Abstract
Mechanosensation is fundamental to many tetrapod limb functions, yet it remains largely uninvestigated in the paired fins of fishes, limb homologues. Here we examine whether membranous fins may function as passive structures for touch sensation. We investigate the pectoral fins of the pictus catfish (Pimelodus pictus), a species that lives in close association with the benthic substrate and whose fins are positioned near its ventral margin. Kinematic analysis shows that the pectoral fins are held partially protracted during routine forward swimming and do not appear to generate propulsive force. Immunohistochemistry reveals that the fins are highly innervated, and we observe putative mechanoreceptors at nerve fibre endings. To test for the ability to sense mechanical perturbations, activity of fin ray nerve fibres was recorded in response to touch and bend stimulation. Both pressure and light surface brushing generated afferent nerve activity. Fin ray nerves also respond to bending of the rays. These data demonstrate for the first time that membranous fins can function as passive mechanosensors. We suggest that touch-sensitive fins may be widespread in fishes that maintain a close association with the bottom substrate.
Keywords: pectoral fin, mechanosensation, swimming, sensory physiology, neuroanatomy, catfish
1. Introduction
The paired fins of fishes, which are homologues to the limbs of tetrapods, serve diverse functions, often involving locomotion but also including defence, station holding, respiration and other roles. While fin movements have been studied extensively, little is known about the sensory roles of fins or their relations to behaviour. Past research on the mechanosensory abilities of fins has focused on a few select species and functions. The pectoral fins of dogfish (Scyliorhinus canicula) are sensitive to pressure and ventral flexion of the fin [1]. The filamentous pelvic rays of hake (Urophycis chuss) [2] and the finger-like pectoral fin rays of sea robins (Prionotus sp.) [2,3], which are used as highly mobile appendages during foraging, respond to tactile and proprioceptive (i.e. movement and position of the fin rays) stimuli. The membranous pectoral fins of more typical ray finned fishes have also been shown to function in a sensory capacity. The fin rays of bluegill sunfish (Lepomis macrochirus), a model species for the study of fin-based propulsion, respond to fin ray deflections and have putative mechanoreceptor structures associated with nerves that run along the fin rays from base to distal tip [4]. The ability of L. macrochirus to interpret the movement and position of their fins, a form of mechanosensation distinct from the sensation of surface touch, is important for navigating complex environments [5] and modulating rhythmic fin movements [6]. While a key feature of the tetrapod somatosensory system is the tactile perception of object movement over the skin, it is unknown whether membranous fins may function as passive touch sensors in the absence of extensive fin movement.
Fishes that commonly interact with the substrate (i.e. demersal or benthic fishes) provide a rich context in which to investigate the prevalence and functional significance of touch-sensitive fins. Feedback from the bottom, contacted objects or water movement could modulate locomotor, orientation and feeding behaviours. Fishes living at the sediment–water interface, including many species of catfishes, gobies and blennies, often exhibit robust and broad pectoral fins [7], which may function in part to increase surface area for sensory feedback.
Here we explore fins as passive sensory surfaces by examining the movement and touch sensation of the membranous pectoral fins of the pictus catfish (Pimelodus pictus), a bottom-dwelling species native to low-visibility regions of the Amazon River [8,9]. The leading-edge fin ray of their pectoral fin is modified into a hardened spine with serrated dentations along both edges (figure 1) [10]. Associated in some species with a poison gland, the spine can be locked into place when threatened, to prevent ingestion [11–13]. Given this morphology, the pectoral fins are presumed to serve a primarily defensive role [14,15], yet they still retain soft rays connected by a membrane behind the leading-edge spine. Pimelodus pictus provide an interesting comparison to the bluegill sunfish, whose pectoral fins are composed entirely of soft rays and act as the primary propulsive structure for low speed locomotion and midwater station holding [16–18]. In bluegills, fin ray bending associated with propulsion is accompanied by the phasic generation of action potentials by fin ray afferents [4]. The spined structure and presumed non-propulsive function of catfish pectoral fins would seem to limit the mechanical stimulation of the rays via bending during locomotion. However, given the fins' close proximity with the substrate, these fishes may be able to feel the immediately local environment in the absence of extensive ray bending.
Figure 1.

Pictus catfish (Pimelodus pictus) pectoral fin and girdle (right fin; dorsal view). At bottom, elements are outlined for clarity: a portion of the pectoral girdle (blue), fin ray afferent (green), leading-edge spine (red) and soft fin rays (yellow). Presumed to function in a primarily defensive role, the pectoral fins retain soft rays connected by a membrane behind the leading-edge spine. Prior to electrophysiology, the dermal layer and connective tissue of the pectoral girdle are removed to expose fin ray afferents (shown in green: bottom) innervating the base of the fin rays. Scale bar: 4 mm.
To address whether fins may be acting as touch sensors, we combine behaviour, neuroanatomy and physiology to examine P. pictus pectoral fin movement and response to touch. We describe positioning of the pectoral fins during swimming, and examine the neural architecture of the fin rays and membrane through antibody staining of nerve fibres and sensory endings. Additionally, we determine mechanoreceptive capabilities of the catfish pectoral fin by exerting touch and bend stimulations of the fin while recording from afferent nerve fibres. By addressing fin sensation in a different functional morphology and behavioural context from previous work, this investigation aims to increase our understanding of the diversity of pectoral fin mechanosensation. In addition, investigations of such systems will help to elucidate how mechanosensory surfaces are organized in biological systems and through the evolution of fins and limbs. In the applied realm, fins and their associated sensory morphology may provide insight into the design of engineered sensory membranes, particularly for use in aquatic environments.
2. Material and methods
(a). Animals
Pimelodus pictus were obtained commercially and housed in 40 l aquaria at the University of Chicago (Chicago, IL, USA) under seasonal day : night light cycles with a water temperature of 25°C. Fish used for immunocytology and physiological experiments were euthanized in a 0.5 g l−1 solution of MS-222 (tricaine methanesulfonate, Sigma-Aldrich, St. Louis, MO, USA) in water.
