Abstract
To date, the study of collective behaviour has mainly focused on intraspecific situations: the collective decision-making of mixed-species groups involving interspecific aggregation–segregation has received little attention. Here, we show that, in both conspecific and heterospecific groups, the larvae of two species (Lucilia sericata and Calliphora vomitoria, calliphorid carrion-feeding flies) were able to make a collective choice. In all groups, the choice was made within a few minutes and persisted throughout the period of the experiment. The monitoring of a focal individual within a group showed that these aggregations were governed by attractive and retentive effects of the group. Furthermore, the similarity observed between the conspecific and heterospecific groups suggested the existence of shared aggregation signals. The group size was found to have a stronger influence than the species of necrophagous larvae. These results should be viewed in relation to the well-known correlation between group size and heat generation. This study provides the first experimental examination of the dynamics of collective decision-making in mixed-species groups of invertebrates, contributing to our understanding of the cooperation–competition phenomenon in animal social groups.
Keywords: amplification, binary choice, gregariousness, marking, mixed-species group, tracking
1. Introduction
The study of collective behaviour has mainly focused on monospecific societies with high levels of sociality. For arthropods, most works concern social insects (eusocieties) [1]. As a result, species with a lower level of social organization, such as those that exhibit gregariousness, have received comparatively little attention. Nevertheless, many species, from bacteria to higher vertebrates, form groups (aggregates) that are more or less stable in time and space in response to environmental heterogeneities and due to attraction between individuals [1–4]. In insect species where gregariousness may be observed at the larval instar and/or adult level [1,5], various signals or cues (e.g. chemical, tactile, and visual) are used to find, form, or split aggregates [1–3,6]. In this context, the self-organization theory has been successfully used to explain how simple interactions (independent of the nature of these interactions) between individuals can generate a diversity of spatial patterns [6–8], collective movement [9,10], specialization [11], and collective decision-making (consensus, collective choice) [5,12–14].
Similar to monospecific groups, heterospecific groups are found in a large range of taxons, from biofilms to mammals [15] and birds [16], but only a few studies have investigated heterospecific groups particularly within the context of collective decision-making [15–18]. These mixed groups can result from different behaviours and interaction between species. For example, in a heterogeneous environment, they can simply result from similar responses to spatial heterogeneities together with an interspecific tolerance. However, more complex mechanisms are required to reach a consensus when choosing between sites of equal value or to aggregate in a homogeneous environment. Different networks of interactions can potentially lead to such heterospecific consensus where, for example, one species is gregarious while the second is not but responds to the cues of the first. Another possibility is that each species is able to reach a consensus and both are attracted to each other (or only one attracts the other). In this case, such heterospecific consensus would suggest that similar aggregation cues are used by individuals regardless of species.
To highlight collective decision-making, the binary-choice test with two equal choices has been successfully used in several studies on monospecific groups (see review in Jeanson et al. [19]). This set-up is also relevant for heterospecific groups. Indeed, comparing the results of experiments with only one species (monospecific groups) to those with mixed groups may highlight the underlying networks of interspecific interactions.
The characteristics of the interaction networks (intra- and interspecific) are linked to their adaptive values. In many conditions, heterospecific groups likely offer similar benefits to those achieved by non-mixed groups, such as protection from predators, efficient foraging behaviour, and stress reduction [1]. However, these benefits and adaptive values can also be slightly different between the associated species. In such cases, commensalism (only one of the species benefits from the association) or social mutualism (both species benefit from each other but with different benefits) are two possible ways of living together.
Most results regarding heterospecific associations in arthropod species have been reported within the context of species coexistence and competition [20], but no study has yet investigated the collective decisions of a mixed group. Aggregations of necrophagous larvae of various blowfly species have been extensively studied, particularly in the forensic entomology literature [21,22], but never under laboratory conditions. Recently, Boulay et al. [23] demonstrated that aggregations of the blowfly Lucilia sericata are active and associated with the chemical cues given. Rivers et al. [21] inventoried the potential benefits of maggot masses, such as heat generation (‘larval-mass effect’) [22] and cooperation for the liquefaction of food and assimilation (exodigestion) [24]. Regarding these benefits, aggregation behaviour facilitates cooperation and therefore permits an acceleration of larval development and an increase in larval survival. Such benefits are a potential source of the Allee effect at the level of the population or even at the multiple species population level [21,25].
This study aims to highlight the collective decision-making process of mixed-species groups of L. sericata and Calliphora vomitoria larvae, two key species in forensic entomology that often feed simultaneously on carrion [26]. These species are often observed on large symmetric cadavers, such as large mammals, which offer several identical spots for larvae to form groups on (electronic supplementary material, S1). Binary-choice tests were performed between two identical food spots for each monospecific and heterospecific group, and video tracking was used to analyse individual movement and collective dynamics.
2. Material and methods
(a). Insect rearing
Larvae (maggots) were obtained from colonies bred at the Forensic Institute of Lille (Nord, France). Adult L. sericata and C. vomitoria were reared in separate cages at an ambient temperature (20 ± 2°C) under daylight (12 light (L) : 12 dark (D)). The flies were fed caster sugar and water ad libitum. Upon the emergence of adult flies (day 0), minced beef liver was added for 7 days (i.e. until day 7) to provide the protein required for vitellogenesis. After 5 days with no food, liver was again provided for use as an oviposition medium (day 12). After 24 h (day 13), eggs of L. sericata and C. vomitoria were separately placed on the breeding substrate (100 g of fresh minced beef liver). Larvae of L. sericata were bred at 17 ± 0.5°C, and after 5–6 days (day 18–19), young third-instar larvae (10 ± 2 mm in length) were sampled for the experiments [27]. Larvae of C. vomitoria were bred at 20 ± 0.5°C, and after 11–12 days (days 24–25), young third-instar larvae (11 ± 2 mm in length) were sampled for the experiments [28]. The number of larvae used in each experiment (40) was sufficiently low to avoid autogenous heat emission (i.e. the larval-mass effect) [29].
