Google Scholar]
- 35.Ekroos J, Oust M, Karell P, Jaatinen K, Kilpi M. 2012. Philopatric predisposition to predation-induced ecological traps: habitat-dependent mortality of breeding eiders. Oecologia 170, 979–986. ( 10.1007/s00442-012-2378-9) [DOI] [PubMed] [Google Scholar]
- 36.Vonesh JR, Kraus JM. 2009. Pesticide alters habitat selection and aquatic community composition. Oecologia 160, 379–385. ( 10.1007/s00442-009-1301-5) [DOI] [PubMed] [Google Scholar]
- 37.Borer ET, Harpole WS, Adler PB, Lind EM, Orrock JL, Seabloom EW, Smith MD. 2014. Finding generality in ecology: a model for globally distributed experiments. Methods Ecol. Evol. 5, 65–73. ( 10.1111/2041-210x.12125) [DOI] [Google Scholar]
- 38.Lawton JH, et al. 1998. Biodiversity inventories, indicator taxa and effects of habitat modification in tropical forest. Nature 391, 72–76. ( 10.1038/34166) [DOI] [Google Scholar]
- 39.Gutzler BC, Butler MJ, Behringer DC. 2015. Casitas: a location-dependent ecological trap for juvenile Caribbean spiny lobsters, Panulirus argus. ICES J. Mar. Sci. 72(suppl. 1), i177–i184. ( 10.1093/icesjms/fsv041) [DOI] [Google Scholar]
- 40.Halfwerk W, Slabbekoorn H. 2015. Pollution going multimodal: the complex impact of the human-altered sensory environment on animal perception and performance. Biol. Lett. 11, 20141051 ( 10.1098/rsbl.2014.1051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sih A, Ferrari MCO, Harris DJ. 2011. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 4, 367–387. ( 10.1111/j.1752-4571.2010.00166.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Underwood AJ, Chapman MG, Connell SD. 2000. Observations in ecology: you can't make progress on processes without understanding the patterns. J. Exp. Mar. Biol. Ecol. 250, 97–115. ( 10.1016/S0022-0981(00)00181-7) [DOI] [PubMed] [Google Scholar]
- 43.Caley MJ, Carr MH, Hixon MA, Hughes TP, Jones GP, Menge BA. 1996. Recruitment and the local dynamics of open marine populations. Annu. Rev. Ecol. Syst. 27, 477–500. ( 10.1146/annurev.ecolsys.27.1.477) [DOI] [Google Scholar]
- 44.Sánchez-Mercado A, Ferrer-Paris JR, García-Rangel S, Yerena E, Robertson BA, Rodríguez-Clark KM. 2014. Combining threat and occurrence models to predict potential ecological traps for Andean bears in the Cordillera de Mérida, Venezuela. Anim. Conserv. 17, 388–398. ( 10.1111/acv.12106) [DOI] [Google Scholar]
- 45.Burgess SC, Treml EA, Marshall DJ. 2012. How do dispersal costs and habitat selection influence realized population connectivity? Ecology 93, 1378–1387. ( 10.1890/11-1656.1) [DOI] [PubMed] [Google Scholar]
- 46.Fletcher RJ, Revell A, Reichert BE, Kitchens WM, Dixon JD, Austin JD. 2013. Network modularity reveals critical scales for connectivity in ecology and evolution. Nat. Commun. 4, 1–7. ( 10.1038/ncomms3572) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article have been uploaded as the electronic supplementary material.