(b). Pectoral fin movement during swimming behaviour
To understand how P. pictus position their pectoral fins during free rhythmic swimming behaviours, fish (n = 5) were filmed in a 20.4 × 20.5 cm tank over a mirror angled at 45°, which allowed for the simultaneous recordings of the lateral and ventral views. Fish ranged in size from 5.0 to 7.9 cm (mean = 6.2 cm, s.d. = 1.18 cm) standard length and required several tail-beat cycles to swim the length of the experimental tank. While a fish swam freely in the tank, video was recorded at 250 frames s−1 with a Basler A504 K high-speed video system (Basler Vision Technologies, Ahrensburg, Germany) and XCAP software (Epix, Buffalo Grove, IL, USA).
For analysis, we restricted swimming trials to swimming during which the fish was moving rhythmically forward without turning and during which all parts of the fish were at least two pectoral spine lengths away from the walls of the tank. For four of the five individuals, we selected the first five bouts of swimming behaviour that met our criteria and analysed the first full tail-beat cycle from each bout. For an additional individual, behaviour consisted of a single extended swimming bout, so we analysed consecutive tail-beat cycles of that one bout. Pectoral fin and tail angles relative to the body axis were measured for each frame of the tail-beat cycle by selecting seven landmarks on the ventral view image of the fish, with a customized digitizing program in Matlab (MathWorks, Natick, MA, USA). The tip of the snout and the midpoint of the body between the pelvic fins represented the body axis. The tail axis was represented by the midpoint of the body between the pelvic fins and the fork of the caudal fin. The distal tip and insertion point of the leading-edge pectoral fin ray were marked on both the left and right pectoral fins. Angles were compared at each eighth of the tail-beat cycle, including the beginning and end of the cycle, for a total of nine time points per cycle. Lateral view videos were used to assess whether there was dorsoventral movement of the fin during swimming. This was done through visual inspection of video that was digitized.
(c). Neuroanatomy of pectoral fins
Antibody staining methods were modified from Thorsen & Hale [19] and Svoboda et al. [20]. Four fish were stained and imaged. Fins were preserved in 4% paraformaldehyde in phosphate-buffered saline (PBS), then dehydrated by stepping into 100% methanol at 4°C for long-term storage through PBS : methanol ratios of 3 : 1, 1 : 1 and 1 : 3. Before staining, fins were rehydrated by stepping up into PBS through PBS : methanol ratios of 1 : 3, 1 : 1 and 3 : 1.
To permeabilize tissues, fins were incubated in deionized water for 60 min at room temperature, then in acetone at −20°C for 7 min. After rinsing in de-ionized water, fins were incubated in collagenase for 60 min then rinsed in PBS. Fins were blocked in 10% bovine serum albumin (BSA) in PBS containing 0.1% Tween-20 (PBST).
Fins were incubated at 4°C in 10% BSA in PBST with both primary antibodies simultaneously. To visualize nerves, the primary antibody was a monoclonal antibody to a neurofilament-associated protein (3A10, Developmental Studies Hybridoma Bank, Iowa City, IA, USA). For putative mechanoreceptors, a monoclonal antibody to cytokeratin 20 was applied (ab76126, Abcam Inc., Cambridge, MA, USA). Cytokeratin 20 has been used in human skin as a marker for Merkel cells, which are specialized mechanoreceptor cells associated with afferent nerves [21]. Each primary antibody was used at a concentration of 1 : 100. After 2 days, fins were rinsed in PBST for approximately 5 h, then incubated in 10% BSA in PBST containing both secondary antibodies simultaneously. The secondary antibody to visualize nerves was goat antimouse antibody conjugated to rhodamine (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). For putative mechanoreceptors, the secondary antibody was goat antirabbit IgG (H + L) conjugated to fluorescein (A-11012, Invitrogen Molecular Probes, Eugene, OR, USA). Each secondary antibody was used at a concentration of 1 : 100. Fins were removed from secondary antibodies after 1–2 days and stored in PBS @ 4°C until they were imaged.
Pectoral fins were imaged using a Zeiss 510 confocal microscope (Thornwood, NY, USA). Images of the ventrolateral surface of the fin were captured as epithelial pigmentation on the dorsomedial surface made nerve structures difficult to visualize.
(d). Physiology of fin ray afferents in response to mechanical stimulation
After exposure to a lethal dose (0.5 g l−1) of MS-222, the pectoral fin and associated musculature of the pectoral girdle were excised from the body. To immobilize the fin for electrophysiology, pins were inserted through the muscle of the pectoral girdle to the bottom of a dish lined with Sylgard (Dow Corning, Midland, MI, USA). Fin rays were clamped down at their proximal ends using small diameter tubing to prevent additional movement. The membrane between each fin ray was cut to isolate individual rays for mechanical stimulation. To maintain responsiveness for electrophysiology, the dish was filled with extracellular solution prepared according to the methods of Masino & Fetcho [22] and refreshed periodically throughout the experiment.
Electrophysiology methods followed Williams et al. [4]. To make electrodes, borosilicate glass capillaries (GC150F-7.5 1.5 mm OD, 0.86 mm ID, Harvard Apparatus, Holliston, MA, USA) were pulled using a Flaming/Brown micropipette puller (model P97, Sutter Instrument Co., Novato, CA, USA) and fire-polished using a microforge (MF-830, Narshige, East Meadow, NY, USA). Electrode diameter ranged from 10 to 30 µm. Electrodes were filled with extracellular solution and mounted on an Axon Instruments CV-7B headstage (Molecular Devices, Foster City, CA, USA). Prior to electrode attachment, afferent nerves on the dorsomedial surface were exposed via dissection of surrounding dermal layer, muscle and connective tissue (figure 1). This orientation provided for more reliable nerve recording, as afferents were more readily accessible with the dorsal fin surface facing up. Multi-unit extracellular recordings were obtained with a Multiclamp 700B amplifier in current-clamp mode. Voltage recordings were digitized with a DigiData 1440A digitizing board (Molecular Devices) and acquired using pClamp 10 software (Molecular Devices). Video of the stimuli was recorded at 125 frames s−1 using a Basler high-speed video camera and synched with the electrical recordings using an external signal.