(b). Experimental set-up
The experimental arena consisted of an 18.5 cm diameter Pyrex® glass Petri dish. The dish was filled to a height of 1 cm with agar–agar (7 g agar–agar in 250 ml of water) with two diametrically opposed food spots (5.5 cm in diameter), each situated 2 cm from the edge of the arena. These spots were designated as the west and east spots. Each spot consisted of a solution of liver (mean nutritional composition: 15.4% proteins, 7.3% carbohydrates, and 4.1% fats (National Sanitary Security Agency)) and water (6 g/50 ml) mixed with agar. This food solution attracted a sufficient number of individuals and permitted clear counts of the number of individuals within each food spot, and each spot was sufficiently large to accommodate all of the larvae used in the trial (personal observation, J. Boulay (2013)). Both food spots were strictly identical in size, composition, and distance from the edge of the arena. The arena was placed in a climatic chamber (Firlabo®) at 25 ± 1°C and monitored by video.
(c). Experimental procedure
Before each trial, the larvae were removed from the feeding substrate, placed in wet pinewood dust and isolated in the dark at 25 ± 1°C for 1 h. This isolation time starved the larvae while the sawdust removed traces of food from their cuticles [30]. Three conditions, including two conspecific conditions and one heterospecific condition, were tested: (1) 40 L. sericata larvae (N = 30; conspecific condition), (2) 40 C. vomitoria larvae (N = 30; conspecific condition), and (3) 20 L. sericata + 20 C. vomitoria larvae (N = 30; heterospecific condition).
(d). Conspecific condition and individual tracking
For each condition, 40 larvae were randomly placed in a fresh arena in a dark room due to their high photophobic behaviour [31]. Based on the locomotion abilities of the larvae (they move quite rapidly), a random dispersion was thus selected as the best option. The arena was lit from below with a fluorescent neon light (8 W; Velopex®) with a red ROSCO™ filter (ref. Roscolux #19 Fire). This filter changed the spectrum of light by transmitting predominantly red wavelengths, which are not perceived by larvae [29]. The aggregation process was video recorded over 60 min using a Bosch Dinion LTC0355 camera. The number of larvae in each of the three zones (the two spots and the area outside of the two spots) was counted visually at 1 min intervals. To be considered located outside of a spot, the individual had to have its entire body; outside of the spot.
In each trial, a single larva was tracked throughout the 60 min trial period. To facilitate tracking, the selected focal individual was 1 mm longer than the other 39 individuals. Using the equation described by Charabidzé et al. [32] to calculate the mean larval velocity, a 1 mm difference in length had negligible influence in our set-up. Videos were down-sampled every 5 s (720 frames per video), and the position of the tracked larva was determined using Avimeca 2.7 freeware. The mid-point of the larval body was used for tracking. For the individual tracking analysis, we virtually enlarged the radius of each spot based on our tracking method (mid-point of the body) and the mean size of the individuals (10 ± 2 mm). Such enlargement was set to 1 cm, corresponding to one larval body length increasing the radius from 2.25 to 3.25 cm.
(e). Heterospecific condition
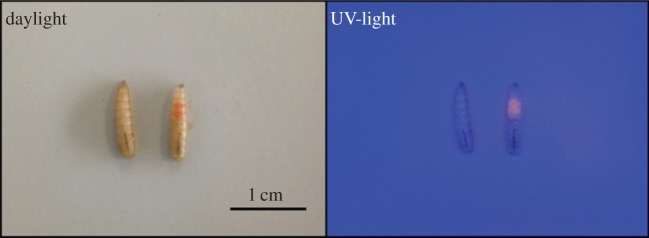
Because the larvae of the two species are very similar in appearance, we marked the individuals according to their species to distinguish them during the heterospecific experiment. One species was marked using Lumicyano™ (Crime Scene Technology©), a cyanoacrylate fluorescent glue that reacts to ultraviolet light (electronic supplementary material, S2) [33]. The marking consisted of a small spot (1 mm²; figure 1) of Lumicyano™ placed on the anterior region of the larva's dorsal surface. Previous observations showed that such marking did not affect larval movement (test of aggregation with 20 marked individuals and 20 unmarked individuals in a monospecific condition; electronic supplementary material, S3). Marking was alternated between the two species between trials. The arena was lit by two ultraviolet lights (2 × 15 W; Omnilux®) because ultraviolet wavelengths are not perceived by larvae [31]. The aggregation process was video recorded for 60 min using a 5 megapixel camera (GoPro Hero 3+™, GoPro Enterprise). No individual tracking was performed during the heterospecific experiments due to the marking method and light conditions. The UV light clouded the arena and made it difficult to precisely follow a given individual, especially when crossing others.
Figure 1.
Lucilia sericata larvae, including non-marked larvae and larvae marked using Lumicyano™. For marking, a small spot of the cyanoacrylate UV-bright glue (1 mm²) was placed on the anterior part of an individual (on the larva's crop). (Online version in colour.)
(f). Data analysis
Trials resulting in an aggregation of individuals outside of either spot (denoted ‘outside trials') were excluded from the analysis (corresponding to two trials of the L. sericata group, four trials of the C. vomitoria group, and six trials of the heterospecific condition (mean proportion of outside trials: 13 ± 7%)). In total, 28 replicates were analysed for the L. sericata group, 26 replicates were analysed for the C. vomitoria group, and 24 replicates were analysed for the heterospecific group.
To verify that our set-up was not biased for the left or right spot, we performed binomial tests. In support of the non-biased set-up hypothesis, no side-related differences were observed between the two spots (west and east) during either the conspecific or heterospecific experiments (binomial tests for L. sericata: p = 0.16; for C. vomitoria: p = 0.18; for the heterospecific condition: p = 0.17). Larvae did not choose one side of the arena more than the other one.
The spot with the largest number of individuals at the end of the experiment was designated the ‘Winner spot’, whereas the other spot was designated the ‘Loser spot’ (binomial test at t = 60 min) [34,35]. If at the end of the experiment, both spots sheltered the same number of individuals, we calculated the mean of individuals present on each spot throughout the experiment. The spot with the higher mean number of individuals was considered as the ‘Winner spot’. To verify the neutrality of the set-up, binomial tests were performed with the hypothesis H0 assuming an equal distribution of individuals between the two spots (west and east). The data were tested for deviance from normality using the Kolmogorov–Smirnov test. Parametric tests were used if data normality and homogeneity of variance were observed; otherwise, corresponding non-parametric tests were used. The statistical tests were two-tailed and performed using GraphPad InStat v. 3.06 for Windows. All of the analyses assumed a significance level of α = 0.05, unless otherwise stated. For Dunn's multiple paired comparison tests, the significance level α was adjusted using the Bonferroni method [36].
3. Results
(a). Conspecific condition
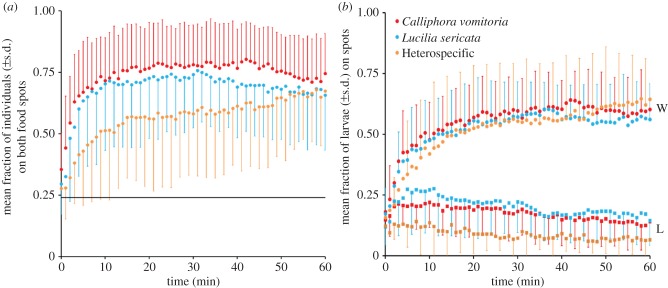
Of the 28 replicates performed with L. sericata, we observed 20 replicates in which one food spot was preferentially chosen over the other at the end of the trial (binomial tests: p = 0.01; electronic supplementary material, S4). For C. vomitoria, we observed 20 replicates out of 26 in which one food spot was preferentially chosen over the other (binomial tests: p = 0.004; electronic supplementary material, S5). On average, more than 50% of the individuals were found on the Winner spot (mean ± standard deviation (s.d.): L. sericata: 50.8 ± 18%; C. vomitoria: 57.2 ± 14%; figure 2b), whereas the Loser spot attracted less than 20% of the individuals (L. sericata: 18.9 ± 12%; C. vomitoria: 19 ± 13%; figure 2b). These percentages reflect a clear trend of collective choice for one spot (i.e. the Winner spot) by both species. Furthermore, the dynamics of these collective choices were similar between the two species. Each collective choice was made within 2 min of the start of the trial, with the majority of the individuals going to one spot (i.e. the Winner spot) (Wilcoxon tests at t = 1 min Winner spot versus Loser spot; L. sericata, W = 160, p = 0.04; C. vomitoria, W = 189, p = 0.003; figure 2b). In both conspecific conditions, these collective choices were made very rapidly and persisted throughout the trial period (figure 2b).
Figure 2.
Aggregation kinetics of conspecific and heterospecific groups. (a) Mean fraction of individuals in conspecific (C. vomitoria (red, N = 26), L. sericata (blue, N = 28)), and heterospecific groups (orange, N = 24) on both food spots over time. The horizontal line corresponds to a random distribution of individuals on the surface, which was calculated according to Canonge et al. [37]. No differences were observed at t = 0 min between a random distribution and the three observed distributions (bilateral tests: C. vomitoria: u = 1.37, p > 0.05; L. sericata: u = 0.69, p > 0.05; heterospecific group: u = 0.42, p > 0.05; ualpha = 1.96). Significant differences from a random distribution were observed 2 min after trial initiation for the L. sericata groups, 1 min after trial initiation for the C. vomitoria groups, and 4 min after trial initiation for the heterospecific groups (using bilateral tests). The two monospecific kinetics presented no differences from t = 0 min to t = 60 min (Mann–Whitney tests). Multiple comparisons among the three conditions showed no significant differences from t = 40 min (Kruskal–Wallis (KW = 5.12, p = 0.08) to t = 60 min (KW = 2.48, p = 0.29). Such comparisons showed that heterospecific groups aggregate more slowly than the two monospecific groups on both spots. (b) Aggregation dynamics of 40 larvae in conspecific (C. vomitoria, N = 26; L. sericata, N = 28) and heterospecific groups (24 replicates; 20 individuals of each species). Mean fraction of individuals 29 (±s.d.) found on the Winner spot (W; represented by circles) and the Loser spot (L; represented by squares). No differences were observed at t = 60 min between the three conditions with respect to the Winner spot (KW = 3.72, p = 0.16) or the Loser spot (Dunn's test, α = 0.017, p > 0.05). (Online version in colour.)
(b). Heterospecific condition
In the 24 replicates performed with the two species together, we observed only three replicates in which no spot was preferentially chosen over the other. Of those trials yielding a clear choice of one spot, the two species chose the same Winner spot in 100% of cases (electronic supplementary material, S6). The aggregation dynamics of the heterospecific groups were similar to those of the conspecific groups but required more time to join the plateau value of intraspecific groups (figure 2a). Indeed, at t = 0 min, no differences were observed between the three conditions (KW = 3.95, p = 0.14; figure 2a). At t = 2 min, the heterospecific groups were significantly different compared with the two monospecific groups (KW = 14.96, p < 0.001; Dunn's test, α = 0.017, p < 0.001; figure 2a). The heterospecific groups re-joined, in terms of the number of individuals, the two monospecific groups at t = 40 min (KW = 5.12, p = 0.08), and this similarity lasted until the end of experiment (t = 60 min, KW = 2.48, p = 0.29; figure 2a). These results show that the heterospecific groups aggregated more slowly on both spots than the two conspecific groups. The Winner spot contained more than 50% of the individuals (53.8 ± 26%; figure 2b), whereas the Loser spot attracted 8.1 ± 7% of the larvae. On each spot, the number of larvae was balanced between the two species (e.g. Winner spot, t = 30 min: L. sericata: 10.1 ± 5.6, C. vomitoria: 10.1 ± 5.5, t = 0.72, p < 0.001; Winner spot, t = 60 min: L. sericata: 12.0 ± 5; C. vomitoria: 11.5 ± 5.1, t = 15.2, p < 0.001; Loser spot, t = 30 min: L. sericata: 1.4 ± 1.6; C. vomitoria: 1.7 ± 1.7, t = 4.3, p < 0.001; Loser spot, t = 60 min: L. sericata: 1.1 ± 1.3; C. vomitoria: 1.9 ± 2, t = 3.7, p < 0.001; electronic supplementary material, S7). This result demonstrates that aggregation on the Winner spot was equally composed of both species over time, as determined based on the number of individuals.