Pectoral fin rays were exposed to mechanical stimulation designed to examine afferent responses to ray bending, pressure applied perpendicularly to the ray and surface brushing along the fin ray surface (electronic supplementary material, figure S1). Stimuli were performed via an actuator mounted on a voice coil positioning stage (VCS10-023-BS-01-M, H2 W Technologies Inc., Valencia, CA, USA) that was controlled by a programmable driver (Intelligent Servo Drive IDM640-8EI, Technosoft, Canton, MI, USA). The Somlab software system (developed by John F. Dammann III, University of Chicago) was used to generate stimuli. To separate the effect of movement distance from velocity, we applied step-and-hold and ramp-and-hold stimuli, respectively. Both types of stimulus consisted of an initial displacement followed by a period of stasis until the probe was withdrawn. During step-and-hold stimuli, the velocity was held constant while the displacement distance varied. Conversely, for ramp stimuli we used five different velocities while the final displacement distance was held constant. The hold period allowed for the examination of afferent activity while the fin rays were held in a fixed deflected position. Multi-unit recordings were taken from the right pectoral fin of five individuals ranging in size from 7.2 to 8.8 cm (mean = 7.56 cm, s.d. = 0.69 cm) standard length, with three to four repetitions of each stimulus type.
Afferent responses to pressure were assessed using the blunt head of a pin (1.4 mm diameter) attached to a force transducer (MLT1030/A; ADInstruments, Colorado Springs, CO, USA). Once positioned perpendicular to the dorsal fin ray surface, we applied a randomly presented series of step-and-hold (0.12, 0.3, 0.6, 1.2, 1.8 mm) and ramp-and-hold (1.2, 2.4, 12, 24, 48 mm s−1; final amplitude of 1.2 mm) stimuli. Data from the force transducer indicate that these indentations imparted on average, a force equivalent to approximately 0.5, 1.5, 3, 6 and 9 g onto the fin ray, respectively. To prevent fin ray damage the hold period was limited to 3 s and the bottom of dish in which these experiments occurred was lined with Sylgard, a highly compliant material. We also exposed fin rays to light surface brushing by passing the eye of a needle along the length of a ray. The probe, positioned near the middle of a fin ray, moved towards the distal tips precisely in the direction of the rays' proximodistal axis via a randomly presented series of extensions at different distances (0.6, 1.2, 2.4, 3.6, 4.8 mm at 2 mm s–1) and velocities (0.6, 1.2, 2.4, 3.6, 4.8 mm s−1; final brushing distance held constant at 2.4 mm). In an effort to examine afferent responses to fin ray bending individual fin rays were connected to the actuator and exposed to a randomly presented series of step-and-hold (1.2, 1.8, 2.4, 3.0 and 3.6 mm) and ramp-and-hold (0.8, 1.2, 2.4, 4.8, 9.6 mm s−1; final displacement fixed at 2.4 mm) bending stimuli. The hold period during fin ray bending trials was 5 s.
(e). Electrophysiology analysis
We used Matlab 7.10.0 (Mathworks) to down-sample (100–10 kHz), filter (second-order elliptical filter) and analyse the physiological data. We performed analysis of variance (ANOVA) and paired t-tests on the full datasets. Linear regressions were performed on data from each individual due to variability among fish. Statistical analyses of the mechanical stimulation data were performed using JMP software (SAS, Cary, NC, USA). Spike rate was calculated as the number of action potentials observed divided by the duration of the relevant observation period in seconds. We defined a burst of action potentials as three or more spikes occurring within 50 ms of each other. The first action potential recorded after the start of a stimulus was considered the beginning of a burst. For the purposes of our statistical analyses, trials that did not record a burst of action potential were coded as 0 for burst duration(s) and activity rate during the burst.
Spike rate analysis during the hold interval varied across trials. The hold period during pressure trials was limited to a 1.5 s portion of the 3 s hold interval. This 1.5 s period began 1 s after the cessation of movement, to prevent inclusion of activity in response to the indentation. This portion of the hold interval was compared with a 0.5 s prestimulus baseline. During fin ray bending trials, the hold period was limited to a 3 s portion of the 5 s hold interval. The 3 s portion of the hold period began 1 s after the step onset and was compared to the prestimulus baseline.
To identify and sort individual units from our extracellular recordings, we used a modified version of the spike sorting software, Wave clus [23]. Wave clus provides an automatic method of spike sorting based on wavelet decomposition and superparamagnetic clustering (SPC). To detect spikes, we set a 1 ms absolute refractory period and used an amplitude threshold five times the standard deviation of the background noise. The minimum cluster size was set to 20 units.
3. Results
(a). Pectoral fin movement during swimming behaviour
Kinematic analysis focused on steady forward swimming with the goal of determining whether the pectoral fins were actuated rhythmically during this behaviour. From simultaneous lateral and ventral views, we observed that pectoral fins were positioned near the ventral surface of the animal, with the distal tip of the fins angled laterocaudally and ventral to the fin base. We found that typical slow swimming included tail-beat frequencies of 4.83 ± 1.25 Hz (mean ± s.d.) and axial oscillations with peak tail angles of 16° ± 9°. In this axial locomotor context, the pectoral fins remained partially protracted over the course of a fin-beat cycle, at a relatively constant protraction angle from the body axis of 35° ± 13° (figure 2). One-way analysis of variance (ANOVA) showed no significant difference among pectoral fin angles at each eighth of the tail-beat cycle (ipsilateral fin, F = 0.09, p > 0.9994; contralateral fin, F = 0.13, p > 0.9974). For comparison, ANOVA showed the tail angle varied significantly over the same time points (F = 40.21, p < 0.0001). At each of these time points, there were no significant differences between pectoral fin angles on opposite sides of the body (p > 0.2959). Although we focused on steady forward swimming, we also noted that when turning or encountering the wall of the tank, there was more pectoral fin movement, suggesting possible roles in braking and manoeuvring.