(c). Individual tracking
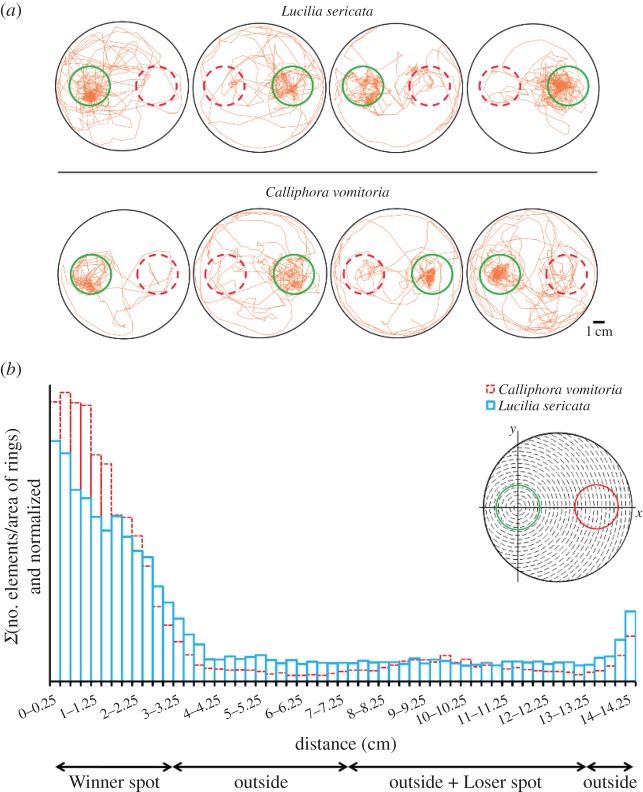
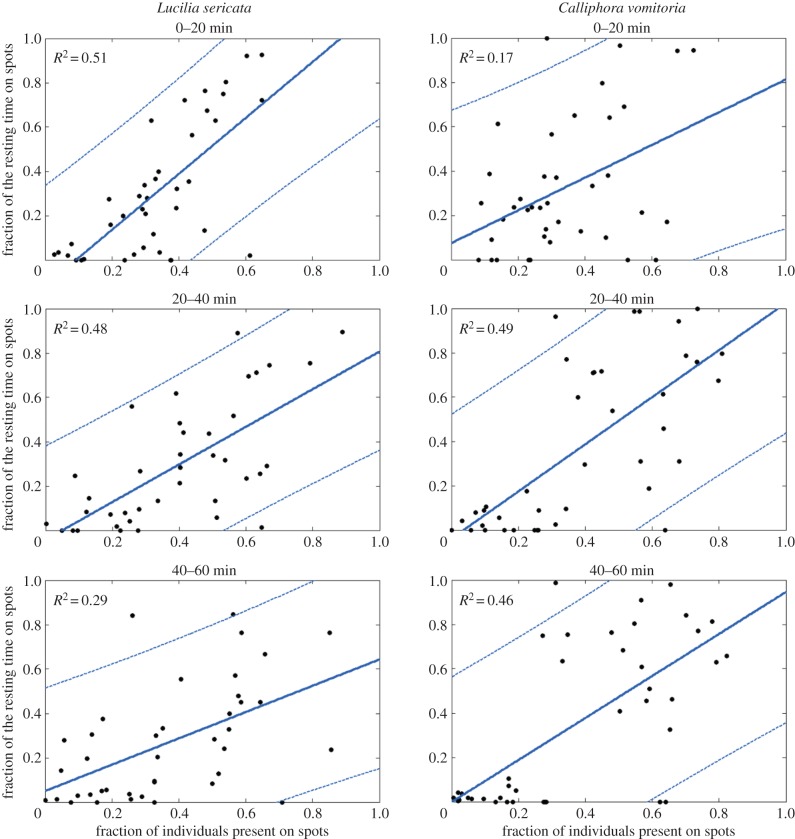
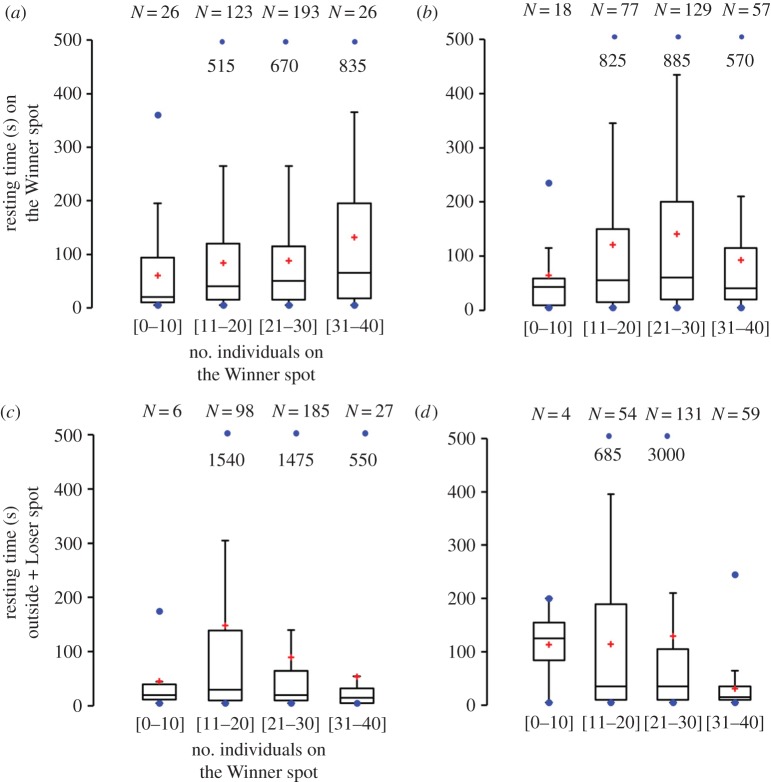
The individual tracking of one larva during each trial was useful for understanding individual behaviour and how collective choice arises from larval displacements. The individual tracks showed that the larvae did not always remain on the Winner spot throughout the trials (figure 3a). The larvae were highly mobile, moving back and forth on the Winner spot as well as exploring the Loser spot (figure 3a). This observation was confirmed by analysing the mean duration of time spent on the Winner spot: larvae remained on it for only a short period of time (L. sericata: 84.6 ± 112 s; C. vomitoria: 137.6 ± 246 s; U = 4 7308, p = 0.03). However, foraging behaviour was clearly oriented towards the Winner spot; although this spot represented only 5.9% of the total test area, the mean time spent by the larvae on the Winner spot was 1 616 ± 750 s for L. sericata and 1 953.5 ± 782 s for C. vomitoria, representing 44% and 55% of the total experiment duration, respectively (bilateral test: u = 0.63, ualpha = 1.96, p = 0.49). Conversely, the larvae also remained on the Loser spot for a short period of time (L. sericata: 46.4 ± 76 s; C. vomitoria: 84.4 ± 209 s; U = 1 1581, p = 0.2). Individuals of L. sericata spent 12% of the total experiment duration on the Loser spot (447.5 ± 351 s), and C. vomitoria individuals spent 15% of their time on this spot (552.8 ± 808 s) (u = 0.28, ualpha = 1.96, p = 0.14). Positive relationships were observed between the time spent on the spots by the tracked individuals and the number of individuals present on the spot during the experiments (figure 4). The larvae tended to stay longer on the Winner spot if the number of individuals on the spot was high, suggesting a retentive effect of the group (figure 4 and figure 5a,b). Moreover, the time spent in the two other zones of the arena (outside + Loser spot) tended to decrease as the number of larvae on the Winner spot increased (figure 5c,d). Such results suggest the existence of an attractive effect of the group.
Figure 3.
Individual behaviour of larvae. (a) Tracks of eight larvae followed for 60 min during the conspecific experiment. The green circles represent the Winner spots, and the dotted red circles represent the Loser spots (diameter: 3.25 cm). (b) Relative density of tracked larvae according to the distance (cm) from the centre of the Winner spot (conspecific experiments, N = 20 for each species). The centre of the Winner spot was used as the reference coordinate (the Winner spot is represented by green, and the Loser spot is shown in red; diameter: 3.25 cm). The sum of the number of elements (distance to the centre of the Winner spot) divided by the ring area (see the illustration) is shown along the Y-ordinate axis. (Online version in colour.)
Figure 4.
Time spent by tracked individuals on each spot according to the number of individuals present. The trial duration was divided into three time periods. For each time period, the fraction of resting time on both spots (Winner and Loser) was plotted as a function of the fraction of individuals present on the corresponding spots. All of the linear regression slopes differed significantly from zero. The dotted lines correspond to the 95% confidence intervals (CIs). (Online version in colour.)
Figure 5.
Evidence of attractive and retentive effects of the group on individual larval behaviour. Resting time (s) of tracked L. sericata larvae on the Winner spot (a) and outside + Loser spot (c) as a function of the number of individuals on the Winner spot. Resting time (s) of tracked C. vomitoria larvae on the Winner spot (b) and outside + Loser spot (d) as a function of the number of individuals on the Winner spot. For all boxplots, the first 10 min were removed to reach a plateau regarding the accumulation of individuals on the Winner spot (figure 2b). The red crosses represent the means. N represents the number of elements. No significant differences were observed in all boxplots according to the species (KW tests: (a) KW = 4.99, p = 0.17; (b) KW = 4.77, p = 0.17; (c) KW = 6.19, p = 0.19; (d) (KW = 13.73, p = 0.003, Dunn's test, p > 0.05 for all multiple paired comparisons). For trials in which aggregations were observed outside of either spot (L. sericata, N = 2; C. vomitoria, N = 4), the mean resting times of the individuals on the Winner spot were 75 ± 112 s for L. sericata and 17.9 ± 13 s for C. vomitoria. On the Loser spot, the corresponding times were 30 ± 22 s for L. sericata and 18.6 ± 10 s for C. vomitoria. The larvae spent less time on a spot when the number of individuals on the spot was low (means ± s.d. throughout the experiment: Winner spot: L. sericata: 6.8 ± 3.5; C. vomitoria: 2 ± 1.5; Loser spot: L. sericata: 3.7 ± 4; C. vomitoria: 2.4 ± 2) (for comparison, see values in the text regarding trials yielding a collective choice for one spot). (Online version in colour.)
Additionally, the probability of returning to the Winner spot was higher than the probability of returning to the Loser spot for both species (the number of transitions from Winner-to-Winner was higher than that from Loser-to-Loser for both species; electronic supplementary material, S8). Moreover, the mean time to exit one spot and return to the same spot was shorter than that found for changing spots (electronic supplementary material, S8). These results are in agreement with the observation that larvae of the two species mostly moved near the edge of the Winner spot (figure 3b) and reinforce the hypothesis of the existence of an attractive effect of the group on the larvae (figure 5).