Figure 2.
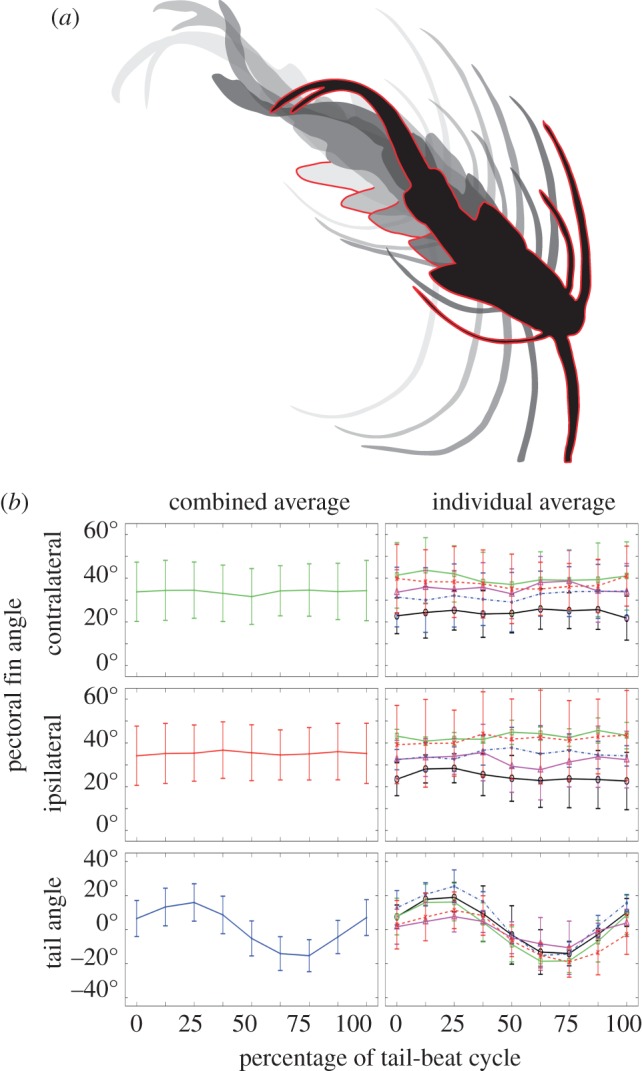
Pectoral fin, tail and body positioning of P. pictus during tail-beat cycle. (a) Ventral silhouettes taken from movie stills to illustrate the position of the tail and pectoral fins over 0% (light grey; far left), 25%, 50%, 75% and 100% (black with red body outline; far right) of the tail-beat cycle. Each right pectoral fin is outlined in red. Total length of tail-beat cycle shown was 0.114 s. (b) Mean pectoral fin and tail angles over the tail-beat cycle. Left column shows the combined mean angles of five fish over a total of 25 tail-beat cycles. Right column shows mean angles for five fish each over five tail-beat cycles, with each fish represented by a different line and symbol. While the tail shows large oscillations indicative of thrust production, the pectoral fins show no regular movement pattern over the tail-beat cycle. Error bars are 1 s.d. above and below mean.
(b). Mechanosensation of pectoral fins
The pectoral fin rays and membrane of P. pictus are heavily innervated, presumably by sensory fibres since these are non-muscular structures. Generally, nerves ran parallel to the fin rays (figure 3) and were observed from the base of the rays to near the distal tips. Individual processes branched from the nerves as they travelled distally. It is still unclear whether each process corresponds to a different neuron or if there are multiple branches associated with each cell. Structures staining with an antibody to cytokeratin 20, a mechanoreceptor marker, were also present throughout the fin. In places, these structures were closely associated with nerve endings (figure 3), often near sites of nerve branching. We attribute the lack of a visible afferent association with all the putative Merkel cell structures shown to imaging limitations due to depth of field and resolution, as well as possible incomplete antibody staining. Along the length of the leading-edge spine, branching nerve structures were observed between the tooth-like serrations.
Figure 3.
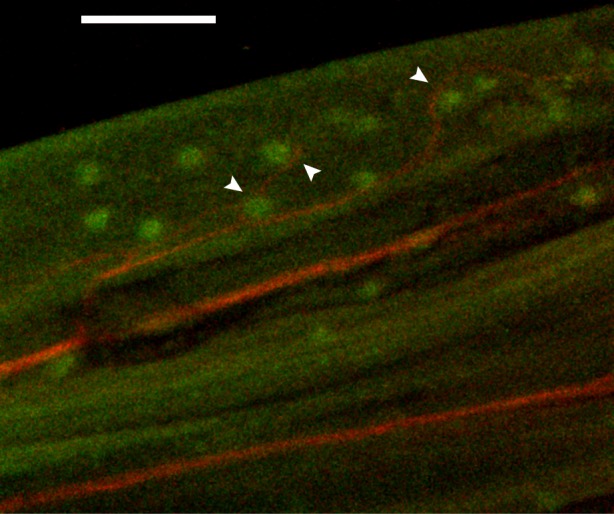
Immunostained pectoral fin rays showing nerves and associated putative mechanoreceptors. Nerves (red) run from the base of the fin to the fin rays' distal tips. Structures (green) present along the nerves stain with an antibody to cytokeratin 20, a mechanoreceptor marker. These putative mechanoreceptor cells are present throughout the fin and in places (denoted by white arrows) are closely associated with nerve endings. Scale bar: 200 µm.
(c). Afferent nerve response to mechanical stimulation of pectoral fins
Pectoral fin ray afferents of P. pictus provide sensory feedback in response to tactile stimulation even in the absence of fin ray bending. In response to localized pressure exerted perpendicular to the dorsal fin ray surface, afferents exhibited bursts of increased spike rate (one-way ANOVA, F = 15.46, p < 0.0005) at both the onset and offset of contact compared with prestimulus baseline rates. The duration of the burst of activity associated with the initial contact increased with increasing force when assessed across all individuals and steps (one-way ANOVA, F = 3.71, p < 0.0204; figure 4). While bursts of action potential were consistently recorded at the largest indentation force of 9 g, only a subset of trials recorded bursts at the lowest contact forces of 0.5 g (1 of 21 trials) and 1.5 g (6 of 21 trials). Afferent firing rate also correlated with the rate at which fin ray contact was made. We found that the spike rate (spikes s−1) during the initial application of pressure increased with increasing velocity when assessed across all individuals and velocities (one-way ANOVA F= 9.35; p < 0.0002). Overall, these data suggest that the catfish fin ray afferents are sensitive to pressure exerted perpendicular to the fin ray surface.
Figure 4.
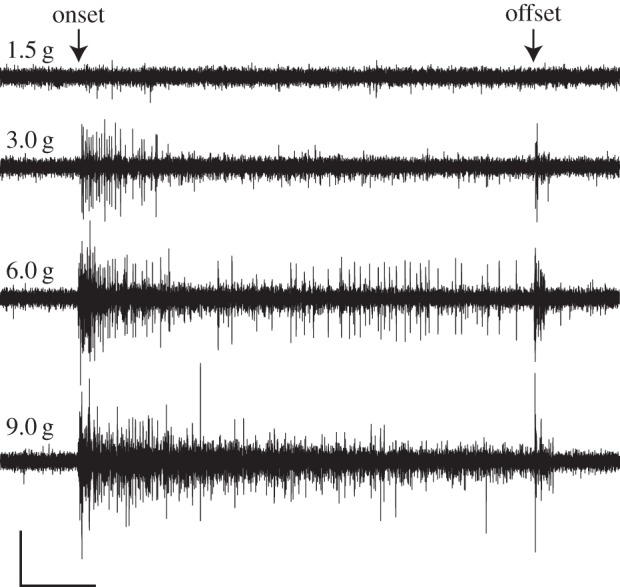
Physiological response of fin ray afferents to pressure exerted perpendicular to the dorsal fin ray surface. Responses to 1.5, 3.0, 6.0 and 9.0 g step indentations are shown. Nerve activity reflects the force of indentation. The duration of the burst of activity associated with the initial contact increased with increasing force when assessed across all individuals and forces (one-way ANOVA, F = 3.71, p < 0.0204). Scale bar: x = 1 s, y = 0.02 mV.
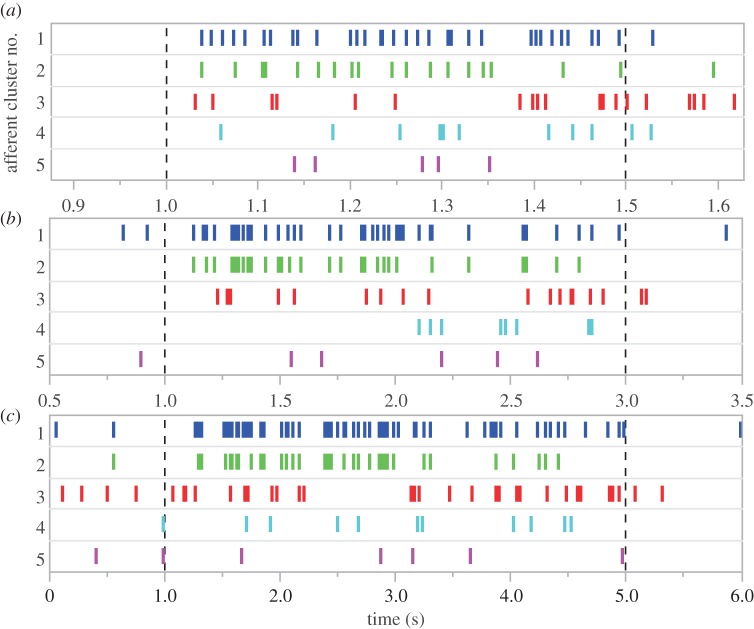
We also found that afferents exhibited a robust response to surface brushing along the proximodistal axis of the fin. During the brush stroke, we recorded an increased spike rate (Student's t-test, t = 5.69, p < 0.0039) compared with prestimulus baseline rates and an increase in the number of stimulus-evoked spikes with increasing stroke distances (one-way ANOVA F= 17.3; p < 0.0001). In four of the five individuals examined in this study, linear regressions show a significant positive correlation exists between afferent activity and brushing velocity (electronic supplementary material, table S1). Spike sorting analysis of 2.4 mm brush strokes conducted at different velocities indicate that as the probe moved along the ray several afferent units were active. We expected to observe the sequential activation of separate units as the probe encountered more distally located mechanoreceptors, but units often showed consistent activity throughout the duration of the 2.4 mm brush stroke (figure 5). As the receptive field of mammalian Merkel cells has been recorded at 2–3 mm [24], we attribute this sustained activity to the probe moving within the receptive field of several sensory endings. Information about stimulus velocity and direction might be encoded by the activation pattern of these discrete afferent units. While we found that the same units were consistently active at all velocities tested, units recorded during a single brush varied in their firing pattern, number of stimulus-evoked spikes and latency times. Despite these differences, however, the spatio-temporal discharge pattern between trials conducted at different velocities was visually similar, further supporting our hypothesis that the pectoral fins of P. pictus are capable of functioning as passive touch sensors (figure 5).
Figure 5.