4. Discussion
Our study highlights the importance of cooperative effects in blowfly larvae through the demonstration of active aggregation behaviour in conspecific and heterospecific groups. Given the choice between two identical spots, both species chose to rest under one of the shelters and behaved as a whole. During the conspecific experiment, the majority of the larvae gathered on one food spot, and their distribution clearly differed from a random distribution (figure 2a). The collective choice of one spot (Winner) out of two occurred rapidly, within 5 min. This larval choice of one aggregation site was a consensus or collective decision [7,11]. Typically, the mechanisms underlying such collective decisions are self-organization and the use of local communication [6]. Previous experimental and theoretical works (e.g. [5]) show that such consensus can be a by-product of competition between amplification processes. Our individual tracking results strongly suggest that the modulation of the resting time (increase of the resting time with the aggregated population on the spots) and the inter-individual attraction were the sources of the amplification processes (figures 4 and 5). In blowfly larvae, local communication likely involves ground-marking signal that is passively left by crawling individuals; this larval signal has been shown to have an attractive/retentive effect on conspecifics [23]. The chemical profile of this cuticular signal has not yet been identified, but a recent study analysing the trail-following behaviour of blowfly larvae confirmed its existence [38]. Preliminary gas chromatography results suggest that a cholesterol-derivate compound could be one of the common signals for both species (unpublished data, J.B. (2015)). According to the aggregation model presented by Amé et al. [39] or Lihoreau et al. [40], it is likely that the interplay between a random exploration of the arena and an increase in the resting time with the sheltering or the feeding population can lead to collective decision-making. Due to random asymmetry, the ground signal on one spot rapidly became stronger over time, which then created a Winner spot due to the progressive increase in the number of larvae on this spot. To support the hypothesis of the existence of this type of social amplification, simulation models similar to those that have been used to investigate the self-organized collective decision-making process should be constructed [39].
Under heterospecific conditions, larvae of the two species also aggregated together on a single (Winner) spot. This result suggests that the aggregation cues given by these two species are very similar and that at least one of the two species can detect and recognize the aggregation cues given by the other. However, a symmetric relationship in which the two species are able to recognize each other's aggregation cues is also possible. It is also interesting to observe that the dynamics of aggregation were slower for the heterospecific group than for the conspecific group (figure 2). Moreover, the mean number of individuals in the heterospecific group present outside the spots was greater than that found in the conspecific groups, whereas the mean number on the Winner spot was the same between the different groups (figure 2b). This result suggests the presence of a common retentive signal combined with a more species-specific attractive signal.
In field conditions, various calliphorid larvae species are often observed in large aggregates composed of thousands of individuals of different instars and species. This strong aggregation behaviour is associated with an emerging property observed in large aggregates that is named the larval-mass effect [22,29]. The gathering of numerous larvae in dense masses can create a local increase in temperature that can reach 20°C above ambient. This modification of the thermal environment by larval groups decreases the duration of larval dependency on carrion (their development time depends on temperature) [21]. Moreover, aggregation behaviour allows larvae to receive benefits from the local increase in enzyme concentration [21,24]. These modifications of the feeding environment by larval groups decrease the duration of larval development on carrion, which is considered one of the main benefits of gregarism in these species [21]. Accordingly, heterospecific aggregation will allow larvae to reach sufficient group size to create such emergent effects, particularly if the monospecific numbers are low. These benefits can lead to an interspecific Allee effect at the population level. We assume that the mean larval fitness decreases when the larval population is too large in front of the size of the food patch (or corpse). However, different mechanisms exist to prevent larval overcrowding: adult females control the number of eggs laid on a given cadaver, especially according to its size/carrying capacity [41,42] and larvae can break the aggregate and move to other parts of the cadaver. Such a splitting is observed under laboratory conditions when too many larvae are bred together in a box (personal observation).
Yet, in natural conditions, the heterospecific aggregation can be affected by heterogeneities of the environment and differences in the preferences among species such as the thermal preferences. According to field observation, our hypothesis is that closely related species that have different thermal preferences should segregate under extreme thermal conditions [43]. A second set of experiments should be designed to show the control of the collective choice by mixed groups when patches/spots differ in attractiveness. It is likely that for small groups, despite the individual preferences, larvae would be socially driven. Both species should rest together, but the frequency of the selected patch may depend on the ratio between species [34]. Only for strong differences between spots would the individuals or a large group with different species-specific thermal preferences be segregated [43].
The notion of gregariousness often implies cooperation and/or competition. These two behaviours are the most fundamental principles that drive the evolution of social structures. The study of mixed-species groups offers biologists an interesting approach for exploring the frontiers between cooperation and competition in animal groups. However, this phenomenon must be analysed in the broader context of population dynamics. Among many problems to be solved is the relationship between the different stages of the life cycle (e.g. adults and larvae). This is particularly evident regarding larval gregariousness and egg-laying strategy, which obviously affects the number of larvae per patch. Interestingly, aggregated oviposition, which favours the formation of locally dense populations, has been demonstrated in many insect species, including calliphorid flies and, notably, L. sericata [44,45].
Finally, our results highlight that forensic entomologists should take into account the social behaviour of Diptera larvae, particularly the group size of larval masses, when estimating the time of death (i.e. post-mortem interval calculation [26]). It also allows a discussion of new perspectives to improve breeding conditions of species living together [46]. The literature on gregarious species [45], which stresses the similarities in the mechanisms of aggregation, leads us to put forward the view that many other multispecies cases would show similar collective decision-making to that described in this article.
Supplementary Material
Acknowledgements
We thank the two anonymous reviewers for their very helpful comments. We thank F. Catteau for helping to track individuals. J.-L. Deneubourg is a Senior Research Associate from the F.R.S.-FNRS.
Data accessibility
Data from: Interspecific shared collective decision-making in two forensically important species. Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.55sh1.
Authors' contributions
J.B. conceived the study, designed the study, collected data, carried out the statistical analyses, and drafted the manuscript; D.C. conceived the study, coordinated the study, and helped draft the manuscript; V.H. gave final approval; J.L.D. carried out the statistical analyses and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
We received no funding for this study.
References
- 1.Costa JT. 2006. The other insect societies. Cambridge, MA: The Belknap Press of Harvard University Press. [Google Scholar]
- 2.Krause J, Ruxton GD. 2002. Living in groups. New York, NY: Oxford University Press. [Google Scholar]
- 3.Sumpter DJT. 2010. Collective animal behavior. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Parrish JK, Edelstein-Keshet L. 1999. Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science 284, 99–101. ( 10.1126/science.284.5411.99) [DOI] [PubMed] [Google Scholar]
- 5.Jeanson R, Deneubourg JL. 2009. Positive feedback, convergent collective patterns, and social transitions in arthropods. In Organization of insect societies: from genome to sociocomplexity (eds Gadau J, Fewell J, Wilson EO), pp. 406–482. Cambridge, MA: Harvard University Press. [Google Scholar]
- 6.Camazine S, Deneubourg JL, Franks NR, Sneyd J, Theraulaz G, Bonabeau E. 2001. Self-organization in biological systems. Princeton, NJ: Princeton University Press. [Google Scholar]
- 7.Deneubourg JL, Lioni A, Detrain C. 2002. Dynamics of aggregation and emergence of cooperation. Biol. Bull. 202, 262–267. ( 10.2307/1543477) [DOI] [PubMed] [Google Scholar]
- 8.Deneubourg JL, Goss S. 1989. Collective patterns and decision-making. Ethol. Ecol. Evol. 1, 295–311. ( 10.1080/08927014.1989.9525500) [DOI] [Google Scholar]
- 9.Bazazi S, Buhl J, Hale JJ, Anstey ML, Sword GA, Simpson SJ, Couzin ID. 2008. Collective motion and cannibalism in locust migratory bands. Curr. Biol. 18, 735–739. ( 10.1016/j.cub.2008.04.035) [DOI] [PubMed] [Google Scholar]
- 10.Buhl J, Sumpter DJT, Couzin ID, Hale JJ, Despland E, Miller ER, Simpson SJ. 2006. From disorder to order in marching locusts. Science 312, 1402–1406. ( 10.1126/science.1125142) [DOI] [PubMed] [Google Scholar]
- 11.Wright CM, Holbrook CT, Pruitt JN. 2006. Animal personality aligns task specialization and task proficiency in a spider society. Proc. Natl Acad. Sci. USA 111, 9533–9537. ( 10.1073/pnas.1400850111) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Conradt L, Roper TJ. 2005. Consensus decision making in animals. Trends Ecol. Evol. 20, 449–456. ( 10.1016/j.tree.2005.05.008) [DOI] [PubMed] [Google Scholar]
- 13.Nicolis SC, Despland E, Dussutour A. 2008. Collective decision-making and behavioral polymorphism in group living organisms. J. Theor. Biol. 254, 580–586. ( 10.1016/j.jtbi.2008.06.028) [DOI] [PubMed] [Google Scholar]
- 14.Dussutour A, Nicolis SC, Despland E, Simpson SJ. 2008. Individual differences influence collective behaviour in social caterpillars. Anim. Behav. 76, 5–16. ( 10.1016/j.anbehav.2007.12.009) [DOI] [Google Scholar]
- 15.Stensland E, Angerbjörn A, Berggren P. 2003. Mixed species groups in mammals. Mammal Rev. 33, 205–223. ( 10.1046/j.1365-2907.2003.00022.x) [DOI] [Google Scholar]
- 16.Farine DR, Aplin LM, Garroway CJ, Mann RP, Sheldon BC. 2014. Collective decision making and social interaction rules in mixed-species flocks of songbirds. Anim. Behav. 95, 173–182. ( 10.1016/j.anbehav.2014.07.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farine DR, Downing CP, Downing PA. 2014. Mixed-species associations can arise without heterospecific attraction. Behav. Ecol. 25, 574–581. ( 10.1093/beheco/aru023) [DOI] [Google Scholar]
- 18.Diamond JM. 1981. Mixed-species foraging groups. Nature 292, 408–409. ( 10.1038/292408a0) [DOI] [Google Scholar]
- 19.Jeanson R, Dussutour A, Fourcassié V. 2012. Key factors for the emergence of collective decision in invertebrates. Front. Neurosci. 6, 133–147. ( 10.3389/fnins.2012.00121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dos Reis SF, Von Zuben CJ, Godoy WAC. 1999. Larval aggregation and competition for food in experimental populations of Chrysomya putoria (Wied.) and Cochliomyia macellaria (F.) (Dipt., Calliphoridae). J. Appl. Entomol. 123, 485–489. ( 10.1046/j.1439-0418.1999.00397.x) [DOI] [Google Scholar]
- 21.Rivers DB, Thompson C, Brogan R. 2011. Physiological trade-offs of forming maggot masses by necrophagous flies on vertebrate carrion. Bull. Entomol. Res. 101, 599–611. ( 10.1017/S0007485311000241) [DOI] [PubMed] [Google Scholar]
- 22.Charabidzé D, Bourel B, Gosset D. 2011. Larval-mass effect: Characterisation of heat emission by necrophageous blowflies (Diptera: Calliphoridae) larval aggregates. Forensic Sci. Int. 1–3, 61–66. ( 10.1016/j.forsciint.2011.04.016) [DOI] [PubMed] [Google Scholar]
- 23.Boulay J, Devigne C, Gosset D, Charabidzé D. 2013. Evidence of active aggregation behaviour in Lucilia sericata larvae and possible implication of a conspecific mark. Anim. Behav. 85, 1191–1197. ( 10.1016/j.anbehav.2013.03.005) [DOI] [Google Scholar]
- 24.Wilson MR, Nigam Y, Jung W, Knight J, Pritchard DI. 2015. The impacts of larval density and protease inhibition on feeding in medicinal larvae of the greenbottle fly Lucilia sericata. Med. Vet. Entomol . ( 10.1111/mve.12138) [DOI] [PubMed] [Google Scholar]
- 25.Courchamp F, Berec L, Gascoigne J. 2008. Allee effects in ecology and conservation. New York, NY: Oxford University Press. [Google Scholar]