Raster plots of spike sorted afferent units in response to light surface brushing. Plots show the identity and timing of spikes corresponding to particular neurons during a 2.4 mm brush stroke conducted at three different velocities: (a) 4.8 mm s−1, (b) 1.2 mm s–1 and (c) 0.6 mm s−1. Each row represented by a different colour shows spikes from a particular neuron. Dotted lines represent the onset and offset of the brushing stimulus. The spatio-temporal discharge pattern between trials conducted at different velocities is visually similar. The same afferent units are active at all velocities tested, but exhibit variation in their firing pattern, number of stimulus-evoked spikes and latency times.
Analysis of physiological responses to fin ray bending indicates that, similar to the bluegill sunfish, P. pictus receive feedback on both fin ray movement and position (electronic supplementary material, figure S2). We observed bursts of increased spike rate during step-and-hold stimuli (one-way ANOVA, F = 32.39, p < 0.001) at both the onset and offset of ray bending compared with prestimulus baseline rates. Furthermore, nerve activity reflected the amplitude of the bend, as the duration of the burst associated with the initial deflection increased with increasing deflection amplitudes when assessed across all individuals and step amplitudes (one-way ANOVA, F = 6.45, p < 0.0017). While bursts of action potential were always recorded at step onset of the largest bending amplitude of 3.6 mm, bursts were only observed in a subset of trials at the lowest bending amplitude of 1.2 mm. Afferent activity also provides feedback on fin ray position as we found that spike rate (spikes s−1) during a 3 s portion of the 5 s hold interval of step-and-hold stimuli was significantly higher than prestimulus baseline rates (Student's t-test, t = 3.65, p < 0.0064).
4. Discussion
Exploration of the kinematics and sensory neurobiology of P. pictus pectoral fins yields three conclusions: (i) pectoral fin kinematics of P. pictus during steady swimming are non-locomotor, with fins held protracted near the fish's ventral margin, conducive for use as passive sensory surfaces; (ii) as in mammalian limbs, P. pictus pectoral fins can sense pressure and object motion; and (iii) the functional capacity of the pectoral fin sensory system includes proprioception, pressure detection and the sensation of surface touch, expanding the known sensory repertoire of paired appendages in fishes.
The electrophysiological response to touch that we observed in fin ray afferents of P. pictus provides evidence of fin mechanoreception in a largely unexplored context. Bardach & Case [2] found that afferents from pectoral fins of searobins (Prionotus sp.) respond to touch, and afferents from the pelvic fins of the hake (Urophycis chuss) respond to touch and small movement of the rays. However, both of these finger-like fins are used by the fish specifically for probing the substrate and are morphologically specialized for this function, so their sensory abilities could conceivably be unique specializations among fishes. Pectoral fin ray afferents of bluegill sunfish (Lepomis macrochirus) provide proprioceptive feedback in response to relatively large fin ray deflections [4]. The results presented here provide the first evidence that touch can be sufficient to produce an afferent nerve response in a fin not specialized as a substrate probe.
As P. pictus catfishes are found in low-visibility riverine environments with steady flow [8,9], mechanosensation may be a particularly important sensory modality during routine swimming as well as at rest. The pectoral fins' lack of abduction and adduction cycles during forward swimming and ventral body positioning facilitate their potential function as passive scanning surfaces for sensory detection. Sensitivity to surface touch on the fins may provide the fish information about nearby environmental features, including conspecifics, refuges or potential food items located lateral and ventral to the body. Catfish have laterally positioned barbels that provide mechanosensory information [10,25], like the pectoral fins, in and around the plane of the fish's ventral surface (figure 2a). Pectoral fins probably complement the barbel mechanosensory system by providing information about the region between the barbels and body as the fins are swept along the substrate during swimming.
In addition to their potential for sensing solid objects, pectoral fins could supplement the lateral line system by providing information about fluid flow around the fish. Sensitivity to small deflections may help pectoral fins stabilize the body or otherwise control body positioning. Additionally, the pectoral fins' position at a distance from lateral line neuromasts on the body wall might allow detection of velocity gradients or vortices. Sensing such hydrodynamic features could aid in complex swimming behaviours. For example, trout have been shown to exploit vortices to reduce energetic costs [26], a behaviour that involves synchronizing body movements to vortex shedding rates behind an object in a flow [27,28]. Examining how barbels, fins and neuromasts function together to represent the external mechanical environment would be an interesting case study of mechanosensory integration.
We suggest that the observed sensory receptors in the fin rays are mechanosensory, since they stain with an antibody to cytokeratin 20, a specific marker for the mechanoreceptive Merkel cell in humans [21] and in rodents [29]. Although we cannot match physiological activity to a particular fibre ending, we believe we are labelling all of the afferent fibres in the rays, and based on their distribution and the putative Merkel cell structures, these nerve endings are the most likely candidates for transmitting mechanical stimulus information to the main afferent. Cytokeratin 20 is currently the most specific marker for Merkel cells [30], but other methods could help confirm the identity of the cells observed in P. pictus. While the size and shape of Merkel cells varies between mammals [31], birds [32], amphibians [33] and fish [34,35], an essential set of morphological criteria exists by which Merkel cells are generally recognized: lobulated nucleus, finger-like protoplasmic protrusions in the part of the cell opposite to the nerve terminal, and dense-core granules in close proximity to the nerve terminal. We propose that future investigations on fin mechanosensation employ electron microscopy to identify Merkel cells using these aforementioned morphological features (reviewed in [36,37]). A full map of these fins' sensory morphology including receptor cells and nerve afferents would also provide insight into the encoding of surface features more broadly.