- 26.Amendt J, Krettek R, Zehner R. 2004. Forensic entomology. Naturwissenschaften 91, 51–65. ( 10.1007/s00114-003-0493-5) [DOI] [PubMed] [Google Scholar]
- 27.Grassberger M, Reiter C. 2002. Effect of temperature on development of the forensically important holarctic blow fly Protophormia terraenovae (Robineau-Desvoidy) (Diptera: Calliphoridae). Forensic Sci. Int. 128, 177–182. ( 10.1016/S0379-0738(02)00199-8) [DOI] [PubMed] [Google Scholar]
- 28.Ames C, Turner B. 2003. Low temperature episodes in development of blowflies: implications for postmortem interval estimation. Med. Vet. Entomol. 17, 178–186. ( 10.1046/j.1365-2915.2003.00421.x) [DOI] [PubMed] [Google Scholar]
- 29.Heaton V, Moffatt C, Simmons T. 2014. Quantifying the temperature of maggot masses and its relationship to decomposition. J. Forensic Sci. 59, 676–682. ( 10.1111/1556-4029.12396) [DOI] [PubMed] [Google Scholar]
- 30.Charabidzé D, Hédouin V, Gosset D. 2013. Discontinuous foraging behavior of necrophagous Lucilia sericata (Meigen 1826) (Diptera: Calliphoridae) larvae. J. Insect Physiol. 59, 325–331. ( 10.1016/j.jinsphys.2012.12.006) [DOI] [PubMed] [Google Scholar]
- 31.Hinnemann A, Niederegger S, Hanslik U, Heinzel HG, Spiess R. 2010. See the light: Electrophysiological characterization of the Bolwig organ's light response of Calliphora vicina 3rd instar larvae. J. Insect Physiol. 56, 1651–1658. ( 10.1016/j.jinsphys.2010.06.010) [DOI] [PubMed] [Google Scholar]
- 32.Charabidzé D, Bourel B, Leblanc H, Hédouin V, Gosset D. 2008. Effect of body length and temperature on the crawling speed of Protophormia terraenovae larvae (Robineau-Desvoidy) (Diptera: Calliphoridae). J. Insect Physiol. 54, 529–533. ( 10.1016/j.jinsphys.2007.11.010) [DOI] [PubMed] [Google Scholar]
- 33.Prete C, Galmiche L, Quenum-Possy-Berry FG, Allain C, Thiburce N, Colard T. 2013. LumicyanoTM: a new fluorescent cyanoacrylate for a one-step luminescent latent fingermark development. Forensic Sci. Int. 233, 104–112. ( 10.1016/j.forsciint.2013.07.008) [DOI] [PubMed] [Google Scholar]
- 34.Halloy J, et al. 2007. Social integration of robots into groups of cockroaches to control self-organized choices. Science 318, 1155–1158. ( 10.1126/science.1144259) [DOI] [PubMed] [Google Scholar]
- 35.Sempo G, Canonge S, Detrain C, Deneubourg JL. 2009. Complex dynamics based on a quorum: decision-making process by cockroaches in a patchy environment. Ethology 115, 1150–1161. ( 10.1111/j.1439-0310.2009.01699.x) [DOI] [Google Scholar]
- 36.Zar JH. 2010. Biostatistical analysis, 5th edn Princeton, NJ: Pearson Prentice-Hall. [Google Scholar]
- 37.Canonge S, Deneubourg JL, Sempo G. 2011. Group living enhances individual resources discrimination: the use of public information by cockroaches to assess shelter quality. PLoS ONE 6, e19748 ( 10.1371/journal.pone.0019748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boulay J, Betremieux C, Hédouin V, Charabidzé D. 2015. A first insight into the scanning behaviour of the presocial blowfly larvae. Physiol. Entomol. 40, 317–324. ( 10.1111/phen.12117) [DOI] [Google Scholar]
- 39.Amé JM, Halloy J, Rivault C, Detrain C, Deneubourg JL. 2006. Collegial decision making based on social amplification leads to optimal group formation. Proc. Natl Acad. Sci. USA 103, 5835–5840. ( 10.1073/pnas.0507877103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lihoreau M, Deneubourg JL, Rivault C. 2010. Collective foraging decision in a gregarious insect. Behav. Ecol. Sociobiol. 64, 1577–1587. ( 10.1007/s00265-010-0971-7) [DOI] [Google Scholar]
- 41.Ives AR. 1989. The optimal clutch size when many females oviposit per patch. Am. Nat. 133, 671–687. [Google Scholar]
- 42.Ives AR. 1991. Aggregation and coexistence in a carrion fly community. Ecol. Monogr. 61, 75–94. ( 10.2307/1943000) [DOI] [Google Scholar]
- 43.Villet MH, Richards CS, Midgley JM. 2010. Contemporary precision, bias and accuracy of minimum post-mortem intervals estimated using development of carrion-feeding insects. In Current concepts in forensic entomology (eds Amendt J, Goff ML, Campobasso CP, Grassberger M), pp. 109–137. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 44.Brodie BS, Wong WHL, VanLaerhoven S, Gries G. 2015. Is aggregated oviposition by the blow flies Lucilia sericata and Phormia regina (Diptera: Calliphoridae) really pheromone-mediated? Insect Sci. 22, 651–660. ( 10.1111/1744-7917.12160) [DOI] [PubMed] [Google Scholar]
- 45.Wertheim B, van Baalen EJA, Dicke M, Vet LEM. 2005. Pheromone-mediated aggregation in nonsocial arthropods: an evolutionary ecological perspective. Annu. Rev. Entomol. 50, 321–346. ( 10.1146/annurev.ento.49.061802.123329) [DOI] [PubMed] [Google Scholar]
- 46.Anderson DM, Fredrickson EL, Estell RE. 2012. Managing livestock using animal behavior: mixed-species stocking and flerds. Animal 6, 1339–1349. ( 10.1017/S175173111200016X) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from: Interspecific shared collective decision-making in two forensically important species. Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.55sh1.