From an evolutionary perspective, the data presented here suggest that putative Merkel cell structures may have arisen in limb structures before the evolution of sarcopterygians. While the presence of Merkel cells has been previously described in the epidermis of several fish species [34], there have been no previous accounts of these mechanosensors in the paired fins of fishes. Whether these sensory structures are primitive to fins or have been secondarily derived from epidermal origins remains unknown. Understanding the function and distribution of this fin-associated sensory morphology also has the potential to inspire membranous sensors for use in engineering contexts. For example, the integration of tactile feedback into the design of underwater robotic devices will facilitate complex tasks involving object manipulation.
Beyond the capacity of fish fins to act as passive mechanosensory surfaces, the results presented here suggest that the pectoral fin ray sensory system has evolved in response to the functional demands associated with a species's ecological niche. Differences in sensitivity to touch among fishes may reflect diversity that exists in mode of life, fin kinematics and behaviour. Given that the sense of touch allows the most direct connection with the immediately local environment, we predict that touch-sensitive fins are widespread among fishes that maintain a close association with the substrate, and may be common beyond just benthic and demersal fishes. Fishes that inhabit low-visibility or deep-sea environments, or exhibit a nocturnal mode of life, may also benefit from touch-sensitive fins. The full extent to which fishes use their pectoral fins for tactile sensation, however, is unknown. Future investigations would benefit from a comparative approach to understanding the function, neuroanatomy and sensory coding dynamics (spatial discrimination of stimuli, receptive field size, etc.) associated with touch-sensitive fins.
Supplementary Material
Acknowledgements
We thank R. Williams IV for guidance on electrophysiology methods and B. Aiello, T. Stewart and H. Katz for valuable discussion. We also thank Dr Sliman Bensmaia, Dr Michael Coates, Dr Michael LaBarbera, Dr Victoria Prince and Dr Mark Westneat for feedback on this research. Two anonymous reviewers provided constructive comments.
Ethics
All experimental, housing and euthanasia protocols were approved by the University of Chicago Institutional Animal Care and Use Committee.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.2c760.
Authors' contributions
All authors contributed to each element of this work.
Funding
This material is based upon work supported by Office of Naval Research grants (nos. N000140910352 and N000141210160) to M.E.H. on fin neuromechanics monitored by Dr Thomas McKenna. A.R.H. was supported by the National Science Foundation under IGERT grant no. DGE-0903637 and GRFP grant no. DGE-1144082. B.M.S. was supported by the Biological Sciences Collegiate Division Research Endowments at the University of Chicago.
Competing interests
We have no competing interests.
References
- 1.Lowenstein O. 1956. Pressure receptors in the fins of the dogfish, Scylliorhinus canicula. J. Exp. Biol. 33, 417–421. [Google Scholar]
- 2.Bardach JE, Case J. 1965. Sensory capabilities of the modified fins of squirrel hake (Urophycis chuss) and Searobins (Prionotus carolinus and P. evolans). Copeia 1965, 194–206. ( 10.2307/1440724) [DOI] [Google Scholar]
- 3.Silver WL, Finger TE. 1984. Electrophysiological examination of a non-olfactory, non-gustatory chemosense in the searobin, Prionotus carolinus. J. Comp. Physiol. 154, 167–174. ( 10.1007/BF00604982) [DOI] [Google Scholar]
- 4.Williams R IV, Neubarth N, Hale ME. 2013. The function of fin rays as proprioceptive sensors in fish. Nat Commun. 4, 1729 ( 10.1038/ncomms2751) [DOI] [PubMed] [Google Scholar]
- 5.Flammang BE, Lauder GV. 2013. Pectoral fins aid in navigation of a complex environment by bluegill sunfish under sensory deprivation conditions. J. Exp. Biol. 216, 3084–3089. ( 10.1242/jeb.080077) [DOI] [PubMed] [Google Scholar]
- 6.Williams R IV, Hale ME. 2015. Fin ray sensation participates in the generation of normal fin movement in the hovering behavior of the bluegill sunfish (Lepomis macrochirus). J. Exp. Biol. 218, 3435–3447. ( 10.1242/jeb.123638) [DOI] [PubMed] [Google Scholar]
- 7.Gosline WA. 1994. Function and structure in the paired fins of scorpaeniform fishes. Environ. Biol. Fishes 40, 219–226. ( 10.1007/BF00002508) [DOI] [Google Scholar]
- 8.Sioli H. 1984. The Amazon limnology and landscape ecology of a mighty tropical river and its basin. Dordrecht, The Netherlands: Dr. Junk Publisher. [Google Scholar]
- 9.Ibarra M, Stewart DJ. 1989. Longitudinal zonation of sandy beach fishes in the Napo River Basin, Eastern Ecuador. Copeia 1989, 364–381. ( 10.2307/1445433) [DOI] [Google Scholar]
- 10.Alexander RM. 1965. Structure and function in the catfish. J. Zool. 148, 88–152. ( 10.1111/j.1469-7998.1966.tb02943.x) [DOI] [Google Scholar]
- 11.Halstead BW, Kuninobu LS, Hebard HG. 1953. Catfish stings and the venom apparatus of the Mexican catfish, Galeichthys felis (Linnaeus). Trans. Am. Microsc. Soc. 72, 297–314. ( 10.2307/3223475) [DOI] [Google Scholar]
- 12.Bosher BT, Newton SH, Fine ML. 2006. The spines of the channel catfish, Ictalurus punctatus, as an anti-predator adaptation: an experimental study. Ethology 112, 188–195. ( 10.1111/j.1439-0310.2006.01146.x) [DOI] [Google Scholar]
- 13.Wright JJ. 2009. Diversity, phylogenetic distribution, and origins of venomous catfishes. BMC Evol. Biol. 9, 282 ( 10.1186/1471-2148-9-282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reed HD, Lloyd TJ. 1916. The nature of the spines in catfishes. T. Am. Fish. Soc. 45, 202–206. ( 10.1577/1548-8659(1915)45%5B202:TNOTSI%5D2.0.CO;2) [DOI] [Google Scholar]
- 15.Reed HD. 1924. The morphology and growth of the spines of siluroid fishes. J. Morphol. 38, 431–451. ( 10.1002/jmor.1050380306) [DOI] [Google Scholar]
- 16.Gibb A, Jayne B, Lauder GV. 1994. Kinematics of pectoral fin locomotion in the bluegill sunfish Lepomis Macrochirus. J. Exp. Biol. 189, 133–161. [DOI] [PubMed] [Google Scholar]
- 17.Peng J, Dabiri JO, Madden PG, Lauder GV. 2007. Non-invasive measurement of instantaneous forces during aquatic locomotion: a case study of the bluegill sunfish pectoral fin. J. Exp. Biol. 210, 685–698. ( 10.1242/jeb.02692) [DOI] [PubMed] [Google Scholar]
- 18.Jones EA, Lucey KS, Ellerby DJ. 2007. Efficiency of labriform swimming in the bluegill sunfish (Lepomis macrochirus). J. Exp. Biol. 210, 3422–3429. ( 10.1242/jeb.005744) [DOI] [PubMed] [Google Scholar]
- 19.Thorsen DH, Hale ME. 2007. Neural development of the zebrafish (Danio rerio) pectoral fin. J. Comp. Neurol. 504, 168–184. ( 10.1002/cne.21425) [DOI] [PubMed] [Google Scholar]
- 20.Svoboda KR, Linares AE, Ribera AB. 2001. Activity regulates programmed cell death of zebrafish Rohon-Beard neurons. Development 128, 3511–3520. [DOI] [PubMed] [Google Scholar]
- 21.Moll I, Kuhn C, Moll R. 1995. Cytokeratin 20 is a general marker of cutaneous merkel cells while certain neuronal proteins are absent. J. Investig. Dermatol. 104, 910–915. ( 10.1111/1523-1747.ep12606183) [DOI] [PubMed] [Google Scholar]
- 22.Masino MA, Fetcho JR. 2005. Fictive swimming motor patterns in wild type and mutant larval zebrafish. J. Neurophysiol. 93, 3177–3188. ( 10.1152/jn.01248.2004) [DOI] [PubMed] [Google Scholar]
- 23.Quiroga RQ, Nadasdy Z, Ben-Shaul Y. 2004. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural. Comput. 16, 1661–1687. ( 10.1162/089976604774201631) [DOI] [PubMed] [Google Scholar]
- 24.Johnson KO. 2001. The roles and functions of cutaneous mechanoreceptors. Curr. Opin. Neurobiol. 11, 455–461. ( 10.1016/S0959-4388(00)00234-8) [DOI] [PubMed] [Google Scholar]
- 25.Fox H. 1999. Barbels and barbel-like tentacular structures in sub-mammalian vertebrates: a review. Hydrobiologia 403, 153–193. ( 10.1023/A:1003778125517) [DOI] [Google Scholar]
- 26.Liao JC, Beal DN, Lauder GV, Triantafyllou MS. 2003. Fish exploiting vortices decrease muscle activity. Science 302, 1566–1569. ( 10.2307/3835788) [DOI] [PubMed] [Google Scholar]
- 27.Liao JC, Beal DN, Lauder GV, Triantafyllou MS. 2003. The Kármán gait: novel body kinematics of rainbow trout swimming in a vortex street. J. Exp. Biol. 206, 1059–1073. ( 10.1242/jeb.00209) [DOI] [PubMed] [Google Scholar]
- 28.Akanyeti O, Liao JC. 2013. The effect of flow speed and body size on Kármán gait kinematics in rainbow trout. J. Exp. Biol. 216, 3442–3449. ( 10.1242/jeb.087502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moll I, Paus R, Moll R. 1996. Merkel cells in mouse skin: intermediate filament pattern, localization, and hair cycle-dependent density. J. Investig. Dermatol. 106, 281–286. ( 10.1111/1523-1747.ep12340714) [DOI] [PubMed] [Google Scholar]
- 30.Boulais N, Misery L. 2007. Merkel cells. J. Am. Acad. Dermatol. 57, 147–165. ( 10.1016/j.jaad.2007.02.009) [DOI] [PubMed] [Google Scholar]
- 31.Kurosumi K, Kurosumi U, Inoue K. 1979. Morphological and morphometric studies with the electron microscope on the Merkel cells and associated nerve terminals of normal and denervated skin. Archivum Histologicum Japonicum 42, 243–261. ( 10.1679/aohc1950.42.243) [DOI] [PubMed] [Google Scholar]
- 32.Saxod R. 1978. Development of cutaneous sensory receptors birds. In Development of sensory systems (ed. Jacobson M.), pp. 337–417. Berlin, Germany: Springer. [Google Scholar]
- 33.Fox H, Whitear M. 1978. Observations on Merkel cells in amphibians. Biol. Cell. 32, 223–232. [Google Scholar]
- 34.Lane EB, Whitear DM. 1977. On the occurrence of Merkel cells in the epidermis of teleost fishes. Cell Tissue Res. 182, 235–246. ( 10.1007/BF00220592) [DOI] [PubMed] [Google Scholar]
- 35.Whitear M, Lane EB. 1981. Fine structure of Merkel cells in lampreys. Cell Tissue Res. 220, 139–151. ( 10.1007/BF00209973) [DOI] [PubMed] [Google Scholar]
- 36.Halata Z, Grim M, Bauman KI. 2003. Friedrich Sigmund Merkel and his ‘Merkel cell’, morphology, development, and physiology: Review and new results. Anat. Rec. A. Discov. Mol. Cell. Evol. Biol. 271A, 225–239. ( 10.1002/ar.a.10029) [DOI] [PubMed] [Google Scholar]
- 37.Moll I, Roessler M, Brandner JM, Eispert AC, Houdek P, Moll R. 2005. Human Merkel cells—aspects of cell biology, distribution and functions. Eur. J. Cell Biol. 84, 259–271. ( 10.1016/j.ejcb.2004.12.023) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.2c760.